Summary
Paediatric asthma exacerbations are often caused by rhinovirus (RV). Moreover, 25(OH)‐vitamin D3 (VitD3) deficiency during infancy was found associated with asthma. Here, we investigated the innate immune responses to RV and their possible modulation by 25(OH)‐VitD3 serum levels in a preschool cohort of children with and without asthma. The innate lymphoid cell type 2 (ILC2)‐associated marker, ST2, was found up‐regulated in the blood cells of asthmatic children with low serum levels of 25(OH)‐VitD3 in the absence of RV in their airways. Furthermore, in blood cells from control and asthmatic children with RV in their airways, soluble (s) ST2 (sST2) protein was found reduced. Asthmatic children with low 25(OH)‐VitD3 in serum and with RV in vivo in their airways at the time of the analysis had the lowest sST2 protein levels in the peripheral blood compared to control children without RV and high levels of 25(OH)‐VitD3. Amphiregulin (AREG), another ILC2‐associated marker, was found induced in the control children with RV in their airways and low serum levels of 25(OH)‐VitD3. In conclusion, the anti‐inflammatory soluble form of ST2, also known as sST2, in serum correlated directly with interleukin (IL)‐33 in the airways of asthmatic children. Furthermore, RV colonization in the airways and low serum levels of 25(OH)‐VitD3 were found to be associated with down‐regulation of sST2 in serum in paediatric asthma. These data indicate a counter‐regulatory role of 25(OH)‐VitD3 on RV‐induced down‐regulation of serum sST2 in paediatric asthma, which is relevant for the therapy of this disease.
Keywords: amphiregulin (AREG), ILC2, paediatric asthma, rhinovirus, vitamin D3
Introduction
Allergic asthma is an increasingly common chronic inflammatory disease of the airways that affects millions of people worldwide 1.
While the classical model of allergy‐induced airway inflammation focuses on a T helper type 2 (Th2)‐driven immune reaction 2, 3, recent studies have noted the role of group 2 innate lymphoid cells (ILC2s) in models of allergic asthma 4, 5, 6. This newly discovered cell population is able to react directly and immediately to an allergen‐induced epithelial cell stimulation without the need for antigen presentation 6. Once stimulated, ILC2s react with the secretion of interleukin (IL)‐5, IL‐9 and IL‐13, known classically as Th2 cytokines 5, 7. In addition, ILC2s release amphiregulin, a protein belonging to the epidermal growth factor family that has the ability to repair damaged epithelial cells, e.g. after an asthma attack and to promote the growth of normal epithelial cells 8. Amphiregulin B (AREGB), the gene that encodes amphiregulin, is a known target gene of 25(OH)‐vitamin D3 (VitD3) 9.
As little is known about how ILC2s can be influenced exogenously, in this study we analysed ILC2‐associated genes and their regulating factors. During an allergen and rhinovirus (RV)‐induced asthma exacerbation in vivo, IL‐33 and IL‐25 (IL‐17E) 10 are released from the infected necrotic airway epithelial cells, leading to the activation of IL‐33‐responsive IL‐33R/ST2+ T cells in general, and specifically to the activation of ILC2s 11, which are key effector cells releasing Th2 cytokines such as IL‐13 and IL‐5, thus linking viral infection and the exacerbation of asthma 12, 13, 14. ST2 is encoded by IL‐1RL1, and after differential gene splicing it can produce a functional membrane‐bound receptor (IL‐33R or ST2L) or soluble decoy receptor (sST2), depending on the splice variant 15. Moreover, it has been demonstrated recently that 25(OH)‐VitD3 enhances production of the soluble ST2 isoform 16. Because soluble sST2 neutralizes the effect of IL‐33, sST2 is considered an anti‐inflammatory factor in asthma 17, 18.
Thus, IL‐33 neutralization may be a novel therapeutic approach for asthma exacerbations 12.
VitD3, also named cholecalciferol, is a type of VitD found in food and is used as a dietary supplement. Cholecalciferol is synthesized in the skin from 7‐dehydrocholesterol under the action of ultraviolet (UV)‐B light. Blood levels of this molecule largely reflect the amount of cholecalciferol produced in the skin combined with any VitD2 or D3 ingested. The hydroxylation of VitD3 in the endoplasmic reticulum of liver hepatocytes to calcifediol (25‐hydroxycholecalciferol) occurs by means of a 25‐hydroxylase. Hydroxylation in the kidneys of calcifediol to calcitriol is induced by a 1‐α‐hydroxylase (CYP27B1), resulting in the production of the biologically active form 1,25‐dihydroxyvitamin D3. As 1,25(OH)‐VitD3 is unstable, the detection of 25(OH)‐VitD3 is one of the highly discussed therapeutic compounds for allergic asthma 19. It is known that 25(OH)‐VitD3‐deficient patients show more asthma exacerbations, longer‐lasting disease 20 and increased corticosteroid use 21. Moreover, recent studies have demonstrated that 25(OH)‐VitD3 supplementation can reduce airway inflammation 22 and inflammatory cell proliferation 23.
To analyse the reciprocal influence of genes associated with ILC2s in allergy development and the anti‐inflammatory effect of 25(OH)‐VitD3, we focused on the influence of 25(OH)‐VitD3 in asthma with a special interest in ST2 12, using human blood samples and looking at the immunomodulatory role of serum levels of 25(OH)‐VitD3 in asthma, as well as RV‐associated asthma, in a paediatric cohort of children.
Materials and methods
Human studies
The present study is part of the prospective study PreDicta (post‐infectious immune reprogramming and its association with persistence and chronicity of respiratory allergic diseases). This study was performed on the WP1‐cohort set up at the Department of Molecular Pneumology in Erlangen in collaboration with the local Children's Hospital. The study in Erlangen was approved by the Ethics Committee of the Universitäts‐klinikum Friedrich‐Alexander Universität Erlangen‐Nürnberg (Re‐no 4435), and is registered in the German Clinical Trials Register (http://www.germanctr.de: DRKS00004914). Parents/guardians of all participants gave their informed consent. All methods in this study were performed in accordance with the relevant guidelines and regulations
Two cohorts of preschool children (aged 4–6 years) with and without asthma were analysed. The recruitment of the subjects, inclusion and exclusion criteria, as well as the time‐scale for clinical visits and data collection, have been described recently 24, 25, 26, and patient characteristics are reported in Table 1.
Table 1.
Clinical data of children at baseline visit.
| Control | Age (years) | Gender | Skinprick test | FEV1% | VitD3 supplement* | Months breastfed |
|---|---|---|---|---|---|---|
| Mean | 4·73 |
Female = 41% Male = 59% |
neg = 90% pos = 10% |
106·9 |
Yes = 36·4% No = 63·6% |
5·84 |
| s.e.m. | 0·18 | 2·67 | 0·88 |
| Asthma | Age (years) | Gender | Asthma severity† | Phenotype‡ | Skin prick test | FEV1% | Treatment | Vitamin D3 supplement* | Months breastfed |
|---|---|---|---|---|---|---|---|---|---|
| Mean | 4·92 |
f = 37·5% m = 62·5% |
I = 62·5% II = 29·2% III = 8·3% |
v+m = 70% u = 16·7% a = 4·2% e = 8·3% |
neg = 15% pos = 85% |
102·75 |
Non‐steroid = 20·9% Steroid = 79·1% |
Yes = 20·8% No = 79·2% |
8·56 |
| s.e.m. | 0·15 | 4·04 | 1·69 |
*Vitamin D3 (VitD3) supplement: Vigantolette, Fluorette. †I = intermittent: forced expiratory volume in 1 s (FEV1) > 80%; MEF > 65%; symptom‐free interval > 2 months; II = mild persistent: FEV1 > 80%; MEF > 65%; symptom‐free interval < 2 months; III = moderate persistent: FEV1 < 80%; MEF < 65%; symptoms several days a week; IV = severe persistent: FEV1 < 60%; symptoms during the day and night; ‡v = virus‐induced; a = allergen‐induced; e = exercise‐induced; u = unresolved; m = mixed (including virus‐induced).
Serum 25(OH)‐VitD3 analysis
At baseline, 25(OH)‐VitD3 was measured in serum during visits to the Department of Paediatrics and Adolescent Medicine of the Friedrich‐Alexander Universität Erlangen‐Nürnberg (Erlangen, Germany) using a liquid chromatography‐tandem mass spectrometry (LC‐MS/MS) MassChrom Kit from Chromsystems Instruments and Chemicals GmbH (Gräfelfing, Germany), according to the manufacturer's protocol.
Isolation of peripheral blood mononuclear cells (PBMCs) from children with and without asthma and RV infection of PBMCs
PBMCs were isolated from heparinized blood with Ficoll using density centrifugation at the time of recruitment (baseline 0) (B0 = day 0) and 2 years later at a follow‐up visit 4 (F4). PBMCs were then adjusted to a concentration of 1 × 106 viable cells/ml in complete culture medium, as described previously, and parts of them were challenged in‐vitro or not with RV (RV1b) and were cultured for an additional 48 h to perform RNA gene array assays and further analyses. The growth of RV1b and the description of the RV1b infection itself have been published previously in detail elsewhere 27.
Gene arrays
To analyse RNA from PBMCs at F4, we performed gene arrays. RNA was reverse‐transcribed after passing high stringency quality controls. Biotinylated cDNA was prepared according to the standard Illumina protocol from 13·9 ng total RNA using an amplification kit Pico WTA (NuGen Technologies, Inc., Leek, the Netherlands; lot no. 1404311‐A/C), followed by labelling 3 µg cDNA using an Encore Biotin IL module kit (NuGen; lot no. 1212262‐D). Bead chips were scanned using the Illumina iScan scanner (Eindhoven, the Netherlands) running Illumina iSCAN control software version 3·3·28. Illumina Genome‐Studio version 2011 was used to analyse the data. Heatmaps were generated by performing supervised clustering on normalized expression data using r version 3·1 and library gplots. In the gene array cultured experiments, cells from five control and seven asthmatic children who were exposed or not to RV1b for 1 h were analysed. Genes related to ILC2 were selected, and the results were reported as log2 ratio (average signal treated/average signal untreated samples) of statistically significantly regulated genes, P ≤ 0·01.
RV detection in vivo
The detection of RV in nasopharyngeal swabs from the children was performed at the Department of Virology, University of Turku (Finland). The description of this method was recently published in detail elsewhere 27.
Enzyme‐linked immunosorbent assay (ELISA)
An ELISA kit from Peprotech (London, UK) was used to detect human interferon (IFN)‐β. ELISA from R&D Systems (Minneapolis, MN, USA) was used to detect IL‐33 and sST2 (Duo Set; R&D Systems) in human serum. Human tumour necrosis factor (TNF)‐α was performed with Duo Set no. DY210 from R&D Systems. ELISAs were performed according to the manufacturer's protocol.
Human RNA isolation from total blood cells and quantitative real‐time polymerase chain reaction (qRT–PCR)
At the baseline visit, whole blood was collected in Tempus® Blood RNA Tubes (Life Technologies™ GmbH, Darmstadt, Germany), and RNA was extracted with the MagMax (ThermoFisher Scientific, Fremont, CA, USA) for the stabilized blood tubes RNA isolation kit. Synthesis of cDNA and, consequently, RT–PCR were performed as described for the murine cells below, with the following primers and sequences: human hypoxanthine‐guanine phosphoribosyltransferase (hHPRT) (5′‐TGA CAC TGG CAA AAC AAT GCA‐3′, 5′‐GGT CCT TTT CAC CAG CAA GCT‐3′), hAREGB (5′‐GCG AAG GAC CAA TTG GAG CC‐3′, 5′‐AGC ATA ATG GCC TGA GCC G‐3′), hST2 (5′‐CAC GGT CAA GGA TGA GCA AG‐3′, 5′‐GCA GAG CAA GTT AGG TTT GAG‐3′).
Forced expiratory volume in 1 s (FEV1)
FEV1 and FVC (forced vital capacity) were measured at the baseline visit (B0) using spirometry. After a period of normal breathing the participant should inhale at maximal capacity, followed directly by maximal and fast exhalation. The volume exhaled in 1 s is FEV1. The total exhaled volume is FVC. The ratio of FEV1/FVC is reported as FEV1%.
Statistical analysis
Differences were evaluated for significance (*P ≤ 0·05; ** P ≤ 0·01, ***P ≤ 0·001) using parametric two‐tailed Student's t‐tests (Prism version 7 for Windows; GraphPad, La Jolla, CA, USA), unless stated otherwise. Data are shown as the mean values ± standard error of the mean (s.e.m.). To examine the correlation of two parameters, GraphPad Prism software (version 7 for Windows) was used.
Results
Clinical characteristics of the cohorts of children analysed in this study
In this study, we analysed 22 control children and 24 preschool children with asthma. The clinical data of these cohorts of preschool children are reported in Table 1. The average age of controls and children with asthma was 4·7 and 4·9 years, respectively. According to the guidelines, 62·5% of the children had intermittent, 29·2% mild–persistent and 8·3% moderate–persistent asthma. Moreover, 72·7% of controls and only 45·8% of the children with asthma had an FEV1 above 100% (Table 1). In 70·8% of the cases, viral infection was a triggering factor for the development of the disease. RV was the most common respiratory virus detected in the airways of these children.
Gene arrays from in‐vitro RV‐infected PBMCs from preschool children with asthma revealed the induction of ILC2‐associated genes
Innate lymphoid cells (ILCs) are detected frequently in the lung, although they originate from the bone marrow 11. Thus, we reasoned that blood‐circulating ILC2 progenitors might be present in the PBMCs of children with asthma. To investigate the relationship between circulating ILC2 and RV, we analysed RNA isolated from PBMCs from preschool children with and without asthma who were recruited in the European study Predicta in Erlangen (WP1‐UKER), and cultured the cells for 48 h after a 1‐h exposure in vitro to RV1b, as described previously 24, 25, 27. After RNA extraction and RNA quality selection for gene arrays, the mRNA from seven children with asthma and five control children were analysed for gene arrays, as shown in Fig. 1a. We then selected genes related to the ILC2 pathway that showed a statistically significant RV‐dependent up‐regulation in PBMCs from asthmatic children. As a control, to create the heatmap we inserted some related genes that were down‐regulated by the RV. We found that several genes inducing ILC2 activation, such as IL‐25 11, IL‐2RA 7 and IL‐7 7, 28, and transmembrane emp24 domain‐containing protein 1 (TMED1) (IL‐1RL‐1) were up‐regulated by RV in PBMCs of asthmatic children (Fig. 1b and Supporting information, Table S1).
Figure 1.
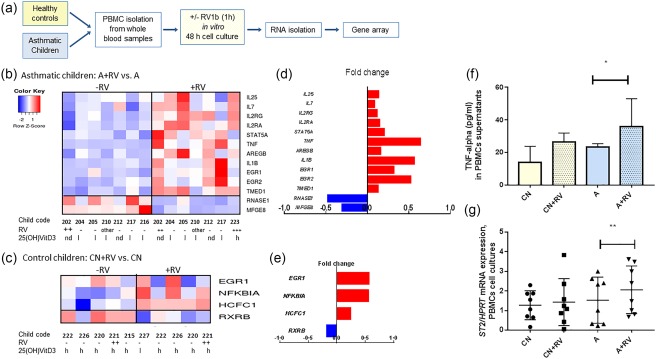
Gene arrays of RNA from in‐vitro rhinovirus (RV)‐infected peripheral blood mononuclear cells (PBMCs) of preschool children with asthma revealed the induction of innate lymphoid cell type 2 (ILC2)‐associated genes. (a) Experimental design for analysis after gene arrays of RNA from PBMCs cultured for 1 h in the presence or absence of the RV1b serotype and then cultured for an additional 48 h. Heatmaps for mRNA isolated from PBMCs of (b) asthmatic and (c) control children exposed (+RV) or not exposed (–RV) to RV. Differential expression analysis of the regulated genes is shown (log2 average +RV/average –RV); n asthma = 7, n control = 5. Horizontal numbers below the heat maps represent the code of the analysed children, the presence (+) or absence (–) of RV or other respiratory viruses (other) in the airways and the levels of serum 25(OH)‐vitamin D3 (VitD3) (n.d. = not determined, l = low, inferior of 20 ng/ml and h = higher than 20 ng/ml. The American Institute of Medicine recommends 25(OH)VitD3 > 20 ng/ml (http://www.nejm.org/doi/full/10.1056/NEJMe1312291). (d,e) On the right‐hand side of the heatmap are the selected genes (red: up‐regulated, blue: down‐regulated genes) regulated significantly by RV in asthmatic (upper graph) and in control children (lower graph). (f) Gene arrays where confirmed at protein and quantitative polymerase chain reaction (qPCR) level by tumour necrosis factor (TNF) enzyme‐linked immunosorbent assay (ELISA) (f) and ST2 at qPCR levels (g).
Moreover, we found increased expression of TNF‐α, a gene stimulated by IL‐33 29, and IL‐1B, a gene involved in the activation of IL‐33 30. Finally, we found that RV induced genes that are targets of 25(OH)‐VitD3, such as amphiregulin, a gene that interacts with EGR 9, 31, and promotes the growth of normal epithelial cells after cell damage. In contrast, lactadherin (Mfge8), which is involved in IL‐33 signalling, was found to be down‐regulated by RV in asthma 32.
In summary, the genes up‐regulated in PBMCs by RV in this paediatric cohort of asthmatic cases suggest an activation of ILC2 development and a down‐regulation of genes that cut the viral RNA, such as RNAse1.
Furthermore, we analysed which genes were regulated by RV in PBMCs isolated from control children. We found an RV‐induced up‐regulation of EGR1, a receptor for amphiregulin 31, in PBMCs. Moreover, NFKB inhibitor‐α (NFKBIA) (also known as hepatic IκBα) is increased by RV. This gene plays an important role in persistent inflammation, and its decrease is associated with 25(OH)‐VitD3 deficiency 33. Conversely, retinoid X receptor β (RXRB), which forms a complex with the VitD receptor (VDR) and would be necessary to activate VDR targets 34, is decreased in the PBMCs of control children by RV. In summary, in PBMCs isolated from control children, RV induces genes that are associated with epithelial damage and chronic inflammation. We next looked at RV in the nasal pharyngeal fluid and 25(OH)‐VitD3 in the serum of these children. As shown below the heatmap, the majority of the asthmatic children analysed had low serum levels of 25(OH)‐VitD3 and few had RV in the airways (Fig. 1b). By contrast, control children had high (h) serum levels of 25(OH)‐VitD3 and, like asthmatic children, generally did not have RV in their airways (Fig. 1c). We next confirmed the gene array results by measuring TNF‐α in the supernatants of the PBMCs using ELISA and found that PBMCs from asthmatic children released increased levels of TNF‐α after RV exposure in vitro (Fig. 1f). RT–PCR showed an up‐regulation of ST2 in PBMCs isolated from asthmatic children after in‐vitro exposure to RV (Fig. 1g).
Reduced IFN‐β levels in serum and increased ST2 mRNA levels in the blood cells of preschool children with asthma compared to control preschool children
To investigate further the role of in‐vivo RV infection on ILC2, we first looked at IFN‐β, an anti‐viral gene regulated by RV and deficient in the airways of asthmatic subjects 35, that was found recently to counter‐regulate ILC2 36. IFN‐β was found to be down‐regulated, although not statistically significantly, in the serum of asthmatic preschool children compared to control children (Fig. 2a). Consistent with the up‐regulation of the ILC2‐associated genes after RV challenge, in a larger cohort of preschool children with asthma we found induction of the ILC2‐associated marker ST2 mRNA in the blood of these preschool children with asthma compared to control children (Fig. 2b). Because ST2 is present in two different spliced variants, we used one set of primers recognizing the total transcript of ST2 (IL1RL1) (Fig. 2b) and an ELISA kit recognizing the soluble sST2 (Fig. 2c,d) protein. No difference in sST2 was observed in serum (Fig. 2c).
Figure 2.
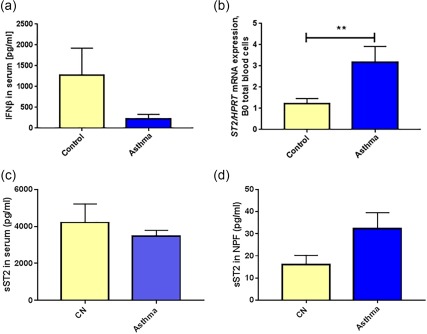
ST2 pathway is activated in preschool children with asthma. (a) Interferon (IFN)‐β protein was measured in the serum of control and asthmatic children (n = 14, B0). (b) ST2/HPRT mRNA values of asthmatic and non‐asthmatic children (control n = 14, asthma n = 16, B0). p = 0·005. (c) Soluble ST2 (sST2) protein in the serum of control and asthmatic preschool children (CN: n = 18; A: n = 12). (d) sST2 protein in the nasopharyngeal fluid in control and in asthmatic children (CN: n = 21; A: n = 24; P = 0·067, paired t‐test).
Increased sST2 protein in the nasopharyngeal fluid (NPF) of preschool children with asthma with RV in the airways
We next wanted to analyse sST2 protein at baseline in the NPF of the two cohorts of children. We found that the asthmatic children had more sST2 in the airways compared to the control children (Fig. 2d). In addition, we found that in serum there is 100 times more sST2 compared to that in the upper airways, indicating that the majority of sST2 is produced by blood cells. These results support the notion that sST2‐producing cells are circulating cells rather than cells present in the airways.
Regulation of ST2 by RV in the airways of asthmatic children
We next considered the presence of RV in the airways in the pathway analysed above. In the presence of RV in the airways in control children, IFN‐β was found to be down‐regulated in serum whereas, independently from RV, IFN‐β was always low in the serum from asthmatic children (Fig. 3a). AREG mRNA was found up‐regulated in mRNA isolated from total blood cells of control children when RV was detected in their airways (Fig. 3b). Moreover, in the absence of RV in the airways, ST2 mRNA was found up‐regulated in blood cells from asthmatic children compared to control children without RV in the airways (Fig. 3c). These data suggest an ST2 induction associated with factors other than RV.
Figure 3.
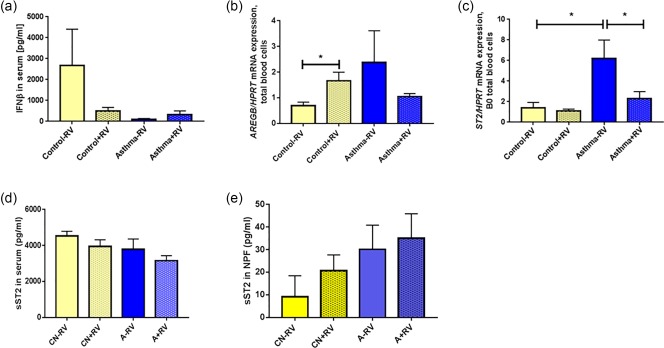
ST2 mRNA is induced in blood cells of asthmatic children without rhinovirus (RV) in the airways. (a) Interferon (IFN)‐β protein of asthmatic and non‐asthmatic children subgrouped by the presence of in‐vivo RV infection in the airways (CN‐RV n = 5, CN+RV, n = 5; A–RV n = 7, A+RV n = 3). (b) Amphiregulin B/hypoxanthine‐guanine phosphoribosyltransferase (AREGB/HPRT) mRNA values of asthmatic and non‐asthmatic children, subgrouped by in‐vivo RV infection in the airways (control –RV n = 6, control +RV n = 8, asthma –RV n = 5, asthma + RV n = 10, B0), P = 0·0326 paired t‐test. (c) ST2/HPRT mRNA values in asthmatic and non‐asthmatic children, subgrouped by the presence of in‐vivo RV infection in the airways (control –RV n = 6, control +RV n = 8, asthma –RV n = 4, asthma +RV n = 10, B0). Control –RV versus asthma –RV, P = 0·0152; asthma –RV versus asthma +RV, P = 0·0259, two‐tailed Student's t‐test. (d). Soluble ST2 in serum in accordance with the presence of RV (+RV) or not (–RV) in the airways: CN–RV n = 8, CN+RV n = 10, A–RV n = 6, A+RV n = 6. (e) Soluble ST2 (sST2) in nasopharyngeal fluid (NPF): CN–RV n = 9, CN+RV n = 11, A–RV n = 11, A+RV n = 12.
We then analysed sST2 protein levels in the serum and NPF considering that RV is present in the airways of these cohorts of preschool children. Although no statistically significant difference was found, as a trend, sST2 protein was reduced in serum and induced in NPF of asthmatic children with RV in their airways (Fig. 3d,e, respectively). These data indicate that sST2 is induced/recruited in the airways of asthmatic children by RV, indicating a role of sST2 in anti‐viral immune responses in the presence of RV in the airways in asthma.
sST2 in serum is correlated directly with IL‐33 in the nasopharyngeal fluid of asthmatic children
Because sST2 neutralizes IL‐33, which we found recently induced in the airways of asthmatic children with Gram‐negative bacteria colonization, we next asked if there was a correlation between sST2 and IL‐33 in the airways. We found that serum sST2 protein correlated directly with IL‐33 in the airways of asthmatic but not in the airways of control children (Fig. 4a,b). No significant correlations were found by comparing IL‐33 and sST2 protein in NPF (Fig. 4c,d).
Figure 4.
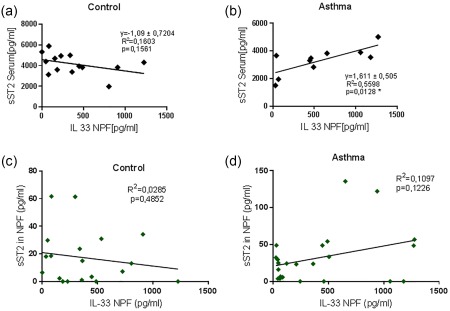
Serum soluble ST2 (sST2) correlated directly with interleukin (IL)‐33 in nasopharyngeal fluid (NPF) in asthmatic children. Linear regressions are reported for sST2 serum and IL‐33 in NPF in control (a) and asthmatic children (b). (c,d) Linear regression for sST2 in NPF and IL‐33 in NPF in controls (c) and in asthmatic children (d).
VitD3 supplementation and 25(OH)‐VitD3 in the serum of asthmatic children
We reasoned further that many genes we found activated by RV were genes that were down‐regulated by 25(OH)‐VitD3, which has a protective role in asthma exacerbations 37.
In Germany, VitD3 is given to infants 38 to avoid immunological suppression, especially in children who did not receive breastfeeding. By analysing the potential effect of this VitD3 supplementation, we analysed the questionnaire completed by the parents/guardians. Here we noted that the percentage of children treated with VitD3 supplement (Fluorette: Colecalciferol and fluorid) was higher in the control group compared to the asthma group (Table 1). We next analysed the role of cholecalciferol, VitD3, supplementation during infancy on serum 25(OH)‐VitD3 levels in our cohort of children with or without asthma. By considering 0 as no VitD3 supplement and 1 as VitD3 supplement taken as an infant, we found that, in contrast to control children (Supporting information, Fig. S1a and Table S2), in asthmatic children increased serum 25(OH)‐VitD3 correlated with having taken a VitD3 supplement during infancy (Supporting information, Fig. S1b). Seventy‐five per cent of asthmatic children and 50% of control children with VitD3 supplement had high 25(OH)‐VitD3 serum levels at the time of recruitment (Supporting information, Table S2). Taken together, these data indicate a strong association between asthma development in childhood, insufficient 25(OH)‐VitD3 in serum and the lack of VitD3 supplementation as an infant.
Symptomatic asthma exacerbations are associated with increased 25(OH)‐VitD3 in serum of asthmatic children
Wheezing is associated with asthma during the first years of life 39. Moreover, RV is the factor that is associated with wheezing in infants 40. Further, the levels of 25(OH)‐VitD3 in serum, considering the presence of RV in the airways, was found down‐regulated in the serum of control children with RV in their airways and in asthmatic children without RV in their airways (Fig. 5a). Moreover, 25(OH)‐VitD3 was found to be induced in the serum of children during asthma exacerbation when, as we have reported previously, RV was present in the airways (Fig. 5b). Altogether, these data indicate a role of the serum level of 25(OH)‐VitD3 in RV‐induced asthma development.
Figure 5.
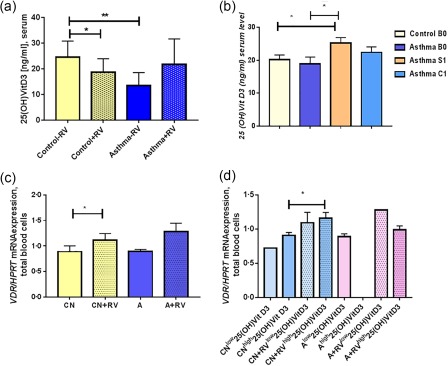
Control children with rhinovirus (RV) in the airways have lower 25(OH)‐vitamin D3 (VitD3) and higher VDR/HPRT mRNA levels while asthmatic children have increased 25(OH)‐VitD3 serum levels during symptomatic visit. (a) 25(OH)‐Vit‐D3 was measured in the sera of children who were subdivided by RV infection in the airways (control –RV n = 6, control +RV n = 12, asthma –RV n = 6, asthma +RV n = 11, B0) control –RV versus control +RV, P = 0·05; control –RV versus asthma –RV, P = 0·009. (b) 25(OH)‐VitD3 in serum: control B0 n = 19; asthma B0 n = 20; asthma S n = 16; asthma C n = 14, P = 0·0257; A–S versus CN B0, P = 0·0229; A–S versus A B0, unpaired t‐test. (c). VitD3 receptor/hypoxanthine‐guanine phosphoribosyltransferase (VDR/HPRT) mRNA expression in blood cells (CN–RV, n = 6, CN+RV n = 8, A–RV n = 2, A+RV n = 5), P = 0·014, unpaired t‐test. (d) Considering RV in the airways and serum levels of 25(OH)VitD3 (from left to the right bars: n = 1, 3 ,4, 2, 2, 0, 1, 2); P = 0·0384, unpaired t‐test.
VDR is induced by RV and serum 25(OH)‐VitD3 levels in control children
We next looked for an explanation of why 25(OH)‐VitD3 serum levels decreased in serum of control children when RV was detected in their airway. As first, we thought that the host would react to RV by transporting the 25(OH)‐VitD3 from blood fluid into blood cells. To prove this, we analysed VitD3 receptor (VDR) expression in total blood cells. We found a significant increase in VDR mRNA in control children with RV in the airways compared to control children without RV in the airways (Fig. 5c). The same trend was observed for VDR in asthmatic children, although not significant. We then questioned if VDR expression would also be influenced by serum levels of 25(OH)‐VitD3; we observed a significant induction of VDR in control children with RV and high serum levels of 25(OH)‐VitD3 (Fig. 5d). In asthmatic children we did not have the group with high 25(OH)‐VitD3 without RV, and therefore we cannot draw any conclusions. Further extended analyses are required.
IL‐33 in the airways correlated directly with 25(OH) in serum in asthma
We then wanted to investigate the role of 25(OH)‐VitD3 and the IL‐33/ST2 pathway. Here, we started by examining the correlation between 25(OH)‐VitD3 in serum and IL‐33 in the NPF. We found that IL‐33 in the NPF was correlated directly with serum 25(OH)‐VitD3 in asthmatic children (Fig. 6a,b) at baseline. This result is in agreement with our previous finding, that sST2 correlated directly with IL‐33, and with the finding that 25(OH)‐VitD3 induces sST2.
Figure 6.
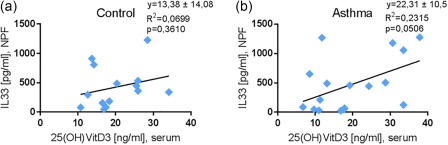
Interleukin (IL)‐33 in nasopharyngeal fluid (NPF) is correlated directly with serum 25(OH)‐vitamin D3 (25(OH)‐VitD3) in asthmatic children. (a,b) Human IL‐33 (hIL‐33) enzyme‐linked immunosorbent assay (ELISA): NPF B0, VitD3 serum B0; control n = 14; asthma n = 17; linear regression.
Low 25(OH)‐VitD3 [low25(OH)‐VitD3] levels are associated with lower IFN‐β levels in serum and high AREG in blood cells in asthmatic children
We next questioned whether IFN‐β was regulated in children with higher levels of 25(OH)‐VitD3 (Fig. 7a). We found that control children with higher serum 25(OH)‐VitD3 levels (higher than 20 ng/ml) had higher serum IFN‐β levels, although this difference was not statistically significant (Fig. 7a); asthmatic children always had lower serum levels of IFN‐β irrespective of their serum of 25(OH)‐VitD3 levels (Fig. 7a).
Figure 7.
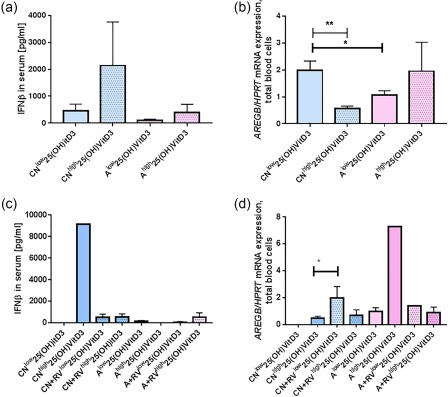
Low serum 25(OH)‐vitamin D3 (VitD3) levels are associated with reduced serum interferon (IFN)‐β and induced amphiregulin (AREG) in the blood cells of control children. (a) IFN‐β protein in serum was measured in asthmatic and non‐asthmatic children with more (highVitD3) or less (lowVitD3) than 20 (ng/ml) 25(OH)‐VitD3. (CNlow n = 5, CNhigh n = 5, Alow n = 7, Ahigh n = 3, B0). (b) AREGB/hypoxanthine‐guanine phosphoribosyltransferase (HPRT) mRNA values were also measured by quantitative polymerase chain reaction (qPCR) in the children's total blood cells (CNlow n = 6, CNhigh n = 6, Alow n = 7, Ahigh n = 6, B0). CNlow versus CNhigh, P = 0·003. CNlow versus Alow, P = 0·028, two‐tailed Student's t‐test. Considering RV in the airways and the serum level of 25(OH)‐VitD3 for (c) IFN‐β in serum from the left to the right bar: n = 0, 1, 5, 4, 4, 1, 3, 3; (d) AREG/HPRT mRNA from the left to the right bar: n = 0, 4, 6, 2, 3, 1, 3, 5, P = 0·025, paired t‐test.
As IFN‐beta limits ILC2 activation, we then analysed AREGB mRNA considering the influence of 25(OH)‐VitD3 and found that, consistent with an increase in IFN‐beta, higher serum levels of 25(OH)VitD3 were associated with the down‐regulation of AREGB mRNA in blood cells of control children (Fig. 7b). By contrast, asthmatic children did not down‐regulate AREGB when 25(OH)‐VitD3 was increased (Fig. 7b), and at low levels of 25(OH)‐VitD3 they had lower AREG compared to the control children, with lower 25(OH)‐VitD3 (Fig. 7b). We next considered the presence of RV in the airways and found that IFN‐beta induction was associated with high 25(OH)‐VitD3 serum levels in control children in the absence of RV in the airways (Fig. 7c). In control children AREG was found to be up‐regulated by RV and low serum 25(OH)‐VitD3 levels (Fig. 7d).
ST2 mRNA is induced in blood cells of asthmatic children with low 25(OH)‐VitD3 serum levels without RV
We next examined the relationship between ST2 mRNA in blood cells and 25(OH)‐VitD3 in serum. We found that asthmatic children with low serum levels of 25(OH)‐VitD3 had significantly higher levels of ST2 mRNA (Fig. 8a) compared to the control children with comparable low serum levels of 25(OH)‐VitD3. By considering the presence of RV in the airways we could confirm the findings that higher ST2 mRNA levels were not associated with the presence of RV in vivo but with lower 25(OH)‐VitD3 serum levels. Reconsidering serum 25(OH)‐VitD3 levels of the children analysed in the gene arrays, we found that the asthmatic children all had lower levels of serum 25(OH)‐VitD3 (Table 2 and number of the children below the heatmaps), whereas the control children had relatively higher serum 25(OH)‐VitD3 levels. In conclusion, we found that the level of 25(OH)‐VitD3 and not RV in the airways is probably the cause of ST2 induction after in‐vitro exposure to RV in PBMCs.
Figure 8.
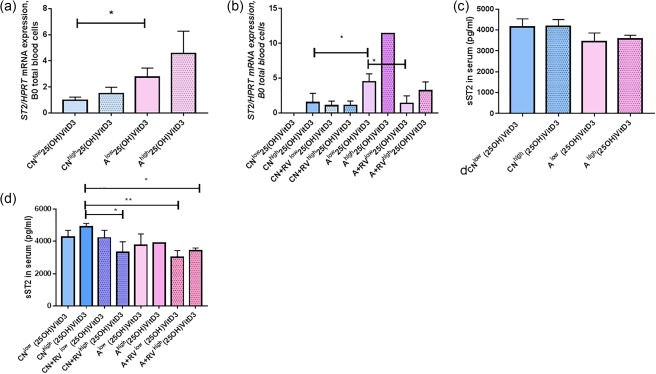
Rhinovirus (RV) in the airways and low serum level of 25(OH) vitamin D3 [25(OH)‐VitD3] associated with decreased serum level of soluble ST2 (sST2) in asthmatic children. (a) ST2/hypoxanthine‐guanine phosphoribosyltransferase HPRT) mRNA values analysed in the same children by quantitative polymerase chain reaction (qPCR) (CNlow n = 6, CNhigh n = 6, Alow n = 7, Ahigh n = 6, B0). CNlow versus Alow, P = 0·048, two‐tailed unpaired t‐test. (b) ST2/HPRT mRNA in total blood cells considering RV in the airways and serum levels of 25(OH)‐VitD3, from the left to the right: n = 0, 5, 5, 2, 3, 1, 3, 5, P = 0·0186 and P = 0·0275, unpaired two‐tailed t‐test. (c) sST2 in serum of control and asthmatic children with low and high 25(OH)‐VitD3 (CNlow n = 9, CNhigh n = 8, Alow n = 9, Ahigh n = 3, B0). (d) sST2 in serum considering RV in the airways and 25(OH)‐VitD3 level (from the left to the right: n = 2, 4, 7, 3, 5, 1, 4, 2, P = 0·0109, P = 0·0065, P = 0·0100, unpaired t‐test).
Table 2.
Analysed data of children at baseline visit
| Virus swab* | 25(OH)‐VitD3† | VDR | |
|---|---|---|---|
| Control | |||
| 208 | – | / | / |
| 211 | RV++ | 16·9 | 0·94 |
| 214 | RV++ | 13·4 | / |
| 215 | – | 26 | 0·82 |
| 218 | RV+++ | 17·4 | / |
| 219 | RV+ | 16·4 | / |
| 220 | – | 22·3 | / |
| 221 | RV++ | 25·5 | 1·10 |
| 222 | – | 34·1 | 0·95 |
| 226 | – | 20·3 | 0·72 |
| 227 | – | 16·5 | / |
| 232 | RV+++ | 13·7 | 1·03 |
| 233 | RV+· other | 25·9 | / |
| 234 | RV++· other | 12·6 | / |
| 235 | RV++ | 14·2 | 1·30 |
| 236 | – | / | 1·00 |
| 237 | Other | 10·7 | / |
| 240 | RV+· other | 18·3 | 1·10 |
| 241 | RV ++ | 25·9 | 1·22 |
| 244 | / | / | / |
| 245 | – | 28·4 | 0·95 |
| 246 | RV+ | 24·4 | / |
| Mean | 20·15 | 1·01 | |
| s.e.m. | 1·46 | 0·050 | |
| Asthma | |||
| 201 | RV++ | 9·69 | / |
| 202 | RV++ | / | 1·26 |
| 203 | – | 6·72 | 0·86 |
| 204 | – | 9·85 | / |
| 205 | – | 11 | / |
| 206 | RV++ | 16·8 | / |
| 207 | – | 17·6 | / |
| 209 | – | / | / |
| 210 | Other | 11·3 | / |
| 212 | – | / | / |
| 213 | RV++ | 17·8 | / |
| 216 | – | 19·9 | 0·92 |
| 217 | – | 15·8 | / |
| 223 | RV+++ | 37·8 | / |
| 224 | RV++· other | 24·3 | / |
| 225 | RV++· other | 33·5 | 1·03 |
| 228 | – | 33·5 | / |
| 229 | RV+++ | / | 1·89 |
| 230 | RV+ | 28·7 | 0·95 |
| 231 | Other | 11·8 | / |
| 238 | RV+++ | 8·5 | 1·28 |
| 239 | RV+ | 13·1 | / |
| 242 | RV+· other | 19·2 | / |
| 243 | RV+++ | 30·6 | / |
| Mean | 18·87 | 1·17 | |
| s.e.m. | 2·11 | 0·13 | |
*Virus swab: RV = rhinovirus; other = other respiratory virus (CoV NL6, Flu A, PIV 4, HBoV, RSV B, HEV, PIV 2, MPV, AdV, Flu B). †25(OH)‐vitamin D3 (VitD3) ng/ml: > 30 ng/ml = sufficient; 20–30 ng/ml = insufficient; < 20 ng/ml = deficient; s.e.m. =standard error of the mean; VDR = VitD3 receptor.
sST2 in serum was reduced by RV in vivo in control and in asthmatic children
It has been demonstrated recently that 25(OH)‐VitD3 enhances production of the soluble ST2 isoform 16.
Soluble ST2, as mentioned above, is an anti‐inflammatory marker capable of neutralizing IL‐33, the inflammatory cytokine also known as the ligand of ST2. Considering the anti‐inflammatory function of 25(OH)‐VitD3, we next questioned the relationship between 25(OH)‐VitD3 RV in the airways and sST2. We found that the highest levels of the anti‐inflammatory isoform sST2 were present in the serum of control children with high levels of 25(OH)‐VitD3 and without RV in the airways (Fig. 8c,d). The presence of RV in the airways was the cause of sST2 reduction both in control and in asthmatic children. The latter, also associated with low levels of 25(OH)‐VitD3, contributed with RV to the reduction of the sST2 in serum.
In conclusion, we found that ST2 is up‐regulated in blood cells of asthmatic children not by RV, but by low levels of serum levels of 25(OH)‐VitD3. By contrast, the anti‐inflammatory sST2 isoform was found down‐regulated by RV and also in asthma by combined low levels of 25(OH)‐VitD3.
Discussion
We found that RV challenge in the airways, which is one of the main factors in asthma exacerbations 41, 42, is associated with reduced expression of the anti‐inflammatory protein sST2 in the blood cells of children with and without asthma. Moreover, we found that the soluble form of ST2, sST2, was down‐regulated in the serum of asthmatic children with RV in the airways and with low serum levels of 25(OH)‐VitD3. 25(OH)‐VitD3 deficiency has been associated with asthma, as in many inflammatory and autoimmune pathologies 43, 44. The highest levels of serum sST2 were observed in the serum of control children without RV in the airways and high serum levels of 25(OH)‐VitD3. Previous studies have shown that ST2 plays an important role in ILC2‐driven allergic asthma 45 and that RV can induce type 2‐ and ILC2‐associated cytokines 12. In addition, the role of sST2 was reported previously in the literature of asthmatics in induced sputum 46. In this study we first observed, by gene arrays in the peripheral blood of preschool children with asthma, an increased expression of ILC2‐related markers such as AREG, and confirmed this finding by correlating with the clinical outcomes of these children. Total ST2 mRNA was found up‐regulated after in‐vitro exposure to RV and in the blood of asthmatic children with low serum levels of 25(OH)‐VitD3 without RV in their airways. sST2 is the ligand of IL‐33, and because it is soluble it can circulate and reach the airways where IL‐33 is released. IL‐33 has also been described to regulate sST2 47. Moreover, IL‐33 and its receptor and sST2 were investigated largely in asthmatic children 48, 49. We thus examined the correlation of IL‐33 in the airways with sST2. We found that the serum levels of sST2 correlated directly with IL‐33 in the airways in asthmatic children. Also, serum levels of 25(OH)‐VitD3 correlated directly with serum sST2 and IL‐33 in the airways of asthmatic children.
Asthmatic children who did not receive VitD3 supplementation as infants had more episodes of asthma exacerbation and those who had RV in vivo in their airways at the time of the analysis had reduced serum 25(OH)‐VitD3 levels associated with less sST2 serum levels and with lower AREG (a protein involved in epithelial cell repair) protein levels in the peripheral blood.
RV infection can damage epithelial cells 50 and delay epithelial cell repair 51.
Several studies have shown that 25(OH)‐VitD3 has the potential to prevent the development of allergic asthma 52, 53 and even has the ability to reduce asthma exacerbations 54, so we investigated further its influence on RV‐induced allergic asthma in preschool children.
RV challenge in the airways is one of the main factors inducing asthma exacerbations 41. Previous studies have shown that ST2 plays an important role in ILC2‐driven allergic asthma 45 and that RV can induce type 2‐ and ILC2‐associated cytokines 12. As little is known about how this new cell type can be influenced exogenously, we chose to focus upon the impact of 25(OH)‐VitD3 on ST2‐driven allergic asthma.
We first found increased ILC2 cell‐related markers in the peripheral blood from asthmatic children after RV challenge, in accordance with the results of former studies 12.
By following‐up this finding we found that AREG is induced, whereas IFN‐β, an anti‐viral IFN that has been discovered recently to inhibit ILC2s, is decreased in the blood of asthmatic children 36. We subgrouped the children further by the presence of RV in their airways in vivo and found that, in the absence of RV in the airways, IFN‐β serum levels of asthmatic children were decreased. We subgrouped the children further according to their serum levels of 25(OH)‐VitD3 and found that only control children with high 25(OH)‐VitD3 serum levels could produce higher amounts of IFN‐β. In conclusion, IFN‐β is induced by high levels of 25(OH)‐VitD3 in control children and was found to be reduced in asthma.
To investigate which mechanism is activated by 25(OH)‐VitD3 in IFN‐β induction, we examined ILC2 markers that have inhibitory functions on IFN‐β production. We found that AREGB, which encodes amphiregulin, a protein that promotes the growth and repair of normal epithelial cells in the airways, was found to be induced by in‐vivo RV infection in control children. This up‐regulation of AREGB may be activated because of the damage that RV causes to the epithelial cells 50 in vivo and, as a result, it contributes to epithelial cell repair 51. We next considered the effect of the serum level of 25(OH)‐VitD3 on AREG mRNA levels in blood and found that it was down‐regulated when higher serum levels of 25(OH)‐VitD3 in control children were measured. In contrast, higher serum levels of 25(OH)‐VitD3 were found to be associated with higher IFN‐β levels in control children. Thus, AREGB is an ILC2 marker that could be considered as a limiting factor, possibly limiting IFN‐β production upon RV infection in control preschool children.
We next examined ST2 and sST2, which we found up‐regulated in asthmatic children. ST2 was found to be up‐regulated in the blood cells of asthmatic children. Considering the presence of RV in the airways, we found a significant induction of ST2 mRNA expression in the blood cells of asthmatic children without RV colonization in the airways. Considering serum levels of 25(OH)‐VitD3, ST2 mRNA was found to be up‐regulated in blood cells of asthmatic children with lower levels of 25(OH)VitD3 without RV in the airways. RV in the airways reduced ST2 mRNA. Additional factors could be responsible for the ST2 induction in the absence of RV in the airways in asthmatic children. One possibility is the decrease in IFN‐β production in this group. Moreover, we reported recently that the bacterial colonization of the airways of these children influences ST2. In fact, ST2 was found to be up‐regulated in asthmatic children with a mixed Gram‐positive and Gram‐negative bacterial colonization in their airways 25. Finally, high 25(OH)‐VitD3 serum levels were found to be associated with higher anti‐inflammatory sST2 in control children without RV in the airways. The presence of RV in vivo both in control and asthmatic children was associated with decreased sST2 serum levels although, in asthmatic children, it was observed at low levels of serum 25(OH)‐VitD3 and in control children at high levels of serum 25(OH)‐VitD3. Thus, it is possible that the down‐regulation of ST2 mRNA observed after RV in asthma relates to sST2.
In summary, low levels of 25(OH)‐VitD3 without RV induced ST2 and low levels of 25(OH)‐VitD3 with RV reduced sST2. These results explain the apparent discrepancy of the gene arrays which do not recognize the soluble form of ST2.
When we examined the asthmatic exacerbations, we found them induced in asthmatic children without VitD3 supplement as infants. Moreover, at the symptomatic visit, a condition always associated with RV in the airways of these children, we found increased serum levels of 25(OH)‐VitD3. By looking at control children, RV in the airways was always associated with decreased serum level of 25(OH)‐VitD3, thus suggesting a dysregulation of this system. By looking at the intracellular carrier for 25(OH)‐VitD3, the VitD3 receptor (VDR), we found it up‐regulated in control children with RV in the airways and with high levels of serum 25(OH)‐VitD3. This mechanism seems to make sense as a transport mechanism induced upon 25(OH)‐VitD3 induction. It remains to be explained why, during symptomatic visits with RV in the airways, asthmatic children have higher serum 25(OH)‐VitD3 levels. One possibility is that in asthmatic children this carrier mechanism is dysregulated.
In conclusion, we suggest that in control children with RV infection 25(OH)‐VitD3 is carried intracellularly, where it binds to the VDR. These data are supported by our VDR expression data showing induction of VDR after RV and at high serum levels of 25(OH)‐VitD3 in control children.
Next, we questioned if there was a relevant influence from factors present in the infancy of the children in our cohort that could have affected their current status of serum 25(OH)‐VitD3 and the development of allergic diseases. Former studies have shown that lower 25(OH)‐VitD3 intake increased the risk of developing allergic asthma 55. Children with asthma who did not receive VitD3 supplements as infants, as reported by their parents, had lower serum 25(OH)‐VitD3 levels.
We analysed further which parameters may have been influenced by the current 25(OH)‐VitD3 serum levels of the children. High 25(OH)‐VitD3 serum levels were found to be associated with an induction of IFN‐β in the serum of control children, which might indicate a possible synergetic effect of 1α, 25(OH)2‐VitD3 and IFN‐β. By contrast, in asthmatic children, even high 25(OH)‐VitD3 levels could not induce any anti‐microbial IFN‐β‐mediated immune response, confirming a defect of IFN‐β in the serum of asthmatic children.
Author contributions
S. F. designed the present study. S. F. and P. H. contributed equally to the writing of the manuscript, to the analysis of the Predicta data and to all the experimental aspects of the Predicta study except for the vitamin D3 measurements, which were performed by M. R. N. G. P. designed the Predicta WP1 project and is the coordinator of the Predicta study. He set up the design of the Predicta study and the questionnaires that were then used by the MP group in Erlangen to analyse the answers of the parents of the children analysed in this study. T. Z. is not are the paediatrician who saw most of the children in Predicta WP1‐UKER and made the medical diagnosis. T. V. analysed the NPF for respiratory viruses. N. P. provided the rhinovirus used in these experiments. H. S. performed and analysed the gene arrays. S. T. W., the Chief of the Translational Genomics Core, Personalized Medicine, Cambridge, MA, coordinated the gene arrays and edited the manuscript.
Disclosure
The authors declare no conflicts of interest on the matter described in this manuscript.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Increased serum levels of 25(OH)‐vitamin D3 (VitD3) asthmatic preschool children are dependent upon VitD3 supplementation (Fluorette, Vigantolette) as infants. Serum levels of 25(OH)‐VitD3 in control (a) and asthmatic (b) preschool children are dependent upon VitD3 supplementation (Fluorette, Vigantolette) as infants (yes 1) or (no 0). VitD3 deficiency is < 20 ng/ml n = 5; VitD3 insufficiency 20–30 ng/ml n = 12; VitD3 sufficiency > 20 ng/ml n = 4.
Table S1. Interleukin (IL)‐C2‐related genes found regulated significantly in gene arrays
Table S2. Analysed data of children at baseline visit
Acknowledgements
The authors thank all children and their parents/guardians who took part in our Europe‐wide study PreDicta. Moreover, the authors are grateful to S. Trump, S. Mittler and B. Klösch at the Division of Molecular Pneumology and to E. Muschiol, I. Jawa and L. Schramm at the Paediatric Pneumology‐Allergology, Department of Paediatrics and Adolescent Medicine, Universitätsklinikum Erlangen, Erlangen Children's Hospital in Erlangen for their technical support. Moreover, the authors thank Drs M. Wölfel, C. Reinhardt, A. Kiefer, A. Neubert and Professor Dr W. Rascher from the Department of Paediatrics and Adolescent Medicine, Universitätsklinikum Erlangen, Erlangen for their assistance in the PreDicta UKER WP‐1 study. Furthermore, we thank R. Stergiou from the Allergy and Clinical Immunology Unit of the National and Kapodistrian University of Athens for the RV1b. This work was supported by the European Grant PreDicta (post‐infectious immune reprogramming and its association with persistence and chronicity of respiratory allergic diseases) in Erlangen and in the European Center in Athens and by the Department of Molecular Pneumology in Erlangen. This work was also supported by a DFG grant SFB 643/TP 12, and P. H. was supported by the European Grant Predicta in Erlangen. This work was supported by the European Grant PreDicta at the Friedrich‐Alexander‐Universität (FAU) Erlangen‐Nürnberg, Universitätsklinikum Erlangen and at the Allergy and Clinical Immunology Unit, 2nd Pediatric Clinic, National and Kapodistrian University of Athens.
References
- 1. Masoli M, Fabian D, Holt S, Beasley R. Global Initiative for Asthma (GINA) Program . The global burden of asthma: executive summary of the GINA dissemination committee report. Allergy 2004; 59:469–78. [DOI] [PubMed] [Google Scholar]
- 2. Robinson DS. The role of the T cell in asthma. J Allergy Clin Immunol 2010; 126:1081–91; quiz: 92–3. [DOI] [PubMed] [Google Scholar]
- 3. Neurath MF, Finotto S, Glimcher LH. The role of Th1/Th2 polarization in mucosal immunity. Nat Med 2002; 8:567–73. [DOI] [PubMed] [Google Scholar]
- 4. Stehle C, Saikali P, Romagnani C. Putting the brakes on ILC2 cells. Nat Immunol 2016; 17:43–4. [DOI] [PubMed] [Google Scholar]
- 5. van Rijt L, von Richthofen H, van Ree R. Type 2 innate lymphoid cells: at the cross‐roads in allergic asthma. Semin Immunopathol 2016; 38:483–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neill DR, Wong SH, Bellosi A et al Nuocytes represent a new innate effector leukocyte that mediates type‐2 immunity. Nature 2010; 464:1367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell‐type cytokines in protease allergen‐induced airway inflammation. Immunity 2012; 36:451–63. [DOI] [PubMed] [Google Scholar]
- 8. Enomoto Y, Orihara K, Takamasu T et al Tissue remodeling induced by hypersecreted epidermal growth factor and amphiregulin in the airway after an acute asthma attack. J Allergy Clin Immunol 2009; 124:913–20.e1–7. [DOI] [PubMed] [Google Scholar]
- 9. Akutsu N, Bastien Y, Lin R, Mader S, White JH. Amphiregulin is a vitamin D3 target gene in squamous cell and breast carcinoma. Biochem Biophys Res Commun 2001; 281:1051–6. [DOI] [PubMed] [Google Scholar]
- 10. Beale J, Jayaraman A, Jackson DJ et al Rhinovirus‐induced IL‐25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci Transl Med 2014; 6:256ra134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim BS, Wojno ED, Artis D. Innate lymphoid cells and allergic inflammation. Curr Opin Immunol 2013; 25:738–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jackson DJ, Makrinioti H, Rana BM et al IL‐33‐dependent type 2 inflammation during rhinovirus‐induced asthma exacerbations in vivo . Am J Respir Crit Care Med 2014; 190:1373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar RK, Foster PS, Rosenberg HF. Respiratory viral infection, epithelial cytokines, and innate lymphoid cells in asthma exacerbations. J Leukoc Biol 2014; 96:391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stolarski B, Kurowska‐Stolarska M, Kewin P, Xu D, Liew FY. IL‐33 exacerbates eosinophil‐mediated airway inflammation. J Immunol 2010; 185:3472–80. [DOI] [PubMed] [Google Scholar]
- 15. De la Fuente M, MacDonald TT, Hermoso MA. The IL‐33/ST2 axis: role in health and disease. Cytokine Growth Factor Rev 2015; 26:615–23. [DOI] [PubMed] [Google Scholar]
- 16. Pfeffer PE, Chen YH, Woszczek G et al Vitamin D enhances production of soluble ST2, inhibiting the action of IL‐33. J Allergy Clin Immunol 2015; 135:824–7.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schmitz J, Owyang A, Oldham E et al IL‐33, an interleukin‐1‐like cytokine that signals via the IL‐1 receptor‐related protein ST2 and induces T helper type 2‐associated cytokines. Immunity 2005; 23:479–90. [DOI] [PubMed] [Google Scholar]
- 18. Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin‐33 signaling in allergic airway inflammation. J Biol Chem 2007; 282:26369–80. [DOI] [PubMed] [Google Scholar]
- 19. Yadav M, Mittal K. Effect of vitamin D supplementation on moderate to severe bronchial asthma. Indian J Pediatr 2014; 81:650–4. [DOI] [PubMed] [Google Scholar]
- 20. Bonanno A, Gangemi S, La Grutta S et al 25‐Hydroxyvitamin D, IL‐31, and IL‐33 in children with allergic disease of the airways. Mediators Inflamm 2014; 2014:520241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DY. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol 2010; 125:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Groot JC, van Roon EN, Storm H et al Vitamin D reduces eosinophilic airway inflammation in nonatopic asthma. J Allergy Clin Immunol 2015; 135:670–5.e3. [DOI] [PubMed] [Google Scholar]
- 23. Song Y, Qi H, Wu C. Effect of 1,25‐(OH)2D3 (a vitamin D analogue) on passively sensitized human airway smooth muscle cells. Respirology 2007; 12:486–94. [DOI] [PubMed] [Google Scholar]
- 24. Bielor C, Sopel N, Maier A et al Role of TGF‐beta in anti‐rhinovirus immune responses in asthmatic patients. J Allergy Clin Immunol 2017; 140: 283–6.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hentschke I, Graser A, Melichar VO et al IL‐33/ST2 immune responses to respiratory bacteria in pediatric asthma. Sci Rep 2017; 7:43426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bergauer A, Sopel N, Kross B et al IFN‐alpha/IFN‐lambda responses to respiratory viruses in paediatric asthma. Eur Respir J 2016; 49:1700006. [Google Scholar]
- 27. Bergauer A, Sopel N, Kroß B et al IFN‐alpha/IFN‐lambda responses to respiratory viruses in paediatric asthma. Eur Respir J 2017; 49:1600969. [DOI] [PubMed] [Google Scholar]
- 28. Bakdash G, van Capel TM, Mason LM, Kapsenberg ML, de Jong EC. Vitamin D3 metabolite calcidiol primes human dendritic cells to promote the development of immunomodulatory IL‐10‐producing T cells. Vaccine 2014; 32:6294–302. [DOI] [PubMed] [Google Scholar]
- 29. Eiwegger T, Akdis CA. IL‐33 links tissue cells, dendritic cells and Th2 cell development in a mouse model of asthma. Eur J Immunol 2011; 41:1535–8. [DOI] [PubMed] [Google Scholar]
- 30. Hosokawa Y, Hosokawa I, Shindo S, Ozaki K, Matsuo T. Calcitriol suppressed inflammatory reactions in IL‐1beta‐stimulated human periodontal ligament cells. Inflammation 2015; 38:2252–8. [DOI] [PubMed] [Google Scholar]
- 31. Cho SJ, Kang MJ, Homer RJ et al Role of early growth response‐1 (Egr‐1) in interleukin‐13‐induced inflammation and remodeling. J Biol Chem 2006; 281:8161–8. [DOI] [PubMed] [Google Scholar]
- 32. Kudo M, Khalifeh Soltani SM, Sakuma SA et al Mfge8 suppresses airway hyperresponsiveness in asthma by regulating smooth muscle contraction. Proc Natl Acad Sci USA 2013; 110:660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang H, Chu X, Huang Y et al Maternal vitamin D deficiency during pregnancy results in insulin resistance in rat offspring, which is associated with inflammation and Iκbα methylation. Diabetologia 2014; 57:2165–72. [DOI] [PubMed] [Google Scholar]
- 34. Inaba Y, Yamamoto K, Yoshimoto N et al Vitamin D3 derivatives with adamantane or lactone ring side chains are cell type‐selective vitamin D receptor modulators. Mol Pharmacol 2007; 71:1298–311. [DOI] [PubMed] [Google Scholar]
- 35. Sykes A, Edwards MR, Macintyre J et al Rhinovirus 16‐induced IFN‐alpha and IFN‐beta are deficient in bronchoalveolar lavage cells in asthmatic patients. J Allergy Clin Immunol 2012; 129:1506–14.e6. [DOI] [PubMed] [Google Scholar]
- 36. Duerr CU, McCarthy CD, Mindt BC et al Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat Immunol 2015; 17:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Castro M, King TS, Kunselman SJ et al Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA 2014; 311:2083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wagner CL, Hulsey TC, Fanning D, Ebeling M, Hollis BW. High‐dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: a 6‐month follow‐up pilot study. Breastfeed Med 2006; 1:59–70. [DOI] [PubMed] [Google Scholar]
- 39. Sigurs N, Gustafsson PM, Bjarnason R et al Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med 2005; 171:137–41. [DOI] [PubMed] [Google Scholar]
- 40. Kotaniemi‐Syrjänen A, Vainionpää R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus‐induced wheezing in infancy – the first sign of childhood asthma? J Allergy Clin Immunol 2003; 111:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Johnston SL, Pattemore PK, Sanderson G et al Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ 1995; 310:1225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Proud D. Role of rhinovirus infections in asthma. Asian Pac J Allergy Immunol 2011; 29:201–8. [PubMed] [Google Scholar]
- 43. Hamzaoui A, Berraies A, Hamdi B, Kaabachi W, Ammar J, Hamzaoui K. Vitamin D reduces the differentiation and expansion of Th17 cells in young asthmatic children. Immunobiology 2014; 219:873–9. [DOI] [PubMed] [Google Scholar]
- 44. Tizaoui K, Berraies A, Hamdi B, Kaabachi W, Hamzaoui K, Hamzaoui A. Association of vitamin D receptor gene polymorphisms with asthma risk: systematic review and updated meta‐analysis of case‐control studies. Lung 2014; 192:955–65. [DOI] [PubMed] [Google Scholar]
- 45. Barlow JL, McKenzie AN. Type‐2 innate lymphoid cells in human allergic disease. Curr Opin Allergy Clin Immunol 2014; 14:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hamzaoui A, Berraies A, Kaabachi W, Haifa M, Ammar J, Kamel H. Induced sputum levels of IL‐33 and soluble ST2 in young asthmatic children. J Asthma 2013; 50:803–9. [DOI] [PubMed] [Google Scholar]
- 47. Demyanets S, Speidl WS, Tentzeris I et al Soluble ST2 and interleukin‐33 levels in coronary artery disease: relation to disease activity and adverse outcome. PLoS One 2014; 9:e95055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Charrad R, Kaabachi W, Berraies A, Hamzaoui K, Hamzaoui A. IL‐33 gene variants and protein expression in pediatric Tunisian asthmatic patients. Cytokine 2017; 104:85–91. [DOI] [PubMed] [Google Scholar]
- 49. Li Y, Wang W, Lv Z et al Elevated expression of IL‐33 and TSLP in the airways of human asthmatics in vivo: a potential biomarker of severe refractory disease. J Immunol 2018; 200:2253–62. [DOI] [PubMed] [Google Scholar]
- 50. Looi K, Troy NM, Garratt LW et al Effect of human rhinovirus infection on airway epithelium tight junction protein disassembly and transepithelial permeability. Exp Lung Res 2016;1–16. [DOI] [PubMed] [Google Scholar]
- 51. Faris AN, Ganesan S, Chattoraj A et al Rhinovirus delays cell repolarization in a model of injured/regenerating human airway epithelium. Am J Respir Cell Mol Biol 2016; 55:487–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gupta S, Singh RK, Dastidar S, Ray A. Cysteine cathepsin S as an immunomodulatory target: present and future trends. Expert Opin Ther Targets 2008; 12:291–9. [DOI] [PubMed] [Google Scholar]
- 53. Martineau AR, Jolliffe DA, Hooper RL et al Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta‐analysis of individual participant data. BMJ 2017; 356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weiss ST, Litonjua AA. Vitamin D dosing for infectious and immune disorders. Thorax 2015; 70:919–20. [DOI] [PubMed] [Google Scholar]
- 55. Erkkola M, Kaila M, Nwaru BI et al Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5‐year‐old children. Clin Exp Allergy 2009; 39:875–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Increased serum levels of 25(OH)‐vitamin D3 (VitD3) asthmatic preschool children are dependent upon VitD3 supplementation (Fluorette, Vigantolette) as infants. Serum levels of 25(OH)‐VitD3 in control (a) and asthmatic (b) preschool children are dependent upon VitD3 supplementation (Fluorette, Vigantolette) as infants (yes 1) or (no 0). VitD3 deficiency is < 20 ng/ml n = 5; VitD3 insufficiency 20–30 ng/ml n = 12; VitD3 sufficiency > 20 ng/ml n = 4.
Table S1. Interleukin (IL)‐C2‐related genes found regulated significantly in gene arrays
Table S2. Analysed data of children at baseline visit


