Abstract
Aims
Up to 50‐fold higher levels of urinary phthalate metabolites have been observed in users of phthalate‐containing drug products compared with non‐users. This is of concern, as phthalates are suspected endocrine disrupters and have been associated with cancer development. This study aims to quantify annual cumulated phthalate exposure from drug products among users of phthalate‐containing oral medications in Denmark throughout the period of 2004–2016.
Methods
We conducted a Danish nationwide cohort study using The Danish National Prescription Registry and an internal database held by The Danish Medicines Agency. These databases hold information on drug products; date of dispensing, and the type and quantity of excipients in drugs with Danish marketing permission. We present the number of users over time and their distribution of exposure to enteric phthalate polymers and ortho‐phthalates.
Results
The annual number of individuals exposed to phthalate‐containing products declined during 2004–2016. The total number of individuals exposed to dibutyl phthalate declined from 21 499 in 2004 to 5400 in 2016. However, among those exposed, the median dibutyl phthalate exposure remained above European regulatory limit of exposure ranging between 380–1710 mg/year throughout the study period. Lithium‐products constituted the majority of dibutyl phthalate exposure. Diethyl phthalate exposure, mainly caused by erythromycin, theophylline and diclofenac products, did not exceed the EMA regulatory limit.
Conclusion
While the number of individuals exposed to phthalates from oral medications during 2004–2016 declined, the use of phthalate‐containing drugs is still considerable.
Keywords: drug utilization, pharmacoepidemiology, pharmacovigilance
What is Already Known about this Subject
Certain types of phthalates are used as excipients in pharmaceutical preparations.
High exposure to phthalates among users of phthalate‐containing drugs compared to non‐users.
Phthalates are associated with harmful effects in animal models.
What this Study Adds
Distribution of individual‐level phthalate exposure throughout an entire population.
Up to 90% of patients treated with dibutyl phthalate‐containing drug products exceeded recommended limit for daily exposure set by European Medicines Agency.
Introduction
The use of phthalates as pharmaceutical excipients has gained interest, as up to a 50‐fold higher exposure has been observed in users of phthalate‐containing drug products compared with non‐users 1. Exposure to some phthalates used in human medications has been associated with harmful effects, especially in animal models. The United States Consumer Product Safety Commission (CPSC) classified http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6295 (DBP) as a reproductive toxicant 2. Data on diethyl phthalate (DEP) exposure are more conflicting, and the CPSC determined that the risk of diethyl phthalate exposure in humans is indeterminate due to lack of data 3. However human data on DEP exposure has been associated with breast cancer development, and maternal DEP exposure has been associated with poorer scores on neurodevelopment as well as shortened anogenital distance in male offspring 3, 4. The controversy regarding the safety of phthalate exposure has led the European Medicines agency (EMA) 5 and the U.S. Food and Drug Administration (FDA) 6 to publish guidelines on patient exposure to DEP and DBP if present in pharmaceutical products. These guidelines propose limits for daily exposure to specific phthalates used in orally administered preparations. Proposed limits for DEP and DBP exposure have been set to 4.0 and 0.01 mg/kg/day, respectively by EMA, corresponding to annual oral exposure of 102 200 mg/year and 255.5 mg/year for a 70‐kg person. A less restrictive limit for DBP exposure has been set to 0.1 mg/kg/day by FDA. Human data on the pharmacokinetics of enteric phthalate polymers are scarce, but this group of phthalates is considered safe due to negligible absorption 7.
Previous studies have focused on phthalate content in marketed pharmaceutical preparations. A recent review concluded that only six drugs among all products licensed in the UK in 2014 would be affected by regulatory limits 8. Another study found that sales of phthalate‐containing drugs in Denmark between 2004–2015 were substantial 9. However, no study has yet determined the distribution of individual‐level exposure to phthalate‐containing medications at a population level.
We quantified the distribution of cumulative individual‐level phthalate exposure from phthalate‐containing oral medications in Denmark throughout the period of 2004–2016.
Method
We conducted a Danish nationwide cohort study using The Danish National Prescription Registry and an internal database maintained by The Danish Medicines Agency.
Data sources
The Danish National Prescription Registry holds data on prescriptions redeemed by Danish residents since 1995 10. These data include type of drug, date of dispensing, fill quantity, the specific Nordic article number (VNR) used for identifying the actual product dispensed, and the Danish personal identification number of the patient 11. Dispensed drugs are classified according to the Anatomical Therapeutic Classification system developed by the World Health Organisation (WHO) 12.
For quantifying pharmaceutical phthalate exposure, we used an internal database maintained by Danish Medicines Agency, which provides detailed information on type and amount of excipients used in drug products with Danish marketing permission from 2004 onwards. Each specific drug product can be identified by the Nordic article number (VNR). This database also holds information on market authorization date, market access and withdrawal dates, as well as changes in phthalate content or type.
All Danish residents receive tax‐supported health care which is administered by the Danish Health Authorities, allowing population‐based register linkage studies covering all inhabitants.
Phthalates
Three substances in the group of enteric phthalate polymers and two substances in the group of ortho‐phthalates were used as excipients in medications marketed in Denmark from 2004–2016. Cellulose acetate phthalate (CAP), hydroxypropyl methylcellulose phthalate (HPMCP) and polyvinyl acetate phthalate (PVAP) formed the group of enteric phthalate polymers. Diethyl phthalate and the DnBP isomer of DBP formed the group of ortho‐phthalates. Content was defined as milligrams of each compound per capsule or tablet.
Analysis
The numbers of users as well as their distribution of cumulated annual exposure to phthalates are presented. Cumulated exposure was calculated for specific phthalates, as well as in combined categories of enteric phthalate polymers and ortho‐phthalates.
Prescriptions redeemed within the period of 1 January 2004 to 31 December 2016 were included. The cumulative phthalate amount was calculated for each dispensing and these cumulative amounts tallied for each individual within each calendar year.
Other
Analyses were performed using STATA release 14.2 (StataCorp, College Station, TX, USA).
According to Danish law, studies based solely on register data do not require ethical approval.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 13, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 14.
Results
In 2004, 157 841 individuals were exposed to DEP through drug products; this number decreased to 21 647 in 2016. The median exposure ranged between 33.75 mg/year and 135 mg/year and the maximum 90th percentile was 2538 mg/year in 2013. None of the individuals exposed to DEP exceeded the EMA limit at 102200 mg/year for a 70‐kg person (Table 1 and Figure 1).
Table 1.
Ortho‐phthalates: Number of exposed individuals, cumulative dibutyl phthalate (DBP) and diethyl phthalate (DEP) exposure (mg/year) in percentiles (p1‐p99) throughout the period of 2004–2016
| DBP | Year | N exposed | p1 | p10 | p25 | p50 | p75 | p90 | p99 |
|---|---|---|---|---|---|---|---|---|---|
| 2004 | 21 499 | 3.8 | 7.6 | 38 | 380 | 2280 | 5280 | 15 840 | |
| 2005 | 20 415 | 3.8 | 7.6 | 38 | 380 | 2090 | 4940 | 17 630 | |
| 2006 | 18 556 | 3.8 | 7.6 | 42.6 | 380 | 2090 | 3960 | 17 160 | |
| 2007 | 14 113 | 3.8 | 7.6 | 38 | 760 | 1900 | 2850 | 12 300 | |
| 2008 | 10 550 | 3.8 | 7.6 | 22.8 | 570 | 1900 | 2660 | 4400 | |
| 2009 | 8935 | 3.8 | 7.6 | 76 | 1140 | 2090 | 2850 | 4560 | |
| 2010 | 8486 | 3.8 | 7.6 | 121 | 1140 | 2090 | 2850 | 4400 | |
| 2011 | 7038 | 3.8 | 30.4 | 570 | 1520 | 2280 | 3040 | 4560 | |
| 2012 | 5972 | 190 | 380 | 950 | 1710 | 2470 | 3040 | 4840 | |
| 2013 | 5959 | 190 | 380 | 950 | 1710 | 2470 | 3040 | 4560 | |
| 2014 | 5726 | 190 | 380 | 760 | 1520 | 2280 | 2850 | 4180 | |
| 2015 | 5435 | 190 | 380 | 950 | 1710 | 2470 | 3040 | 4370 | |
| 2016 | 5400 | 190 | 380 | 950 | 1710 | 2470 | 3040 | 4370 |
| DEP | Year | N exposed | p1 | p10 | p25 | p50 | p75 | p90 | p99 |
|---|---|---|---|---|---|---|---|---|---|
| 2004 | 157 841 | 0.06 | 4 | 12 | 33.75 | 78.2 | 210 | 3609 | |
| 2005 | 153 674 | 0.06 | 6.6 | 12 | 40 | 78.2 | 259 | 3184 | |
| 2006 | 87 723 | 0.06 | 0.45 | 30 | 78.2 | 150 | 480 | 4445 | |
| 2007 | 78 517 | 0.06 | 0.32 | 30 | 78.2 | 156 | 480 | 4445 | |
| 2008 | 64 957 | 0.06 | 0.45 | 30 | 78.2 | 156 | 436 | 4445 | |
| 2009 | 55 668 | 0.06 | 11.25 | 30 | 78.2 | 156 | 518 | 4445 | |
| 2010 | 31 359 | 0.45 | 11.25 | 30 | 78.2 | 234 | 750 | 4653 | |
| 2011 | 17 244 | 0.45 | 11.25 | 11.25 | 56.25 | 241 | 1270 | 5080 | |
| 2012 | 14 754 | 0.45 | 11.25 | 11.25 | 78.75 | 522 | 1905 | 5220 | |
| 2013 | 12 653 | 0.45 | 11.25 | 11.25 | 135 | 635 | 2538 | 6264 | |
| 2014 | 17 967 | 0.45 | 11.25 | 11.25 | 90 | 597 | 2088 | 6264 | |
| 2015 | 23 154 | 0.45 | 11.25 | 11.25 | 45.45 | 315 | 1269 | 6055 | |
| 2016 | 21 647 | 0.45 | 11.25 | 11.25 | 45 | 180 | 1270 | 6264 |
Figure 1.
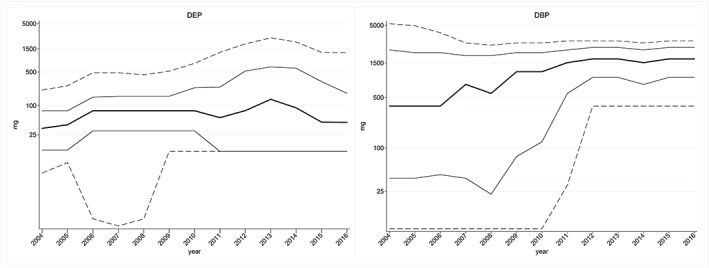
Percentiles (p10‐p90) of annual cumulated exposure to diethyl phthalate (DEP) (left) and dibutyl phthalate (DBP) (right)
Among the 21 499 individuals exposed to DBP through drug products, the median exposure was 380 mg/year in 2004 with a 90th percentile at 5280 mg/year. In 2016, only 5400 were exposed but the median exposure increased to 1710 mg/year with a 90th percentile at 3040 mg/year (Table 1 and Figure 1).
In 2004, a total of 110 657 individuals were exposed to enteric phthalate polymers ‐containing products, with a median exposure of 2400 mg/year. Individuals above the 90th percentile were exposed to more than 15 830 mg/year. In 2016, the number of individuals exposed to enteric phthalate polymers was 79 003, with a median exposure of 3042 mg/year. Individuals above the 90th percentile were exposed to more than 12 168 mg/year. Regarding ortho‐phthalates, the number of exposed individuals was 177 837 in 2004 with a median exposure of 40 mg/year. Individuals above the 90th percentile were exposed to more than 518 mg/year. In 2016, 26 990 individuals were exposed to ortho‐phthalates, with a median exposure of 90 mg/year. The individuals above the 90th percentile were exposed to more than 2538 mg/year (Table 2 and Figure 2).
Table 2.
Number of exposed individuals, cumulative exposure to entericphthalate polymers and ortho‐phthalates (mg/year) in percentiles (p1‐p99) throughout the period of 2004–2016
| Enteric phthalate polymers | Year | N exposed | p1 | p10 | p25 | p50 | p75 | p90 | p99 |
|---|---|---|---|---|---|---|---|---|---|
| 2004 | 116 057 | 142 | 621 | 781 | 2400 | 3694 | 15 830 | 70 400 | |
| 2005 | 129 662 | 60 | 469 | 781 | 2088 | 3448 | 12 704 | 63 180 | |
| 2006 | 140 786 | 35.5 | 469 | 781 | 1989 | 3448 | 8800 | 56 064 | |
| 2007 | 147 729 | 4.54 | 284 | 781 | 1719 | 3410 | 7773 | 55 880 | |
| 2008 | 137 596 | 4.54 | 384 | 781 | 2032 | 3410 | 7773 | 48 000 | |
| 2009 | 122 955 | 4.54 | 384 | 781 | 1539 | 2895 | 8912 | 50 400 | |
| 2010 | 93 289 | 4.54 | 284 | 568 | 1243 | 3042 | 14 400 | 56 064 | |
| 2011 | 87 475 | 4.54 | 384 | 769 | 1539 | 3042 | 12 528 | 50 400 | |
| 2012 | 84 512 | 4.54 | 567 | 1705 | 2842 | 3463 | 7200 | 43 200 | |
| 2013 | 76 462 | 4.54 | 113 | 1326 | 2557 | 3448 | 7200 | 43 200 | |
| 2014 | 67 837 | 4.54 | 384 | 1539 | 3042 | 4176 | 12 970 | 43 200 | |
| 2015 | 83 403 | 4.54 | 113 | 1136 | 3093 | 5079 | 12 478 | 38 400 | |
| 2016 | 79 003 | 4.54 | 276 | 1112 | 3042 | 5219 | 12 168 | 43 200 |
| Ortho‐phthalates | Year | N exposed | p1 | p10 | p25 | p50 | p75 | p90 | p99 |
|---|---|---|---|---|---|---|---|---|---|
| 2004 | 177 873 | 0.06 | 6.6 | 12 | 40 | 93.84 | 518 | 6600 | |
| 2005 | 172 645 | 0.06 | 6.6 | 12 | 43.2 | 99.3 | 530 | 6560 | |
| 2006 | 105 283 | 0.06 | 0.64 | 30 | 78.2 | 234 | 1230 | 6600 | |
| 2007 | 92 105 | 0.06 | 0.45 | 30 | 78.2 | 225 | 1200 | 4445 | |
| 2008 | 75 181 | 0.06 | 0.45 | 30 | 78.2 | 190 | 950 | 4445 | |
| 2009 | 64 358 | 0.06 | 11.25 | 43.2 | 78.2 | 252 | 1269 | 4445 | |
| 2010 | 39 741 | 0.45 | 11.25 | 45 | 90 | 440 | 1900 | 4560 | |
| 2011 | 24 214 | 0.45 | 11.25 | 30 | 135 | 1140 | 2540 | 5080 | |
| 2012 | 20 677 | 0.45 | 11.25 | 45 | 281 | 1520 | 2660 | 5080 | |
| 2013 | 18 571 | 0.45 | 11.25 | 45 | 522 | 1710 | 2850 | 5715 | |
| 2014 | 23 627 | 0.45 | 11.25 | 45 | 298 | 1194 | 2660 | 5742 | |
| 2015 | 28 538 | 0.45 | 11.25 | 22.5 | 135 | 950 | 2470 | 5220 | |
| 2016 | 26 990 | 0.45 | 11.25 | 11.25 | 90 | 1044 | 2538 | 5715 |
Figure 2.
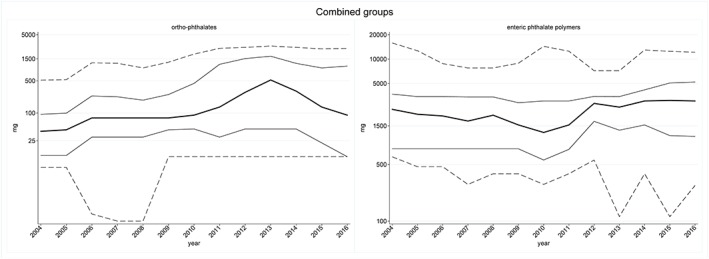
Percentiles (p10‐p90) of annual cumulated exposure to ortho‐phthalates (left) and enteric phthalate polymers (right)
Data on each of the three individual enteric phthalate polymers are presented in the supplementary material (Supplementary Table S1 and Figure S1).
Data on Danish marketed drugs ‐ phthalate type and content throughout the period of 2004–2015 have previously been reported 9.
Discussion
The annual number of individuals exposed to enteric phthalate polymers and ortho‐phthalates decreased during 2004–2016. Increased exposure was observed among those in the highest exposure groups. Fewer individuals were exposed to DEP through drug products during the study period, and no one exceeded the EMA regulatory limit. However, half of all individuals exposed to DBP from drug products exceeded EMA exposure limits in 2004, and this proportion increased to 90% of all exposed individuals in 2016. Within each calendar year, those who did exceed limits were exposed to larger quantities.
The number of individuals exposed decreased markedly over the study period likely as a consequence of more initiatives aiming at reducing phthalate use. National and European legislation restricting the use of some phthalates in consumer products was introduced in 1999 and 2006 15, 16. Additionally, FDA and EMA guidelines were released in 2012 and 2015 respectively 5, 6. EMA guidance on phthalate exposure limits came into effect in June 2015. However, for existing authorised medicinal products a time limit of 3 years (after coming into force of the final guideline) was set for the implementation of formulation changes and consequential regulatory applications, as necessary. Consequently the data reported here on phthalate exposure collected in 2016 may not be representative of the situation following expiry of the June 2018 deadline for existing products.
This is the first study quantifying individual phthalate exposure from orally administered drugs throughout an entire population thus determining some of the consequences of above mentioned initiatives. The main strength of the study is the population‐based design using complete registers which supports the validity of data. The weakness of this study design is the use indirect measures of exposure judged via dispensed prescriptions. The degree of non‐adherence to prescribed medications was not accounted for in this study, leading to a possible over‐estimation of exposure. However, by using dispensed prescriptions rather than issued prescriptions, we eliminated the influence of primary non‐adherence 17.
Estimates of DEP exposure from environmental sources ranges between 0.0023 and 0.012 mg/kg bodyweight daily for an average adult person corresponding to 58–306 mg/year for a 70‐kg person 18, 19, 20. The magnitude of DEP exposure in this study did not exceed EMA exposure limit at 4 mg/kg/day. Regulatory limits have been defined using conservative No‐Observed‐Adverse‐Events‐Levels (NOAELs) reported on reproductive and developmental outcomes, since these outcomes are considered the most relevant in assessing the safety of phthalate exposure 21. Overall developmental NOAELs vary between 197–267 mg/kg/day DEP daily based on data obtained in rodents 5. The magnitude of DEP exposure from orally administered drug products in our study is similar to average population exposure estimates of DEP and notably lower than conservative NOAELs. In the period of 2004–2015, fourteen drug products on the Danish market contained DEP. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1456, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=413 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2714 constituted the majority of the sales among DEP‐holding drug products 9.
Estimates of average population DBP exposure from environmental sources ranges between 0.001–0.005 mg/kg bodyweight daily, corresponding to 48–127 mg/year for a 70‐kg person 21, 22. An U.S. biomonitoring study indicated that the median of average population exposure to DBP was less than 0.001 mg/kg/day with a 95th percentile at 0.004 mg/kg/day 23. We found that the median DBP exposure from orally administered drug products exceeded the maximum estimate of average population exposure from environmental sources up to more than 10‐fold. Further the median exposure for DBP exceeded the EMA limit up to 6‐fold, but stayed below the FDA limit throughout the study period for a 70‐kg person. The 99‐percentile exceeded EMA and FDA limits of DBP exposure up to more than 70‐fold and 7‐fold respectively for a 70‐kg person. In our studyabout 50–90% of exposed individuals exceeded EMA limit and about 10% exceeded FDA limit of DBP exposure. This was mainly driven by http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5212 products. Lithium products containing DBP accounted for 64% of all lithium sales in Denmark during the period of 2004–2015 9. Diclofenac, multienzymes and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2700 preparations also contributed a considerable fraction of the DBP exposure in this period.
Current data on toxicity are predominantly from animal studies using a variety of endpoints. Recommendations regarding the safety of exposure are therefore based on extrapolations and interpretation. This issue is reflected in guidelines indicating diverse recommendations e.g. the U.S. Consumer Product Safety Commission DBP‐exposure limit at 0.2 mg/kg/day and the DBP‐exposure limit of 0.3 mg/kg/day in the draft IRIS reassessment 2, 24.
Regulatory authorities have set values for maximum daily exposure to DEP and DBP from medicinal products. The recommendations for DBP exposure published by EMA and FDA are different, which reflects different approaches for preparing the guidelines. The FDA recommendations regarding DBP exposure from medications are based on the Environmental Protection Agency recommended reference dose (RfD). The RfD is the estimated tolerable oral exposure throughout lifetime not expected to cause harmful effects in humans, including sensitive populations. The EPA RfD is based on a dose–response assessment determining the point of departure for the outcome of interest and following extrapolating for relevance to human exposure. Deriving the DBP RfD, the U.S. EPA used the NOAEL of 30 mg/kg/day for the outcome “decrease in fetal rat testis testosterone concentration” reported by Lehmann et al. 2004 25. No recommendations regarding DEP exposure was published by FDA. The European Medicines Agency derived recommendations for DBP and DEP exposure. European recommendations are based on Permitted Daily Exposure (PDE), defined in the EMA guideline “ICH Topic Q3C (R4) Impurities: Guideline for Residual Solvents” 26. This term describes the pharmaceutically acceptable exposure. It was defined to avoid confusion with the established terms “tolerable daily intake” (TDI) and“ acceptable daily intake” (ADI). Calculation of exposure limits are conducted according to standards published by Pharmacopeial Forum (Pharmacopeial Forum, nov‐dec 1989). The proposed DBP exposure limit published by EMA is based on a Lowest‐Observed‐Adverse‐Event‐Level (LOAEL) at 2 mg/kg/day reported by Lee et al. 27 on the outcome increased incidence of alveolar atrophy in male rat mammary glands and decreased number of spermatocytes in the seminiferous tubules. However, this LOAEL was considered an outlier and was discarded by the U.S. EPA in the DBP RfD calculations. Further a quality assessment of the study by Lee et al. revealed methodological and statistical issues 28.
The recommendations concerning pharmaceutically acceptable DEP exposure published by EMA are based on a NOAEL of 197 mg/kg/day reported by Fujii et al. 29. No regulatory limits exist for any of the enteric phthalate polymers. It is assumed that absorption of enteric phthalate polymers is negligible 7. Further, lack of consistency in type of outcomes, lack of reproducibility and high doses complicated interpretations of animal toxicity studies 5. In this study the exposure to enteric phthalate polymers originated from three different compounds in the period of 2004–2015. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4840 and theophylline products represented most CAP exposure. Multienzymes and erythromycin products constituted the main part of HPMCP exposure. The PVAP exposure was represented by http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7009 products entirely 9. There are no studies on CAP or HPMCP of local toxic effect in the gastric ventricle. Such studies may be hypothesized because of direct contact of enteric phthalate polymers with enterocytes. Chronic PVAP toxicity studies study conducted in rats and dogs found chronic inflammatory changes of the colon and small intestines of both species in dose ranges 2000–3000 mg/kg/day 30.
Phthalates are acid‐stable and this property is utilized in the production of sustained or delayed release preparations, where phthalates are used as coating material excipients, preventing tablets from disintegrating in the ventricle 31. Acetyl tributyl citrate is used in pharmaceutical preparations as an alternative to phthalates. Exposure data on this compound are very limited, but acetyl tributyl citrate exposure in rodents has been demonstrated to induce sensitization and affect the central nervous system 32. Human data on effects of acetyl tributyl exposure are scarce.
Several treatment regimens imply possible chronic exposure to phthalates, but no recommendations concerning duration of exposure to DEP or DBP from pharmaceutical preparations exist in the EMA guidelines whereas the recommendations in the FDA guidelines are based on the RfD for DBP. Although exposure limits for phthalates can be derived from animal data FDA advises minimization of patient exposure as a precautionary measure. Further, there is some controversy regarding the additive effect of concomitant exposure to more than one type of phthalate 32, 33. Several drug products contain more than one type of phthalate 34 and patients may take more than one drug product containing phthalates in addition to the exposure from environmental or occupational sources. Dose‐additivity between DBP and diethyl hexyl phthalate (DEHP) on androgen‐sensitive developmental outcomes has been demonstrated in rats. However, the implications for human health are still unclear 35. However, human data has shown that increased exposure to monobutyl phthalate (MBP), a DBP metabolite, is associated with increased levels of the hormones and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5011 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1157 (FSH) and reduced sperm parameters. In addition, increased levels of sex hormone binding globuline and increased ratio of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1159 (LH) and testosterone has been linked to DBP exposure. Exposure to MBP has also been associated with reduced thyroid hormones T4 and free T4 (FT4) in pregnant women 2. Increased urinary levels of mono‐ethyl phthalate (MEP), a DEP metabolite, has been associated with reduced sperm parameters, low levels of LH, decreased forced vital capacity (FVC) and decreased forced expiratory volume at 1 second (FEV1) in males 3. Further high MEP exposure has been associated with breast cancer development with an OR at 2.2 (95%CI: 1.33–3.63) when comparing women in the highest tertile of exposure to those in the lowest tertile of exposure 4. The above‐mentioned issues are crucial when assessing the safety of phthalate use in pharmaceutical preparations.
Conclusion
While the total number of individuals exposed to enteric phthalate polymers and in particular ortho‐phthalates decreased during 2004–2016, the use of phthalate‐containing drugs remained considerable. Among patients treated with DBP‐containing drug products 50–90% exceeded EMA exposure limits, but 90% of all patient remained below the less restrictive FDA exposure limits. Little is known about the potential effect of phthalate exposure from drugs and future pharmacoepidemiological studies could help uncover implications of pharmaceutical phthalate exposure.
Competing Interests
There are no competing interests to declare.
This study is funded by The Danish Cancer Society, grant ID: R90‐A6066.
Contributors
All authors have made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data. They have all participated in drafting of the paper or revising it critically.
Supporting information
Table S1 Phthalates in the group of enteric phthalate polymers – Polyvinyl acetate phthalate (PVAP), cellulose acetate phthalate (CAP) and hydroxypropylmethylcellulose phthalate (HPMCP) exposure (mg/year) in percentiles (p1‐p99) and yearly number of exposed individuals throughout the period of 2004‐2016
Figure S1 Percentiles (p10‐p90) of annual cumulated polyvinyl acetate phthalate (PVAP), cellulose acetate phthalate (CAP) and hydroxypropylmethylcellulose phthalate (HPMCP) exposure
Ennis, Z. N. , Broe, A. , Pottegård, A. , Ahern, T. P. , Hallas, J. , and Damkier, P. (2018) Cumulative exposure to phthalates from phthalate‐containing drug products: a Danish population‐wide study. Br J Clin Pharmacol, 84: 1798–1805. 10.1111/bcp.13614.
Zandra Nymand Ennis is the Principal Investigator of the study.
References
- 1. Hernández‐Díaz S, Mitchell AA, Kelley KE, Calafat AM, Hauser R. Medications as a potential source of exposure to phthalates in the U.S. population. Environ Health Perspect 2009; 117: 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Consumer Product Safety Commission . Toxicity review for Di‐n‐butyl phthalate [internet]. Available at https://www.cpsc.gov/s3fs-public/ToxicityReviewOfDBP.pdf (last accessed 21 Dec 2017).
- 3. Consumer Product Safety Commission . Toxicity review for diethyl phthalate [internet]. Available at https://www.cpsc.gov/s3fs-public/ToxicityReviewOfDEP.pdf (last accessed 19 January 2018).
- 4. López‐Carrillo L, Hernández‐Ramírez RU, Calafat AM, Torres‐Sánchez L, Galván‐Portillo M, Needham LL, et al Exposure to phthalates and breast cancer risk in northern Mexico. Environ Health Perspect 2010; 118: 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. European Medicines Agency . Guideline on the use of phthalates as excipients in human medicinal products. EMA/CHMP/SWP/362974/2012 corr 2 [homepage on the internet]. European Medicines Agency; 2012. Available at http://www.ema.europa.eu (last accessed 28 August 2017).
- 6. U.S. Food and Drug Administration . Guidance for industry limiting the use of certain phthalates as excipients in CDER‐regulated products. [homepage on the internet]. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER); 2012. Available at http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm (last accessed 28 August 2017).
- 7. Carter WD. Defining “phthalates.” Environ Health Perspect 2012; 120: A416 author reply A416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jamieson L, McCully W. Review: UK medicines likely to be affected by the proposed European medicines Agency's guidelines on phthalates. BMC Pharmacol Toxicol 2015; 16: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Broe A, Ennis ZN, Pottegård A, Hallas J, Ahern T, Damkier P. Population exposure to phthalate‐containing drugs. Basic Clin Pharmacol Toxicol 2017; 121: 153–158. [DOI] [PubMed] [Google Scholar]
- 10. Pottegård A, Schmidt SAJ, Wallach‐Kildemoes H, Sørensen HT, Hallas J, Schmidt M. Data resource profile: the Danish National Prescription Registry. Int J Epidemiol 2017; 46: 798–798f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmidt M, Pedersen L, Sørensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol 2014; 29: 541–549. [DOI] [PubMed] [Google Scholar]
- 12. WHO Collaborating Centre for Drug Statistics Methodology . Guidelines for ATC classification and DDD assignment 2017 [homepage on the internet]. 2017. Available at https://www.whocc.no/filearchive/publications/2017_guidelines_web.pdf (last accessed 13 Jun 2017).
- 13. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD, et al The Concise Guide to PHARMACOLOGY 2017/18: Overview. Br J Pharmacol 2017; 174: S1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ministry of Environment and Food of Denmark . Fact sheet: PVC and phthalates [internet]. Available at http://eng.mst.dk/chemicals/chemicals-in-products/legislation-on-chemicals/fact-sheets/fact-sheet-pvc-and-phthalates/ (last accessed 19 January 2018).
- 16. European Commission , REACH ‐ legislation [internet]. European Commission. Available at http://ec.europa.eu/environment/chemicals/reach/legislation_en.htm (last accessed 12 January 2018).
- 17. Pottegård A, dePont CR, Houji A, Christiansen CB, Paulsen MS, Thomsen JL, et al Primary non‐adherence in general practice: a Danish register study. Eur J Clin Pharmacol 2014; 70: 757–763. [DOI] [PubMed] [Google Scholar]
- 18. Calafat AM, McKee RH. Integrating biomonitoring exposure data into the risk assessment process: phthalates [diethyl phthalate and di(2‐ethylhexyl) phthalate] as a case study. Environ Health Perspect 2006; 114: 1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koch HM, Calafat AM. Human body burdens of chemicals used in plastic manufacture. Philos Trans R Soc Lond B Biol Sci 2009; 364: 2063–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tefre de Renzy‐Martin K, Frederiksen H, Christensen JS, Boye Kyhl H, Andersson A‐M, Husby S, et al Current exposure of 200 pregnant Danish women to phthalates, parabens and phenols. Reproduction 2014; 147: 443–453. [DOI] [PubMed] [Google Scholar]
- 21. Kamrin MA. Phthalate risks, phthalate regulation, and publichealth: a review. J Toxicol Environ Health B Crit Rev 2009; 12: 157–174. [DOI] [PubMed] [Google Scholar]
- 22. ATSDR (Agency for Toxic Substances and Disease Registry) . Toxicological profile for Di‐n‐Butyl Phthalate. Atlanta, GA: US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, 2002. [Google Scholar]
- 23. Wormuth M, Scheringer M, Vollenweider M, Hungerbühler K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal 2006; 26: 803–824. [DOI] [PubMed] [Google Scholar]
- 24. Assessment UENC for E . IRIS toxicological review of Dibutyl phthalate (DBP) (external review draft) 2006. [Internet]. Available at https://cfpub.epa.gov/ncea/cfm/iris/recordisplay.cfm?deid=15570724 (last accessed 5 January 2018).
- 25. Lehmann KP, Phillips S, Sar M, Foster PMD, Gaido KW. Dose‐dependent alterations in gene expression and testosterone synthesis in the fetal testes of male rats exposed to di (n‐butyl) phthalate. Toxicol Sci 2004; 81: 60–68. [DOI] [PubMed] [Google Scholar]
- 26. European Medicines Agency . ICH topic Q3C (R4) impurities: guideline for residual solvents ‐ CPMP/ICH/283/95 [internet]. 2009. Available at http://www.ema.europa.eu (last accessed 3 January 2018).
- 27. Lee K‐Y, Shibutani M, Takagi H, Kato N, Takigami S, Uneyama C, et al Diverse developmental toxicity of di‐n‐butyl phthalate in both sexes of rat offspring after maternal exposure during the period from late gestation through lactation. Toxicology 2004; 203: 221–238. [DOI] [PubMed] [Google Scholar]
- 28. U.S. Environmental Protection Agency . External peer review summary for the Dibutyl phthalate human health assessment: FINAL REPORT [internet]. 2006. Available at https://www.epa.gov/ (last accessed 3 January 2018).
- 29. Fujii S, Yabe K, Furukawa M, Hirata M, Kiguchi M, Ikka T. A two‐generation reproductive toxicity study of diethyl phthalate (DEP) in rats. J Toxicol Sci 2005; 30Spec No.: 97–116. [DOI] [PubMed] [Google Scholar]
- 30. Schoneker DR, DeMerlis CC, Borzelleca JF. Evaluation of the toxicity of polyvinylacetate phthalate in experimental animals. Food Chem Toxicol 2003; 41: 405–413. [DOI] [PubMed] [Google Scholar]
- 31. Aulton ME, Taylor K, eds. Aulton's pharmaceutics: the design and manufacture of medicines, 4th edn. Edinburgh: Churchill Livingstone/Elsevier, 2013; 894. [Google Scholar]
- 32. The Danish Environmental Protection Agency . Environmental and health assessment of alternatives to phthalates and to flexible PVC [homepage on the internet]. 2001. Available at http://www2.mst.dk/udgiv/publications/2001/87-7944-407-5/pdf/87-7944-408-3.pdf (last accessed 3 July 2017).
- 33. Hannas BR, Furr J, Lambright CS, Wilson VS, Foster PMD, Gray LE. Dipentyl phthalate dosing during sexual differentiation disrupts fetal testis function and postnatal development of the male Sprague‐Dawley rat with greater relative potency than other phthalates. Toxicol Sci 2011; 120: 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Katdare A, Chaubal M. Exipient Development for Pharmaceutical, Biotechnology and Drug Delivery. Boca Raton: CRC Press, 2006; 474. [Google Scholar]
- 35. Howdeshell KL, Rider CV, Wilson VS, Gray LE. Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in male laboratory rats. Environ Res 2008; 108: 168–176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Phthalates in the group of enteric phthalate polymers – Polyvinyl acetate phthalate (PVAP), cellulose acetate phthalate (CAP) and hydroxypropylmethylcellulose phthalate (HPMCP) exposure (mg/year) in percentiles (p1‐p99) and yearly number of exposed individuals throughout the period of 2004‐2016
Figure S1 Percentiles (p10‐p90) of annual cumulated polyvinyl acetate phthalate (PVAP), cellulose acetate phthalate (CAP) and hydroxypropylmethylcellulose phthalate (HPMCP) exposure


