Abstract
Introduction
The objective of this manuscript is to describe the methodology that will be used to test the comparative effectiveness, feasibility, and acceptability of three formats of family problem solving therapy (F-PST) for improving functional outcomes of complicated mild to severe adolescent TBI.
Methods
Three-arm comparative effectiveness, randomized clinical trial (RCT) design. We describe the protocol of a three-arm RCT comparing the effectiveness of three modalities of F-PST to reduce executive dysfunction and behavior problems following TBI in adolescence. The RCT will compare the relative effectiveness among face-to-face; online and self-directed; and therapist-supported online modes of treatment.
Ethics and dissemination
It is anticipated that findings from this work will inform future clinical care practices, with implications for treatment of other patient populations of youth with psychological symptoms arising from neurological conditions. Institutional review board approval will be obtained from all sites prior to commencement of the study.
Clinical Trials Registration: NCT:02368366
Keywords: Pediatric traumatic brain injury, Telehealth, Problem solving, Behavior, Executive function
1. Introduction
Traumatic brain injury (TBI) is a world-wide health problem, one of the most common causes of acquired disability in youth and a source of significant morbidity and family burden [[1], [2], [3], [4], [5], [6]]. TBI results in 7843 deaths, 46,260 hospitalizations, and 1,083,122 emergency department visits in children and young adults yearly in the United States [6]. Early injuries can have a life-long impact [7]. TBI often results in deficits in cognition, behavior, and social development [[8], [9], [10]]. Novel behavior problems are among the most common and problematic consequences [[11], [12], [13]], yet many youth fail to receive needed psychological services due to lack of identification and access [14]. Linking youth with TBI to effective treatments could improve functional outcomes, reduce family burden, and increase treatment satisfaction.
There is also a bidirectional influence of the TBI and family functioning on outcomes [15]. Parent and family functioning are adversely affected by TBI [[16], [17], [18]] and parental distress and poor parent-child interactions are associated with poorer recovery over time after childhood TBI [12,[19], [20], [21], [22], [23], [24]]. The adverse effects of TBI on parents/families and the central role of family functioning in child recovery highlight the need for interventions designed to facilitate positive family and parental functioning following pediatric TBI.
A number of barriers could prevent families from seeking or receiving services for children's behavioral problems after TBI [25]. Outpatient services may be unavailable altogether or families may have to travel significant distances to obtain appropriate care. Access to professionals with experience in treating patients with pediatric TBI and their families is even more limited, with only a small subset of providers having sufficient training in both TBI and behavioral intervention programs. The use of internet technology makes it possible to deliver interventions online without a negative impact on adherence to or satisfaction with treatment [26,27].
In pediatric TBI, several studies demonstrated the potential effectiveness of web-based family problem-solving focused interventions on improving behavioral and social outcomes after pediatric brain injury by working with both the injured child and family [[28], [29], [30]]. The overarching aim of this paper is to describe a protocol for comparing the effectiveness, feasibility, and acceptability of three formats of family problem solving therapy (F-PST) to improve functional outcomes after complicated mild to severe adolescent TBI: Face-to-face F-PST; therapist-guided online F-PST; self-guided online F-PST. Therapist-guided, online F-PST has shown promise in reducing behavior problems in older adolescence following TBI when delivered to the right individuals, youth having existing problems and environmental adversity [31,32]. The comparative acceptability and effectiveness relative to traditional face-to-face treatment is unknown, and it is unclear if families could also benefit from online F-PST without therapist support. We describe the methodology that will be used to test the comparative effectiveness of these modalities for delivering F-PST interventions in anticipation that findings from this work will inform future clinical care practices. Overall, it was hypothesized that participants in the therapist-guided online F-PST group compared to the face-to-face and self-guided conditions will report the greatest improvements in teen-, parent-, and family-level outcomes.
2. Methods
2.1. Overview and study design
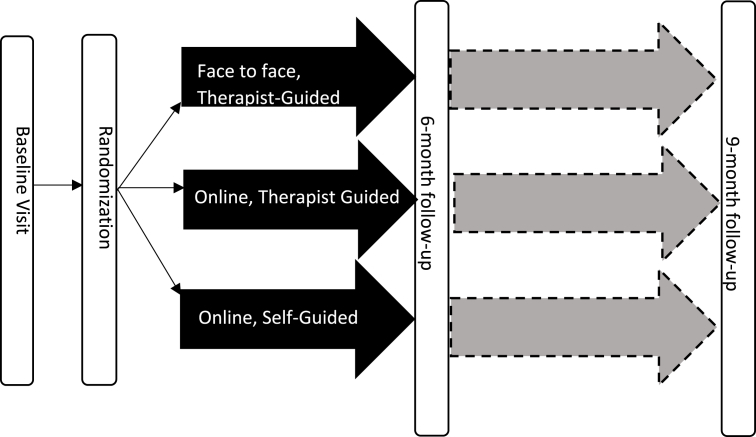
A randomized clinical trial (RCT) design will be used to examine the comparative effectiveness of three versions of F-PST in improving/ameliorating patient- and caregiver-reported behavioral outcomes (See Fig. 1). The three groups (therapist-guided face-to-face F-PST; therapist-guided online F-PST; and self-guided online F-PST) have equivalent content but vary in the mode of delivery (face-to-face versus web-based) and the degree of therapist involvement. We will assess patient treatment modality preferences to assess how preferences influence treatment effectiveness. Although we considered a partially randomized patient preference trial design [33], we opted for a traditional RCT design given the equipoise among the treatment groups and uncertainty regarding the proportion of patients who would decline randomization. The face-to-face arm reflects the current standard of care that families are likely to receive following TBI across the country. If families prove unable to participate in the face-to-face arm, we will have critical new information about the feasibility of current standards of care. The study is registered on clinicaltrials.gov (NCT: 02368366).
Fig. 1.
STUDY DESIGN. Study design is a randomized clinical trial (RCT) that examines the comparative effectiveness of three versions of family problem solving therapy (F-PST): Therapist-guided face-to-face F-PST; therapist-guided online F-PST; and self-guided online F-PST.
2.2. Sample characteristics
Participants will include families of approximately 160 youth aged 14–19 years with complicated mild to severe TBI as defined below (see Recruitment). Adolescents will need to have persistent behavior symptoms for at least one month post injury. We chose not to impose a maximum time since injury, given that research [13,[34], [35], [36]], and feedback from families suggest that concerns may not become apparent until months or years post injury and many problems tend to persist or worsen over time. Given substantial differences in management and recovery trajectory between mild TBI/concussion (i.e., typical recovery in <2 weeks) and more severe TBI, we excluded adolescents with uncomplicated mild TBI. Inclusion/exclusion criteria are detailed under Recruitment.
The study will be conducted at five hospitals affiliated with academic institutions in Ohio and Colorado to ensure that the sample size is adequate, ethnically diverse, and representative of children with TBI. Cincinnati Children's maintains a Level I Trauma Center and is one of the few inpatient pediatric rehabilitation programs accredited by the Commission on the Accreditation of Rehabilitation Facilities in the state of Ohio. MetroHealth Medical Center in Cleveland, Ohio includes a Level I trauma center with pediatric commitment. Rainbow Babies & Children's Hospital maintains a Level I Trauma Center and serves as the pediatric hospital for the University Hospital Health System in Cleveland, Ohio. Children's Hospital Colorado (CHCO) is the Rocky Mountain region's only Level 1 Pediatric Trauma Center. Nationwide Children's hospital in Columbus, Ohio is also a level I Trauma Center. We anticipate recruiting 80 children per year in years 1 and 2 across the sites. Males and females will be recruited for participation in the study consistent with the demographics of patients treated for TBI at the participating sites.
3. Procedures
3.1. Recruitment
Potentially eligible children will be identified either during hospitalization or after discharge based on trauma registry information, during an outpatient medical visit, via referral or letter from their physician at participating hospitals, or via ClinicalTrials.gov. Recruitment from multiple access points for families will allow inclusion of a widely representative sample of adolescents with TBI and will broaden the potential impact of the intervention. Eligible children must have been hospitalized for a complicated mild to severe TBI. Consistent with previous investigations, severe TBI will be defined as a Glasgow Coma Scale (GCS) score of 8 or less; moderate TBI as a GCS score between 9 and 12; and complicated mild TBI as a GCS score > 12 [37] accompanied by an Abbreviated Injury Scale (AIS) [38] score of the head region of >3 or abnormal neuroimaging. Exclusionary criteria include: primary language other than English, parent hospitalization for psychiatric reasons during the previous year, or child hospitalization for psychiatric reasons prior to their TBI. Prior psychiatric hospitalization is included as a proxy variable for severe psychiatric disorders. Caregivers with these disorders are relatively uncommon but may compromise capacity to consent or to participate in the intervention. Likewise, children who had psychiatric hospitalizations prior to their injury likely posed a danger to themselves (suicidal) or others (homicidal) and as such would not make good candidates for brief intervention. We elected not to exclude youth who were hospitalized for psychiatric reasons after their injuries because the TBI itself may result in dysregulated behavior that leads to hospitalization. Additionally, children with moderate or severe cognitive disability prior to the injury and those who have not recovered sufficiently to participate verbally in the intervention (e.g., minimally responsive state, severe aphasia) will be excluded. We will include children with nonblunt trauma or a history of child abuse/non-accidental trauma. To be eligible, children must be living in the home with a clearly identified parent/legal guardian who consents to participate. There will be no upper limit on time since injury for recruitment because cognitive and behavioral problems may develop soon after injury and persist long-term after injury [35,36,39].
3.2. Screening
Potential participants must complete their in-hospital medical or rehabilitation care and demonstrate persistent problems (>1 month duration) on the Impact section of the Strengths and Difficulties Questionnaire [40] at the time of enrollment, as defined by parent ratings of child problems that interfere with functioning “only a little” in two or more domains, or “medium amount” or “a great deal” in at least one domain. Potential participants that meet screening criteria will be offered enrollment in the study and a baseline interview.
3.3. Baseline interview and assessment
After consent is obtained, baseline assessment measures, including child and parent treatment preferences, patient/parent-reported measures of teen functioning and quality of life, and parent/family functioning, will be completed either in the medical clinic or the family's home, depending on family preference, by a research assistant naïve to group assignment. The entire baseline assessment is anticipated to take 75–90 min. Additionally, siblings and secondary caregivers will be invited to participate when interested.
3.4. Random assignment
After completion of baseline assessments, families will be randomized based on their distance from the hospital. Patients who live >25 miles from the hospital/clinic will be randomly assigned to one of the two online treatment arms (therapist-guided online F-PST or self-guided online F-PST). Patients who live ≤25 miles will be randomly assigned to one of the three arms (therapist-guided face-to-face F-PST; therapist-guided online F-PST; and self-guided online F-PST) in a two to one ratio, with a higher proportion assigned to the face-to-face arm. This randomization scheme will allow for better matching of treatment modalities to family needs and constraints. Families will be given a sealed envelope with their treatment group assignment based on a computer-generated randomization sequence.
3.5. Computer installation and orientation
If a family does not have an existing home computer and is assigned to one of the two online arms, the research coordinator (not naïve to group assignment) will go to the family's home to set up the computer and Internet connections. A research coordinator will provide all families assigned to an internet treatment group with instructions and a hands-on demonstration of how to get to the study website, and an overview of how to access treatment modules. Research coordinators will also ensure that families assigned to the therapist guided online intervention group have access to a video-conference program (i.e. Skype or Facetime), and make a call with the family to their assigned therapist to introduce the family to the therapist, ensure the family has the therapist's contact information, and schedule the family's first session. The research coordinator will follow-up by phone during the first week to ensure that the family is able to access the site on their own, and will schedule a second home visit if necessary to help the family get online.
3.6. Family problem solving therapy (F-PST)
The F-PST interventions were developed through a series of iterative, stakeholder-driven studies funded by the National Institute on Disability and Rehabilitation Research (NIDRR: 90RT5004-001-00), the National Institute of Child Health and Human Development (NICHD: R01HD04279), and the National Institute of Mental Health (NIMH: R01MH76764). Guided by a participatory action research framework [41], each study has been informed by stakeholder, specifically family, input prior to initiation and subsequently refined based on feedback regarding intervention content, structure, timing, and duration [42,43]. Youth with TBI and their families in all three treatment arms will receive 10 sequential sessions providing training in staying positive/cognitive reframing, problem-solving, communication, and self-regulation/anger management. Core and supplemental sessions are listed in Table 1. The steps of problem solving (Aim, Brainstorm, Choose, Do It, Evaluate; ABCDE) are introduced in the problem solving sessions and the youth with TBI and his/her family are encouraged to apply the problem-solving heuristic to a personal problem or goal at each subsequent session. During sessions with the therapist, lasting approximately 60 min, didactic information, practice skills pertinent to the session, and the problem solving heuristic will be reviewed. Based on individual family concerns, families in the therapist-guided arms can receive up to four additional sessions completed with the therapist focusing on areas of concern for their particular family. Supplemental sessions were generated based on suggestions/input from patients and families and include: Just for Siblings, Parents and Siblings - for Parents, Marital Communication, Talking with your Teen, TBI and Seizures, Sleep and Your Teen, After High School, Pain Management, Crisis Management, Guilt, Grief and Caregiving, and Memory Session. The online-therapist guided and online self-guided interventions will include all of the supplemental sessions on the website, so participants and families can review these sessions on their own. The Face-to-Face intervention will include four additional sessions to be reviewed with the therapist and content from additional sessions will be available from the therapist upon request. Families in the Online Therapist Guided and Face-to-Face arm will receive a total of 10–14 sessions with their therapist.
Table 1.
| Core sessions |
| Session 1: Getting Started |
| Session 2: Problem Solving |
| Session 3: Getting Organized |
| Session 4: Working with the School after TBI |
| Session 5: Staying in Control |
| Session 6: Controlling Your Anger & Improving Communication |
| Session 7: Listening, Talking, Reading Non-Verbal Cues |
| Session 8: Social Behavior and Joining a Group |
| Session 9: Taking Care of You |
| Session 10: Brining It All Together |
| Supplemental sessions |
| Session 11: Just for Siblings |
| Session 12: Parents and Siblings - for Parents |
| Session 13: Marital Communication |
| Session 14: Talking with your Teen |
| Session 15: TBI and Seizures |
| Session 16: Sleep and Your Teen |
| Session 17: After High School |
| Session 18: Pain Management |
| Session 19: Crisis Management |
| Session 20: Guilt, Grief and Caregiving |
| Session 21: Memory Session |
3.7. Face-to-face F-PST (Table 2)
Table 2.
Format, structure, and content of each of the interventions.
| Key Characteristics | Face-to-Face | Therapist-Guided Online | Self-Guided Online |
|---|---|---|---|
| Sessions with Therapistsa | 10-14 sessions | 10-14 sessions | None |
| Online Didactic Modulesa | noneb | 15-21 sessions | 10-21 sessions |
| Total Possible Sessionsa | 10–14 | 10–21 | 10–21 |
| Treatment Components | Education about TBI | Education about TBI | Education about TBI |
| Problem Solving | Problem Solving | Problem Solving | |
| Communication skills | Communication Skills | Communication skills | |
| Anger Management | Anger Management | Anger Management | |
| Self-Regulation | Self-Regulation | Self-Regulation | |
| Who Directs Treatment | Therapist and Family | Therapist and Family | Self and Family |
Sessions refer to the core and supplemental session numbers listed in Table 1.
Families in the face-to-face arm will receive the didactic information in the form of handouts that will be included in a Family Problem Solving Workbook provided at the beginning of treatment.
The goal of the study is to involve caregivers, as well as siblings when available, as participants in therapy sessions. At least one caregiver and the adolescent must participate in all sessions. Families assigned to this arm will meet with the therapist in person at the medical center clinics. Ideally, sessions will occur weekly for the first month and then taper to biweekly for the next 3 months. Therapists will provide the family with a family workbook that contains a printed version of the online content for each session (core and supplemental sessions), and content from additional supplemental sessions are available from the therapist upon request.
3.8. Online therapist-guided F-PST (Table 2)
Family members assigned to this arm will receive a password enabling them to access the online intervention materials throughout the course of the intervention. Each session of online F-PST consists of a self-guided online portion providing didactic content regarding the desired skill (i.e., problem-solving), video clips showing individuals and families modeling the skill, and exercises and assignments giving the family an opportunity to practice the skill. New materials will be released to families upon completion of each session with the therapist. All materials will be encrypted and password protected. Families will be able to obtain immediate corrective feedback regarding online exercises and assignments through the Web pages. Exercises completed by the family online will be stored for review by the therapist prior to the synchronous session. Synchronous, videoconference sessions with the therapist will be ideally scheduled weekly for the first month and then biweekly for the next three months of the intervention, for a total of 10–14 sessions. During these sessions, the therapist will review the online materials and, beginning in session 3, practice the problem-solving process using a problem that the family has identified.
3.9. Self-guided online F-PST (Table 2)
In the self-guided, online F-PST arm, adolescents and their families will receive a password enabling them to access the online intervention materials throughout the course of the intervention. They will receive access to the same web-modules as the therapist-guided group, but will review them on their own without therapist support. Participants in this group will be encouraged to complete web modules at the same schedule (initially weekly then biweekly) as participants in the other groups. If the family fails to log on or complete web modules, they will receive reminders via phone, text, or e-mail.
3.10. Assessment of treatment effects
To evaluate treatment effects, the baseline assessments will be re-administered at six months post baseline, which should correspond to two weeks post treatment completion for most families. We will also collect data regarding parent and adolescent satisfaction with the intervention they received at the post-treatment assessment. An additional follow-up will be conducted three months later to assess the maintenance of and/or other changes in treatment effects. When possible, data will be collected from secondary caregivers (i.e., both mothers and fathers) and satisfaction data from participating siblings. These follow-up assessments will be conducted via secure online link or mail, if the participant does not have internet access. To minimize attrition, research coordinators will contact participants by phone or during a face-to-face visit to complete any questionnaires that the family does not complete independently.
3.11. Therapist training, supervision, and treatment integrity
Study therapists will have a PhD in clinical psychology with experience in treating pediatric TBI and in delivering cognitive behavioral therapy. An intervention training will be conducted by the principal investigator for all sites prior to the beginning of the intervention. A comprehensive treatment manual will also be developed. The treatment manual will outline the objectives for each session and provide detailed sample scripts for working with families to achieve these objectives. Weekly supervision will be provided via conference call among the therapists and the study principal investigator to maintain treatment fidelity across sites. Through the treatment website, there will be an electronic record of the content reviewed during each session. Additionally, the therapist will be asked to chart the content of each session and their notes will be reviewed during weekly supervision to ensure that the treatment manual is being followed. Fidelity to self-guided online F-PST will be evaluated by examining number of sessions completed, amount of time spent on each session, number of family members participating, timing between sessions, and total duration of the intervention.
3.12. Management of threats to study validity
Given that all three groups are receiving identical treatment content delivered in differing formats, contamination across conditions is unlikely. Threats to internal validity include differential assignment to the face-to-face group based on site (e.g. higher proportion of online at some sites), differential family nonadherence across conditions, and differential attrition. We will address threats to validity arising from recruitment and retention problems through several strategies including: 1) use of stakeholder involvement to determine how best to engage and retain families; 2) multiple efforts to contact families to schedule follow-up appointments, including home visits to re-engage them when necessary; 3) communicating with participants and families using their preferred means of communication (e-mail, text, phone, etc.) and 4) collection of alternative phone numbers and contacts in the event that phones are disconnected or addresses change.
If randomization varies by enrollment site, we will include this variable as a covariate in the primary analyses. It is possible that families will terminate their participation if assigned to the face-to-face arm due to feasibility issues, which would provide important information about the viability of this treatment approach for teens with TBI.
3.13. Intervention stopping rules
If throughout the course of treatment a participant is determined to meet any of exclusionary criteria, then they will be discontinued from the study. Additionally, adverse events and safety events will be tracked throughout the course of the study. If there are any events directly attributable to the intervention, then participation will be stopped. Also, families may withdraw from the study at any time.
3.14. Timeline
This study will be conducted over a 3-year period. Participant recruitment will occur in months 0–24, with 20 participants recruited per city (20 Cincinnati, 20 Denver, 20 Cleveland, 20 Columbus) in each of the initial two years of the study. Treatment will be conducted in months 3–30, post treatment follow-ups will be conducted in months 9–30, and 3-month extended follow-ups will be conducted in months 12–33. Data entry, coding, and cleaning will occur throughout the study. The final three months of the study are reserved for statistical analyses and manuscript preparation.
4. Overview of measures
Previous research identified a number of patient-reported outcome measures that are sensitive to treatment-related improvements in behavior and distress. Prior to starting the trial and selecting measures to be used in the study, we obtained feedback from patient/stakeholder groups regarding which patient-reported outcomes were most important to them. The measures described in Table 3 are those selected for the trial based on iterative input from patients/potential participants and their parents/caregivers.
Table 3.
Primary and secondary outcomes and covariates.
| Measure | Domain Assessed | Informant | Time (minutes) | Baseline | 6 Months | 9 Months |
|---|---|---|---|---|---|---|
| Screening | ||||||
| SDQ – impact screening questions | Behavioral functioning | Parent/CG | 5 | |||
| Teen Outcomes | ||||||
| BRIEF | Executive dysfunction | Parent/CG, Youth | 10 | x | x | x |
| SDQ | Behavioral functioning | Parent | 10 | x | x | x |
| Peds QL | Quality of life | Parent/CG, Youth | 5 | x | x | x |
| PHQ-9 | Depression Rating | Youth | 5 | x | x | x |
| HBI | Symptom Ratings of TBI | Parent/CG, Youth | 10 | x | x | x |
| Caregiver Outcomes | ||||||
| CES-D | Depression | Parent/CG | 5 | x | x | x |
| BSI | Psychological Distress | Parent/CG | 5 | x | x | x |
| Family Outcomes | ||||||
| Problem-Solving Discussion Rating | Family functioning | Parent/CG, Youth | 10 | x | x | x |
| Satisfaction | ||||||
| Utilization and Preference Questionnaire | Preference for treatment | Parent/CG, Youth | 10 | x | ||
| Utilization and Satisfaction Questionnaire | Satisfaction of treatment | Parent/CG, Youth | 10 | x | x | |
| Potential Moderators | ||||||
| B&F | Demographics, income | Parent/CG | 10 | x | x | x |
| OSU TBI | History of TBI | Parent/CG | 5 | x | ||
CG=Caregiver; HBI = Health Behavior Inventory; Peds QL = pediatric quality of life inventory and children's activity and participation evaluation; PHQ-9 = Patient Health Questionnaire; CES-D = Center for Epidemiology Scale for Depression; BSI= Brief Symptom Inventory, B&F = Background & Family Form; SDQ= Strength and Difficulties Questionnaire.
4.1. Primary outcome
Parents and youth with TBI will complete the Behavior Rating Inventory of Executive Function (BRIEF), a rating of the child's executive functioning abilities [44]. This measure has high levels of internal consistency (ranging from 0.80 to 0.98) and stability and acceptable levels of both inter-rater and test-retest reliability (ranging from 0.72 to 0.92) [44,45]. The General Executive Composite (GEC), or total score, will be utilized as a broad measure of executive ability/self-regulation. The BRIEF GEC will serve as a primary outcome.
4.2. Secondary outcomes
The Strengths and Difficulties Questionnaire (SDQ) is a parent rating of child behavior over the previous 6 months [40]. The SDQ is scored on a likert scale and includes 25 items, providing at total score and five subscale scores: Emotional Symptoms (ES), Conduct Symptoms (CS), Hyperactivity-Inattention (HYP), Peer Problems (PP), Prosocial Behavior (PSB). The SDQ has good validity and reliability [46]. The SDQ was selected over the Child Behavior Checklist (CBCL) based on stakeholder feedback related to assessment length and National Institute of Neurologic Disease and Stroke common data elements recommendations [47].
The Pediatric Quality of Life Inventory (PedsQL) generic core will be used to assess quality of life. The PedsQL includes 23 items measuring physical, emotional, social, and school function [[48], [49], [50]]. Self-report forms are validated for children 5–18 years and parent report forms are available for children 2–18 years. The PedsQL has been used in pediatric TBI as a quality of life outcome [[51], [52], [53], [54]].
Child depression will be assessed using the Patient Health Questionnaire (PHQ-9), a 9- item self-report rating of depression symptoms [55,56]. The PHQ-9 has good sensitivity and specificity for depression and has been modified for use in adolescents [55]. When pertinent items regarding suicide are elevated, participants will be given a handout that includes information on how to access suicide prevention resources. Additionally, if participants are actively considering suicide they will be referred directly to the emergency room for evaluation.
The Health and Behavior Inventory (HBI) is a 50-item questionnaire that assesses cognitive, somatic, emotional, and behavioral symptoms of TBI [57]. The HBI requires the adolescent and parent to rate the frequency of occurrence of each symptom over the past week on a 4-point scale, ranging from “never” to “often.” The HBI is one of the core common data element (CDE) measures for symptom rating for TBI research in children according to the National Institute of Neurological Diseases and Stroke [47].
4.3. Parent/guardian self-report measures
The Center for Epidemiological Studies Depression Scale (CES-D) [58] will be administered to assess for parental depression. Scores on the CES-D range from 0 to 60, with higher scores reflecting higher levels of depression. Raw scores of 16 and higher are generally used in clinical settings to designate significant depressive symptomatology [58]. The Brief Symptom Inventory (BSI) [59] is a 53-item, self-report inventory in which participants rate the extent to which they have been bothered in the past week by a range of psychiatric symptoms. The BSI has nine subscales designed to assess individual symptom groups and includes three scales that capture global psychological distress.
4.4. Family functioning
The Problem-Solving Discussion Rating Scale (PSDRS) is a 28-item checklist that is completed by parents and teens to evaluate the frequency and severity of parent-teen conflicts [60]. The parent and teen report how often they argue or become upset for each of the 28 conflict areas assessed and also rate the top three conflict areas. Responses range from “never” to “all the time.”
4.5. Moderators and covariates
Lifetime history of TBI will be considered as a covariate. The Ohio State University (OSU) Traumatic Brain Injury (TBI) Identification Method (OSU TBI-ID) is a standardized procedure for eliciting a person's lifetime history of TBI via a 3–5 min structured interview. Validation research indicates that summary indices from this measure have good psychometric properties [61]. Reliability has been demonstrated by both inter-rater and test/re-test reliability [61,62]. Predictive validity has been documented by associations between indices of lifetime history and measures of cognitive performance, affective status, interpersonal functioning and aggression [[61], [62], [63], [64], [65]].
4.6. Preference questionnaire
The Preference Questionnaire will be given to participants at baseline to assess their preference for the varying treatment options. We will examine whether a match (or non-match) in patient-reported preference to group assignment moderates treatment response. Mounting evidence suggests that patients who are randomly assigned to a treatment that they prefer have better outcomes on both patient-reported and objective indices of improvement [33].
4.7. Satisfaction questionnaire
Both qualitative and quantitative methods will be used to assess the family's experience with the treatment that they received. First, parents/caregivers, adolescents with TBI and participating siblings will complete satisfaction surveys. Parents and adolescents will also provide open-ended, qualitative feedback to gain further insight into their experiences with the various treatments.
4.8. Website use
For participants in the online arms, we will collect data on each family's usage of the study website. These data will be used to determine if outcomes vary as a function of website use.
4.9. Psychosocial data
Measures of sociodemographic status will include race and ethnicity, and parent-reported education and income level [66]. Parent-reported education and income level will be used as a proxy for socioeconomic status. These measures will serve as covariates in analyses and will also be examined as potential moderators of intervention effects.
5. Hypotheses
We hypothesize that participants in the therapist-guided online F-PST group compared to the face-to-face and self-guided conditions will report the greatest improvements in teen-, parent-, and family-level outcomes over time owing to the greater treatment intensity. We further anticipate that teen and family characteristics will moderate treatment response, with families with fewer resources (i.e., lower socioeconomic status) and higher levels of stress demonstrating the greatest improvements in the therapist-guided online F-PST [43,[67], [68], [69]]. Additionally, we anticipate that participants assigned to their preferred treatment will report greater improvements in patient outcomes than those who are assigned to a non-preferred treatment group (significant group × preference interaction). We anticipate that the advantage of therapist-guided online F-PST will be evident in terms of both dimensional reductions of behavior problems (i.e., reduced symptom ratings) and reduced proportions of clinically elevated symptoms. Based on previous findings and patient/stakeholder feedback, the major outcomes of interest are: adolescent behavior and emotional functioning (e.g., BRIEF – GEC and SDQ and associated subscales scores) and quality of life (PedsQL). In addition to examining group differences in these measures, we will also assess the extent to which primary and secondary outcomes change in a positive direction from the baseline to post-treatment and maintenance assessments. We also hypothesize that treatment adherence and intensity will be related to patient-reported outcomes, with those completing the greatest number of sessions reporting the greatest improvements in outcomes.
6. Statistical analyses and data management plan
6.1. Data management and reduction
The data management center at Cincinnati Children's Hospital Medical Center will provide the operational infrastructure to facilitate the completeness and accuracy of the collected data, while maintaining subject confidentiality. In order to be as ‘user-friendly’ to the respondents as possible, data will be directly entered in a survey format using the Research Electronic Data Capture (REDCap) system. Monthly data reports will be generated examining dates and ranges of values for outliers or missing data. If discrepancies are found, a query form will be sent to the site for resolution. The answer to the query will be noted on the form and sent back to the coordinating site. Measures of central tendency, variability, and association will be calculated for all variables. Frequency counts will be used to examine variables that are dichotomous or polytomous in nature and histograms will be used to describe continuous measures. Statistical methods will be employed that are appropriate for the distributional properties of the outcome variables. Associations between demographic and outcome variables at baseline will be assessed using correlations (either bi-serial or Pearson's correlations, depending on variable type). Data will be analyzed using Statistical Analysis Software, SAS®, v9.3 (SAS Institute Inc., Cary, NC).
6.2. Analytic plan
Mixed modeling will be used to compare the three treatment conditions on changes over time on primary and secondary outcomes from baseline to the 6- and 9-month assessments. Some models may include baseline scores as a covariate in the analysis along with the two randomization strata (i.e., distance and investigational site). Additional covariates considered for inclusion in the model will be sex, race/ethnicity, age at injury, time since injury, and socioeconomic status. Time since injury will be considered as a covariate in analysis to control for the possibility of recovery of function due to processes other than treatment. Appropriate contrasts will be used to compare treatment groups at baseline (to test for group differences) and at both 6 months (to determine the effectiveness of treatment) and 9 months (to determine maintenance of treatment effects). The primary pairwise comparison will be between the two online treatment groups. Secondary comparisons will be between each of the online treatment groups and the face-to-face group. If less than 14 patients are randomized to the face-to-face group, this group will not be included in any inferential statistical analyses and only descriptive statistics will be presented for this group. The time-by-treatment interaction will determine if the time trends differ among the treatment groups. To determine if there are any moderators of the treatment effects, interaction terms of the potential modifiers with treatment will be examined. The link function appropriate for the distribution of the outcome measure will be used (e.g. log for binary, identity for continuous, log for Poisson). P-values < 0.05 will be considered significant, but may be corrected due to multiple comparisons.
To test the hypothesis that treatment adherence and intensity will be related to improvements in patient-reported outcomes, we will examine total amount of treatment as a mediator of reductions in patient-reported teen behavior problems and parent-reported distress using procedures described by Preacher and Hayes [70,71].
Within a modified intent-to-treat framework [72], we will include baseline and follow-up data from all participants even if they failed to complete the intervention sessions. To evaluate potential attrition bias, drop-outs will be compared to those who remain in follow-up on background characteristics and outcomes measured prior to drop-out. Any factors related to drop out will be considered as cofactors in the analysis, and the possibility of non-random missing data will be examined by means of pattern mixture analysis and suspected biases taken into account in interpreting the results [30,73,74]. As we were able to retain 85–95% of participants in previous investigations [68], we do not anticipate that attrition will pose substantial threats to study validity.
6.3. Power calculations
Power calculations were based on comparing the two online intervention groups on the change from baseline to 6 months for CBCL total and the BRIEF GEC. As noted above, the SDQ was substituted for the CBCL for the current study per stakeholder input. Assuming a standard deviation of 7 units in the change from baseline score to 6 months for each treatment group and that a clinically relevant difference to detect between the two treatment groups is 5 units [75,76], a sample size of 43 subjects in each of the two online intervention groups is needed for 90% power at α = 0.05. Assuming a dropout rate of 15%, 50 subjects will be enrolled into each of the three treatment groups. Because of uncertainty regarding the number of participants willing or able to be randomized to the face-to-face intervention, the primary treatment comparison will be between the two online intervention groups. Recruitment will thus proceed until there are at least 50 subjects assigned to each of the two online treatment groups. Comparison will also be made between each of the online intervention groups and the face-to-face intervention group if there are at least 14 subjects randomized to the face-to-face intervention group. A sample size of at least 14 patients in the face-to-face group and 43 subjects in each of the online groups will provide at least 80% power to detect a difference of 6.5 units assuming a standard deviation of 7 units at α = 0.05. Our planned recruitment of 160 participants across the four sites will allow us to reach these sample size goals with a conservative 15% drop-out rate based on our prior studies.
6.4. Confidentiality
The trial has been approved by the institutional review board (IRB) at all participating sites, and standards for maintaining confidentiality as required by the IRB will be followed. All participants will be assigned a study identification (ID) number at the time of enrollment. This will be kept separate from all identifiable information, and the document linking the study ID to the participant contact information will be kept in a password protected file which is stored in a network drive that is only accessible to those involved in the study, and maintained behind the institutions secure firewall. All paper data will be kept in locked filing cabinets, with signed informed consent documents kept in a physically separate locked cabinet. Once the dataset has been finalized it will be de-identified, and only those with authorization will have access to the dataset.
6.5. Data monitoring/protocol adherence
While all sites will obtain IRB approval, the IRB at the primary site will serve as the IRB of record and function to ensure and maintain protocol adherence for the study. All adverse events related to the study and instances of protocol deviations will be reported to the IRB. All sites will participate in weekly conference calls to discuss recruitment, participant enrollment, data collection, and ensure adherence to protocol. A data monitor and safety board (DSMB) will also be used in the study. The DSMB will act in an advisory capacity to the investigators and the funding agency (Patient-Centered Outcomes Research Institute-PCORI) to monitor the progress, data quality, confidentiality, and patient safety for this clinical study. The DSMB will meet semi-annually and will consist of members independent from the study sponsor and investigative team. There are no plans for interim analyses, unless recommend by the DSMB during the course of the study. A complete de-identified copy of the final dataset will be made available upon request.
7. Discussion
7.1. Summary/plan for dissemination
The present manuscript discusses the rationale and details of a comparative effectiveness trial of F-PST for adolescents with TBI and their families. To our knowledge, this is the only large multi-site RCT examining the comparative effectiveness of F-PST. Prior research has demonstrated the feasibility and efficacy of F-PST delivered via a telehealth medium; however, the generalizability to typical clinical settings is unclear. Additionally, a comprehensive understanding of factors that influence treatment response or non-response is currently lacking. This study evaluates the comparative effectives of F-PST delivered in three different manners. The findings from this trial are expected to inform clinical use and future implementation of F-PST in real-world clinical settings.
We have identified a number of outlets for dissemination. Findings will be conveyed to families and caregivers, clinical care professionals working with these patients, related professionals, and the community more broadly. As with our previous work, we will aggressively disseminate the results from the proposed research at local, state, national and international conferences and through peer-reviewed publications in a wide variety of journals.
A priority of the framework for this trial was to include a high level of patient and family participation in the design and dissemination. Therefore, we will also plan to publish a family newsletter to ensure that participating families are aware of study progress and findings. Our family advisory board will work with us to disseminate the findings to parent and family advocacy groups such as the Brain Injury Association of America. Thus, findings will be disseminated through both traditional (peer-reviewed publications) and contemporary (websites) avenues to diverse audiences. By soliciting input from families about how to best get the information to them, we will put the findings into the hands of all relevant stakeholders.
To further facilitate reproducibility, we are committed to sharing our treatment protocols and providing training and ongoing technical assistance to both clinicians and investigators who may want to use them in their clinical practice or research. This technical assistance may be delivered via e-mail, phone, videoconference, or face-to-face consultation. We will work with interested parties to facilitate collection of similar patient-reported outcomes to allow for comparisons across implementation sites and populations.
7.2. Conclusion
This will be the first randomized, multi-center, clinical trial evaluating the comparative effectiveness of three different delivery modes of F-PST for adolescents with persistent behavioral problems after TBI. It is anticipated that findings from this work will inform future clinical care practices. These data could potentially be translated to other patient populations of youth with psychological symptoms arising from neurological conditions.
Contributorship statement
Each author has made substantial contributions to study design, implementation, analysis, and/or write-up. All authors accept responsibility for reported research, and all authors have participated in the concept and design, analysis and interpretation of data, drafting or revising of the manuscript, and have approved the manuscript as submitted.
Concept and design
Brad G. Kurowski, MD, MS, Terry Stancin PhD, MA, H. Gerry Taylor, PhD, Kelly A. McNally, PhD, Michael W. Kirkwood PhD, MA, Amy Cassedy PhD, Eileen King PhD, Shari L. Wade, PhD.
Acquisition, analysis, or interpretation of data (as planned)
Brad G. Kurowski, MD, MS, Terry Stancin PhD, MA, H. Gerry Taylor, PhD, Kelly A. McNally, PhD, Michael W. Kirkwood PhD, MA, McKenna Sklut BA, Megan E Narad PhD, Shari L. Wade, PhD.
Drafting of the manuscript
Brad G. Kurowski, MD, MS, McKenna Sklut BA, Megan E Narad PhD, Shari L. Wade, PhD.
Critical revision of the manuscript for important intellectual content
Terry Stancin PhD, MA, H. Gerry Taylor, PhD, Kelly A. McNally, PhD, Michael W. Kirkwood PhD, MA, Amy Cassedy PhD, Eileen King PhD, Shari L. Wade, PhD.
Statistical analysis plan and design
Amy Cassedy PhD, Eileen King PhD.
Obtained funding
Shari L. Wade, PhD Administrative, technical, or material support: McKenna Sklut, BA.
Supervision
Terry Stancin PhD, MA, H. Gerry Taylor, PhD, Kelly A. McNally, PhD, Michael W. Kirkwood PhD, MA, Shari L. Wade, PhD.
Conflicts of interest
The authors declare that there are no conflicts or competing interests to disclose.
Funding
Funding for this study was provided by the Patient Center Outcomes Research Institute. Information reported in this work/publication was funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (PCORI-CER-1306-02435). The views, statements, opinions in this work/publication are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.conctc.2018.04.001.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Maas A.I., Stocchetti N., Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7(8):728–741. doi: 10.1016/S1474-4422(08)70164-9. [published Online First: 2008/07/19] [DOI] [PubMed] [Google Scholar]
- 2.Styrke J., Stalnacke B.M., Sojka P. Traumatic brain injuries in a well-defined population: epidemiological aspects and severity. J. Neurotrauma. 2007;24(9):1425–1436. doi: 10.1089/neu.2007.0266. [published Online First: 2007/09/26] [DOI] [PubMed] [Google Scholar]
- 3.Tagliaferri F., Compagnone C., Korsic M. A systematic review of brain injury epidemiology in Europe. Acta Neurochir. 2006;148(3):255–268. doi: 10.1007/s00701-005-0651-y. discussion 68. [published Online First: 2005/11/29] [DOI] [PubMed] [Google Scholar]
- 4.Langlois J., Rutland-Brown W., Thomas K. US Department of Health and Human Servicies, CDC; Atlanta, GA: 2004. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. [Google Scholar]
- 5.Faul M., Xu L., Wald M. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta, GA: 2010. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002-2006. [Google Scholar]
- 6.Taylor C.A., Bell J.M., J B.M. Traumatic brain injury–related emergency department visits, hospitalizations, and deaths — United States, 2007 and 2013. MMWR Surveill. Summ. 2017;66(9):1–18. doi: 10.15585/mmwr.ss6609a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babikian T., Merkley T., Savage R.C. Chronic aspects of pediatric traumatic brain injury: review of the literature. J. Neurotrauma. 2015;32(23):1849–1860. doi: 10.1089/neu.2015.3971. [DOI] [PubMed] [Google Scholar]
- 8.Anderson V., Brown S., Newitt H. Long-term outcome from childhood traumatic brain injury: intellectual ability, personality, and quality of life. Neuropsychology. 2011;25(2):176–184. doi: 10.1037/a0021217. [published Online First: 2011/01/12] [DOI] [PubMed] [Google Scholar]
- 9.Anderson V., Catroppa C., Godfrey C. Intellectual ability 10 years after traumatic brain injury in infancy and childhood: what predicts outcome? J. Neurotrauma. 2012;29(1):143–153. doi: 10.1089/neu.2011.2012. [published Online First: 2011/10/26] [DOI] [PubMed] [Google Scholar]
- 10.Karver C.L., Wade S.L., Cassedy A. Age at injury and long-term behavior problems after traumatic brain injury in young children. Rehabil. Psychol. 2012;57(3):256–265. doi: 10.1037/a0029522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman L.A., Wade S.L., Walz N.C. Clinically significant behavior problems during the initial 18 months following early childhood traumatic brain injury. Rehabil. Psychol. 2010;55(1):48–57. doi: 10.1037/a0018418. 2010-03250-006 [pii] 10.1037/a0018418 [published Online First: 2010/02/24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz L., Taylor H.G., Drotar D. Long-term behavior problems following pediatric traumatic brain injury: prevalence, predictors, and correlates. J. Pediatr. Psychol. 2003;28:251–263. doi: 10.1093/jpepsy/jsg013. [DOI] [PubMed] [Google Scholar]
- 13.Ganesalingam K., Sanson A., Anderson V. Self-regulation and social and behavioral functioning following childhood traumatic brain injury. J. Int. Neuropsychol. Soc.: JINS. 2006;12(5):609–621. doi: 10.1017/S1355617706060796. S1355617706060796 [pii] [published Online First: 2006/09/12] [DOI] [PubMed] [Google Scholar]
- 14.Slomine B.S., McCarthy M.L., Ding R. Health care utilization and needs after pediatric traumatic brain injury. Pediatrics. 2006;117(4):e663–e674. doi: 10.1542/peds.2005-1892. [published Online First: 2006/03/15] [DOI] [PubMed] [Google Scholar]
- 15.Taylor H.G., Yeates K.O., Wade S.L. Bidirectional child-family influences on outcomes of traumatic brain injury in children. J. Int. Neuropsychol. Soc. 2001;7(6):755–767. doi: 10.1017/s1355617701766118. [DOI] [PubMed] [Google Scholar]
- 16.Wade S.L., Gerry Taylor H., Yeates K.O. Long-term parental and family adaptation following pediatric brain injury. J. Pediatr. Psychol. 2006;31(10):1072–1083. doi: 10.1093/jpepsy/jsj077. [published Online First: 2005/09/10] [DOI] [PubMed] [Google Scholar]
- 17.Rivara J.B., Jaffe K.M., Polissar N.L. Predictors of family functioning and change 3 years after traumatic brain injury in children. Arch. PM&R (Phys. Med. Rehabil.) 1996;77(8):754–764. doi: 10.1016/s0003-9993(96)90253-1. [DOI] [PubMed] [Google Scholar]
- 18.Wade S.L., Taylor H.G., Drotar D. A prospective study of long-term caregiver and family adaptation following brain injury in children. J. Head Trauma Rehabil. 2002;17(2):96–111. doi: 10.1097/00001199-200204000-00003. [published Online First: 2002/03/23] [DOI] [PubMed] [Google Scholar]
- 19.Raj S.P., Wade S.L., Cassedy A., Taylor H.G., Stancin T., Brown T.M., Kirkwood M.W. Parent psychological functioning and communication predict externalizing behavior problems after pediactric traumatic brain injury. J. Pediatr. Psychol. 2014;39(1):84–95. doi: 10.1093/jpepsy/jst075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor H.G., Yeates K.O., Wade S.L. Bidirectional child-family influences on outcomes of traumatic brain injury in children. J. Int. Neuropsychol. Soc.: JINS. 2001;7(6):755–767. doi: 10.1017/s1355617701766118. [published Online First: 2001/09/29] [DOI] [PubMed] [Google Scholar]
- 21.Max J.E., Lindgren S.D., Knutson C. Child and adolescent traumatic brain injury: correlates of disruptive behaviour disorders. Brain Inj. 1998;12(1):41–52. doi: 10.1080/026990598122845. [DOI] [PubMed] [Google Scholar]
- 22.Yeates K.O., Taylor H.G., Drotar D. Preinjury family environment as a determinant of recovery from traumatic brain injuries in school-age children. J. Int. Neuropsychol. Soc.: JINS. 1997;3(6):617–630. [published Online First: 1998/02/04] [PubMed] [Google Scholar]
- 23.Kinsella G., Ong B., Murtagh D. The role of the family for behavioral outcome in children and adolescents following traumatic brain injury. J. Consult. Clin. Psychol. 1999;67(1):116–123. doi: 10.1037//0022-006x.67.1.116. [published Online First: 1999/02/24] [DOI] [PubMed] [Google Scholar]
- 24.Taylor H.G., Yeates K.O., Wade S.L. Influences on first-year recovery from traumatic brain injury in children. Neuropsychology. 1999;13(1):76–89. doi: 10.1037//0894-4105.13.1.76. [published Online First: 1999/03/06] [DOI] [PubMed] [Google Scholar]
- 25.Owens P.L., Hoagwood K., Horwitz S.M. Barriers to children's mental health services. J. Am. Acad. Child Adolesc. Psychiatr. 2002;41(6):731–738. doi: 10.1097/00004583-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Newman M.G. Technology in psychotherapy: an introduction. J. Clin. Psychol. 2004;60(2):141–145. doi: 10.1002/jclp.10240. [DOI] [PubMed] [Google Scholar]
- 27.Thomas K.M., Hunt R.H., Vizueta N. Evidence of developmental differences in implicit sequence learning: an fMRI study of children and adults. J. Cognit. Neurosci. 2004;16(8):1339–1351. doi: 10.1162/0898929042304688. [DOI] [PubMed] [Google Scholar]
- 28.Wade S.L., Walz N.C., Carey J. A randomized trial of teen online problem solving for improving executive function deficits following pediatric traumatic brain injury. J. Head Trauma Rehabil. 2010;25(6):409–415. doi: 10.1097/HTR.0b013e3181fb900d. [published Online First: 2010/11/16] [DOI] [PubMed] [Google Scholar]
- 29.Wade S.L., Walz N.C., Carey J.C. Preliminary efficacy of a Web-based family problem-solving treatment program for adolescents with traumatic brain injury. J. Head Trauma Rehabil. 2008;23(6):369–377. doi: 10.1097/01.HTR.0000341432.67251.48. [published Online First: 2008/11/27] [DOI] [PubMed] [Google Scholar]
- 30.Wade S.L., Taylor H.G., Yeates K.O. Long-term parental and family adaptation following pediatric brain injury. J. Pediatr. Psychol. 2006;31(10):1072–1083. doi: 10.1093/jpepsy/jsj077. [DOI] [PubMed] [Google Scholar]
- 31.Wade S.L., Taylor H.G., Cassedy A. Long-term behavioral outcomes after a randomized, clinical trial of counselor-assisted problem solving for adolescents with complicated mild-to-severe traumatic brain injury. J. Neurotrauma. 2015;32(13):967–975. doi: 10.1089/neu.2014.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wade S.L., Kurowski B.G., Kirkwood M.W. Online problem-solving therapy after traumatic brain injury: a randomized controlled trial. Pediatrics. 2015 doi: 10.1542/peds.2014-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King M., Nazareth I., Lampe F. Impact of participant and physican intervention preferences on randomized trials. J. Am. Med. Assoc.: JAMA. 2009;293(9):1089–1099. doi: 10.1001/jama.293.9.1089. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz L., Taylor H.G., Drotar D. Long-term behavior problems following pediatric traumatic brain injury: prevalence, predictors, and correlates. J. Pediatr. Psychol. 2003;28(4):251–263. doi: 10.1093/jpepsy/jsg013. [DOI] [PubMed] [Google Scholar]
- 35.Max J.E., Smith W.L., Jr., Sato Y. Traumatic brain injury in children and adolescents: psychiatric disorders in the first three months. J. Am. Acad. Child Adolesc. Psychiatr. 1997;36(1):94–102. doi: 10.1097/00004583-199701000-00022. [published Online First: 1997/01/01] [DOI] [PubMed] [Google Scholar]
- 36.Max J.E., Wilde E.A., Bigler E.D. Psychiatric disorders after pediatric traumatic brain injury: a prospective, longitudinal, controlled study. J. Neuropsychiatry Clin. Neurosci. 2012;24(4):427–436. doi: 10.1176/appi.neuropsych.12060149. [published Online First: 2012/12/12] [DOI] [PubMed] [Google Scholar]
- 37.Teasdale G., Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. S0140-6736(74)91639-0 [pii] [published Online First: 1974/07/13] [DOI] [PubMed] [Google Scholar]
- 38.Baker S.P., O'Neill B., Haddon W., Jr. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J. Trauma. 1974;14(3):187–196. [PubMed] [Google Scholar]
- 39.Max J.E., Castillo C.S., Robin D.A. Posttraumatic stress symptomatology after childhood traumatic brain injury. J. Nerv. Ment. Dis. 1998;186(10):589–596. doi: 10.1097/00005053-199810000-00001. [published Online First: 1998/10/27] [DOI] [PubMed] [Google Scholar]
- 40.Bourdon K.H., Goodman R., Rae D.S. The strengths and difficulties questionnaire: U.S. Normative data and psychometric properties. J. Am. Acad. Child Adolesc. Psychiatr. 2005;44(6):557–564. doi: 10.1097/01.chi.0000159157.57075.c8. [DOI] [PubMed] [Google Scholar]
- 41.Baum F., MacDougall C., Smith D. Participatory action research. J. Epidemiol. Community. 2006;60(10):854–857. doi: 10.1136/jech.2004.028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wade S.L., Wolfe C., Pestian J.P. A web-based problem solving intervention for families of children with traumatic brain injury. Behav. Res. Meth. Inst. Comput. 2004;36:261–269. doi: 10.3758/bf03195572. [DOI] [PubMed] [Google Scholar]
- 43.Wade S.L., Michaud L., Brown T.M. Putting the pieces together: preliminary efficacy of a family problem-solving intervention for children with traumatic brain injury. J. Head Trauma Rehabil. 2006;21(1):57–67. doi: 10.1097/00001199-200601000-00006. [published Online First: 2006/02/04] [DOI] [PubMed] [Google Scholar]
- 44.Gioia G., Isquith P.K., Guy S.C. Psychological Assessment Resources, Inc; Lutz, FL: 2000. BRIEF: Behavior Rating Inventory of Executive Function. [Google Scholar]
- 45.Gioia G.A., Isquith P.K., Guy S.C. Behavior rating inventory of executive function. Child Neuropsychol. 2000;6(3):235–238. doi: 10.1076/chin.6.3.235.3152. [published Online First: 2001/06/23] [DOI] [PubMed] [Google Scholar]
- 46.Goodman R., Meltzer H., Bailey V. The strengths and difficulties questionnaire: a pilot study on the validity of the self-report version. Eur. Child Adolesc. Psychiatr. 1998;7:125–130. doi: 10.1007/s007870050057. [DOI] [PubMed] [Google Scholar]
- 47.McCauley S.R., Wilde E.A., Anderson V.A. Recommendations for the use of common outcome measures in pediatric traumatic brain injury research. J. Neurotrama. 2012;29(4):678–705. doi: 10.1089/neu.2011.1838. [published Online First: Mar 1 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varni J.W., Burwinkle T.M., Seid M. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul. Pediatr. 2003;3(6):329–341. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [published Online First: 2003/11/18] [DOI] [PubMed] [Google Scholar]
- 49.Varni J.W., Seid M., Kurtin P.S. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med. Care. 2001;39(8):800–812. doi: 10.1097/00005650-200108000-00006. [published Online First: 2001/07/27] [DOI] [PubMed] [Google Scholar]
- 50.Varni J.W., Seid M., Rode C.A. The PedsQL: measurement model for the pediatric quality of life inventory. Med. Care. 1999;37(2):126–139. doi: 10.1097/00005650-199902000-00003. [published Online First: 1999/02/19] [DOI] [PubMed] [Google Scholar]
- 51.McCarthy M.L., MacKenzie E.J., Durbin D.R. The Pediatric Quality of Life Inventory: an evaluation of its reliability and validity for children with traumatic brain injury. Arch. Phys. Med. Rehabil. 2005;86(10):1901–1909. doi: 10.1016/j.apmr.2005.03.026. [published Online First: 2005/10/11] [DOI] [PubMed] [Google Scholar]
- 52.McCarthy M.L., MacKenzie E.J., Durbin D.R. Health-related quality of life during the first year after traumatic brain injury. Arch. Pediatr. Adolesc. Med. 2006;160(3):252–260. doi: 10.1001/archpedi.160.3.252. 160/3/252 [pii] [published Online First: 2006/03/08] [DOI] [PubMed] [Google Scholar]
- 53.Rivara F.P., Vavilala M.S., Durbin D. Persistence of disability 24 to 36 months after pediatric traumatic brain injury: a cohort study. J. Neurotrauma. 2012;29(15):2499–2504. doi: 10.1089/neu.2012.2434. [published Online First: 2012/07/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aitken M.E., McCarthy M.L., Slomine B.S. Family burden after traumatic brain injury in children. Pediatrics. 2009;123(1):199–206. doi: 10.1542/peds.2008-0607. [DOI] [PubMed] [Google Scholar]
- 55.Johnson J.G., Harris E.S., Spitzer R.L. The Patient Health Questionnaire for Adolescents: validation of an instrument for the assessment of mental disorders among adolescent primary care patients. J. Adolesc. Health. 2002;30(3):196–204. doi: 10.1016/s1054-139x(01)00333-0. Pii S1054-139x(01)00333-0. [DOI] [PubMed] [Google Scholar]
- 56.Kroenke K., Spitzer R.L., Williams J.B.W. The PHQ-9-Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barry C.T., Taylor H.G., Klein S. Validity of neurobehavioral symptoms reported in children with traumatic brain injury. Child Neuropsychol. 1996;2(3):213–226. [Google Scholar]
- 58.Radloff L.S. The CES-d scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1(3):385–401. [Google Scholar]
- 59.Derogatis L.R. National Computer Systems; Minneapolis, MN: 1993. The Brief Symptom Inventory (BSI) Administration, Scoring, and Procedures Manual. [Google Scholar]
- 60.Oregon Social Learning Center (OSLC. Project LIFT: Problem Solving Discussion Rating,response Format Revised by the Conduct Problems Prevention Research Group (1996), as the Problem Solving Discussion Rating – (Grade 5+) 1996. [Google Scholar]
- 61.Bogner J., Corrigan J.D. Reliability and predictive validity of the Ohio State University TBI identification method with prisoners. J. Head Trauma Rehabil. 2009;24(4):279–291. doi: 10.1097/HTR.0b013e3181a66356. [published Online First: 2009/07/25] [DOI] [PubMed] [Google Scholar]
- 62.Corrigan J.D., Bogner J. Initial reliability and validity of the Ohio state university TBI identification method. J. Head Trauma Rehabil. 2007;22(6):318–329. doi: 10.1097/01.HTR.0000300227.67748.77. [published Online First: 2007/11/21] [DOI] [PubMed] [Google Scholar]
- 63.Corrigan J.D., Bogner J., Holloman C. Lifetime history of traumatic brain injury among persons with substance use disorders. Brain Inj. 2012;26(2):139–150. doi: 10.3109/02699052.2011.648705. [DOI] [PubMed] [Google Scholar]
- 64.Corrigan J.D., Yang J., Singichetti B. Lifetime prevalence of traumatic brain injury with loss of consciousness. Inj. Prev. 2017 doi: 10.1136/injuryprev-2017-042371. injuryprev-2017-042371. [DOI] [PubMed] [Google Scholar]
- 65.Dams-O'Connor K., Mellick D., Dreer L.E. Rehospitalization over 10 Years among survivors of TBI: a national Institute on disability, independent living, and rehabilitation research traumatic brain injury model systems study. J. Head Trauma Rehabil. 2017;32(3):147–157. doi: 10.1097/HTR.0000000000000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hauser R.M., Warren J.R. Socioeconomic indexes for occupations: a review, update, and critique. Socio. Meth. 1997;27(27):177–298. [Google Scholar]
- 67.Wade S.L., Carey J., Wolfe C.R. An online family intervention to reduce parental distress following pediatric brain injury. J. Consult. Clin. Psychol. 2006;74(3):445–454. doi: 10.1037/0022-006X.74.3.445. [DOI] [PubMed] [Google Scholar]
- 68.Wade S.L., Carey J., Wolfe C.R. The efficacy of an online cognitive-behavioral, family intervention in improving child behavior and social competence following pediatric brain injury. Rehabil. Psychol. 2006;51(3):179–189. [Google Scholar]
- 69.Wade S.L., Walz N.C., Carey J. A randomized trial of teen online problem solving: efficacy in improving caregiver outcomes after brain injury. Health Psychol. 2012;31(6):767. doi: 10.1037/a0028440. [DOI] [PubMed] [Google Scholar]
- 70.Holmbeck G.N. Toward terminological, conceptual, and statistical clarity in the study of mediators and moderators: examples from the child-clinical and pediatric psychology literatures. J. Consult. Clin. Psychol. 1997;65(4):599–610. doi: 10.1037//0022-006x.65.4.599. [DOI] [PubMed] [Google Scholar]
- 71.Hayes A.F., Preacher K.J. Statistical mediation analysis with a multicategorical independent variable. Br. J. Math. Stat. Psychol. 2014;67:451–470. doi: 10.1111/bmsp.12028. [DOI] [PubMed] [Google Scholar]
- 72.Gupta S.K. Intention-to-treat concept: a review. Perspect. Clin. Res. 2011;2(3):109–112. doi: 10.4103/2229-3485.83221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hedeker D., J.S R. The natural history of smoking: a pattern-mixture random-effects regression model. In: Rose J.S., Chassin L., Sherman S.J., editors. Multivariate Applications in Substance Use Research: New Methods for New Questions. Lawrence Erlbaum Associates; Mahwah, NJ: 2000. pp. 79–112. [Google Scholar]
- 74.Schafer J.L. Chapman & Hall/CRC; Boca Raton, FL: 1997. Analysis of Incomplete Multivariate Data. [Google Scholar]
- 75.Wade S.L., Stancin T., Kirkwood M. Counselor-assisted problem solving (CAPS) improves behavioral outcomes in older adolescents with complicated mild to severe TBI. J. Head Trauma Rehabil. 2013 doi: 10.1097/HTR.0b013e31828f9fe8. [published Online First: 2013/05/04] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kurowski B.G., Wade S.L., Kirkwood M.W. Online problem-solving therapy for executive dysfunction after child traumatic brain injury. Pediatrics. 2013;132(1):e158–e166. doi: 10.1542/peds.2012-4040. [published Online First: 2013/06/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.