Abstract
Purpose
To evaluate the effectiveness of high‐dose progesterone supplementation for women who are undergoing a frozen‐thawed embryo transfer (FET).
Methods
Among the 2010 FET cycles that were included in the present study, 1188 were 1200 mg/d of vaginal progesterone, while 822 were 900 mg/d. The dose of progesterone that was used was decided by the treatment period and additional progesterone supplementation was used when the serum progesterone levels were <9 ng/mL on luteal day 5.
Results
The clinical pregnancy rate was higher in the 1200 mg group than in the 900 mg group. The mean serum progesterone level on luteal day 5 in the 1200 mg and 900 mg groups was 12.6 ng/mL and 13.4 ng/mL, respectively. The rate of additional progesterone supplementation was higher in the 1200 mg group. A logistic regression analysis identified a younger age (≤37 years) and the use of 1200 mg progesterone as independent predictive factors for the clinical pregnancy outcome. The analysis of the infant outcomes revealed no significant difference in the distribution of birth ages and weights.
Conclusion
High‐dose transvaginal progesterone of 1200 mg/d as luteal support contributed to good pregnancy outcomes.
Keywords: assisted reproductive technology, embryo transfer, luteal phase, pregnancy outcome, vaginal administration
1. INTRODUCTION
Progesterone supplementation in the luteal phase is known to improve fertility outcomes and has become the standard for artificial reproductive technology (ART). It is based on the theory that the need for progesterone supplementation compensates for the iatrogenic luteal phase defect that is induced by the gonadotropin‐releasing hormone agonist and antagonist that are used in standard in vitro fertilization (IVF) protocols.1 A meta‐analysis that evaluated 18 randomized trials showed that luteal support by the i.m. administration of progesterone or human chorionic gonadotropin (hCG) improved the pregnancy rates over those with the placebo.2
There are three main progesterone supplementation routes: oral, i.m., and transvaginal. The oral route is the most convenient for patients. However, oral progesterone is degraded by the hepatic first‐pass effect, resulting in lower implantation and pregnancy rates than those with the i.m. or transvaginal routes.3, 4, 5 Therefore, the use of oral progesterone for luteal phase support during IVF or intracytoplasmic sperm injection cycles has become less common recently.6 The transvaginal route results in a greater bioavailability, with less relative variability, than the oral route.7 Previous studies compared the outcomes of the i.m. and vaginal routes and mostly reported no significant difference in pregnancy outcomes.8, 9, 10 A meta‐analysis revealed that the i.m. and vaginal routes promote clinical pregnancy to a similar extent.11 Therefore, transvaginal progesterone has become the most popular route for luteal support, avoiding the risk of pain and local reactions at the injection site that are caused by i.m. administration.6 However, a recent study reported that in frozen embryo transfer (FET) cycles, clinical pregnancy and live birth rates were significantly improved by the additional use of i.m. progesterone.12
According to a pharmacokinetic study, the transvaginal route results in a markedly higher progesterone concentration in the endometrial tissue, but a markedly lower serum concentration than by the i.m. route.13, 14 Therefore, the combination of i.m. and transvaginal progesterone administration will increase the serum and endometrial concentrations.
Based on these findings, high‐dose progesterone administration might result in more intensive progesterone support and thus be of benefit to FET cycles. However, there is no agreement currently on the standard dose of transvaginal progesterone for luteal phase support. Previous studies were conducted using vaginal progesterone, including micronized capsules with concentrations ranging between 200 mg and 1200 mg15; however, a direct comparison has not yet been performed for dose‐dependent outcomes. Therefore, the aim of the present study was to compare the outcomes of FET cycles in women who used 900 mg vs 1200 mg transvaginal progesterone for the luteal phase and early pregnancy.
2. MATERIALS AND METHODS
This was a retrospective study that included consecutive cases of 2010 FET cycles with estradiol and progesterone supplementation that were treated at the authors’ clinic. The dose of progesterone that was used was decided by the treatment period; 1200 mg/d of vaginal progesterone was used for patients who were treated between January, 2012 and March, 2014 (1200 mg group), while 900 mg/d was used between April, 2014 and November, 2015 (900 mg group). No significant difference was observed in the treatment strategy, except for the progesterone dose between the two groups. The analyses were limited to FET cycles using hormone replacement cycles with estradiol and progesterone supplementation and also were limited to blastocysts that were graded 3BB or higher. This study was approved by the authors’ Institutional Ethical Committee in accordance with ethical principles that have their origin in the Declaration of Helsinki. All the patients were well informed and written informed consent was obtained prior to the treatment period. The type of treatment that is investigated in the present study already has been discussed in other studies that have shown positive outcomes.
After excluding confounding medical issues, luteal phase support was initiated with transdermal estradiol patches (Estrana® TAPE 0.72 mg; Hisamitsu Pharmaceutical Co., Tokyo, Japan). Briefly, estradiol patches were started with two patches every other day on days 2, 4, 6, and 8 of the menstrual cycle and increased to three, four, and six on days 10, 12, and 14. After 14 days of estradiol administration, transvaginal progesterone supplementation (Utorogestan® 100mg; Besins Manufacturing Belgium, Brussels, Belgium) was added at the doses described above (four or three capsules three times daily). The estradiol patches then were decreased to three every other day and continued until day 30 of the menstrual cycle. Five days after the initiation of progesterone supplementation, frozen embryos were warmed and used for embryo transfer (ET). If the serum progesterone level was <9 ng/mL on the day of the ET, additional progestin medication, such as chlormadinone acetate or hydroxy progesterone capronate, was used. The primary endpoint of clinical pregnancy was defined as the presence of an intrauterine gestational sac. Chemical spontaneous abortion was defined as decreased β‐hCG serum levels before the detection of a gestational sac.
Birth information was obtained by query letters or phone calls to the obstetrics institutions to which the patients were transferred after pregnancy. Cases with full information on infants, including their sex, birthweight, and gestational age at birth were included in the present study.
Statistical comparisons of the clinical parameters between the 1200 mg and 900 mg groups were performed by the Student's t test and chi‐squared test. For the parameters that were analyzed by the chi‐square test, odds ratios were calculated in addition to P‐values. Univariate and multivariate logistic regression analyses were conducted in order to analyze the relationships between several parameters and clinical pregnancy. In the logistic regression analysis, a clinically acceptable threshold was calculated when sensitivity and specificity were at the maximum for the continuous variables of age and serum progesterone levels. All the statistical analyses were performed with EXCEL (2016; Microsoft, Santa Rosa, CA, USA) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (R Foundation for Statistical Computing, Vienna, Austria).16 P‐values of <.01 were considered to be significant in the present study.
3. RESULTS
Table 1 summarizes the baseline characteristics of the cases that were examined in the present study. Among the 2010 cases, 1188 were treated with 1200 mg, while 822 were treated with 900 mg of progesterone. Differences in the numbers were based on the study duration (27 and 20 months) and no significant difference was noted in the age or the number of ART experiences between the two groups. Among the indications for ART, the tubular factor was the most common in both groups and similar ratios were observed among all factors.
Table 1.
Baseline characteristics of the full set of patients
| Variable | 1200 mg | 900 mg | P‐value |
|---|---|---|---|
| Study period | Jan. 2012‐Mar. 2014 | Apr. 2014‐Nov. 2015 | — |
| (duration) | (27 months) | (20 months) | |
| FET cycles | 1188 | 822 | — |
| Agea | 34.4 ± 3.8 | 34.9 ± 3.5 | .13 |
| No. of patients aged >37 years (%) | 290 (24.4) | 212 (25.8) | .48 |
| No. of ART experiencesa | 1.4 ± .97 | 1.4 ± .94 | .90 |
| Indications for ART (%) | |||
| Tubal factors | 683 (57.5) | 683 (51.7) | — |
| Endometriosis | 116 (9.8) | 78 (9.5) | — |
| Male factors | 324 (27.3) | 230 (28.1) | — |
| Immune factors | 18 (1.5) | 7 (0.9) | — |
| Unexplained infertility | 206 (17.4) | 197 (24.0) | — |
| Othersb | 455 (38.3) | 290 (35.4) | — |
ART, assisted reproductive technology; FET, frozen‐thawed embryo transfer.
Indications for ART allowed multiple answers and included duplicated cases among factors.
Values are expressed as the average ± SD.
Includes ovulation disorders, corpus luteum incompetence, uterine fibroids, and ovarian insufficiency.
Table 2 shows the clinical outcomes, including clinical pregnancy, chemical spontaneous abortion, live birth, serum progesterone level on luteal day 5, and the number of cases that were administered additional progesterone (serum progesterone levels on luteal day 5 were <9 ng/mL). The total clinical pregnancy and live birth rates were higher in the 1200 mg group (63.2% vs 57.5%, P < .01 and 40.4% vs 34.8%, P < .01, respectively). Conversely, the serum progesterone level on luteal day 5 was lower in the 1200 mg group (12.6 ± 5.4 vs 13.4 ± 4.1, P < .01). As a result, the rate of additional progesterone administration was higher in the 1200 mg group (23.5% vs 3.5%, P < .01). No significant difference was observed in the chemical spontaneous abortion rate between the two groups. A subgroup analysis revealed that the clinical pregnancy rates were not significantly different among the cohort of additional progesterone use or not (66.2% vs 63.5%, P = .41 in the 1200 mg group and 57.5% vs 58.6%, P = .91 in the 900 mg group).
Table 2.
Clinical outcomes between the 1200 mg group and the 900 mg group
| Variable | Overall | Age ≤37 years | Age >37 years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1200 mg n = 1188 | 900 mg n = 822 | OR | P‐value | 1200 mg n = 898 | 900 mg n = 610 | OR | P‐value | 1200 mg n = 290 | 900 mg n = 212 | OR | P‐value | |
| Clinical pregnancy (N, %) | 753.0 (63.2) | 473.0 (57.5) | 1.28 | <.01b | 590.0 (65.7) | 376.0 (61.6) | 1.19 | .10 | 163.0 (56.2) | 97.0 (45.8) | 1.5 | .02 |
| Chemical spontaneous abortion (N, %) | 168.0 (14.1) | 131.0 (15.9) | .81 | .10 | 110.0 (12.2) | 90.0 (14.7) | .83 | .43 | 58.0 (20.0) | 41.0 (19.3) | 0.97 | .98 |
| Live birth (N, %) | 480.0 (40.4) | 294.0 (34.8) | 1.22 | <.01b | 413.0 (46.0) | 240.0 (39.3) | 1.31 | .02 | 88.0 (30.3) | 56.0 (26.4) | 1.21 | .36 |
| Serum progesterone level (ng/mL)a | 12.6 ± 5.4 | 13.4 ± 4.1 | — | <.01b | 12.6 ± 5.4 | 13.4 ± 4.1 | — | <.01b | 12.7 ± 5.6 | 13.4 ± 4.1 | — | <.01b |
| No. of additional progesterone uses (N, %) | 281.0 (23.5) | 29.0 (3.5) | 8.3 | <.01b | 209.0 (23.2) | 23.0 (3.8) | 7.70 | <.01b | 72.0 (24.8) | 6.0 (2.8) | 11.3 | <.01b |
OR, odds ratio.
Additional progesterone was administered when the serum progesterone level on embryo transfer (luteal day 5) was <9 ng/mL.
Values are expressed as the mean ± standard deviation.
P < .01, which was a significant difference.
When the clinical outcomes were compared between the cohorts of patients who were aged 37 years or younger and 37 years or older, the younger patients showed more favorable outcomes in both groups (a higher clinical pregnancy rate, higher live birth rate, and lower chemical spontaneous abortion rate). Conversely, no significant difference was observed in the serum progesterone level or the rate of additional progesterone administration between the younger and older patients.
Univariate and multivariate regression analyses subsequently were conducted in order to identify the factors related to clinical pregnancy. As shown in Table 3, the univariate analysis identified age and the progesterone dose as significant predictors of clinical pregnancy and both appeared to be independently associated with clinical pregnancy in the multivariate analysis.
Table 3.
Univariate and multivariate analyses of several parameters as regulators of clinical pregnancy
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P‐value | Odds ratio | 95% CI | P‐value | |
| No. of ART experiences (1 vs more) | 1.22 | .99‐1.48 | .06 | — | — | — |
| ART method (IVF vs ICSI) | .92 | .75‐1.13 | .45 | — | — | — |
| Additional progesterone use (“Yes” vs “No”) | 1.25 | .96‐1.63 | .09 | — | — | — |
| Serum progesterone level (>20.4 vs <20.4) | 1.30 | .88‐1.93 | .17 | — | — | — |
| Age (years) (≤37 vs >37) | 1.61 | 1.35‐2.08 | <.01 | 1.67 | 1.35‐2.24 | <.01a |
| Progesterone dose (1200 mg vs 900 mg) | 1.27 | 1.06‐1.53 | <.01 | 1.27 | 1.06‐1.53 | <.01a |
ART, assisted reproductive technology; CI, confidence interval; ICSI; intracytoplasmic sperm injection; IVF, in vitro fertilization.
P < .01, which means a significant difference.
The profile of 758 infants were analyzed: 587 (318 male and 269 female) and 171 (85 male and 86 female) were born to mothers of the 1200 mg and 900 mg groups, respectively. As shown in Figure 1A, no significant difference was observed in the distribution of the gestational age at birth between the two groups. The birthweight of the infants naturally increased in proportion to their gestational age at birth and no significant difference was noted between the two groups, regardless of their sex (Figure 1B,C).
Figure 1.
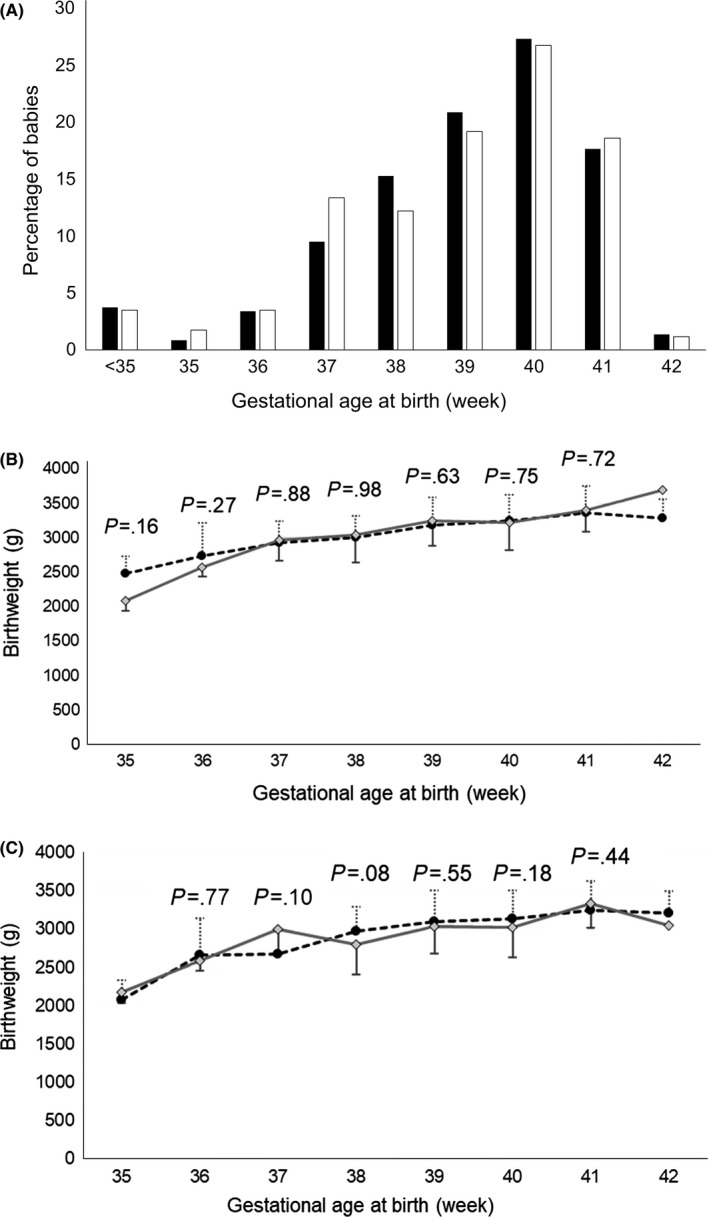
A, Distribution of the gestational ages at birth among the 1200 mg group (■) and the 900 mg group (□). B, Comparison of the birthweight of the male babies by gestational age from 35‐42 weeks between the 1200 mg group and the 900 mg group. ● and ◊ represent the mean values and standard deviations of the 1200 mg group and 900 mg group, respectively, with the P‐values calculated by an unpaired t test in each period. C, Comparison of the birthweight of the female babies by gestational age from 35‐42 weeks between the 1200 mg group and the 900 mg group. ● and ◊ represent the mean values and standard deviations of the 1200 mg group and 900 mg group, respectively, with the P‐values calculated by an unpaired t test in each period
4. DISCUSSION
Progesterone supplementation in the luteal phase has become the standard in ART and many types of medications are available for use. Historically, the i.m. route was the most widely used for luteal phase support because of earlier evidence of higher clinical pregnancy and delivery rates than those with the vaginal route.17 However, with the accumulation of evidence that the vaginal route is at least equally as effective as the i.m. route, transvaginal progesterone preparations became more popular and the most widely used route in most countries.6, 18 The vaginal route has some advantages over the i.m. route, including less pain, fewer adverse effects, and as a result, better compliance. In addition, vaginal progesterone supplementation is considered to result in high progesterone levels in the uterine endometrium, which might result in favorable effects on pregnancy outcomes.19 Conversely, the serum progesterone concentrations were reported to be lower with the vaginal route than with the i.m. route.20 However, currently it remains unclear whether these different pharmacokinetic characteristics affect the results of ART and thus there is no established dosing method for the transvaginal route that is optimal for luteal phase support in ART.21 In contrast, some drugs of other dosage forms have the optimal dose for luteal phase support in ART. Patients need to be presented with a range of preparations for use, based on their effectiveness, convenience, and cost considerations. Therefore, a comparative analysis based on the different dose settings for transvaginal progesterone should have clinical value, so as to provide evidence for the optimal dosage for luteal support in ART.
In the present study, the serum progesterone concentrations were higher in the 900 mg group. This result prompted the authors to speculate that higher serum progesterone concentrations might negatively affect pregnancy outcomes. However, many patients with high serum progesterone levels became pregnant and the logistic regression analysis revealed that the serum progesterone concentrations were not associated with clinical pregnancy. Therefore, differences in the serum progesterone concentrations were not considered to be a significant factor when the two groups were compared.
In the present study, additional progesterone supplementation was administered when the serum progesterone levels on luteal day 5 were <9 ng/mL and this might have had an impact on the results that were obtained. The proportion of patients who used additional progesterone supplementation was significantly higher in the 1200 mg group, indicating the beneficial effects of additional progesterone. However, no significant difference was observed in the clinical outcomes when additional progesterone was administered or not in the 1200 mg and 900 mg groups. (The odds ratio was 1.17 vs 1.05 and the P‐value was 0.26 vs 0.94 in the 1200 mg and 900 mg groups, respectively.) Similar results were confirmed by the logistic regression analysis, showing that additional progesterone supplementation was not associated with pregnancy outcomes.
These results indicate that the factor that resulted in better outcomes in the 1200 mg group was simply higher endometrium progesterone concentrations. To date, several studies have reported transvaginal progesterone doses in the range of 200‐1200 mg.22, 23, 24 The most popular dose that was used until recently was 600 mg/d.18, 20 In the present study, a double dose was used in the 1200 mg group. Although it is not appropriate to directly compare the results because of the inclusion criterion of blastocysts that were graded 3BB or higher, the present study revealed higher pregnancy outcomes without any major adverse events, indicating the high efficacy of high‐dose transvaginal progesterone supplementation. It currently remains unclear whether a dose that is >1200 mg could result in better outcomes. Although no study has used >1200 mg of progesterone, it might be possible in theory. However, using more than four capsules three times daily might be inconvenient for patients and thus 1200 mg daily appears to be the upper limit for a practical dose.
In its present form, ART cannot compensate for all births that are lost by the natural decline in fertility with age.25 Similarly, in this series, a younger age was another significant factor, besides the progesterone dose, that was associated with favorable outcomes in ART. The important result of the present study is that the clinical outcomes showed that a transvaginal progesterone dose of 1200 mg was more effective than 900 mg in both the younger and older cohorts. Furthermore, the serum progesterone level on luteal day 5 was similar in the younger and older cohorts, regardless of the progesterone dose. This result indicates that the metabolic reactions of transvaginal progesterone are not related to age and thus the same dose needs to be administered, regardless of age.
In the present study, the effects on birth outcomes also were investigated. The administration of progesterone generally has been reported to reduce the incidence of premature birth under specific conditions, including ART.26 Preterm birth rates have been reported to account for between 5% and 7% of births in developed countries.27 In the present study, the preterm birth rates were 7.9% and 8.7% in the 1200 mg and 900 mg groups, respectively, which were slightly higher than that of the general population. However, considering the increased risk of preterm birth in ART,28 this slight difference is within allowable levels. Progesterone also exerts some effects on the growth of an infant by increasing the mother's appetite during pregnancy. In the present study, the birthweights were within normal limits at all gestational ages on the normal Japanese birthweight chart.29 These results indicate that high‐dose progesterone supplementation does not influence infant profiles.
There were some limitations in the present study. This study only included Asian populations, mostly Japanese, who have been shown to have different characteristics regarding medication doses from non‐Asian populations; therefore, it is difficult to apply the present results to the entire cohort of patients going through ART. Furthermore, this study was a non‐randomized, retrospective study and the patients were divided by the treatment period. Although there was no significant difference in the treatment strategy, other than the progesterone dose between periods, unknown factors might have affected the obtained results. Therefore, a larger, randomized, prospective study is needed in order to reach more concrete conclusions.
In conclusion, the results of the present study suggest that high‐dose transvaginal progesterone of 1200 mg/day as luteal support contributed to higher pregnancy and live birth rates and no major side‐effects were observed in the patients or infants.
DISCLOSURES
Conflict of interest: The authors declare no conflict of interest. Human Rights Statement and Informed Consent: This study was approved by the Ethical Committee of Hanabusa Women's Clinic, which consists of members that are chosen by the institute and a third‐party medical institute (Approval No. 2017‐09). All the procedures that were followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all the patients to be included in the study. Animal studies: This article does not contain any study with animal participants that have been performed by any of the authors.
ACKNOWLEDGEMENTS
The authors would like to thank the laboratory staff at Hanabusa Women's Clinic, Kobe City, Japan, who helped to collect the data.
Enatsu Y, Enatsu N, Kishi K, et al. Effectiveness of high‐dose transvaginal progesterone supplementation for women who are undergoing a frozen‐thawed embryo transfer. Reprod Med Biol. 2018;17:242–248. 10.1002/rmb2.12096
REFERENCES
- 1. Ebrahimi M, Asbagh FA, Darvish S. The effect of luteal phase support on pregnancy rates of the stimulated intrauterine insemination cycles in couples with unexplained infertility. Int J Fertil Steril. 2010;4:51‐56. [Google Scholar]
- 2. Soliman S, Daya S, Collins J, Hughes EG. The role of luteal phase support in infertility treatment: a meta‐analysis of randomized trials. Fertil Steril. 1994;61:1068‐1076. [DOI] [PubMed] [Google Scholar]
- 3. Pritts E, Atwood A. Luteal phase support in infertility treatment: a meta‐analysis of the randomized trials. Hum Reprod. 2002;17:2287‐2299. [DOI] [PubMed] [Google Scholar]
- 4. Licciardi FL, Kwiatkowski A, Noyes NL, Berkeley AS, Krey LL, Grifo JA. Oral versus intramuscular progesterone for in vitro fertilization: a prospective randomized study. Fertil Steril. 1999;71:614‐618. [DOI] [PubMed] [Google Scholar]
- 5. Friedler S, Raziel A, Schachter M, Strassburger D, Bukovsky I, Ron‐El R. Luteal support with micronized progesterone following in‐vitro fertilization using a down‐regulation protocol with gonadotrophin‐releasing hormone agonist: a comparative study between vaginal and oral administration. Hum Reprod. 1999;14:1944‐1948. [DOI] [PubMed] [Google Scholar]
- 6. Vaisbuch E, de Ziegler D, Leong M, Weissman A, Shoham Z. Luteal‐phase support in assisted reproduction treatment: real‐life practices reported worldwide by an updated website‐based survey. Reprod Biomed Online. 2014;28:330‐335. [DOI] [PubMed] [Google Scholar]
- 7. Levine H, Watson N. Comparison of the pharmacokinetics of crinone 8% administered vaginally versus Prometrium administered orally in postmenopausal women(3). Fertil Steril. 2000;73:516‐521. [DOI] [PubMed] [Google Scholar]
- 8. Dal Prato L, Bianchi L, Cattoli M, Tarozzi N, Flamigni C, Borini A. Vaginal gel versus intramuscular progesterone for luteal phase supplementation: a prospective randomized trial. Reprod Biomed Online. 2008;16:361‐367. [DOI] [PubMed] [Google Scholar]
- 9. Yanushpolsky E, Hurwitz S, Greenberg L, Racowsky C, Hornstein MD. Comparison of crinone 8% intravaginal gel and intramuscular progesterone supplementation for in vitro fertilization/embryo transfer in women under age 40: interim analysis of a prospective randomized trial. Fertil Steril. 2008;89:485‐487. [DOI] [PubMed] [Google Scholar]
- 10. van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev. 2015;(7):CD009154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zarutskie PW, Phillips JA. A meta‐analysis of the route of administration of luteal phase support in assisted reproductive technology: vaginal versus intramuscular progesterone. Fertil Steril. 2009;92:163‐169. [DOI] [PubMed] [Google Scholar]
- 12. Feinberg EC, Beltsos AN, Nicolaou E, Marut EL, Uhler ML. Endometrin as luteal phase support in assisted reproduction. Fertil Steril. 2013;99:174‐178. e1. [DOI] [PubMed] [Google Scholar]
- 13. Miles RA, Paulson RJ, Lobo RA, Press MF, Dahmoush L, Sauer MV. Pharmacokinetics and endometrial tissue levels of progesterone after administration by intramuscular and vaginal routes: a comparative study. Fertil Steril. 1994;62:485‐490. [DOI] [PubMed] [Google Scholar]
- 14. Ficicioglu C, Gurbuz B, Tasdemir S, Yalti S, Canova H. High local endometrial effect of vaginal progesterone gel. Gynecol Endocrinol. 2004;18:240‐243. [DOI] [PubMed] [Google Scholar]
- 15. Ghanem ME, Al‐Boghdady LA. Luteal phase support in ART: an update. Enhancing success of assisted reproduction. Intech. 2012;7:155‐172. [Google Scholar]
- 16. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lightman A, Kol S, Itskovitz‐Eldor J. A prospective randomized study comparing intramuscular with intravaginal natural progesterone in programmed thaw cycles. Hum Reprod. 1999;14:2596‐2599. [DOI] [PubMed] [Google Scholar]
- 18. Mitwally MF, Diamond MP, Abuzeid M. Vaginal micronized progesterone versus intramuscular progesterone for luteal support in women undergoing in vitro fertilization–embryo transfer. Fertil Steril. 2010;93:554‐569. [DOI] [PubMed] [Google Scholar]
- 19. Cicinelli E, De Ziegler D, Bulletti C, Matteo MG, Schonauer LM, Galantino P. Direct transport of progesterone from vagina to uterus. Obstet Gynecol. 2000;95:403‐406. [DOI] [PubMed] [Google Scholar]
- 20. Cicinelli E, Schonauer LM, Galantino P, Matteo MG, Cassetta R, Pinto V. Mechanisms of uterine specificity of vaginal progesterone. Hum Reprod. 2000;15(Suppl. 1):159‐165. [DOI] [PubMed] [Google Scholar]
- 21. Hubayter ZR, Muasher SJ. Luteal supplementation in in vitro fertilization: more questions than answers. Fertil Steril. 2008;89:749‐758. [DOI] [PubMed] [Google Scholar]
- 22. Farhi J, Ben‐Haroush A, Sapir O, Fisch B, Ashkenazi J. High‐quality embryos retain their implantation capability in overweight women. Reprod Biomed Online. 2010;21:706‐711. [DOI] [PubMed] [Google Scholar]
- 23. Tay P, Lenton E. The impact of luteal supplement on pregnancy outcome following stimulated IVF cycles. Med J Malaysia. 2005;60:151. [PubMed] [Google Scholar]
- 24. de Oliveira SA, Calsavara VF, Cortés GC. Final oocyte maturation in assisted reproduction with human chorionic gonadotropin and gonadotropin‐releasing hormone agonist (dual trigger). JBRA Assist Reprod. 2016;20:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leridon H. Can assisted reproduction technology compensate for the natural decline in fertility with age? A model assessment Hum Reprod. 2004;19:1548‐1553. [DOI] [PubMed] [Google Scholar]
- 26. Khandelwal M. Vaginal progesterone in risk reduction of preterm birth in women with short cervix in the midtrimester of pregnancy. Int J Womens Health. 2012;4:481‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lawn JE, Cousens SN, Darmstadt GL, et al. 1 year after The Lancet Neonatal Survival Series – was the call for action heard? Lancet. 2006;367:1541‐1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dunietz GL, Holzman C, McKane P, et al. Assisted reproductive technology and the risk of preterm birth among primiparas. Fertil Steril. 2015;103:974‐979. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Itabashi K, Miura F, Uehara R, Nakamura Y. New Japanese neonatal anthropometric charts for gestational age at birth. Pediatr Int. 2014;56:702‐708. [DOI] [PubMed] [Google Scholar]


