Abstract
Aims
Nephrotic syndrome (NS) is the most common clinical manifestation of glomerular disease in children. Currently, tacrolimus (TAC) is widely used in children with NS. However, pharmacokinetic data in children with nephrotic syndrome is limited. This study was intended to evaluate the population pharmacokinetics (PPK) of TAC in paediatric NS and to optimize dosing regimen.
Methods
Blood samples from NS children treated with TAC were collected and the blood concentrations of TAC were detected using HPLC‐MS/MS. A PPK model was developed using NONMEM software. Pharmacogenetic analysis was carried out in the CYP3A5 gene.
Results
The data from 28 children were used for PPK analysis. A one‐compartment model and first‐order elimination were accorded with the TAC data in paediatric NS. A covariate analysis showed that body weight and CYP3A5 genotype significantly affected TAC pharmacokinetics. Monte Carlo simulation indicated that NS children with CYP3A5*3/*3 receiving 0.10 mg kg−1 dose−1 twice daily and NS children with CYP3A5*1 receiving 0.25 mg kg−1 dose−1 twice daily TAC could achieve the target concentrations of 5–10 ng ml−1.
Conclusion
The PPK of TAC was estimated in children with NS and a CYP3A5 genotype‐based dosing regimen was set up based on simulations.
Keywords: children, nephrotic syndrome, pharmacokinetics, tacrolimus
What is Already Known about this Subject
Tacrolimus is used in the treatment of children with nephrotic syndrome.
CYP3A5 gene polymorphism significantly affected tacrolimus pharmacokinetics and clinical outcomes in solid organ transplantation patients.
What this Study Adds
Body weight and CYP3A5 genotype had significant impact on tacrolimus pharmacokinetics in children with nephrotic syndrome.
A CYP3A5 genotype‐based dosing regimen in children with nephrotic syndrome was formulated.
Introduction
Nephrotic syndrome (NS) is the most common glomerular disease in childhood with clinical manifestation of proteinuria, hypoalbuminaemia, hyperlipidaemia, and oedema 1, 2. Minimal change nephropathy (MCN) is the most common pathological type of NS in paediatrics. Although more than 90% of children with MCN were reported to achieve remission with oral corticosteroid therapy, recurrence is common, bringing about an increase in morbidity, complications, treatment costs and a decline in quality of life 3. In addition, the majority of children with focal segmental glomerulosclerosis (FSGS), the second most common pathological type, do not respond to corticosteroids 4.
Tacrolimus (TAC), a calcineurin inhibitor, was labelled to prevent or treat rejection in organ transplant patients. In clinical practice, TAC is also commonly used for the treatment of nephrotic syndrome in an off‐label manner. The first published reports can be found as early as the 1990s in adults and 2000s in paediatric patients 5, 6, 7, 8.
Several studies suggest that the immune system plays a vital role in NS 9, 10. TAC inhibits the activation of a necessary transcription factor in T cells, which is indispensable for the transcription of cytokine genes, resulting in a decreased production of cytokines such as IFN‐γ and IL‐2 11, 12. Recently, it is believed that podocyte injury is the central event of nephrotic syndrome 13. Studies have shown that TAC can inhibit the specific phosphatase activity of podocyte, stabilize the actin skeleton of podocyte, and reduce proteinuria 14, 15.
Although the efficacy and safety data are available in children with NS, the clinical application of TAC remains hampered by its narrow therapeutic index (TI) and high inter‐ and intra‐individual pharmacokinetic variability 16, 17. Both under‐ and overexposure to TAC may have serious consequences, increasing the risk of treatment failure or toxic side effects 18.
TAC is extensively metabolized in the liver and undergoes biliary excretion. http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=263#1338 is the predominant enzyme for metabolism of TAC 19, 20. CYP3A5 gene polymorphism significantly affects TAC pharmacokinetics and clinical outcomes in solid organ transplantation patients 21, 22, 23, 24, 25, 26. Although the pharmacokinetics of TAC have been evaluated in paediatric organ transplant patients, these data cannot be directly used in NS patients because the organ transplant patients had hypohepatia and/or rapid change of renal function after transplant and the liver enzyme activity showed a modest derangement during TAC treatment 27. It has been reported that time post‐transplantation in days, hematocrit, liver weight and liver function influenced tacrolimus elimination 28, 29, 30, 31. Nephrotic syndrome induces proteinuria, hypoalbuminemia, hypertriglyceridemia and hypercholesterolemia 32, 33. These characteristics may have an impact on TAC pharmacokinetics. Gut oedema secondary to hypoproteinaemia may affect drug absorption, while hypoalbuminaemia can reduce protein binding of the drug and thus alter volume of distribution (V) or clearance (CL), as TAC is highly bound to plasma proteins 34. All these clinical features in organ transplant patients lead to their unique pharmacokinetic characteristics, which cannot be directly extrapolated to children with NS 35.
Given the limited available pharmacokinetic data in paediatric patients with NS and unmet need in clinical practice to optimize TAC therapy, we conducted this study aiming (i) to develop a PPK model of TAC in children with NS; (ii) to evaluate impacts of CYP3A5 genotype as well as patient characteristics on TAC pharmacokinetic parameters; and (iii) to optimize TAC dosing regimens based on the developed model.
Methods
Study design
This pharmacokinetic study was a prospective, open label trial, carried out in our department of paediatric nephology from 2015 to 2017 in the Children's hospital of Hebei province, China. The inclusion criteria included: children under 18 years of age with NS; treated with TAC as initial immunosuppressant. The exclusion criteria included: children with a concomitant medical condition, whose participation, according to the opinion of the Investigators, may lead to unacceptable additional risks. The study, designed in accordance with requirements of the law and the Declaration of Helsinki, was approved by the institutional ethics board, and registered at http://clinicaltrials.gov (ID number NCT03347357). Informed consent was signed by the children's parents or guardians.
Dosing regimen and pharmacokinetic sampling
Tacrolimus (Prograf, Astellas, Japan), was administered orally at a dose of 0.05 mg kg−1 dose−1 twice daily. A full concentration–time profile was confirmed when a steady‐state condition was achieved during hospitalization. Blood samples were extracted before and at 1, 2, 3, 6, 9 and 12 h after taking TAC. Precise medication times and sampling times were recorded. Each blood sample used for pharmacokinetic analysis had a volume of 0.2 ml.
Analytical method of tacrolimus and genotyping
Tacrolimus blood concentrations were determined by high‐performance liquid chromatography with tandem mass spectrometry. The range of calibration curve was from 2.0 to 100 ng ml−1, and the lower limit of quantification (LOQ) was 2.0 ng ml−1. The intraday and interday coefficients of variation were 4.4 and 7.2%, respectively.
Total genomic DNA was extracted from blood samples using a TIANamp Blood Clot DNA Kit (TIANGEN Biotech, Beijing, China) according to the manufacturer's protocol. CYP3A5 A6986G (rs776746) polymorphism was determined using the TaqMan (Thermo Fisher Scientific, MA, USA) allelic discrimination technique with 3′‐minor groove binding quencher probes on a Bio‐Rad Fluorescence Quantitative PCR (Hercules, CA, USA) according to manufacturer's instructions.
Population pharmacokinetic modelling of tacrolimus
The PPK analysis was conducted by the nonlinear mixed‐effects modelling program NONMEM (Version 7.2, Icon Development Solutions, USA). The first‐order conditional estimation (FOCE) with interaction method was selected throughout the model‐building procedure to estimate the pharmacokinetic (PK) parameters and their variability.
An exponential model was used to estimate interindividual variability of the PK parameters and could be expressed as follows:
where θ mean is the typical population value of the parameter, θ i is the individual pharmacokinetic parameter value of the ith subject, and ηi is the difference between the ith individuals' θ and the predicted values. The values of ηi are random, independent, identically distributed variables, following the normal distribution with mean 0 and variance ω2.
The selection of covariates followed a forward‐selection process and a backward‐elimination process. The effect of each variable on parameters was tested using likelihood ratio. Body weight, age and CYP3A5 genotype were investigated as potential variables affecting PK parameters. In the forward‐selection process, a covariate was added to the model if the objective function value (OFV) significantly (P < 0.05) decreased (reduction>3.84) from the basic model. All the statistically significant covariates were included in the full model. Then, in order to re‐evaluate the importance of these variables, each was independently removed from the full model. The covariate was retained in the final model only if the increase (increase > 3.84) of OFV was significant (P < 0.05).
The final model was validated based on statistical and graphical criteria. Goodness‐of‐fit was evaluated using diagnostic scatter plots, including: (i) observed (DV) vs. population predicted concentrations (PRED); (ii) DV vs. individual predicted concentrations (IPRED); (iii) conditional weighted residuals (CWRES) vs. time; (iv) CWRES vs. PRED. The stability of the final model was evaluated by the nonparametric bootstrap with re‐sampling and replacement. Re‐sampling was repeated 1000 times. The values of estimated parameters from the bootstrap procedure, such as the medians and SEs, were compared with those estimated from the original dataset. The final model was also evaluated using normalized prediction distribution errors (NPDE) 36, 37. The dataset was simulated 1000 times using the final model parameters. The NPDE is expected to follow the N(0, 1) distribution. NPDE results were summarized graphically and the following graphs were plotted by using the NPDE R package (version 1.2) 38: (i) QQ‐plot of the NPDE; (ii) histogram of the NPDE.
Dosing regimen optimization
Monte Carlo simulations were carried out using the parameter estimates from the final population model 39, 40. The aim was to determine an optimal dosing regimen and achieve the target trough concentration (C0) of 5–10 ng ml−1 41. The dose of TAC was simulated on a 0.05, 0.10, 0.15, 0.20, 0.25, 0.30 mg kg−1 dose−1 twice daily basis according to different CYP3A5 genotype groups. One thousand simulations were carried out using the initial dataset, and steady‐state C0 of each simulated subject was calculated. An optimal dosing regimen of tacrolimus was then established based on the median of simulated C0 in each CYP3A5 genotype group.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 42, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 43.
Results
Study population
A total of 28 patients were included in final pharmacokinetic analysis. All the participants met the inclusion criteria and signed informed consent. The mean (SD) age and body weight of the 28 participants were 9.5 (4.4) (range 2.7–17.3) years and 36.5 (17.4) (range 12.9–81.0) kg, respectively. The patient features are listed in Table 1.
Table 1.
Characteristics of the 28 paediatric nephrotic syndrome patients
| Patient characteristic | Number | Mean | SD | Median | Range |
|---|---|---|---|---|---|
| No. of patients | 28 | ||||
| Male/female | 19/9 | ||||
| Age (year) | 9.5 | 4.4 | 9.4 | 2.7–17.3 | |
| Body weight (kg) | 36.5 | 17.4 | 30.0 | 12.9–81.0 | |
| Tacrolimus dose (mg, twice daily) | 3.8 | 2.2 | 4.0 | 1.0–8.0 | |
| Tacrolimus dose (mg kg −1 , twice daily) | 0.1199 | 0.0860 | 0.0909 | 0.0222–0.3876 | |
| Pharmacokinetic data | |||||
| Samples (n) | 148 | ||||
| Concentrations (ng ml −1 ) | <2–38.7 | ||||
| Samples per patient | 1–7 | ||||
| CYP3A5 | |||||
| *3/*3 | 21 | ||||
| *1/*3 | 6 | ||||
| *1/*1 | 1 |
Model building
For PPK modelling, a total of 148 TAC concentrations were obtained from 28 children. The TAC concentrations ranged from <2.0 (n = 11) to 38.7 ng ml−1. Half of the LOQ value was used to handle concentrations below the LOQ in PK modelling. The curve of concentration varying with time is shown in Figure 1.
Figure 1.
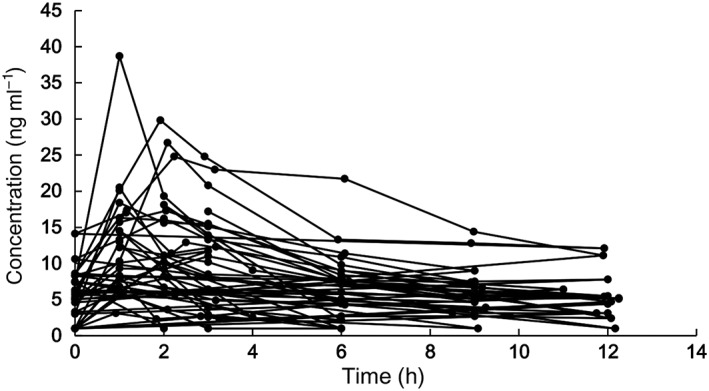
The concentration vs. time profile
A one‐compartment model and first‐order elimination were more suitable for describing TAC concentrations in paediatric NS. The proportional model best described residual variability. The model was parameterized in terms of clearance (CL), volume of distribution (V) and absorption rate constant (k a) of TAC. The inter‐individual variability (IIV) was modelled using the exponential error model and was then estimated for CL, V and k a.
Covariate analysis
The allometric size approach was used by implementing the body weight into the basic model (the allometric coefficients fixed at 0.75 for CL and 1 for V). After implementing body weight, OFV significantly decreased by 5.462 units. A further drop in the OFV of 4.961 points occurred when CYP3A5 genotype was incorporated on CL. Parameter estimates of the final PK model are shown in Table 2. The median (range) of estimated CL and V at steady state were 0.595 (0.211–1.933) l h−1 kg−1 and 4.688 (0.924–39.389) l kg−1, respectively. CL of TAC in children with NS increased allometrically with body weight.
where FLAG1 = 1 for the CYP3A5*1 allele; FLAG1 = 0 for CYP3A5*3/*3.
Table 2.
Population pharmacokinetic parameters of tacrolimus and bootstrap validation (n = 1000)
| PK parameters | SE (%) | Bootstrap | |||
|---|---|---|---|---|---|
| Median | 5th | 95th | |||
| Absorption rate constant (h −1 ) k a | 5.21 | 17.1 | 5.23 | 2.68 | 7.08 |
| Volume of distribution (L) V/F | |||||
| V/F = θ1 × (bodyweight /70) | |||||
| θ1 | 411 | 20.9 | 413 | 296 | 609 |
| Oral clearance (l h −1 ) CL/F | |||||
| CL/F = θ2 × (bodyweight/70) 0.75 × F CYP3A5 | |||||
| θ2 | 30.9 | 9.2 | 30.9 | 26.3 | 35.8 |
| If CYP3A5 *1/*1, F CYP3A5 = θ3 | |||||
| If CYP3A5 *1/*3 F CYP3A5 = θ3 | |||||
| If CYP3A5 *3/*3, F CYP3A5 = 1 | |||||
| θ3 | 1.60 | 23.8 | 1.64 | 1.11 | 2.42 |
| Inter‐individual variability (%) | |||||
| k a | 79.1 | 52.8 | 75.1 | 38.9 | 142.5 |
| V/F | 99.4 | 34.3 | 96.7 | 70.4 | 126.7 |
| CL/F | 43.8 | 33.0 | 40.6 | 29.2 | 52.3 |
| Residual variability (exponential) (%) | 25.9 | 22.2 | 25.6 | 21.2 | 30.7 |
CL/F was significantly lower in children with CYP3A5*3/*3 compared with children with the CYP3A5*1 allele (0.567 ± 0.216 vs. 1.050 ± 0.641 l h−1 kg−1, P = 0.005).
Model validation
Acceptable goodness‐of‐fit for the final model of TAC was shown by model diagnostics. Figures 2A and 2B show that predictions are unbiased. No trends were observed (Figures 2C and 2D) in the diagnostic plots of CWRES vs. time and PRED. Furthermore, as shown in Table 2, the median parameter estimates obtained by the bootstrap process are consistent with the respective values of the final model, demonstrating that the final population model is stable, and it can reconfirm the estimated value of the PPK parameters.
Figure 2.
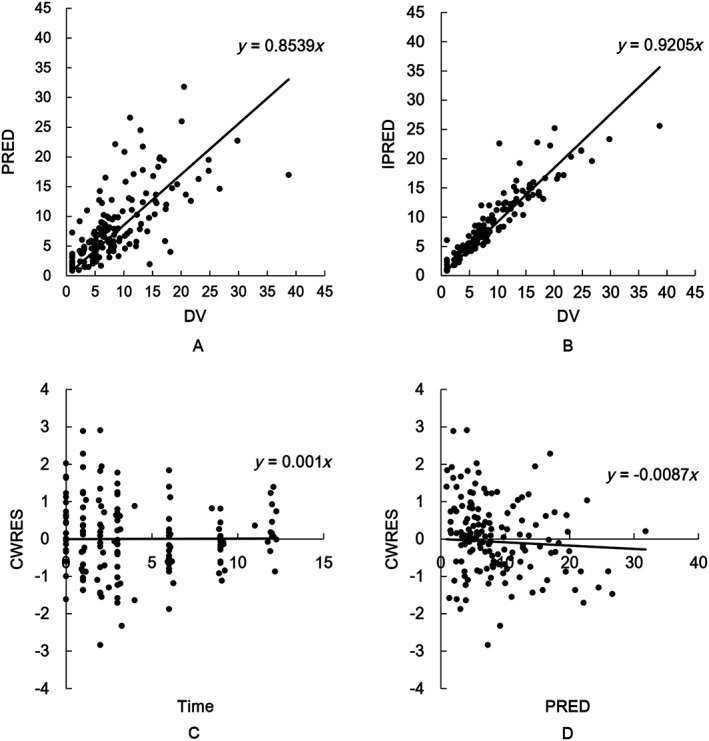
Diagnostic goodness‐of‐fit plots for the final population pharmacokinetic model of tacrolimus, including (A) observed (DV) vs. population prediction (PRED); (B) DV vs. individual prediction (IPRED); (C) time vs. conditional weighted residuals (CWRES); and (D) PRED vs. CWRES
The NPDE results are shown in Figure 3. The mean of NPDE was 0.0185 (Wilcoxon signed rank test P = 0.902) and the variance was 0.871 (Fisher variance test = 0.263). The NPDE distribution and histogram accorded with the theoretical N(0, 1) distribution and density, showing that the model fitted the individual data.
Figure 3.
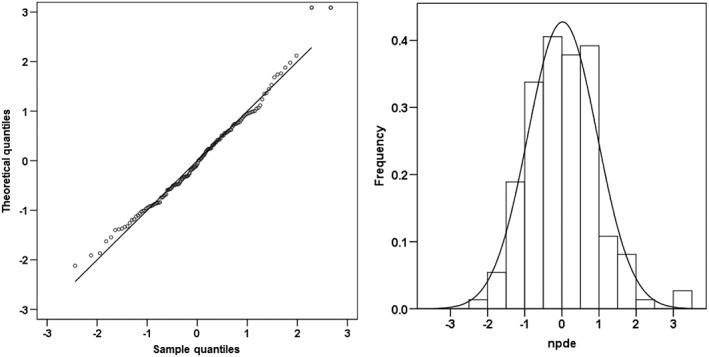
Normalized prediction distribution errors (NPDE) analysis for the tacrolimus final model. NPDE: QQ‐plot of the distribution of the NPDE versus the theoretical N(0,1) distribution (left). Histogram of the distribution of the NPDE, with the density of the standard Gaussian distribution overlaid (right)
Dosing regimen optimization
Monte Carlo simulation showed that patients with CYP3A5*3/*3 receiving tacrolimus 0.10 mg kg−1 twice daily reached a median steady state C0 of 8.1 ng ml−1 and patients with CYP3A5*1 receiving 0.25 mg kg−1 twice daily had a median C0 of 7.6 ng ml−1. The simulation results are presented in Figure 4.
Figure 4.
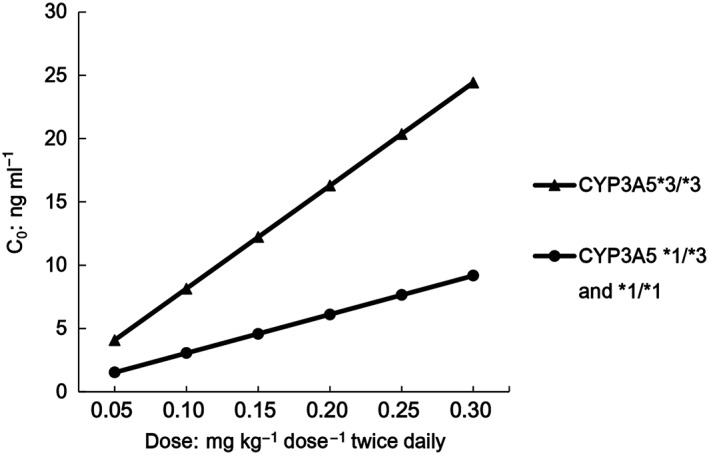
Simulation of the median for tacrolimus steady state C0 obtained in patients of two genetic backgrounds
Discussion
Population pharmacokinetic study of TAC was first conducted in children with NS. The purpose of this study was to assess the PK parameters of TAC and to assess the effects of biological, demographic and clinical factors on TAC disposition. A one‐compartment model with first‐order elimination with body weight and CYP3A5 genotype as covariates was established.
In the present model, the mean CL/F of TAC is 0.69 l h−1 kg−1, which is consistent with the value in kidney transplant children (0.76 l h−1 kg−1) 44. However, the half‐life in paediatric nephrotic syndrome patients in our study (about 9 h) seems to be much shorter than observed in patients that have undergone renal transplantation (about 24 h). TAC is highly bound to plasma proteins. Nephrotic syndrome may cause severe hypoproteinaemia. The percentage of unbound TAC in the NS patients will be higher than that in renal transplantation patients 45. Therefore, patients with nephrotic syndrome have lower apparent distribution volume and a greater elimination rate constant (k e) than those with renal transplantation.
After forward‐selection and backward‐elimination processes, body weight and CYP3A5 gene polymorphisms were identified as significant covariates associated with interindividual variability. An allometry‐based PPK model was developed for TAC: body weight was a biological factor with allometric coefficients of 0.75 for CL and 1 for V, respectively. These quarter‐power models have observational and theoretical bases in biology 46, 47. The same model was used for children with kidney transplant 48, 49.
As CYP3A5 plays a vital role in TAC metabolism, a possible association was investigated between CYP3A5 polymorphism and TAC CL/F. It has been reported that kidney and liver microsomes from donors with a CYP3A5*1/*3 genotype had a higher TAC clearance than those with CYP3A5*3/*3 genotype 19. Our results demonstrated that the weight normalized CL/F of tacrolimus was significantly higher in expressers (CYP3A5*1 allele) than in non‐expressers (CYP3A5*3/*3). CL/F in CYP3A5*1 carriers was 1.6‐fold higher than in CYP3A5*3/*3 carriers (non‐expressers) in our study. Similarly, the ratio was 1.66, 1.8 and 1.45 in paediatric and adolescent kidney transplant recipients 48, 49, 50.
Tacrolimus has gained acceptance in the treatment of NS in children 7, 51, 52, 53. The recommended target C0 was 5–10 ng ml−1 in children with NS 8, 54, which was lower than 10–20 ng ml−1 in the immediate post‐transplantation period used for all types of paediatric organ transplantations 55. There is growing evidence to support a potential benefit for CYP3A5 genotyping before the initiation of tacrolimus‐based immunosuppressive therapy in children undergoing organ transplantation 56, 57. We have proved that a standard TAC dosage of 0.10 mg kg−1 dose−1 twice daily may result in underexposure to TAC in NS children with the CYP3A5*1 allele, and that the higher dosage (0.25 mg kg−1 dose−1 twice daily) should be suggested.
There are some limitations in our study. Due to the limited number of participants, the PPK model of TAC was established and internally validated only. External validation will be carried out after applying it in clinical practice. Randomized controlled studies are definitely required prior to implementing a personalized approach as a ‘standard of care’. Ultimately, the CYP3A5 genotype‐based dose regimen must be applied in clinical practice to identify its benefits in children with NS.
Conclusion
The PPK model of tacrolimus was established in children with nephrotic syndrome. Body weight and CYP3A5 genotype significantly affected pharmacokinetics of tacrolimus. A CYP3A5 genotype‐based dosing regimen was developed based on the PPK analysis.
Competing Interest
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author). None of the authors have any other relationships or activities that could appear to have influenced the submitted work. This work is supported by the National Natural Science Foundation of China (81503163), National Science and Technology Major Projects for Major New Drugs Innovation and Development (2017ZX09304029‐002) and Young Taishan Scholars Program and Young Scholars Program of Shandong University.
Hao, G.‐X. , Huang, X. , Zhang, D.‐F. , Zheng, Y. , Shi, H.‐Y. , Li, Y. , Jacqz‐Aigrain, E. , and Zhao, W. (2018) Population pharmacokinetics of tacrolimus in children with nephrotic syndrome. Br J Clin Pharmacol, 84: 1748–1756. 10.1111/bcp.13605.
References
- 1. Pal A, Kaskel F. History of nephrotic syndrome and evolution of its treatment. Front Pediatr 2016; 4: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet 2003; 362: 629–639. [DOI] [PubMed] [Google Scholar]
- 3. Tarshish P, Tobin JN, Bernstein J, Edelmann CM Jr. Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol 1997; 8: 769–776. [DOI] [PubMed] [Google Scholar]
- 4. Uwaezuoke SN. Steroid‐sensitive nephrotic syndrome in children: triggers of relapse and evolving hypotheses on pathogenesis. Ital J Pediatr 2015; 41: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schweda F, Liebl R, Riegger GA, Krämer BK. Tacrolimus treatment for steroid‐ and cyclosporin‐resistant minimal‐change nephrotic syndrome[J]. Nephrol Dial Transplant 1997; 12: 2433–2435. [DOI] [PubMed] [Google Scholar]
- 6. Roberti I, Vyas S. Long‐term outcome of children with steroid‐resistant nephrotic syndrome treated with tacrolimus. Pediatr Nephrol 2010; 25: 1117–1124. [DOI] [PubMed] [Google Scholar]
- 7. Yang EM, Lee ST, Choi HJ, Cho HY, Lee JH, Kang HG, et al Tacrolimus for children with refractory nephrotic syndrome: a one‐year prospective, multicenter, and open‐label study of Tacrobell®, a generic formula. World J Pediatr 2016; 12: 60–65. [DOI] [PubMed] [Google Scholar]
- 8. Loeffler K, Gowrishankar M, Yiu V. Tacrolimus therapy in pediatric patients with treatment‐resistant nephrotic syndrome. Pediatr Nephrol 2004; 19: 281–287. [DOI] [PubMed] [Google Scholar]
- 9. Pereira W de F, Brito‐Melo GE, Guimarães FT, Carvalho TG, Mateo EC, Simões e Silva AC. The role of the immune system in idiopathic nephrotic syndrome: a review of clinical and experimental studies. Inflamm Res 2014; 63: 1–12. [DOI] [PubMed] [Google Scholar]
- 10. Colucci M, Corpetti G, Emma F, Vivarelli M. Immunology of idiopathic nephrotic syndrome. Pediatr Nephrol 2018; 33: 573–584. [DOI] [PubMed] [Google Scholar]
- 11. Ho S, Clipston N, Timmermann L, Northrop J, Graef I, Fiorentino D, et al The mechanism of action of cyclosporine A and FK506. Clin Immunol Immunopathol 1996; 80: S40–S45. [DOI] [PubMed] [Google Scholar]
- 12. Westhoff TH, Giet MVD. Tacrolimus in the treatment of idiopathic nephrotic syndrome. Expert Opin Investig Drugs 2007; 16: 1099–1110. [DOI] [PubMed] [Google Scholar]
- 13. Bierzynska A, Saleem M. Recent advances in understanding and treating nephrotic syndrome. F1000Res 2017; 6: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wen Y, Liu L, Zhou P, Li H, Wang Z, Zhang Y, et al Tacrolimus restores podocyte injury and stabilizes the expression of Cabin1 in 5/6 nephrectomized rats. Ren Fail 2016; 38: 1. [DOI] [PubMed] [Google Scholar]
- 15. Becknell B, Greenbaum LA, Smoyer WE. A new ‘tac’ for childhood nephrotic syndrome. Kidney Int 2012; 82: 1049–1051. [DOI] [PubMed] [Google Scholar]
- 16. Goodall DL, Willicombe M, McLean AG, Taube D. High intrapatient variability of tacrolimus levels and outpatient clinic nonattendance are associated with inferior outcomes in renal transplant patients. Transplant Direct 2017; 3: e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Antignac M, Barrou B, Farinotti R, Lechat P, Urien S. Population pharmacokinetics and bioavailability of tacrolimus in kidney transplant patients. Br J Clin Pharmacol 2007; 64: 750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nguyen TH, Mouksassi MS, Holford N, Al‐Huniti N, Freedman I, Hooker AC, et al Model evaluation of continuous data pharmacometric models: metrics and graphics. CPT Pharmacometrics Syst Pharmacol 2017; 6: 87–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dai Y, Hebert MF, Isoherranen N, Davis CL, Marsh C, Shen DD, et al Effect of CYP3A5 polymorphism on tacrolimus metabolic clearance in vitro. Drug Metab Dispos 2006; 34: 836–847. [DOI] [PubMed] [Google Scholar]
- 20. Kamdem LK, Streit F, Zanger UM, Brockmöller J, Oellerich M, Armstrong VW, et al Contribution of CYP3A5 to the in vitro hepatic clearance of tacrolimus. Clin Chem 2005; 51: 1374–1381. [DOI] [PubMed] [Google Scholar]
- 21. Alvarez‐Elías AC, García‐Roca P, Velásquez‐Jones L, Valverde S, Varela‐Fascinetto G, Medeiros M. CYP3A5 genotype and time to reach tacrolimus therapeutic levels in renal transplant children. Transplant Proc 2016; 48: 631–634. [DOI] [PubMed] [Google Scholar]
- 22. Mac Guad R, Zaharan NL, Chik Z, Mohamed Z, Peng NK, Adnan WA. Effects of CYP3A5 genetic polymorphism on the pharmacokinetics of tacrolimus in renal transplant recipients. Transplant Proc 2016; 48: 81–87. [DOI] [PubMed] [Google Scholar]
- 23. Kato H, Usui M, Muraki Y, Tanemura A, Murata Y, Kuriyama N, et al Long‐term influence of CYP3A5 gene polymorphism on pharmacokinetics of tacrolimus and patient outcome after living donor liver transplantation. Transplant Proc 2016; 48: 1087–1094. [DOI] [PubMed] [Google Scholar]
- 24. Xue F, Han L, Chen Y, Xi Z, Li Q, Xu N, et al CYP3A5 genotypes affect tacrolimus pharmacokinetics and infectious complications in Chinese pediatric liver transplant patients. Pediatr Transplant 2014; 18: 166–176. [DOI] [PubMed] [Google Scholar]
- 25. Chen JS, Li LS, Cheng DR, Ji SM, Sun QQ, Cheng Z, et al Effect of CYP3A5 genotype on renal allograft recipients treated with tacrolimus. Transplant Proc 2009; 41: 1557–1561. [DOI] [PubMed] [Google Scholar]
- 26. Hooper DK, Fukuda T, Gardiner R, Logan B, Roy‐Chaudhury A, Kirby CL, et al Risk of tacrolimus toxicity in CYP3A5 nonexpressors treated with intravenous nicardipine after kidney transplantation. Transplantation 2012; 93: 806–812. [DOI] [PubMed] [Google Scholar]
- 27. Tarantino G, Palmiero G, Polichetti G, Perfetti A, Sabbatini M, Basile V, et al Long‐term assessment of plasma lipids in transplant recipients treated with tacrolimus in relation to fatty liver. Int J Immunopathol Pharmacol 2010; 23: 1303–1308. [DOI] [PubMed] [Google Scholar]
- 28. Jalil MH, Hawwa AF, McKiernan PJ, Shields MD, McElnay JC. Population pharmacokinetic and pharmacogenetic analysis of tacrolimus in paediatric liver transplant patients. Br J Clin Pharmacol 2014; 77: 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Musuamba FT, Guy‐Viterbo V, Reding R, Verbeeck RK, Wallemacq P. Population pharmacokinetic analysis of tacrolimus early after pediatric liver transplantation. Ther Drug Monit 2014; 36: 54–61. [DOI] [PubMed] [Google Scholar]
- 30. Yang JW, Liao SS, Zhu LQ, Zhao Y, Zhang Y, Sun XY, et al Population pharmacokinetic analysis of tacrolimus early after Chinese pediatric liver transplantation. Int J Clin Pharmacol Ther 2015; 53: 75–83. [DOI] [PubMed] [Google Scholar]
- 31. Garcia Sanchez MJ, Manzanares C, Santos‐Buelga D, Blazquez A, Manzanares J, Urruzuno P, et al Covariate effects on the apparent clearance of tacrolimus in paediatric liver transplant patients undergoing conversion therapy. Clin Pharmacokinet 2001; 40: 63–71. [DOI] [PubMed] [Google Scholar]
- 32. Kaysen GA. Plasma composition in the nephrotic syndrome. Am J Nephrol 1993; 13: 347–359. [DOI] [PubMed] [Google Scholar]
- 33. Jahan A, Prabha R, Chaturvedi S, Mathew B, Fleming D, Agarwal I. Clinical efficacy and pharmacokinetics of tacrolimus in children with steroid‐resistant nephrotic syndrome. Pediatr Nephrol 2015; 30: 1961–1967. [DOI] [PubMed] [Google Scholar]
- 34. Iwasaki K, Miyazaki Y, Teramura Y, Kawamura A, Tozuka Z, Hata T, et al Binding of tacrolimus (FK506) with human plasma proteins re‐evaluation and effect of mycophenolic acid. Res Commun Mol Pathol Pharmacol 1996; 94: 251–257. [PubMed] [Google Scholar]
- 35. Zhao W, Elie V, Baudouin V, Bensman A, André JL, Brochard K, et al Population pharmacokinetics and Bayesian estimator of mycophenolic acid in children with idiopathic nephrotic syndrome. Br J Clin Pharmacol 2010; 69: 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brendel K, Comets E, Laffont C, Laveille C, Mentré F. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res 2006; 23: 2036–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction‐corrected visual predictive checks for diagnosing nonlinear mixed‐effects modes. AAPSJ 2011; 13: 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Comets E, Brendel K, Mentré F. Computing normalised prediction distribution errors to evaluate nonlinear mixed‐effect models: the npde add‐on package for R. Comput Methods Programs Biomed 2008; 90: 154–166. [DOI] [PubMed] [Google Scholar]
- 39. Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model‐based drug development. CPT Pharmacometrics Syst Pharmacol 2012; 1: e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bonate PL. A brief introduction to Monte Carlo simulation. Clin Pharmacokinet 2001; 40: 15–22. [DOI] [PubMed] [Google Scholar]
- 41. Butani L, Ramsamooj R. Experience with tacrolimus in children with steroid‐resistant nephrotic syndrome. Pediatr Nephrol 2009; 24: 1517–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acid Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E, et al The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 2017; 174 (Suppl 1): S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhao W, Elie V, Roussey G, Brochard K, Niaudet P, Leroy V, et al Population pharmacokinetics and pharmacogenetics of tacrolimus in de novo pediatric kidney transplant recipients. Clin Pharmacol Ther 2009; 86: 609–618. [DOI] [PubMed] [Google Scholar]
- 45. Gugler R, Shoeman DW, Huffman DH, Cohlmia JB, Azarnoff DL. Pharmacokinetics of drugs in patients with the nephrotic syndrome. J Clin Invest 1975; 55: 1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Anderson BJ, Holford NH. Mechanism‐based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicology 2008; 48: 303–332. [DOI] [PubMed] [Google Scholar]
- 47. West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science 1997; 276: 122–126. [DOI] [PubMed] [Google Scholar]
- 48. Andrews LM, Hesselink DA, van Gelder T, Koch BCP, Cornelissen EAM, Brüggemann RJM, et al A population pharmacokinetic model to predict the individual starting dose of tacrolimus following pediatric renal transplantation. Clin Pharmacokinet 2018; 57: 475–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Prytuła AA, Cransberg K, Bouts AH, van Schaik RH, de Jong H, de Wildt SN, et al The effect of weight and CYP3A5 genotype on the population pharmacokinetics of tacrolimus in stable paediatric renal transplant recipients. Clin Pharmacokinet 2016; 55: 1129–1143. [DOI] [PubMed] [Google Scholar]
- 50. Zhao W, Fakhoury M, Baudouin V, Storme T, Maisin A, Deschênes G, et al Population pharmacokinetics and pharmacogenetics of once daily prolonged‐release formulation of tacrolimus in pediatric and adolescent kidney transplant recipients. Eur J Clin Pharmacol 2013; 69: 189–195. [DOI] [PubMed] [Google Scholar]
- 51. Gulati A, Sinha A, Gupta A, Kanitkar M, Sreenivas V, Sharma J, et al Treatment with tacrolimus and prednisolone is preferable to intravenous cyclophosphamide as the initial therapy for children with steroid‐resistant nephrotic syndrome. Kidney Int 2012; 82: 1130–1135. [DOI] [PubMed] [Google Scholar]
- 52. Bock ME, Cohn RA, Ali FN. Treatment of childhood nephrotic syndrome with long‐term, low‐dose tacrolimus. Clin Nephrol 2013; 79: 432–438. [DOI] [PubMed] [Google Scholar]
- 53. Supavekin S, Surapaitoolkorn W, Kurupong T, Chaiyapak T, Piyaphanee N, Pattaragarn A, et al Tacrolimus in steroid resistant and steroid dependent childhood nephrotic syndrome. J Med Assoc Thai 2013; 96: 33–40. [PubMed] [Google Scholar]
- 54. Pediatrics Kidney Science Group of Chinese Medical Association . Guidelines for the diagnosis and treatment of steroid resistant nephrotic syndrome (2016). Chin J Pediatr 2017; 55: 805–809. [DOI] [PubMed] [Google Scholar]
- 55. Lancia P, Jacqz‐Aigrain E, Zhao W. Choosing the right dose of tacrolimus. Arch Dis Child 2015; 100: 406–413. [DOI] [PubMed] [Google Scholar]
- 56. Ferraresso M, Tirelli A, Ghio L, Grillo P, Martina V, Torresani E, et al Influence of the CYP3A5 genotype on tacrolimus pharmacokinetics and pharmacodynamics in young kidney transplant recipients. Pediatr Transplant 2007; 11: 296–300. [DOI] [PubMed] [Google Scholar]
- 57. Zong YP, Wang ZJ, Zhou WL, Zhou WM, Ma TL, Huang ZK, et al Effects of CYP3A5 polymorphisms on tacrolimus pharmacokinetics in pediatric kidney transplantation: a systematic review and meta‐analysis of observational studies. World J Pediatr 13: 421–426. [DOI] [PubMed] [Google Scholar]


