Abstract
Purpose
To determine whether the cycle regimens that are used for endometrial preparation are associated with the birthweight (BW) after assisted reproductive technology (ART) using frozen‐thawed embryo transfer (FET).
Methods
The BW of singletons who were born by ART using FET was compared retrospectively, according to whether a FET was conducted in a hormone replacement therapy cycle (HRT, n = 403) or an ovulatory cycle (OVL, n = 117). The BW after timed intercourse (NAT, n = 162) also was investigated.
Results
There were no significant differences in the age of the mothers, percentage of primiparas, gestational periods, Body Mass Index, and sex ratio between the HRT and OVL cycles. The average BW from HRT was significantly greater than that of OVL. The BW from HRT was also greater, compared with NAT, while statistical significance was not achieved between OVL and NAT. The putative factors affecting the BW, such as ovarian stimulation protocols, endometrial thickness, and the stage and quality of embryos, could not explain the difference in the BW between the HRT and OVL cycles.
Conclusion
An increased BW from ART using FET seems to be ascribable to conditions of the endometrium, but not cryopreservation procedures per se, which might provide a mechanistic framework for understanding heavier neonates who are born by FET.
Keywords: assisted reproductive technology, birthweight, endometrial preparation, frozen embryo, hormone replacement therapy
1. INTRODUCTION
In the face of growing demand for assisted reproductive technology (ART), the potential health impact on babies who are conceived through ART remains a still unresolved concern. To date, a large number of previous works have provided data to indicate that the average birthweight after ART using a fresh embryo transfer is lower in comparison with that after natural conception.1, 2, 3, 4 This seems to be unchanged when an analysis is restricted to singleton babies.5
The first successful pregnancy using a frozen‐thawed embryo transfer (FET) was documented in 1983.6 The FET enables surplus embryos to be stored and the number of embryos per transfer to be reduced, which leads to a lowering of the risk of multiple pregnancies. Hence, the FET is now the most common way as an adjunct to in vitro fertilization (IVF) or IVF/intracytoplasmic sperm injection. Interestingly, there is increasing evidence to suggest that frozen embryo transfers lead to heavier babies, compared with fresh embryo transfers.2, 7, 8
In the present study, the aim was to gain insight into a possible mechanism of a link between ART practices using frozen‐thawed embryos and a heavier birthweight relative to fresh embryos. The FETs were performed using different cycle regimens; that is, ovulatory cycles with or without ovulation‐inducing agents or artificially prepared cycles by the administration of estrogen alone, followed by estrogen in combination with progestin, which involves ovulation suppression (hormone replacement therapy [HRT] cycles). In this connection, it is interesting to note that the quantitative morphology of the placenta in pregnancies conceived through FET is apparently distinct between the cycle regimens; that is, ovulatory cycles vs HRT cycles.9 In view of the placenta playing crucial roles for fetal growth, it was asked whether the birthweight from pregnancies using a FET is related to the cycle regimens for embryo transfer.
2. MATERIALS AND METHODS
A retrospective study was carried out on 523 women from 26‐45 years old who conceived by ART and gave birth at ≥37 weeks of gestation. Every woman who participated in this study had no apparent medical complication other than infertility. The sample was restricted to singleton live births. The ART procedures were performed in Women's Clinic Oizumigakuen, Tokyo, Japan, between September, 2006 and July, 2016, during which time the program's protocols for ART were basically similar. The main reasons for infertility were unexplained infertility, including a low ovarian reserve that was mainly due to ovarian aging, male infertility, female factor infertility, such as endometriosis and tubo‐peritoneal factor. Donor oocytes were not used. For comparison, 162 women who achieved a singleton pregnancy after timed intercourse, with or without fertility medication, during the same period at the clinic, and who gave birth at ≥37 weeks of gestation were investigated.
The women who conceived by ART underwent a FET in either a HRT cycle (406 cases) or an ovulatory cycle (117 cases). As no apparent differences in the implantation rate and pregnancy outcome were found between the HRT and OVL cycles,10 the choice between HRT and OVL was left to the request of the women after being given sufficient information of the methods for endometrial preparation. When using HRT, it is easier to plan the day of embryo transfer; thus, a considerable proportion of women preferred HRT. In addition, women with anovulation, irregular cycles, and older women were recommended to choose HRT.
The stage of the embryos that were transferred was either at the cleavage stage or blastocyst stage. The HRT cases included 38 cleavage‐stage embryo transfers and 368 blastocyst transfers, while the OVL cases consisted of 30 cleavage‐stage embryo transfers and 87 blastocyst transfers. For egg collection, the following ovarian stimulation protocols were used: clomiphene citrate, clomiphene citrate + gonadotropin, gonadotropin + gonadotropin‐releasing hormone (GnRH) agonist, or gonadotropin + GnRH antagonist. In a small number of cases, the eggs were collected in the natural cycle.
For artificially controlled endometrial preparation, estrogen and progestin were administered consecutively. Specifically, estrogen administration was started from day 2 in the menstrual cycle onward every day. It was given until the endometrium reached a thickness of 8 mm, as measured by a transvaginal ultrasound, followed by progestin being combined to initiate the secretory changes. Estrogen was administered as oral tablets of estradiol valerate or estradiol transdermal plasters in increasing doses so as to suppress dominant follicle development, which was confirmed on ultrasonic examination. Both estrogen (estradiol transdermal plasters) and progestins were administered as luteal phase support. The serum concentrations of progesterone on the day of embryo transfer in the OVL cases exceeded well above the physiological concentrations that are found in the luteal phase, while those in the HRT cases were comparable to those corresponding to the same period of the natural cycle, as was confirmed by measuring the hormone. From the day of embryo transfer, chlormadinone acetate was added to vaginal progesterone. Chlormadinone acetate was replaced with dydrogesterone after confirming a positive pregnancy test. Luteal phase support was sustained until 9 weeks’ gestation ended. In the OVL cases, drugs to promote the development of the ovarian follicles, such as clomiphene citrate, gonadotropin etc. were not given, except human chorionic gonadotropin (hCG) administration to trigger ovulation. The luteal phase was supported by vaginal progesterone from the day of the hCG injection with dydrogesterone being co‐administered on the day of the FET onward. If a pregnancy test was positive, luteal phase support continued until the 9 weeks’ gestation was over.
The maximal endometrial thickness was defined as the thickness of the endometrium on the last day of estrogen‐alone administration in the HRT cases and on the day of hCG injection in the OVL cases. The endometrial thickness was measured in the mid‐sagital plane by a transvaginal ultrasound.
The frozen‐thawed embryos that were used in the present study were either early‐stage embryos or blastocysts. The blastocysts that were transferred were graded based on Gardner's classification.11 The cleavage‐stage embryos were classified based on the criteria introduced by Veeck12. A single embryo transfer was used as the basic procedure. However, multiple embryos were transferred in limited particular cases. When multiple embryos were transferred, a top‐quality embryo was regarded as the embryo grade of the case.
The data were analyzed by using EZR software (a modified version of R commander). P < .05 was considered to be statistically significant. The study was approved by the institutional ethics committee managed in Lenia Medical Corporation. All the patients gave informed consent to participate in this study.
3. RESULTS
The maternal characteristics and perinatal outcomes by different cycle regimens are shown in Table 1. The average birthweight in the HRT cases was 3133.0 ± 374.6 g, which was significantly heavier than that in the OVL cases (2996.9 ± 304.9 g; P < .01). The average birthweight in the HRT cases was significantly heavier than that in the NAT cases (3040.9 ± 354.8 g; P < .05). In contrast, the average birthweight in the OVL cases tended to be smaller than that in the NAT cases, but a statistical significance was not reached. No significant difference in the maternal age at delivery was noted between the HRT cases and the OVL cases. The duration of gestation is one of the determinants of the birthweight. The average gestational periods of the HRT cases and the OVL cases were comparable ie, (276.0 ± 8.8 days and 274.8 ± 7.3 days, respectively). It is known that the birthweight with primiparous women is lighter, compared with that of multiparous women. The percentage of primiparous women was 71.4% for the HRT cases and 73.5% for the OVL cases; no significant difference was observed. Parity bias, therefore, was unable to explain the observed difference between the two groups. The maternal Body Mass Index (BMI) is known to have an association with the birthweight. In the present study, the mean maternal BMI value was 20.8 for the HRT cases and 20.4 for the OVL cases, there being no significant difference. Maternal complications might affect the birthweight. The incidence rate of gestational diabetes mellitus was 2.8% for the HRT cases and 3.2% for the OVL cases; no difference was observed. In contrast, the incidence rate of pregnancy‐induced hypertension was 13.0% for the HRT cases, which was significantly higher compared with the OVL cases (6.5%). However, even if analyzed by excluding the cases with these complications, a significant difference in the birthweight between the two groups was still noted.
Table 1.
Maternal and perinatal characteristics according to the treatment modality for infertility
| Variable | Cryopreserved ET | Natural pregnancy | |
|---|---|---|---|
| HRT cycle | Ovulatory cycle | ||
| Number of patients | 406 | 117 | 162 |
| Birthweight (g) | 3133.0 ± 374.6a,c | 2996.9 ± 304.9b | 3040.9 ± 354.8d |
| Age at delivery | 36.2 ± 3.4a | 35.6 ± 3.4c | 34.2 ± 3.7b,d |
| Gestational age (days) | 276.0 ± 8.8 | 274.8 ± 7.3 | 275.9 ± 7.9 |
| Number of primiparous women (%) | 290 (71.4) | 86 (73.5) | 119 (73.5) |
| Maternal BMI | 20.8 ± 2.8 | 20.4 ± 2.2 | 20.4 ± 2.3 |
BMI, body mass index; ET, embryo transfer; HRT, hormone replacement therapy.
Values are expressed as the mean ± standard deviation.
a vs b, P < .01; c vs d, P < .05.
The body weight of the neonates who were born through FET in the HRT cases vs the OVL cases was investigated by the length of gestation (Figure 1). First of all, it was confirmed that there was no difference in the distribution of the number of births for the respective number of gestational weeks between the two groups. Besides, the distribution of the number of births between the two groups was not significantly different, as compared with the distribution of the cases conceived by timed intercourse. Next, the average birthweight in the HRT cases vs that in the OVL cases was investigated at each gestational period, ranging from 37 weeks to ≥40 weeks. The neonates in the HRT cases seemed to be heavier at respective gestational weeks, compared with the OVL cases, with a significant difference being observed at ≥40 weeks (P < .01). The weight of the neonates who were born following timed intercourse was further examined. The neonates in the HRT cases were heavier at any gestational period relative to the NAT cases, with a statistically significant difference noted at 38 weeks’ gestation (P < .05). No discernible difference in the birthweight was seen for each gestational week between the OVL cases and the NAT cases.
Figure 1.
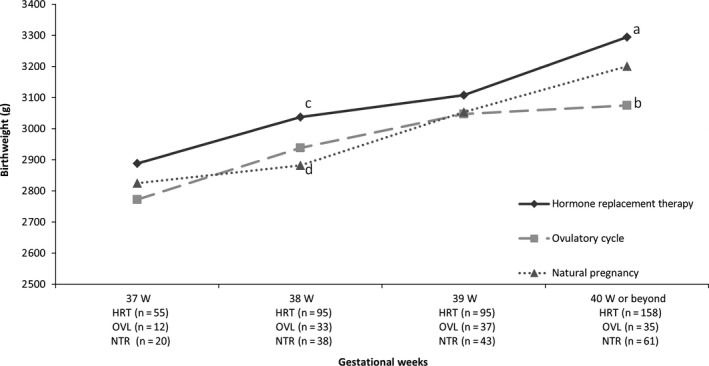
Comparison of the mean birthweight of neonates who were born by assisted reproductive technology (ART) using frozen‐thawed embryo transfer (FET) in hormone replacement therapy (HRT) cycles or ovulatory (OVL) cycles and neonates without ART. The birthweight is shown for each gestational week. a vs b, P < .01; c vs d, P < .05. NTR, natural pregnancy
The endometrial thickness has been shown to be an ART‐related factor that influences newborns’ weight.13 Therefore, the maximal endometrial thickness was compared between the HRT cases vs the OVL cases. The average maximal endometrial thickness was 10.5 ± 2.1 mm in the HRT cases and 10.6 ± 2.3 mm in the OVL cases, with no difference according to the cycle regimen. Table 2 provides a comparison of the birthweight in each protocol for ovarian stimulation between the HRT cases vs the OVL cases. The birthweight for a particular protocol was never significantly heavier than that for other protocols within the HRT group or the OVL group. But, when compared with the birthweight between the groups, the birthweight of the HRT group tended to be heavier in all protocols, compared with those of the OVL group, a significant difference being observed for the GnRH antagonist protocol (P <. 01).
Table 2.
Mean birthweight in each ovarian stimulation protocol
| Category | Cycle | N (%) | Birthweight |
|---|---|---|---|
| Natural | HRT | 9 (1.7) | 3201.3 ± 478.6 |
| OVL | 1 (0.2) | 2902.0 | |
| Clomiphene citrate | HRT | 10 (1.7) | 3056.3 ± 394.5 |
| OVL | 6 (1.2) | 3022.3 ± 544.5 | |
| Clomiphene citrate + gonadotropin | HRT | 102 (19.5) | 3160.9 ± 350.9 |
| OVL | 15 (2.9) | 3012.5 ± 228.3 | |
| GnRH agonist | HRT | 111 (21.2) | 3060.5 ± 350.8 |
| OVL | 39 (7.5) | 3022.9 ± 268.4 | |
| GnRH antagonist | HRT | 172 (32.9) | 3167.6 ± 393.5a |
| OVL | 56 (10.7) | 2973.7 ± 323.6b |
GnRH, gonadotropin‐releasing hormone; HRT, hormone replacement therapy; OVL, ovulatory.
Values are expressed as the mean ± standard deviation.
a vs b, P < .01.
Two cases in the HRT group who were undergoing other ovarian stimulation protocols are deleted from the table.
There exist conflicting data as to whether or not the embryo stage to be transferred (ie, the cleavage stage vs the blastocyst stage) impacts on the birthweight.14, 15 In this study, the birthweight of neonates who were born from a cleavage‐stage embryo transfer vs a blastocyst transfer was compared (Table 3). There was no significant difference in the birthweight between the neonates who were born from cleavage‐stage embryos vs blastocysts in the HRT cases and the OVL cases. Focusing on the blastocyst transfers, the average birthweight of the HRT cases was significantly heavier than that of the OVL cases (P < .01). Furthermore, the average birthweight from the blastocyst transfers in the HRT cases was significantly heavier, compared with that from the cleavage‐stage embryo transfer in the OVL cases (P < .01). When looking at the cleavage‐stage embryo transfers, the average birthweight of the HRT cases was heavier, compared with that of the OVL cases, but it did not reach a significant difference.
Table 3.
Comparison of birthweights between the cleavage‐stage embryos and blastocysts in different cycle regimens
| Stage | Cycle | N (%) | Birthweight |
|---|---|---|---|
| Cleavage | HRT | 38 (9.4) | 3082.4 ± 287.7 |
| OVL | 30 (25.6) | 2959.3 ± 411.4 | |
| Blastocyst | HRT | 368 (90.6) | 3138.3 ± 382.4a |
| OVL | 87 (74.4) | 3009.9 ± 260.1b |
HRT, Hormone replacement therapy; OVL, ovulatory.
Values are expressed as the mean ± standard deviation.
a vs b, P < .01.
Although a better embryo quality is known to be correlated with an increase in implantation and live birth rates,11, 16 it is still an open question whether the grade of blastocyst development at transfer is related to the birthweight or not. Blastocysts were graded according to the criteria of Gardner (Table 4). The inner cell mass (ICM) grade A accounted for 38.0% in the HRT cases and 26.4% in the OVL cases, with the rest being non‐A (B or C). The ratio of A was significantly higher in the HRT cases (P <. 05). As for trophectoderm (TE) grading, the ratio of A was 30.4% in the HRT cases and 19.5% in the OVL cases, there being a significant difference (P < .05). Unexpectedly, the transfer of blastocysts with ICM grade non‐A was associated with a heavier birthweight, compared with those with ICM grade A (P < .05). In contrast, the TE grade (ie, A or non‐A) was not related to the birthweight in both groups. Regarding the cleavage‐stage embryos, the percentage of grade 1 was 31.6% in the HRT cases and 26.7% in the OVL cases, the difference lacking statistical significance. No appreciable difference was found in the birthweight between the grade 1 embryos and the embryos other than grade 1 in each group. Thus, as far as frozen embryo transfers are concerned, the grading of cleavage‐stage embryos seems to have no correlation with the birthweight.
Table 4.
Comparison of mean birthweight by embryo grade in pregnancies after frozen‐thawed blastocyst transfer
| Variable | Cycle | N (%) | Birthweight |
|---|---|---|---|
| ICM Grade | |||
| A | HRT | 140 (38.0) | 3088.7 ± 387.8a |
| Non‐A | 228 (62.0) | 3168.7 ± 376.6b | |
| A | OVL | 23 (26.4) | 2937.5 ± 253.3 |
| Non‐A | 64 (73.6) | 3036.0 ± 259.5 | |
| A | Total | 163 (35.8) | 3067.4 ± 374.9a |
| Non‐A | 292 (64.2) | 3139.6 ± 358.1b | |
| TE Grade | |||
| A | HRT | 148 (40.2) | 3156.4 ± 415.6 |
| Non‐A | 220 (59.8) | 3126.1 ± 358.8 | |
| A | OVL | 22 (25.3) | 3041.5 ± 216.1 |
| Non‐A | 65 (74.7) | 2999.2 ± 274.1 | |
| A | Total | 170 (37.4) | 3141.5 ± 396.9 |
| Non‐A | 285 (62.6) | 3097.1 ± 345.0 | |
HRT, Hormone replacement therapy; ICM, inner cell mass; OVL, ovulatory; TE, trophectoderm.
Values are expressed as the mean ± standard deviation.
a vs b; P < .05.
In general, a female newborn is lighter in weight than a male newborn. The male‐to‐female ratio was 1:22 with the HRT cases and 1:34 with the OVL cases. Although no significant difference was found between the two groups, the ratio of boys tended to be higher with the OVL cases, which could rather discount the possibility that the sex ratio bias could be an explanation for the observed difference between the two groups.
4. DISCUSSION
Here it is demonstrated that the birthweight that arises from ART using frozen‐thawed embryos is related to the cycle regimens of the FET. More precisely, the babies who are born from a FET in HRT cycles are heavier, compared with those born from a FET in ovulatory cycles. Thus far, several papers stated that babies who were born through frozen‐thawed embryos were heavier, compared with fresh embryos. Frozen‐thawed embryos are transferred in either an ovulatory cycle or a HRT cycle. In most of the previous articles that examined the birthweight of babies who were born after a FET, the data, however, were analyzed by combining the cases of both the ovulatory cycle transfers and the HRT cycle transfers together. Currently, a FET is mostly conducted in a HRT cycle, while a fresh embryo is transferred in an egg‐collecting cycle; that is, a non‐HRT cycle. In the present study, though babies who were born from fresh embryos were not looked at, the data presented here might help to explain why babies who are born using frozen‐thawed embryos are heavier than those born using fresh embryos. Put differently, the present results raise a possibility that the heavier birthweight of babies who are born by the use of frozen‐thawed embryos, in comparison with fresh embryos, could be, at least in part, explained by the difference in endometrial preparation for embryo transfer; that is, a HRT cycle vs a non‐HRT cycle.
There is accumulating evidence to suggest that infertile women, if they get pregnant with or without medical assistance, including ovulation induction, ART using fresh embryos, and so on have smaller babies, compared with non‐infertile women.5 This implies that ART using a fresh embryo transfer does not seem to solve the perplexing problem that babies who are born of infertile women tend to be smaller. Interestingly, the nationwide study in Japan showed that babies who were born through a FET were heavier, as compared to those who were born through fresh embryo transfers and all the babies who were born in Japan during the same period.2 In this study, all the babies who were born in Japan included babies born of both infertile and non‐infertile women, but most babies were thought to be born of the non‐infertile women. Thus, one might expect that ART by using a FET could be one of the solutions to the problem of smaller babies that is inherent in pregnancies after infertility.
In the above‐mentioned article from the study in Japan that examined the birthweight of babies who were born after a FET, they, however, did not clearly distinguish the birthweight data according to different cycle protocols; that is, HRT cycles or ovulatory cycles.2 Nevertheless, regarding the neonates who were born after a FET, they notably documented that the weight of neonates in pregnancies after treatment with estrogen combined with progesterone for luteal phase support was heavier, compared with that without any luteal phase support or those undergoing luteal phase support without estrogen. In this context, treatment with estrogen combined with progesterone and no treatment or treatment without estrogen during the luteal phase probably could be regarded as the cases with a HRT cycle transfer and ovulatory cycle transfer, respectively. Viewed in this light, the article from Japan seems to be in line with this study's results.
There is an article from China that compared the birthweight of pregnancies achieved through frozen‐thawed embryos that were transferred in natural cycles vs hormonally stimulated cycles.17 The article concluded that there was no difference in the birthweight between the two groups. However, there are several differences in the study methods between the article and this study. For example, most of the embryos were transferred as blastocysts in this study, while the Chinese group used exclusively cleavage‐stage embryos. Furthermore, they analyzed the data by combining term babies with preterm babies and stimulated the endometrium only in those women with a menstrual irregularity. Therefore, the average gestational period in the study from China was shorter for 7‐10 days, compared with the current study's data. When considering menstrual irregularity, polycystic ovary syndrome (PCOS) is the most representative disorder manifesting the symptom. Several lines of evidence have implicated that PCOS often is accompanied with an endometrial abnormality.18, 19 If this is actually the case, such an endometrium might not acquire sufficient receptivity, even if the endometrium is stimulated by exogenous sex steroids. Collectively, it seems difficult to compare this study's data with those from the Chinese group head‐to‐head.
The birthweight is affected by multiple factors, such as maternal age, being infertile before conception, parity, duration of gestation, medical complications of the mother, the baby's sex, chromosomal abnormalities of the baby etc. A significant difference in the birthweight between the HRT group and the ovulatory group was found. This finding seems to be solid, considering that the known confounding factors that influence the birthweight were well balanced in the two groups. In contrast, the proportion of low‐birthweight neonates (<2500 g) did not differ between the two groups. Therefore, it is conceivable that the difference in birthweight could not be explained by the delay in the development occurring in certain limited fetuses, but it is rather better to interpret that a slight developmental delay appears to occur in a considerable proportion of fetuses.
Various literature has documented the methods for endometrium preparation for a FET. As a whole, no significant difference could be found among the different cycle regimens in terms of the clinical pregnancy rate, live birth rate, and so on.20 At present, however, regarding the birthweight, no conclusion has been drawn to indicate that one particular regimen is superior to another. In the authors’ clinic, estrogen was administered that started on day 2 in the menstrual cycle as oral tablets of estradiol valerate or estradiol transdermal plasters in increasing doses so as to suppress dominant follicle development, followed by the co‐administration of progesterone as vaginal suppositories in addition to synthetic progestins. At present, it remains to be determined concerning the relationship between how to manipulate the endometrium and newborns’ weight.
So far, mixed reports have existed regarding the birthweight after ART in relation to the length of culture for the transferred embryos. A previous article suggested that the birthweight was likely to be decreased with a blastocyst transfer, compared with a cleavage‐stage embryo transfer.14 According to a recent article, this finding could be true for a frozen embryo transfer, but not for a fresh embryo transfer.21 In stark contrast, the article from the Japanese group described that a higher risk of a low birthweight was associated with a cleavage‐stage embryo transfer, compared with a blastocyst transfer, in ART using a fresh embryo transfer, but this was not found with a FET.2 The data presented here showed that as far as frozen‐thawed embryos were used, no apparent difference in birthweight was noted between the blastocysts and cleavage‐stage embryos. To make matters more complicated, the present results demonstrated that, limiting the discussion to a frozen‐thawed blastocyst transfer, the birthweight of the HRT cycle group was significantly heavier, as compared with that of the ovulatory cycle group, implying that the birthweight through whichever cleavage‐stage embryo transfer or blastocyst transfer might be further affected by the endometrial status, in addition to whether the embryos are fresh or frozen‐thawed. At present, it is difficult to explain the reason for the inconsistent results from different study groups. Perhaps, it might be that the stage of the embryo could be associated with the birthweight to such an extent that it might be considerably perturbed or readily canceled in the context of a variety of ART procedures.
Here it was asked whether this study's observation that the difference in birthweight between the HRT group and the ovulatory group being found only with a blastocyst transfer, but not with a cleavage‐stage embryo transfer, has a pivotal meaning or an incidental finding. When focusing on the cleavage‐stage embryo transfer cases, the ratio of the average birthweight in the ovulatory group to that in the HRT group was 0.96, which was almost the same with the blastocyst transfer cases. Accordingly, a possible reason for a non‐significant difference in the birthweight for the cleavage‐stage transfer between the two different cycle regimens might be the number of cleavage‐stage transfer cases being smaller in comparison with that of the blastocyst transfer cases. Viewed this way, the authors feel that manipulation of the endometrium could affect the weight of neonates, regardless of the culturing period of the embryo.
Although it is known that the transfer of embryos with a good quality leads to an increased implantation rate, it is an open question as to how the embryo quality is associated with the birthweight. In this connection, a previous study that examined 224 ART cases who were undergoing a single fresh blastocyst transfer reported that the blastocysts with a more advanced inner cell mass (ICM) went on to become heavier babies, compared with those with a less advanced ICM. In contrast, the trophectoderm grade was not related to the birthweight.22 In this study, when the ICM grade of the blastocysts was divided into “A” and “non‐A,” the percentage of grade non‐A was 62.0% for the HRT group and 73.6% for the ovulatory group. Unexpectedly, the blastocysts of non‐A grade were rather associated with a heavier birthweight relative to the A grade. That is, despite a lower percentage of non‐A for the HRT group, compared with the ovulatory group, the birthweight of the HRT group was greater, which could reduce the possibility that the greater birthweight of the HRT group would be, at least in part, explained by a biased ratio of the ICM grade of the blastocysts that were transferred. The key question is how the grade of ICM is related to the birthweight. The cited study found a relationship between the ICM grade and the birthweight when a single fresh embryo was transferred.22 But, this was not the case with frozen‐thawed blastocysts, possibly because of the low number of cases. In this study, only the cases with a FET were dealt with and, therefore, the status of the endometrium differed between the two studies. These considerations imply that both studies should not be taken as contradicting each other. In addition, the difference in birthweight between grade A and non‐A that was observed in the present study was of borderline significance. Accordingly, it is possible that an association of the non‐A grade with a heavier birthweight might be a random finding.
The placenta plays a variety of roles to support fetal growth and development, carrying gas, nutrition, waste materials across a mother and her fetus, and serving as an immunological barrier between them. The present findings leave open the possibility that there might be differences in the functions of the placenta between HRT cycle transfers and ovulatory cycle transfers. In view of the pleiotropic functions of the placenta, it is extremely difficult to explore this possibility. One way to address the question is histological observations of the placenta. In this regard, the findings from the laboratory of Hamamatsu University in Japan are noteworthy. They revealed that the morphology of the placental basal plate in pregnancies following a FET that was conducted in HRT cycles differs when compared with a FET that was conducted in ovulatory cycles.9 More precisely, compared with a FET in ovulatory cycles, the placenta that was obtained from a FET in HRT cycles exhibited the thickened Rohr fibrinoid layer that is located between the villous structure and the extravillous trophoblast layer, concomitant with thinning of the decidual layer. At present, it is far from clear how to relate the morphological findings to the differential birthweight between the different cycle regimens for embryo transfer. But, what the authors would like to emphasize here is that using frozen embryos was common in both HRT cycle cases and ovulatory cycle cases, implying that the differences in the tissue construction of the placenta might be attributed to different endometrial preparation for transfer. In other words, the endometrial status around the implantation period could influence the formation of the placenta, the biological phenomenon occurring a long time after the implantation period.
The decidua, the maternal part of the placenta, participates in regulating trophoblast invasion into the uterine wall, thus avoiding trophoblast cells going too deep beyond the decidual layer into the myometrium, a pathological condition known as “placenta accreta.” In contrast, a shallow invasion of the trophoblast cells results in preeclampsia. In either circumstance, the decidua is underdeveloped in association with restricted fetal growth.23 One article indicated the association of the thinned decidual layer with pregnancies after a FET in HRT cycles.9 This seemed to stand in contrast to the current findings that the birthweight of the HRT group was higher relative to the ovulatory group. One tentative explanation for this inconsistency might be that the underdevelopment of the deciduas, within the extent of causing obstetrical complications, such as placenta accreta, preeclampsia, and so on might rather allow the trophoblast cells to proliferate and invade to a higher degree, resulting in the birth of heavier babies. This interpretation requires future verification because so far there is no surety as to whether current obstetrical knowledge could be applied to the discussion of feto‐maternal interactions and he pathophysiology of the placenta in pregnancies that are achieved after too much artificial manipulation, including controlled ovarian hyperstimulation, freezing and thawing of embryos, and their transfer in completely hormonally controlled cycles.
A question remains as to why babies arising from a FET in HRT cycles are born heavier in comparison with those who are born from ovulatory cycles. As already mentioned, the babies who are born of infertile women in general tend to be lighter, as compared with non‐infertile women. The endometrial histology of infertile women, even though the menstrual cycle is seemingly ovulatory, often exhibits the finding unfavorable for implantation, which might be a reflection of subtle ovarian dysfunction.24 These findings led to the following hypothesis. The endometrial abnormalities could be causally related to infertility on the one hand and, if pregnancy were achieved, could have a mild influence on the development of the fetus on the other hand. If this actually could be the case, administering sufficient amounts of sex steroid hormones sequentially after menstruation to infertile women might correct the endometrial abnormalities that are often associated with infertile women. However, this remains open to criticism at the moment.
Based on the data presented herein, it is surmised that a FET in hormonally regulated cycles could mitigate the negative side that is related to ART using fresh embryos. This is, however, highly speculative at the present time because it still remains to be seen whether infants born by a FET in hormonally regulated cycles actually could be associated with better health outcomes. In addition, another point of discussion should be taken into consideration when it comes to the dissemination of a FET in hormonally regulated cycles. For instance, it is of note that this technology was suggested to be linked with an increased amount of bleeding during birth.9 Besides, a higher incidence of placenta accreta and pregnancy‐induced hypertension in association with a FET was pointed out.25 Actually, in most of the ART cases using frozen embryos, the embryos have been transferred in HRT cycles. These considerations might pose the problem of obstetric risks that is intrinsic to the procedure of embryo transfer in HRT cycles. The benefit of a FET in HRT cycles (ie, a heavier newborn weight) must be weighed against the putative obstetric risks that are associated with the procedure. To obtain a definitive conclusion as to whether a FET in HRT cycles could be really good for both mothers and children, large‐scale, prospective, randomized studies are required.
This article, dealing with the clinical outcomes of ART, entails some limitations because of the inherent biases that are common to observational studies. Furthermore, the allocation of women to the HRT or OVL group was not in a random fashion, with certain women being inevitably assigned to HRT. Thus, the background of both groups might not be equivalent, implying that unknown biases might have brought about the difference in the birthweight.
DISCLOSURES
Conflict of interest: The authors declare no conflict of interest. Human rights statement and informed consent: All the procedures were followed in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all the patients to be included in the study. Institutional review board of Lenia Medical Corporation approved the study's protocol and its consent form; informed consent was obtained from all the participants in this study. Animal studies: This article does not contain any study with animal participants that were performed by any of the authors.
Ishii R, Shoda A, Kubo M, et al. Identifying a possible factor for the increased newborn size in singleton pregnancies after assisted reproductive technology using cryopreserved embryos, in comparison with fresh embryos. Reprod Med Biol. 2018;17:307–314. 10.1002/rmb2.12206
REFERENCES
- 1. Kondapalli LA, Perales‐Puchalt A. Low birth weight: is it related to assisted reproductive technology or underlying infertility? Fertil Steril. 2013;99:303‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nakashima A, Araki R, Tani H, et al. Implications of assisted reproductive technologies on term singleton birth weight: an analysis of 25,777 children in the national assisted reproduction registry in Japan. Fertil Steril. 2012;99:450‐455. [DOI] [PubMed] [Google Scholar]
- 3. Kalra SK, Ratcliffe SJ, Coutfaris C, et al. Ovarian stimulation and low birth weight in infants conceived through in vitro fertilization. Obstet Gynecol. 2011;118:863‐871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cooper AR, O'Neill KE, Allsworth JE, et al. Smaller fetal size in singletons after infertility therapies: the influence of technology and the underlying infertility. Fertil Steril. 2011;96:1100‐1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shieve LA, Meicke SF, Ferre C, et al. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med. 2002;346:731‐737. [DOI] [PubMed] [Google Scholar]
- 6. Zeilmaker GH, Alberda AT, van Gent I, et al. Two pregnancies following transfer of intact frozen‐thawed embryos. Fertil Steril. 1984;42:293‐296. [DOI] [PubMed] [Google Scholar]
- 7. Pinborg A, Loft A, Aaris Henningsen AK, et al. Infant outcome of 957 singletons born after frozen embryo replacement: the Danish National Cohort Study 1995‐2006. Fertil Steril. 2010;94:1320‐1327. [DOI] [PubMed] [Google Scholar]
- 8. Litzky JF, Boulet SL, Esfandiari N, et al. Effect of frozen/thawed embryo transfer on birth weight, macrosomia, and low birth weight rates in US singleton infants. Am J Obstet Gynecol. 2018;218:433; e1‐433.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakamura Y, Yaguchi C, Itoh H, et al. Morphological characteristics of the placental basal plate in in vitro fertilization pregnancies: a possible association with the amount of bleeding in delivery. Hum Pathol. 2015;46:1171‐1179. [DOI] [PubMed] [Google Scholar]
- 10. Gelbaya TA, Nardo LG, Hunter HR, et al. Cryopreserved‐thawed embryo transfer in natural or down‐regulated hormonally controlled cycle: a retrospective study. Fertil Steril. 2006;85:603‐609. [DOI] [PubMed] [Google Scholar]
- 11. Gardner DK, Lane M, Stevens J, et al. Blastocyst score affects implantation and pregnancy outcome: toward a single blastocycyt transfer. Fertil Steril. 2000;73:1155‐1158. [DOI] [PubMed] [Google Scholar]
- 12. Veeck LL. Preembryo grading and degree of cytoplasmic fragmentation In: An Atlas of Human Gametes and Conceptuses: An Illustrated Reference for Assisted Reproductive Technology. New York, NY: Parthenon Publishing; 1999:46‐51. [Google Scholar]
- 13. Moffat B, Beutler S, Schötzau A, et al. Endometrial thickness influences neonatal birth weight in pregnancies with obstetric complications achieved after fresh IVF‐ICSI cycles. Arch Gynecol Obstet. 2017;296:115‐122. [DOI] [PubMed] [Google Scholar]
- 14. Kallen B, Finnström O, Lindam A, et al. Blastocyst versus cleavage stage tansfer in in vitro fertilization: differences in neonatal outcome? Fertil Steril. 2010;94:1680‐1683. [DOI] [PubMed] [Google Scholar]
- 15. Grinström EE, Bergh C, Khatibi KB, et al. Neonatal and maternal outcome after blastocyst transfer: a population‐based registry study. Am J Obstet Gynecol. 2016;214:378; e1‐378.e10. [DOI] [PubMed] [Google Scholar]
- 16. Heitmann RJ, Hill MJ, Richter KS, et al. The simplified SART embryo scoring system is highly correlated to implantation and live birth in single blastocyst transfers. J Assist Reprod Genet. 2013;30:563‐567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ding J, Zhang Y, Yin T, Yang J. Effects of different endometrial preparation on birth weight of frozen‐thawed embryo transfer. Int J Clin Exp Med. 2016;9:14922‐14928. [Google Scholar]
- 18. Piltonen TT. Polycystic ovary syndrome: endometrial markers. Best Pract Res Clin Obstet Gynecol. 2016;37:66‐79. [DOI] [PubMed] [Google Scholar]
- 19. Shang K, Jia X, Qiao J, et al. Endometrial abnormality in women with polycystic ovary syndrome. Reprod Sci. 2012;19:674‐683. [DOI] [PubMed] [Google Scholar]
- 20. Groenewoud ER, Cantineau AE, Kollen BJ, et al. What is the optimal means of preparing the endometrium in frozen‐thawed embryo transfer cycles? A systematic review and meta‐analysis. Hum Reprod Update. 2013;19:458‐470. [DOI] [PubMed] [Google Scholar]
- 21. De Vos A, Santos‐Ribeiro S, Van Landuyt L, et al. Birth weight of singletons born after cleavage‐stage or blastocyst transfer in fresh and warming cycles. Hum Reprod. 2018;33:196‐201. [DOI] [PubMed] [Google Scholar]
- 22. Licciardi F, McCaffrey C, Oh C, et al. Birth weight is associated with inner cell mass grade of blastocysts. Fertil Steril. 2015;103:382‐387. [DOI] [PubMed] [Google Scholar]
- 23. Seet EL, Kay HH, Wu S, et al. Placenta accreta: depth of invasion and neonatal outcomes. J Matern Fetal Neonatal Med. 2012;25:2042‐2045. [DOI] [PubMed] [Google Scholar]
- 24. Deligdisch L. Hormonal pathology of the endometrium. Mod Pathol. 2000;13:285‐294. [DOI] [PubMed] [Google Scholar]
- 25. Ishihara O, Araki R, Kuwahara A, et al. Impact of frozen‐thawed single‐blastocyst transfer on maternal neonatal outcome: an analysis of 277,042 single‐embryo transfer cycles from 2008 to 2010 in Japan. Fertil Steril. 2014;101:128‐133. [DOI] [PubMed] [Google Scholar]


