Summary
Acute ischaemic stroke can induce secondary brain injury by activating an inflammatory response that contributes to clinical impairment. As a specific inhibitor of the immunoproteasome subunit low molecular weight polypeptide 7 (LMP7), PR‐957 may participate in regulating pathophysiological and inflammatory responses in multiple diseases of the central nervous system (CNS). We investigated the neuroprotective properties of PR‐957 in a mouse model of stroke, induced by middle cerebral artery occlusion (MCAO). After MCAO and injections of PR‐957 or vehicle, we evaluated mice behaviourally using modified Neurological Severity Scores (mNSS) and sensorimotor tests, including the adhesive‐removal test, a foot‐fault test and an inclined plane test. Infarct volume was measured 24 and 72 h after MCAO. Infiltration by different lymphocyte subpopulations was evaluated by flow cytometry and immunofluorescent staining of brain tissue from the penumbral area. Quantitative real‐time polymerase chain reaction analysis and enzyme‐linked immunosorbent assay were used to measure the expression of proinflammatory cytokines: interkeukin (IL)‐1α, IL‐1β, IL‐2, IL‐4, IL‐6, IL‐10, IL‐12, IL‐17A, interferon (IFN)‐γ, tumour necrosis factor (TNF)‐α, granulocyte colony‐stimulating factor (GCSF) and granulocyte–macrophage colony‐stimulating factor (GM‐CSF). Expression of phosphorylated signal transducer and activator of transcription 3 (pSTAT‐3) protein levels in brain was measured by immunoblot. MCAO mice treated with PR‐957 showed a significant decrease in infarct volume and had mild neurological deficits compared to vehicle‐treated mice. PR‐957 administration also significantly decreased IL‐1β, IL‐6, IL‐12, IL‐17A and TNF‐α. PR‐957 provides neuroprotection via inhibiting T lymphocyte infiltration and decreasing T helper type 17 (Th17) cell differentiation in MCAO mice, which may result from the reduced expression of pSTAT‐3. The neuroprotective effect of PR‐957 indicates its potential utility as anti‐inflammatory therapy for ischaemic stroke.
Keywords: inflammation, MCAO, RORγt, STAT‐3, Th17 cell
Introduction
As the second leading cause of death and the leading cause of adult disability worldwide, stroke poses a serious threat to public health 1. Ischaemic stroke and the associated inflammatory response, in particular, account for approximately 70–80% of neurological impairment associated with stroke. Activation of the immune system plays an important role in the pathophysiology of the brain injury that occurs after stroke, as demonstrated in both human and animal studies 2. The extensive inflammatory response after stroke leads to severe brain injury and poor long‐term outcomes 3. Therefore, a better understanding of the inflammatory consequences of stroke can inform stroke management by focusing therapeutic strategies to reduce inflammation and to improve functional recovery.
Middle cerebral artery occlusion (MCAO) in mice is the classic animal model used for studying the consequences of ischaemia reperfusion and associated inflammation 4. This model is often used to assess ischaemic stroke and is valuable in estimating the effects of treatment strategies 5. T cells, particularly certain subtypes of T cells [T helper type 1 (Th1), Th2, Th17 and regulatory T cells (Treg cells)] play a critical role in inflammation resulting from ischaemic brain injury and are associated closely with outcomes following stroke 6, 7, 8, 9, 10. Thus, one strategy to protect the brain following stroke‐associated inflammation is to enhance the beneficial Th2 and Treg response, and the second is to prevent the destructive responses of Th1 and Th17.
Another therapeutic strategy is to target immunoproteasomes. Immunoproteasomes are specialized proteasomes expressed mainly in haematopoietic cells 11, and are well known for processing proteins for major histocompatibility complex (MHC) class I presentation. Moreover, immunoproteasomal subunits have been shown to be up‐regulated in a mouse model of ischaemic stroke 12, 13, suggesting that they might be involved in the pathophysiology of the damage occurring after ischaemic stroke. Immunoproteasomes contain three major catalytic subunits: low molecular weight polypeptide 2 (LMP2) (β1i), multi‐catalytic endopeptidase complex subunit (MECL) (β2i) and LMP7 (β5i). Increasing evidence indicates that immunoproteasomes are essential for T cell expansion and survival 14, inflammatory cytokine production 15, 16, 17 and T cell differentiation 18. Immunoproteasomes influence T cells through the nuclear factor kappa B (NF‐κB) or signal transducer and activator of transcription (STAT) pathway and underlie the pathogenesis of multiple diseases. Blockade of the LMP7 subunit by PR‐957 (also named ONX 0914), an immunoproteasome‐specific inhibitor, strongly attenuates brain injury and suppresses activity of proinflammatory cytokines 15, 19.
Apart from their role in experimental rheumatoid arthritis and inflammatory bowel disease, immunoproteasomes are also associated with degenerative neurological diseases of the central nervous system (CNS) 20, 21, such as Alzheimer's disease. Immunoproteasomal subunits are up‐regulated in a mouse model of ischaemic stroke 12, 13, suggesting that they might be involved in the pathophysiology following ischaemic stroke. However, the molecular mechanisms mediating immunoproteasome activity during cerebral ischaemia are complicated.
Here, we conducted a detailed investigation of what role immunoproteasomes play during ischaemic stroke using the LMP7 inhibitor, PR‐957. We hypothesize that this compound acts as an anti‐inflammatory agent, whereby it modulates T cell responses in an experimental model of stroke. Our results point to a therapeutic opportunity to exploit the effects of this drug to mitigate brain injury after ischaemic stroke.
Materials and methods
Experimental animals
Adult male mice of C57BL/6 background (6–8 weeks old, 18–20 g) were obtained from Vital River Laboratories (Beijing, China). All the mice were housed in a temperature‐ and humidity‐controlled room under 12‐h light/12‐h dark cycles. In our experiments, 50 mice were used for dose optimization of PR‐957 treatment (Supporting information, Table S1). In addition, 324 adult male mice with a C57BL/6 background were assigned into three groups: sham‐operated group, vehicle control group (MCAO + vehicle) and PR‐957‐treated group (MCAO + PR‐957) (Supporting information, Table S2). During the experimental period, mice were provided food and water ad libitum and acclimated to the housing conditions for 1 week prior to the experiments. The number of animals needed for each experiment was calculated to be the minimum necessary to produce valid results. That calculation was the result of power analysis using G‐Power software (G*Power version 3.1; Heinrich‐Heine‐Universitat Düsseldorf, Düsseldorf, Germany). To achieve α = 0·05 at β = 0·2 (power 80%) with a mean 20% standard deviation, results from sample size calculations show that the sample size should be at least six per group. All animal procedures were performed according to national regulations and were approved by the Animal Experiments Ethical Committee of Tianjin Medical University General Hospital. These are consistent with Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines 22.
Protocol for MCAO
Transient cerebral ischaemia was induced according to the methods reported by Longa et al. 4. Briefly, this involved occlusion of the middle cerebral artery (MCA) using aseptic procedures. After anaesthetizing the mouse with 10% chloral hydrate [30 mg/kg, intraperitoneally (i.p.)], a ventral mid‐line neck incision was made and the left common carotid artery (CCA), internal carotid artery (ICA) and the external carotid artery were exposed and isolated. A 0·16‐mm diameter monofilament (tip diameter, 0·20 mm; Xinong, Beijing, China) was inserted into the ICA through the cut end of CCA and gently advanced 7–8 mm to block blood flow into the MCA. Sixty min after vessel occlusion, the monofilament was withdrawn to achieve ICA blood reperfusion. Regional cerebral blood flow (rCBF) corresponding to the perfusion territory of left MCA was monitored by a laser Doppler flowmeter (PF5010; Perimed AB, Beijing, China). Mice showed an 85% reduction of rCBF after occlusion was included in the following experiments. Body temperature was maintained by a heating pad. For the sham‐operated group, mice were subjected to the same surgical protocol without insertion of monofilament. Buprenorphine (0·03 mg/kg, i.p.) was given every 12 h within the first 24 h for pain management after surgery.
Treatment with PR‐957
PR‐957, purchased from Selleckchem (catalogue no. S7172; Shanghai, China), was dissolved in an aqueous mixture of 10% (w/v) sulphobutylether‐β‐cyclodextrin and 10 mM sodium citrate (pH 6). In a dose optimization study, mice were randomly administered a single i.p. injection of 5, 10, 20 or 30 mg/kg. We determined the optimum dose to be 20 mg/kg. This was based on results of 3 days of behavioural testing and infarct volume, which was measured 72 h after PR‐957 treatment.
Three groups were composed of randomly assigned mice: a sham‐operated group; a MCAO group (MCAO + vehicle); and a PR‐957‐treatment group (MCAO + PR‐957). In the PR‐957‐treatment group, mice received a dose of 20 mg/kg PR‐957; the sham‐operated and MCAO groups of mice were given the same volume of vehicle [10% (w/v) sulphobutylether‐β‐cyclodextrin and 10 mM sodium citrate (pH 6)]. The corresponding solutions were injected i.p. into all mice at the beginning of ischaemia/reperfusion (I/R); that is, after 60 min of MCAO.
Neurological assessment following MCAO
All three groups of mice – MCAO group (MCAO + vehicle), PR‐957‐treatment group (MCAO + PR‐957) and sham‐operated group (vehicle only) (n = 10 each) – underwent neurobehavioural testing 24, 48 and 72 h after MCAO. These tests included the modified Neurological Severity Score (mNSS) 23, the adhesive‐removal test, the foot‐fault test and the inclined plane test. For all testing, the observer was blinded to the experimental grouping. mNSS is a comprehensive evaluation method that assesses motor, sensory, reflex and balance function. It is graded on a scale of 0–18, with higher scores reflecting more severe deficits.
The adhesive‐removal test 24, 25, 26 evaluates somatosensory and motor deficits. All mice were trained for 3 consecutive days before undergoing MCAO. After the mice were familiarized with the testing environment, the tester attached two adhesive paper dots gently to the distal region of each forelimb. The mice would naturally attempt to remove the paper dots from their limbs. After 3 days of training, all mice were able to remove both paper dots from their forelimbs. The elapsed time spent tearing off the paper dots from each forelimb was recorded for later statistical analysis. Three trials were performed using an intertrial interval of 5 min. The mice were then separated into different groups for other assessments.
In the foot‐fault test 27, a horizontal grid floor is used to assess locomotor function as the mouse crosses the surface. Briefly, paw misplacements (fault foots) were recorded for each run of the test (2 min). When the mouse's forelimb or hind limb fell into one of the grid openings, a foot fault was recorded. The number of foot faults for the three groups was evaluated statistically.
The inclined plane test was used to test the motor functions 28. Mice were placed on a grooved, 1‐mm‐thick rubber surface and coaxed down an inclined plane platform. The incline of the platform was increased gradually until the mouse crept down from its starting position. The last incline position that the mouse maintained for 5 s without sliding down was recorded.
Infarct volume measurement
To assess the extent of anatomical damage after 60 min of vessel occlusion in our MCAO procedure, we perfused mice 24 or 72 h after MCAO surgery. Mice were anaesthetized with chloral hydrate (30 mg/kg, i.p.), and then perfused with 20 ml cold phosphate‐buffered saline (PBS) (pH 7.4, 4°C) through the left ventricle. The brains were removed rapidly for measurement of infarct volumes, as described previously 29. Briefly, the brain was cut into five coronal blocks (2 mm thick), starting from the rostral tip of the cerebrum to the caudal tip. The five blocks were stained with 2% 2, 3, 5‐tripenyltetrazolium chloride (TTC; Sigma, St Louis, MO, USA) at 37°C for 20 min, followed by fixation in 4% paraformaldehyde at 4°C for 1 h. After TTC staining, undamaged tissue appeared deep red, and infarcted tissue remained white. Image‐Pro‐Plus, version 6.0 image analysis software (Media Cybernetics, Washington, DC, USA) was used for determining the infarct volumes 30. Infarct volumes are shown as a percentage of the volume of the corresponding areas in the whole brain to the infarct.
Immunofluorescent staining and analysis
Mice were anaesthetized with chloral hydrate (30 mg/kg, i.p.), and then perfused intraventricularly with PBS (pH 7.4, 4°C). Brains were removed rapidly and then immersed in 30% sucrose with 4% paraformaldehyde overnight at 4°C. After this whole‐brain fixation and cryoprotection procedure, the brains were whole‐frozen in liquid nitrogen. A standard cryostat (Leica Microsystems LM3050S, Shanghai, China) was used to slice coronal sections (8 μm), which were mounted on slides and stored at −80°C until staining. After 30 min at room temperature, the mounted sections were blocked in 3% bovine serum albumin (BSA) for 30 min at 37°C. The sections were then incubated with primary antibodies overnight at 4°C. Primary antibodies included rabbit anti‐interleukin (IL)‐17 (1 : 200; Abcam, Cambridge, UK) and mouse anti‐CD4 (1 : 200; Santa Cruz Biotechnology, Dallas, TX, USA). Sections were next washed in cold PBS three times and incubated with secondary antibodies for 1 h at room temperature. Secondary antibodies included: Alexa Fluor® 594‐conjugated donkey anti‐rabbit IgG (H + L) (1 : 1000; Thermo Fisher Scientific, Waltham, MA, USA) and Alexa Fluor® 488‐conjugated donkey anti‐mouse IgG (H + L) (1 : 1000; Thermo Fisher Scientific). After five washes in cold PBS (10 min each), the sections were coverslipped, using fluoro‐shield mounting medium containing 4′,6‐diamidino‐2‐phenylindole (DAPI) (Abcam) to counterstain cell nuclei. The sections were imaged using a fluorescence microscope (Nikon, Sendai, Ishinomaki, Japan). The number of positively stained CD4 cells and IL‐17 cells were determined per mm2 in the penumbra of the hemisphere ipsilateral to the MCAO from three randomly selected microscopic fields (×200 magnification), and the average results were used for analysis 31.
Flow cytometric analysis [fluorescence activated cell sorter (FACS)]
Seventy‐two h after MCAO, single‐cell suspensions were prepared from the brains of mice from each group (six per group) for flow cytometric analysis. Natural killer (NK) cells (CD3−NK1.1+) and lymphocyte subpopulations (CD4+ T cells, CD8+ T cells and B cells) were counted by FACS. First, brains were mechanically homogenized separately by passing the tissues through a 40‐μm nylon cell strainer (Becton Dickinson, San Jose, VA, USA) in cold PBS on ice. Then the cell homogenates were collected and centrifuged at 400 g for 10 min. The pellets were then resuspended and subjected to gradient centrifugation in 5 ml of 30% Percoll (GE Healthcare Bio Science AB, Björkgatan, Uppsala, Sweden), underlain with 5 ml of 60% Percoll. The gradient suspension was then centrifuged at 500 g for 20 min. Cells were recovered from the boundary of the slope layers and washed with PBS in preparation for FACS.
All antibodies for FACS analyses were purchased from Biolegend (San Diego, CA, USA). Before staining, 1 × 106 of the recovered cells were first suspended in tubes in 100 μl of 1% BSA/PBS. For staining, we used anti‐mouse phycoerythrin‐cyanin 7 (PE‐Cy7)‐conjugated CD3 (1 : 100) and fluorescein isothiocyanate (FITC)‐conjugated CD4 (1 : 200) for CD4+ T cells, PE‐Cy7‐conjugated CD3 (1 : 100) and FITC‐conjugated CD8 (1 : 200) for CD8+ T cells, PE‐Cy7‐conjugated CD3 (1 : 100) and FITC‐conjugated CD19 (1 : 100) for B cells and PE‐Cy7‐conjugated CD3 (1 : 100) and peridinin chlorophyll (PerCP)‐conjugated NK1.1 (1 : 100) for NK cells.
For assessing CD4+CD25+ Treg cells, brain leucocytes were stained for the surface markers with PerCP‐conjugated anti‐CD4 (1 : 200) and FITC‐conjugated anti‐CD25 (1 : 100) antibodies for 30 min at room temperature. The cells were fixed with fixation buffer, washed twice with permeabilization buffer (eBioscience, San Diego, CA, USA), and then stained with PE‐conjugated anti‐forkhead box protein 3 (FoxP3) (1 : 100) antibody in permeabilization buffer for 30 min at room temperature.
For Th1, Th2 and Th17 cell analysis, brain leucocytes were obtained as before and placed into 24‐well plates (1 × 106 cells/well), where they were stimulated with 2 µl of cell activation cocktail (with brefeldin A) (Biolegend) for 4 h at 37°C. Each vial of the cocktail contained 40·5 µM phorbol‐12‐myristate 13‐acetate, 669·3 µM ionomycin and 2·5 mg/ml brefeldin A in dimethylsulphoxide (DMSO). After stimulation, the cells were collected into 15‐ml conical polypropylene centrifuge tubes and washed twice with PBS. The cells were transferred to polystyrene flow cytometry tubes, and then centrifuged at 400 g for 5 min. The resulting pellet was resuspended in 1% BSA and surface‐stained with PerCP‐conjugated anti‐CD4 (1 : 200) antibody for 30 min at room temperature. After washing and fixation, cells were again washed twice with permeabilization buffer, and then stained with the following antibodies dissolved in permeabilization buffer for 30 min at room temperature: FITC‐conjugated anti‐IFN‐γ (1 : 100) for Th1 cells, PE‐conjugated anti‐IL‐17 (1 : 100) for Th17 cells or FITC‐conjugated anti‐IL‐4 (1 : 100) antibody for Th2 cells. Cells stained with isotype‐control antibody were used to create background staining and to set analysis quadrants before calculating the percentage of positive cells. Flow cytometric data were acquired with a FACS Aria™ flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and analysed with FlowJo software, version 7.6.1 (http://www.flowjo.com; Tree Star Inc., Ashland, OR, USA).
Western blot
Total protein was obtained from brain tissues with Trizol (Ambion, Waltham, MA, USA). Protein concentration was measured using a Protein Assay Kit (Beyotime, Hangzhou, China). Samples were separated on 12% sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) gels from 80 to 120 V, and then transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA) for 1·5 h at 4°C, 80 V. Membranes were blocked in 5% BSA solution for 1 h at room temperature, followed by incubation with primary antibody overnight at 4°C. The membranes were then washed three times (10 min each), incubated with a horseradish peroxidase‐coupled secondary antibody for 1 h, and washed again three times (10 min each). All incubations were performed in Tris‐buffered saline (TBS buffer) with 0·1% Tween‐20. The primary antibodies were rabbit monoclonal antibody (mAb) phosphorylated signal transducer and activator of transcription 3 (pSTAT‐3) (Tyr705) (1 : 2000; Cell Signaling Technology, Danvers, MA, USA); mouse mAb STAT‐3 (1 : 1000; Cell Signaling Technology) and mouse anti‐glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) antibody (1 : 5000; Abcam). The next day, membranes were washed and incubated with goat anti‐rabbit immunoglobulin (Ig)G or goat anti‐mouse IgG conjugated to horseradish peroxidase (1 : 5000; Transgen Biotech, Beijing, China) for 1 h at room temperature. The protein‐specific signals were captured with a Bio‐Rad 721BR08844 Gel Doc Imager (Bio‐Rad, Hercules, CA, USA).
Enzyme‐linked immunosorbent assay (ELISA) for determining cytokine profiles
Brain tissue was taken from ischaemic regions. Simultaneous quantitation of 12 cytokines (IL‐1α, IL‐1β, IL‐2, IL‐4, IL‐6, IL‐10, IL‐12, IL‐17A, interferon (IFN)‐γ, tumour necrosis factor (TNF)‐α, granulocyte colony‐stimulating factor (G‐CSF) and granulocyte–macrophage colony‐stimulating factor (GM‐CSF) was accomplished using a multi‐analyte ELISArray Kit (Qiagen, Düsseldorf, Germany). Procedures followed the manufacturer's protocol. Each sample was analysed in triplicate from individual mouse brains (six mice per group), and results are expressed as mean optical density (OD) ± standard error of the mean (s.e.m.).
Quantitative reverse transcription–polymerase chain reaction (qRT–PCR)
Total RNA was extracted from the ischaemic hemisphere using Trizol reagent (Ambion), according to the manufacturer's instructions. RNA concentration was quantified with ultraviolet spectrophotometry at 260/280 nm. Then, cDNA was transcribed from 5 μg of RNA with TransScript First‐Strand cDNA Synthesis SuperMix (Transgen Biotech). PCR was performed on an Opticon 2 real‐time PCR detection system (Bio‐Rad) with SYBR gene PCR Master Mix (Roche, Basel, Switzerland). The primers are listed in Table 1. Samples were run in duplicate at 95°C for 10 min, then 40 cycles at 95°C for 15 s, and finally at 60°C for 1 min. Following automatic analysis with an ABI Stepone Plus instrument (Thermo Fisher), the results were calculated using the 2–ΔΔCt method. The expression levels of the mRNAs are reported as fold changes versus control (six mice per group).
Table 1.
Primer sequences for quantitative reverse transcription–polymerase chain reaction (qRT–PCR)
| Genes | Primers (forward and reverse) |
|---|---|
| GAPDH | (F) TCATTGACCTCAACTACATGGT |
| (R) CTAAG CAGTTGGTGGTGCAG | |
| IL‐1β | (F) GGAGAAGCTGTGGCAGCTA |
| (R) GCTGATGTACCAGTTGGGGA | |
| IL‐6 | (F) CCGGAGAGGAGACTTCACAG |
| (R) TGGTCTTGGTCCTTAGCCAC | |
| TNF‐α | (F) GACCCTCACACTCAGATCAT |
| (R) TTGAAGAGAACCTGGGAGTA | |
| IL‐12 | (F) ATGATGACCCTGTGCCTTGG |
| (R) CCTTTGGGGAGATGAGATGT | |
| IL‐23 | (F) AGCGGGACATATGAATCTACTAAGAGA |
| (R) GTCCTAGTAGGGAGGTGTGAAGTTG | |
| IL‐17A | (F) CTCCAGAAGGCCCTCAGACTAC |
| (R) GCTTTCCCTCCGCATTGACACAG | |
| RORγt | (F) GTG GAC TTC GTT TGA GGA AAC |
| (R) ACT TCC TCT GGT AGC TGG TCA C |
GAPDH = glyceraldehyde 3‐phosphate dehydrogenase; IL = interleukin; TNF = tumour necrosis factor; RORγt = retinoic acid‐related orphan receptor gamma T.
Statistical analysis
All data are expressed as mean values ± s.e.m. Statistical analyses were performed using Student's t‐test for comparison of two groups and one‐way analysis of variance (anova), followed by the Bonferroni post‐hoc test for multiple group comparisons (GraphPad Prism version 5.0; La Jolla, CA, USA); P < 0·05 was considered significant.
Results
PR‐957 reduces neurological deficits and infarct volume
Initially, mice subjected to MCAO received PR‐957 at doses of 5, 10, 20 or 30 mg/kg administered as a single i.p. injection, and then the effect of each dose was assessed. Both the 20 and 30 mg/kg doses reduced the clinical score and infarct volumes significantly compared to the vehicle group (Fig. 1a,b; P < 0·05). However, there were no significant differences in mNSS scores or infarct volumes between animals receiving the 20 mg/kg dose and of those receiving the 30 mg/kg dose. We determined that a PR‐957 dose of 20 mg/kg was the optimal dose, as it produced the most significant improvement in neurobehavioural tests. The 20 mg/kg dose also does not exceed the maximum tolerated dose of PR‐957 19. Thus, all subsequent experiments were performed with 20 mg/kg PR‐957 administered post‐MCAO.
Figure 1.
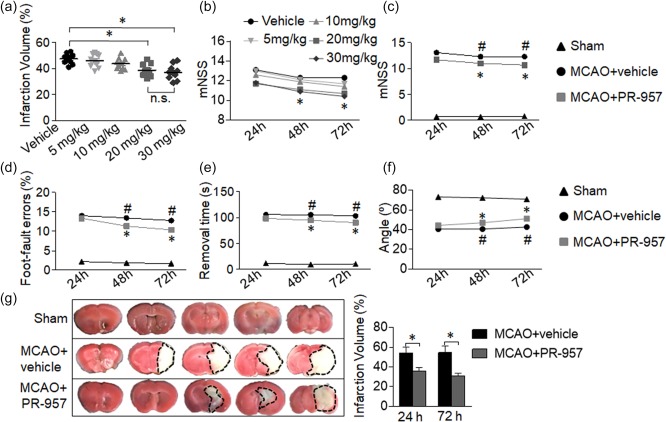
PR‐957 treatment alleviates neurological behavioral deficits and reduces infarct volumes after middle cerebral artery occlusion (MCAO) and reperfusion. Multiple behavioural and anatomical tests were performed in mice that underwent 60 min of ischaemia by MCAO, followed by 24, 48 or 72 h of reperfusion. (a,b) Infarct volumes were determined histologically at 72 h after MCAO, and modified Neurological Severity Score (mNSS) scores were assessed 24, 48 or 72 h after mice received the indicated doses of PR‐957. (a) Dispersion of infarct volume percentages of individual mice for each group. The optimum dose was 20 mg/kg in our experiments (n = 10 mice per group). (c) mNSS was used to evaluate neurological deficits after MCAO, with or without PR‐957 treatment or vehicle (n = 10 mice per group). (d–f) Mean behavioural results of sensorimotor coordination tests, including foot‐fault (d), adhesive removal (e) and inclined plane (f) tests at the indicated times after MCAO (n = 10 mice per group). (g) Representative 2, 3, 5‐tripenyltetrazolium chloride (TTC) staining of coronal brain sections at five rostral–caudal planes for the indicated treatment groups. Infarct brain tissue appears white, whereas the non‐infarcted region is red. Quantitation of brain infarct volume (n = 6 mice per group). MCAO, MCAO + vehicle administration; MCAO + PR‐957, MCAO with PR‐957 administration. Data are presented as means ± standard error of the mean (s.e.m.); *P < 0·05, MCAO + PR‐957 compared to MCAO + vehicle; # P < 0·05, MCAO + vehicle compared to sham. The experiment was repeated twice with similar results.
After MCAO, reperfusion took place for 24, 48 or 72 h. Neurological function was then evaluated after each of these time‐points. In the PR‐957‐treated group, neurological function scores 72 h after MCAO were better than those in the MCAO + vehicle group (Fig. 1c–f). We observed large deficits in the MCAO + vehicle group compared to the sham‐operated group, and PR‐957 treatment reduced these deficits. Results from the mNSS tests exemplified this improvement (Fig. 1c; P < 0·05 at 48 h; P < 0·01 at 72 h). The MCAO group had more foot‐faults compared to the sham‐operated group. When PR‐957 was administered to MCAO mice, we observed markedly improved foot‐fault performance (Fig. 1d; P < 0·05 at 48 h; P < 0·01 at 72 h). In the MCAO group, mice spent more time trying to remove the paper dots on their forelimbs than the mice in the sham‐operated group. Administration of PR‐957 shortened the average removal time (Fig. 1e; P < 0·05 at 48 h; P < 0·01 at 72 h). MCAO animals were impaired compared to sham‐operated animals, and PR‐957 prevented the motor function impairment seen after MCAO (Fig. 1f; P < 0·01).
To evaluate the effects of PR‐957 on brain injury after focal ischaemia and reperfusion in mice, we measured infarct volumes 24 and 72 h after MCAO. TTC staining clearly distinguished infarct areas from unaffected areas. As shown in Fig. 1, treatment with PR‐957 after ischaemic injury reduced the infarct volume significantly 24 h after MCAO (Fig. 1g; P < 0·05). At 72 h, the MCAO + PR‐957 group yielded nearly the same result (Fig. 1g; P < 0·01). These results indicate that PR‐957 treatment effectively attenuates the severity of neurological damage from brain ischaemia, and therefore appears to render some neuroprotection in MCAO mice.
PR‐957 suppresses CD4+ T cell infiltration and Th17 differentiation in brains of MCAO mice
With the following assays, we sought to understand the mechanism by which PR‐957 protects against brain damage after MCAO. First, we performed flow cytometric analysis of cellular components from the brains of sham‐operated mice, MCAO + vehicle mice and MCAO + PR‐957 mice. The gating strategy for leucocytes appears in Fig. 2a. Then, counts of CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD8+), NK cells (CD3−NK1·1+) and B cells (CD3−CD19+) from the brains of sham‐operated mice, MCAO + vehicle mice, and MCAO + PR‐957 mice obtained at 72 h after MCAO were analysed (Fig. 2b–e). While fewer CD4+ T cells were present in the MCAO + PR‐957 group (Fig. 2b; P < 0·01), the numbers of CD8+ T cells showed no obvious change (Fig. 2c). No change in the counts of B cells (Fig. 2d) or NK cells (Fig. 2e) was observed 72 h after MCAO among these three groups.
Figure 2.
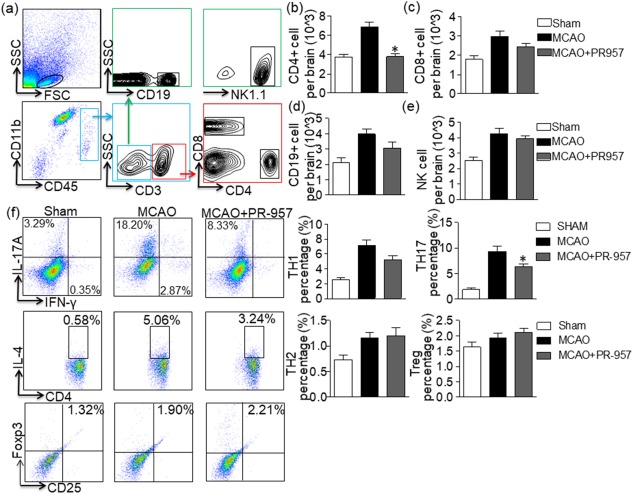
PR‐957 decreases the number of CD4+ T cells in the brain after middle cerebral artery occlusion (MCAO) and suppresses T helper type 17 (Th17) differentiation. At 72 h after MCAO, cells were harvested from the brains of mice treated with PR‐957 (MCAO + PR‐957), vehicle (MCAO + vehicle) and sham operation (sham), and flow cytometric analysis was conducted. (a) Gating strategy for leucocytes isolated from the brain. (b–e) Counts of CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD8+), natural killer (NK) cells (CD3−NK1.1+), and B cells (CD3−CD19+) in the indicated groups. Flow cytometric analysis showed a significant decline in CD4+ T cells in the mice treated with PR‐957 group compared to the group treated with vehicle. PR‐957 had no significant effect on CD8+ T, B or NK cells. Dot‐plots representing the distribution of different cell types counted during fluorescence activated cell sorter (FACS) for individual cytokines. The areas enclosed in boxes indicate the percentages of CD4+interferon (IFN)‐γ+ cells, CD4+interleukin (IL)‐17+ cells, CD4+IL‐4+ cells and forkhead box protein 3 (FoxP3+) CD25+ cells among the CD4+ T cells, which are presented quantitatively in the bar graphs of panel (f). Data are presented as means ± standard error of the mean (s.e.m.); # P < 0·05, compared with sham; *P < 0·05, compared with MCAO + vehicle; n = 6 mice per group. The experiment was repeated twice with similar results.
Next, we determined to investigate the influences of PR‐957 on CD4+ T cell subpopulations in the brain 72 h after MCAO. Using the Th cell marker CD4 and IFN‐γ, IL‐4, IL‐17, or FoxP3, we completed flow cytometric analysis of cellular components from the brains of MCAO mice and sham‐operated mice. PR‐957 treatment decreased the proportion of Th17 (CD4+IL‐17+) cells 72 h after MCAO, but there was no significant change in the counts of Th1, Th2 or Treg cells (Fig. 2f; P < 0·05 for Th17 cells).
Few CD4+ T cells infiltrated the CNS in the sham‐operated group. Compared to the MCAO + vehicle group, double‐immunofluorescent staining of brain tissue from cortical and subcortical regions (see Fig. 3b) of MCAO + PR‐957 mice showed decreased expression of Th17 (Fig. 3a–c; P < 0·01). In summary, PR‐957 suppresses CD4 T cell infiltration and Th17 differentiation after ischaemic stroke.
Figure 3.
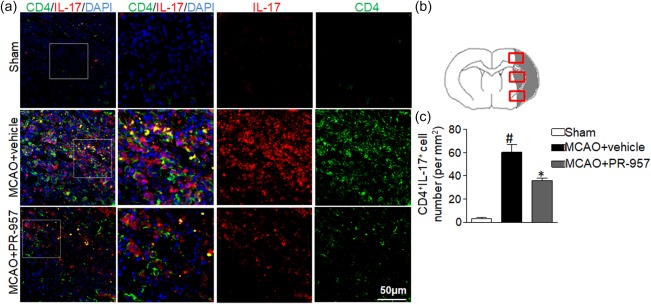
PR‐957 lessens T helper type 17 (Th17) cell infiltration in brains of middle cerebral artery occlusion (MCAO) mice. (a) Brain sections from the three groups of mice were stained for interleukin (IL)‐17 and CD4 using double‐fluorescent immunohistochemistry to analyse Th17 cell brain infiltration after MCAO [4′,6‐diamidino‐2‐phenylindole (DAPI) counterstain]. (b) Schematic representation of a coronal brain section with analysis fields overlain in the cortex and striatum, from which Th17‐positive cells per mm2 were counted. In the sham‐operated group, the IL‐17‐positive staining was difficult to detect as very few IL‐17‐positive cells had infiltrated. However, in the ipsilateral ischaemic hemisphere 72 h after MCAO, IL‐17 cells became activated. The PR‐957‐treated group had fewer Th17‐positive cells compared with the MCAO + vehicle group. (c) Quantitation of brain‐infiltrated Th17 cells in the indicated groups. There were significantly fewer Th17‐positive cells in the PR‐957‐treated group than in the MCAO + vehicle group. Bars and error bars represent means ± standard error of the mean (s.e.m.); # P < 0·05, compared with sham‐operated; *P < 0·05, compared with MCAO + vehicle; n = 6 mice in each group. Experiments were repeated three times, yielding similar results. Same labelling conventions as previous figures.
PR‐957 reduces pSTAT‐3 protein levels and retinoic acid‐related orphan receptor gamma T (RORγt) mRNA levels in brains of MCAO mice
To determine whether PR‐957 affects STAT‐3 and RORγt, two important transcriptional regulators of Th17 cell differentiation, we analysed STAT‐3 and RORγt expression in the brains of mice 72 h after MCAO. We measured pSTAT‐3 (Tyr705) protein levels and RORγt mRNA levels via Western blotting (Fig. 4a–c) and qRT–PCR (Fig. 4d), respectively. Western blotting revealed that the MCAO + PR‐957 group expressed less pSTAT‐3 (Tyr705) compared to the MCAO + vehicle group (Fig. 4b; P < 0·05). However, STAT‐3 protein level did not differ significantly throughout these groups (Fig. 4c). Furthermore, RORγt mRNA expression decreased significantly in the brains of MCAO + PR‐957 mice compared to the brains of MCAO + vehicle mice (Fig. 4d; P < 0·05). Overall, PR‐957 reduced the phosphorylation of STAT‐3 at the protein level, causing a reduction of RORγt at the transcriptional level after MCAO.
Figure 4.
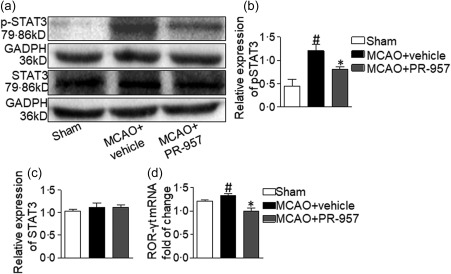
PR‐957 treatment reduces phosphorylated signal transducer and activator of transcription 3 (pSTAT‐3) protein levels and retinoic acid‐related orphan receptor gamma T (RORγt) mRNA levels in brains of middle cerebral artery occlusion (MCAO) mice. In the brain, the penumbra area (or complementary region in sham‐operated mice) of each mouse was processed for Western blotting analysis. (a) Western blots of pSTAT‐3 (Tyr705) and STAT‐3 protein compared to glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) standard. Compared with the MCAO + vehicle group, the PR‐957‐treated group expressed less pSTAT‐3, while the expression of STAT‐3 did not differ in the three groups. (b,c) Quantitation of Western blots for pSTAT‐3 and STAT‐3. Relative expression of pSTAT‐3 in the MCAO + PR‐957 group was reduced significantly compared to that in the MCAO + vehicle groups, whereas the level of STAT‐3 was not significantly different. (d) The mRNA expression of RORγt in the PR‐957‐treated group was significantly lower than in the MCAO + vehicle groups. Bars and error bars represent means ± standard error of the mean (s.e.m.); # P < 0·05, compared with Sham; *P < 0·05, compared with MCAO; n = 6 mice per group. The experiments were repeated three times, yielding similar results. Same labelling conventions as previous figures.
PR‐957 moderates the inflammatory milieu in post‐ischaemic brain
We studied the cytokine profiles in the mouse brain by ELISA and qRT–PCR. We assessed the levels of the following inflammatory cytokines: IL‐1α, IL‐1β, IL‐2, IL‐4, IL‐6, IL‐10, IL‐12, IL‐17A, IFN‐γ, TNF‐α, G‐CSF and GM‐CSF. IL‐1β, IL‐6, IL‐12, IL‐17A, IFN‐γ and TNF‐α were reduced significantly in the MCAO + PR‐957 group compared to those in the MCAO + vehicle group (Fig. 5a; P < 0·05 for IL‐1β, IL‐6, IL‐12, IL‐17A and IFN‐γ; P < 0·01 for TNF‐α). However, protein levels of IL‐1α, IL‐2, IL‐4, IL‐10, G‐CSF and GM‐CSF were not significantly different between the MCAO + vehicle group and the MCAO + PR‐957 group.
Figure 5.
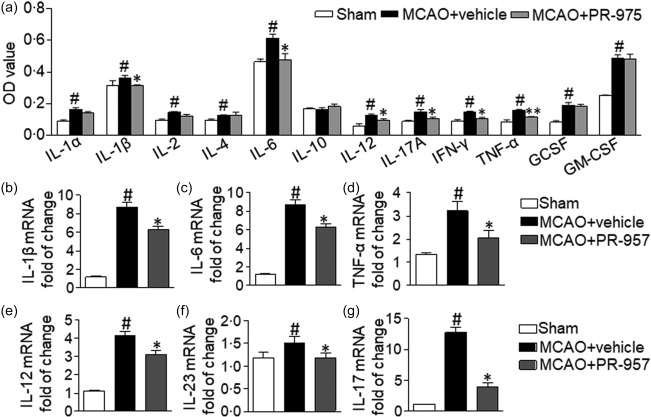
Effect of PR‐957 on inflammatory cytokines in ischaemic brain following middle cerebral artery occlusion (MCAO). (a) Cytokines in the supernatants of brain tissues from the penumbra area (or complementary region in sham‐operated mice) were analysed using enzyme‐linked immunosorbent assay (ELISA). Interleukin (IL)‐1α, IL‐1β, IL‐2, IL‐4, IL‐6, IL‐10, IL‐12, IL‐17A, interferon (IFN)‐γ, tumour necrosis factor (TNF)‐α, granulocyte colony‐stimulating factor (GCSF) and granulocyte–macrophage colony‐stimulating factor (GM‐CSF) were included in the panel of analysed factors (n = 6 mice per group). (b–g) Quantitative reverse transcription–polymerase chain reaction (qRT–PCR) was used to detect mRNA expression of IL‐1β (b), IL‐6 (c), TNF‐α (d), IL‐12 (e), IL‐23 (f) and IL‐17 (g), in the indicated groups. Same labelling conventions as previous figures (n = 6 mice per group). Bars and error bars represent means ± standard error of the mean (s.e.m.); # P < 0·05 compared with sham; *P < 0·05 versus the MCAO + vehicle group. The experiment was repeated twice with similar results.
We also studied the cytokine profiles in the mouse brain by qRT–PCR. The mRNA levels of IL‐1β, IL‐6, IL‐12, IL‐17, IL‐23 and TNF‐α were decreased in the MCAO + PR‐957 group compared to those in the MCAO + vehicle group (Fig. 5b–g; P < 0·05). These results suggest that PR‐957 could alleviate inflammatory milieu after ischaemic stroke. It appears that PR‐957 mediated the anti‐inflammatory effect after experimentally induced stroke and reperfusion. Presumably, therefore, PR‐957 is involved in regulating the balance between proinflammatory and anti‐inflammatory Th cells in MCAO mice via inhibiting the STAT‐3/RORγt pathway (Fig. 6).
Figure 6.
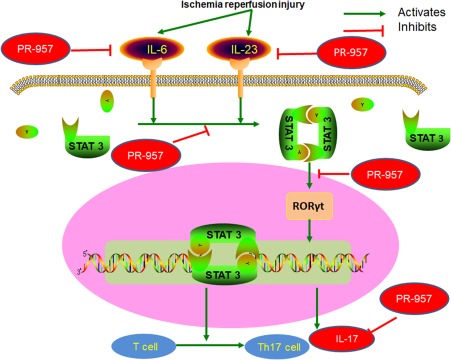
Possible mechanism to explain the effect of PR‐957 on T helper type 17 (Th17) cell differentiation. Schematic diagram of PR‐957's interaction with T cells and cytokines in brain tissue after stroke. PR‐957 exerts neuroprotection by phenotypical modulation of inflammatory cytokines and suppression of signal transducer and activator of transcription 3 (pSTAT‐3)/retinoic acid‐related orphan receptor gamma T (RORγt) signalling.
Discussion
We hypothesized that PR‐957, a selective inhibitor of the immunoproteasome subunit LMP7 and an anti‐inflammatory agent, might mitigate inflammation‐associated neuronal damage after ischaemic stroke. The results of the present study support the hypothesis that PR‐957 is neuroprotective in MCAO mice. Our experiments with a classic animal model of I/R injury, caused by transient MCAO, demonstrate strongly that PR‐957 is capable of reducing infarct volume, diminishing the associated neurological deficits, and attenuating the inflammatory reaction. The neuroprotective effect of PR‐957 aligns well with our observation in the present study that lymphocyte concentration and Th17 cell accumulation were inhibited substantially in brains undergoing such injury and with the suppression of proinflammatory cytokines. PR‐957 treatment in MCAO mice inhibited the STAT‐3 signalling pathway, which corresponded with decreased RORγt levels. This, in turn, contributed to a decline of Th17 cells in the brain. Moreover, protein and mRNA levels of proinflammatory cytokines were down‐regulated significantly in the brains of MCAO mice after PR‐957 treatment.
Previous studies have emphasized that T lymphocyte‐mediated inflammation plays a central role during ischaemic brain injury. Following acute ischaemic stroke, T lymphocytes become activated, infiltrate through the brain–blood barrier and accumulate in ischaemic core tissue 6. T lymphocytes begin to infiltrate into this core 24 h after ischaemic stroke and reach their peak 3 days after onset 32, 33. After ischaemic stroke an inflammatory cascade breaks out, in which T cells and their subpopulations take part in inflammation‐mediated brain injury 7, 34. Prior research shows that IL‐17 plays a more important role in cerebral I/R injury than IFN‐γ 35. IL‐17 is a proinflammatory cytokine produced by Th17 cells and γδT cells. The γδT cells infiltrate the infarct boundary zones in an early phase after ischaemic stroke, and then release a large amount of IL‐17. After a few days, Th17 cells are the chief source of IL‐17 36. Also, IL‐17A secreted by Th17 cells may aggravate brain injury after ischaemic stroke. IL‐23 released by macrophages may also stimulate the production of IL‐17 37, 38. Besides this response, increased levels of TNF‐α and IL‐1 can also stimulate IL‐17 secretion 39.
In the present study, improvements of MCAO mice treated with PR‐957 were associated with significantly fewer Th17 cells in their brains compared to their vehicle‐treated counterparts. PR‐957 also reduced mRNA expression of IL‐1β, IL‐6, TNF‐α, IL‐12, IL‐17 and IL‐23 in the brains of MCAO mice. ELISAs of brain supernatants from MCAO mice showed the same downward trend in proinflammatory cytokines. Our results suggest that the PR‐957‐associated improvement of the inflammatory environment after MCAO was the basis for the neuroprotective effect documented here.
Considerable evidence indicates that CD4+CD25+FoxP3+ T cells play a key role in moderating immune responses under physiological and pathological conditions in the CNS through anti‐inflammatory cytokines 7, 40. However, our PR‐957‐treatment group did not differ in Treg cell counts compared to the vehicle‐treated control group (MCAO + vehicle) at 72 h post‐MCAO reperfusion. Additionally, studies show that LMP7 inhibition is effective in preventing differentiation of naive Th cells to polarized Th1 cells and Th17 cells in vivo and vitro 16, 17, 18, 19. Our flow cytometric results show that Th17 expression is reduced in MCAO brains from mice treated with PR‐957 compared to the control group (MCAO + vehicle). Similarly, double‐immunofluorescence staining of brain tissue from mice treated with PR‐957 also showed a reduction of IL‐17 expression compared to the control group. Nevertheless, the number of Th1 or Th2 cells was not statistically different in the latter experiment, similar to the flow cytometric results.
In a study of experimental autoimmune encephalomyelitis in which PR‐957 was administered, differentiation to Th17 or Th1 cells was impaired 16. This result is at variance with the present study. One possible reason for this may be related to the different animal models used in the two studies and/or differences in technical methods. Other research exploring the mechanism of PR‐957's involvement in the control of T cell expansion 18, 19 proposed initially that LMP7 is a regulator of cytokine production accompanying NF‐κB‐independent pathways 19. Additionally, the STAT‐3 pathway has been studied in a model of colitis, which is relevant to Th17 differentiation. This report posited that the down‐regulation by PR‐957 of RORγt mRNA production is a factor in Th17 differentiation, due most probably to reduced phosphorylation of STAT‐3 18. Although the molecular mechanism governing Th17 cell differentiation is somewhat imprecise, IL‐6 and IL‐23 may affect Th17 cell differentiation synergistically 41, 42. Furthermore, STAT‐3 may serve as a selective STAT protein in cytokine‐mediated Th17 cell polarization in the presence of IL‐6 and IL‐23 43. In the present study, PR‐957 not only down‐regulated the expression of IL‐6 and IL‐23, but also decreased STAT‐3 phosphorylation in naive CD4+ T cells. These changes might result from a reduction of RORγt, the key transcription factor for Th17 cell differentiation and IL‐17 production. As shown in Fig. 6, the reduction of STAT‐3 phosphorylation may suppress Th17 cell differentiation directly, thereby supporting the participation of PR‐957 in the STAT‐3 pathway. In our study, PR‐957 reduced the phosphorylation of STAT‐3 at the protein level, causing a reduction of RORγt and IL‐17 at the transcriptional level.
In accord with a demonstration that inhibition of LMP2 and LMP7 significantly alleviates inflammatory reactions and protects against focal cerebral ischaemia injury in MCAO rats 13, our present study demonstrated that PR‐957 is a selective immunoproteasome inhibitor in a murine model of MCAO. Our results also support the idea that inhibition of LMP7 provides neuroprotection after MCAO. However, in contrast with that former study, which involved the regulation of NF‐κB activation in ischaemic stroke, we provide new evidence that PR‐957 inhibits the STAT‐3 pathway and, thereby, plays a central role in regulating the balance between proinflammatory and anti‐inflammatory Th cells in MCAO mice.
In summary, PR‐957 exerted a neuroprotective effect against ischaemic stroke through modulating Th17 differentiation and regulating the cytokine profile of immune responses in the brain. This demonstration that PR‐957 attenuates inflammation in MCAO mice might offer a strategy for targeting the immune system with therapeutic intervention after stroke.
Author contributions
X. M. and Y. G. designed the experiments and wrote the manuscript. Y. G., X. C. and D. L. carried out the general studies and contributed to writing the paper. H. L., Y. D., R. H., and Y. S. conducted the experiments. All authors reviewed and approved the final manuscript.
Disclosure
None.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Table S1. Numbers of mice used to for dose optimization of PR‐957 treatment.
Table S2. Numbers of mice used in the formal experiments.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81401361 to X.F.M.).
References
- 1. Kim AS, Johnston SC. Temporal and geographic trends in the global stroke epidemic. Stroke 2013; 44:S123–5. [DOI] [PubMed] [Google Scholar]
- 2. Macrez R, Ali C, Toutirais O et al Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol 2011; 10:471–80. [DOI] [PubMed] [Google Scholar]
- 3. Worthmann H, Tryc AB, Goldbecker A et al The temporal profile of inflammatory markers and mediators in blood after acute ischaemic stroke differs depending on stroke outcome. Cerebrovasc Dis 2010; 30:85–92. [DOI] [PubMed] [Google Scholar]
- 4. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989; 20:84–91. [DOI] [PubMed] [Google Scholar]
- 5. Bahor Z, Liao J, Macleod MR et al Risk of bias reporting in the recent animal focal cerebral ischaemia literature. Clin Sci 2017; 131:2525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brait VH, Arumugam TV, Drummond GR, Sobey CG. Importance of T lymphocytes in brain injury, immunodeficiency, and recovery after cerebral ischemia. J Cereb Blood Flow Metab 2012; 32:598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arumugam TV, Granger DN, Mattson MP. Stroke and T‐cells. Neuromolecular Med 2005; 7:229–42. [DOI] [PubMed] [Google Scholar]
- 8. Appel SH. CD4+ T cells mediate cytotoxicity in neurodegenerative diseases. J Clin Invest 2009; 119:13–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kramer JM, Gaffen SL. Interleukin‐17: a new paradigm in inflammation, autoimmunity, and therapy. J Periodontol 2007; 78:1083–93. [DOI] [PubMed] [Google Scholar]
- 10. Liesz A, Suri‐Payer E, Veltkamp C et al Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med 2009; 15:192–9. [DOI] [PubMed] [Google Scholar]
- 11. Basler M, Mundt S, Bitzer A, Schmidt C, Groettrup M. The immunoproteasome: a novel drug target for autoimmune diseases. Clin Exp Rheumatol 2015; 33:S74–9. [PubMed] [Google Scholar]
- 12. Lu L, Wang H. Transient focal cerebral ischemia upregulates immunoproteasomal subunits. Cell Mol Neurobiol 2012; 32:965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen X, Zhang X, Wang Y et al Inhibition of immunoproteasome reduces infarction volume and attenuates inflammatory reaction in a rat model of ischaemic stroke. Cell Death Dis 2015; 6:e1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moebius J, van den Broek M, Groettrup M, Basler M. Immunoproteasomes are essential for survival and expansion of T cells in virus‐infected mice. Eur J Immunol 2010; 40:3439–49. [DOI] [PubMed] [Google Scholar]
- 15. Basler M, Beck U, Kirk CJ, Groettrup M. The antiviral immune response in mice devoid of immunoproteasome activity. J Immunol 2011; 187:5548–57. [DOI] [PubMed] [Google Scholar]
- 16. Basler M, Mundt S, Muchamuel T et al Inhibition of the immunoproteasome ameliorates experimental autoimmune encephalomyelitis. EMBO Mol Med 2014; 6:226–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Basler M, Dajee M, Moll C, Groettrup M, Kirk CJ. Prevention of experimental colitis by a selective inhibitor of the immunoproteasome. J Immunol 2010; 185:634–41. [DOI] [PubMed] [Google Scholar]
- 18. Kalim KW, Basler M, Kirk CJ, Groettrup M. Immunoproteasome subunit LMP7 deficiency and inhibition suppresses Th1 and Th17 but enhances regulatory T cell differentiation. J Immunol 2012; 189:4182–93. [DOI] [PubMed] [Google Scholar]
- 19. Muchamuel T, Basler M, Aujay MA et al A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nat Med 2009; 15:781–7. [DOI] [PubMed] [Google Scholar]
- 20. Fietta P, Delsante G. Proteasomes and immunoproteasomes. Riv Biol 2010; 103:29–50. [PubMed] [Google Scholar]
- 21. Orre M, Kamphuis W, Dooves S et al Reactive glia show increased immunoproteasome activity in Alzheimer's disease. Brain 2013; 136:1415–31. [DOI] [PubMed] [Google Scholar]
- 22. Karp NA, Meehan TF, Morgan H et al Applying the ARRIVE Guidelines to an In Vivo Database. PLOS Biol 2015; 13:e1002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen J, Sanberg PR, Li Y et al Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 2001; 32:2682–8. [DOI] [PubMed] [Google Scholar]
- 24. Bouet V, Boulouard M, Toutain J et al The adhesive removal test: a sensitive method to assess sensorimotor deficits in mice. Nat Protoc 2009; 4:1560–4. [DOI] [PubMed] [Google Scholar]
- 25. Freret T, Bouet V, Leconte C et al Behavioral deficits after distal focal cerebral ischemia in mice: usefulness of adhesive removal test. Behav Neurosci 2009; 123:224–30. [DOI] [PubMed] [Google Scholar]
- 26. Zhang Y, Xing S, Zhang J et al Reduction of beta‐amyloid deposits by gamma‐secretase inhibitor is associated with the attenuation of secondary damage in the ipsilateral thalamus and sensory functional improvement after focal cortical infarction in hypertensive rats. J Cereb Blood Flow Metab 2011; 31:572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou Y, Lekic T, Fathali N et al Isoflurane posttreatment reduces neonatal hypoxic‐ischaemic brain injury in rats by the sphingosine‐1‐phosphate/phosphatidylinositol‐3‐kinase/Akt pathway. Stroke 2010; 41:1521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pajoohesh‐Ganji A, Byrnes KR, Fatemi G, Faden AI. A combined scoring method to assess behavioral recovery after mouse spinal cord injury. Neurosci Res 2010; 67:117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu G, Zhang F, Hu Y et al Genetic variants and multiple sclerosis risk gene SLC9A9 expression in distinct human brain regions. Mol Neurobiol 2016; 54:6820–6. [DOI] [PubMed] [Google Scholar]
- 30. Yao Y, Chen L, Xiao J et al Chrysin protects against focal cerebral ischemia/reperfusion injury in mice through attenuation of oxidative stress and inflammation. Int J Mol Sci 2014; 15:20913–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu H, Wan C, Ding Y et al PR‐957, a selective inhibitor of immunoproteasome subunit low‐MW polypeptide 7, attenuates experimental autoimmune neuritis by suppressing Th17‐cell differentiation and regulating cytokine production. FASEB J 2017; 31:1756–66. [DOI] [PubMed] [Google Scholar]
- 32. Jander S, Kraemer M, Schroeter M, Witte OW, Stoll G. Lymphocytic infiltration and expression of intercellular adhesion molecule‐1 in photochemically induced ischemia of the rat cortex. J Cereb Blood Flow Metab 1995; 15:42–51. [DOI] [PubMed] [Google Scholar]
- 33. Schroeter M, Jander S, Witte OW, Stoll G. Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion. J Neuroimmunol 1994; 55:195–203. [DOI] [PubMed] [Google Scholar]
- 34. Gu L, Xiong X, Zhang H, Xu B, Steinberg GK, Zhao H. Distinctive effects of T cell subsets in neuronal injury induced by cocultured splenocytes in vitro and by in vivo stroke in mice. Stroke 2012; 43:1941–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shichita T, Sugiyama Y, Ooboshi H et al Pivotal role of cerebral interleukin‐17‐producing gammadeltaT cells in the delayed phase of ischaemic brain injury. Nat Med 2009; 15:946–50. [DOI] [PubMed] [Google Scholar]
- 36. Swardfager W, Winer DA, Herrmann N, Winer S, Lanctot KL. Interleukin‐17 in post‐stroke neurodegeneration. Neurosci Biobehav Rev 2013; 37:436–47. [DOI] [PubMed] [Google Scholar]
- 37. Chen Y, Langrish CL, McKenzie B et al Anti‐IL‐23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest 2006; 116:1317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Langrish CL, Chen Y, Blumenschein WM et al IL‐23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005; 201:233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)‐1 in the induction of IL‐17‐producing T cells that mediate autoimmune encephalomyelitis. J Exp Med 2006; 203:1685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jin R, Yang G, Li G. Inflammatory mechanisms in ischaemic stroke: role of inflammatory cells. J Leukoc Biol 2010; 87:779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Burke MK. How does adaptation sweep through the genome? Insights from long‐term selection experiments. Proc Biol Sci 2012; 279:5029–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stephanou A, Isenberg DA, Akira S, Kishimoto T, Latchman DS. The nuclear factor interleukin‐6 (NF‐IL6) and signal transducer and activator of transcription‐3 (STAT‐3) signalling pathways co‐operate to mediate the activation of the hsp90beta gene by interleukin‐6 but have opposite effects on its inducibility by heat shock. Biochem J 1998; 330:189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang XO, Panopoulos AD, Nurieva R et al STAT3 regulates cytokine‐mediated generation of inflammatory helper T cells. J Biol Chem 2007; 282:9358–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Table S1. Numbers of mice used to for dose optimization of PR‐957 treatment.
Table S2. Numbers of mice used in the formal experiments.


