Abstract
Venous thromboembolism is a major complication in cancer patients. The basis for the strong association between cancer and thrombosis remains incompletely understood, and the optimal approaches to both the treatment and the prevention of cancer‐associated thrombosis are evolving. Here we review several important topics related to cancer‐associated thromboembolism, including the pathogenesis, prevention, and management of this disease. Wherever possible, we include evidence from clinical trials, including the results of recently published trials that compared direct oral anticoagulants to low‐molecular‐weight heparin for the treatment of cancer‐associated thrombosis.
Keywords: anticoagulation, cancer‐associated thrombosis, deep vein thrombosis, malignancy, pulmonary embolism, Venous thromboembolism
Essentials.
Thombosis is a major complication in cancer patients, but optimal management remains unclear.
We summarize the literature of pathogenesis, risk factors, prevention, and treatment of cancer‐associated thrombosis.
We include the most recent data on the use of direct oral anticoagulants for cancer‐associated thrombosis.
More research is needed for the management in challenging cancer population.
1. INTRODUCTION
Malignancy is a strong risk factor for venous thromboembolism (VTE), shown to be associated with 20%‐30% of incident VTE in population studies.1 Cancer patients have a four‐ to seven‐fold increased risk of VTE and a two‐fold increased risk of major hemorrhage on anticoagulation when compared with patients without cancer,2 and therefore, VTE is the second leading cause of death in the cancer population, right behind cancer itself.3 VTE and its treatment could impact the quality of life in cancer patients, delay cancer treatment, and have complications including recurrent VTE and/or bleeding. Therefore, the optimal prevention and treatment of VTE are crucial components of patient care in this population. Low‐molecular‐weight heparin (LMWH), when compared with warfarin, has been shown to reduce the risk of recurrent VTE in patients with cancer‐associated thrombosis (CAT),4 and is therefore the standard treatment for acute CAT for the past 15 years. However, the high cost and significant lifestyle burden associated with LMWH have led many clinicians and investigators to wonder whether the direct oral anticoagulants (DOACs) might be a better choice for primary and/or secondary VTE prevention in patients with cancer. Here we review literature on important topics in the prevention and treatment of CAT. Our management recommendations are based on available evidence whenever possible. For clinical situations in which there is no high‐quality evidence, we provide management suggestions that are based on our experience and opinion.
2. PATHOGENESIS OF CANCER‐ASSOCIATED THROMBOSIS
Multiple mechanisms of CAT have been identified, which could vary depending on the type of malignancy. A recent review has summarized this topic in detail.5 Traditionally, CAT is thought to represent the intersection of the “Virchow's triad” with chronic disseminated intravascular coagulation (DIC) from malignancy, venous stasis from central venous catheter placement, and endothelial injury from antineoplastic chemotherapy.6 More recent translational research suggests that the vascular microenvironment, including tissue factor, platelets, and neutrophils, explains a great deal of the prothrombotic tendencies.
Tumor derived tissue factor (TF) and TF‐positive microparticles (MPs), especially those from pancreatic cancer cells, can enhance the development of VTE in vivo.7 Retrospective and prospective clinical studies have also demonstrated associations between high levels of TF‐positive MPs and VTE.8, 9 There is ongoing interest to study TF and TF‐positive MPs as potential predictive biomarkers in cancers with high risk of VTE.10 .
Increased soluble P‐selectin, a marker of platelet activation, has been shown to be associated with higher incidence of VTE.11 Increased endothelial expression of P‐selectin appears to be important for CAT, possibly by promoting leukocyte adhesion.7, 12 Platelet‐leukocyte interactions may also be important because neutrophil extracellular traps (NETs) can both promote platelet aggregation and activate the coagulation cascade.13, 14 At least one ongoing clinical trial (NCT02285738) aims to examine the role of anti‐platelet agents in the prevention of CAT.
3. RISK FACTORS OF CANCER‐ASSOCIATED THROMBOSIS
Many risk assessment models have been developed in attempt to identify patients with higher risk of CAT to strategize the optimal therapy. The Khorana Risk Score is one well‐known tool that can help to assess the risks of first VTE in cancer patients undergoing treatment with chemotherapy.15 Based on five prechemotherapy clinical characteristics (site of cancer, platelet count, hemoglobin level, leukocyte count, and body mass index), patients are divided into three risk categories: low, intermediate, and high‐risk, with significantly different risks of VTE in 3 months (0.3%, 2%, and 6.7% in the validation cohort, respectively). Subsequently many other studies have independently validated this risk assessment tool while improving its predictive accuracy by incorporating biomarkers such as D‐dimer and soluble P‐selectin.16 Of note, this risk prediction model does not apply to many hematological malignancies such as multiple myeloma and leukemia because they were excluded from the original study. The Vienna Cancer and Thrombosis study group has recently proposed an alternative risk prediction scheme that uses only two factors (tumor site and D‐dimer) to estimate VTE risk. Their derivation and validation data suggest this simpler model may have a higher C‐statistic than the Khorana score.17
As for recurrent VTE, the Ottawa score has been developed to identify risk factors for recurrent VTE in cancer patients.18 The researchers identified four factors—gender, type of cancer, stage of cancer, and history of VTE for risk assessment. A score ≤0 corresponds with a low risk (≤4.5%) and score ≥1 corresponds with a high risk (≥19%) of VTE recurrence within 6 months. Attempts to validate this model in other populations have yielded conflicting results.19, 20 A subsequent small prospective study showed that baseline soluble P‐selection, but not D‐dimer, was associated with increased risk of recurrent cancer‐associated thrombosis.20 Other studies have identified additional risk factors for recurrent VTE. For example, in the Olmstead County population based cohort study, cancer type, stage, stage progression, and leg paresis were significant risk factors,21 while elevated tissue factor, venous compression, and hepatobiliary cancer were identified as risk factors in the CATCH study.22 More studies are needed to delineate the most useful risk assessment tool to help clinicians to identify patients with high risk of recurrent VTE.
4. PRIMARY PREVENTION OF THROMBOSIS IN CANCER PATIENTS
4.1. Prevention of thrombosis in cancer patients undergoing surgery
Multiple randomized controlled trials have explored the role and optimal duration of thromboprophylaxis in cancer patients undergoing surgery. A recent meta‐analysis included 39 studies comparing perioperative pharmacological thromboprophylaxis in cancer patients undergoing surgery with no pharmacological prophylaxis (including mechanical prophylaxis or no prophylaxis), and demonstrated 50% reduction in the rate of deep vein thrombosis (DVT) with pharmacological prophylaxis, with an acceptable increase in the risk of bleeding, without a difference in mortality and pulmonary embolism (PE).23 In addition, a systemic review and meta‐analysis on seven randomized studies (encompassing 4807 patients) showed that extended thromboprophylaxis (2‐6 weeks) after an abdominopelvic cancer surgery significantly reduced the risk of all VTE and proximal DVT by approximately 50%, when compared with conventional duration of thromboprophylaxis (<2 weeks).24 No difference was found in the incidence of symptomatic PE, major bleeding events, and 3‐month all‐cause mortality.24 Given this evidence, pharmacological thromboprophylaxis is recommended for most cancer patients undergoing surgery, with extended prophylaxis (4 weeks) for patients undergoing abdominopelvic cancer surgery.25, 26
4.2. Prevention of thrombosis in the ambulatory outpatient setting
Randomized controlled studies and meta‐analyses have demonstrated that prophylactic LMWH in ambulatory cancer patients receiving chemotherapy can reduce the risk of VTE.27, 28 However, given the high number needed to treat (NNT) of 40 to 50 in the general population of cancer patients, major guidelines continue to recommend against routine VTE prophylaxis for all ambulatory cancer patients. Ongoing clinical studies of “targeted thromboprophylaxis” aim to determine whether limiting prophylactic anticoagulation to high‐risk patients may result in a lower NNT for this subgroup. The results of one such study (NCT00876915) recently became available.29 After 12 weeks of prophylactic dalteparin in cancer patients with Khorana score ≥3 undergoing chemotherapy, there was a nonsignificant reduction in the risk of VTE (12% on dalteparin vs 21% on placebo, hazard ratio [HR] of 6.9). Major bleeding was low and comparable in both groups, although the dalteparin group had statistically significantly increased risk of clinically relevant bleeding. Of note, the study was underpowered because it was terminated prematurely due to slow accrual. The ongoing Cassini trial (low‐dose rivaroxaban vs placebo, NCT02555878) and the Avert trial (low‐dose apixaban vs placebo, NCT02048865), if positive, may have a higher likelihood of changing clinical practice in high‐risk cancer patients since primary prophylaxis with an oral agent would be less burdensome than LMWH.
5. TREATMENT OF CANCER‐ASSOCIATED THROMBOSIS
5.1. Acute treatment (within 3‐6 months)
Several pivotal randomized controlled studies have shaped our treatment strategy for CAT. In Tables 1 and 2, we summarize and compare the key patient characteristics as well as clinical outcomes across major studies for the treatment of acute CAT (CLOT, CATCH, Hokusai VTE Cancer, Select‐D, and DALTECAN studies).
Table 1.
Comparison of baseline patient characteristics of CLOT, CATCH, Hokusai Cancer VTE, Select‐D, and Daltecan study
| Study | CLOT4 | CATCH33 | Hokusai VTE Cancer45 | Select‐D47 | Daltecan50 , a |
|---|---|---|---|---|---|
| N | 676 | 900 | 1046 | 406 | 334 |
| Age, years (mean) | 62.5 | 59.2 | 64 | 67 (median) | 63.8 |
| Male | 51.5% | 40.6% | 51.6% | 51% | 48.8% |
| Solid tumor | 89.6% | 89.6% | 89.1% | 97% | 91.6% |
| Metastatic disease | 67.3% | 54.7% | 59% | 59% | 62.6% |
| ECOG ≥ 2 | 36.7% | 23.2% | 23.8% | 23.5% | 21% |
| Cancer treatment at randomization | 77.7% | 52.9% | 72.4% | 69% | N/A |
| Incidental | 0% | 0% | 32.5% | 53% | N/A |
| History of VTE | 11% | 6.3% | 10.7% | N/A | N/A |
ECOG, Eastern Cooperative Oncolology Group performance status; N, total number of patients enrolled; N/A, not available; VTE, venous thromboembolism.
Daltecan study is the only nonrandomized prospective cohort study included here.
Table 2.
Rates of 6‐month recurrent venous thromboembolism, major bleeding, and mortality in both arms of CLOT, CATCH, Hokusai VTE Cancer, Select‐D, and Daltecan study
| Study | CLOT4 | CATCH33 | Hokusai VTE Cancer45 | Select‐D47 | Daltecan50 , a | ||||
|---|---|---|---|---|---|---|---|---|---|
| Arms | Warfarin | Dalteparin | Warfarin | Tinzaparin | Edoxaban | Dalteparin | Rivaroxaban | Dalteparin | Dalteparin |
| Recurrent VTE | 53/336 (15.8%) | 27/336 (8.0%) | 45/451 (10.5%) | 31/449 (6.9%) | 34/522 (6.5%) | 46/524 (8.8%) | 8/203 (3.9%) | 18/203 (8.9%) | 29/334 (8.7%) |
| Major bleeding | 12/335 (3.6%) | 19/338 (5.6%) | 11/451 (2.4%) | 12/449 (2.7%) | 29/522 (5.6%) | 17/524 (3.2%) | 11/203 (5.4%) | 6/203 (3%) | 26/334 (7.8%) |
| Mortality | 136/336 (40.5%) | 130/336 (38.7%) | 138/451 (30.6%) | 150/449 (33.4%) | 140/522 (26.8%) | 127/524 (24.2%) | 25%b | 30%b | 116/334 (33.8%) 12 months |
VTE, venous thromboembolism.
Daltecan study is a nonrandomized prospective cohort study.
The numbers of patients are not available.
5.1.1. Low‐molecular‐weight heparin
Low‐molecular‐weight heparin (LMWH) has been the standard of care for treatment of acute CAT for at least 15 years, based on the results of several randomized controlled trials dedicated to patients with CAT. In these studies, LMWH was compared to vitamin K antagonists (VKA) for the treatment of acute VTE in cancer patients. The pivotal CLOT study showed a significant reduction in VTE recurrence,4 while three other smaller studies showed no difference in efficacy or safety outcomes,30, 31, 32 and the most recent CATCH study33 showed a nonsignificant reduction in VTE recurrence, but a significantly decreased risk of clinically relevant non‐major bleeding (CRNMB) in the LMWH arm. We performed a meta‐analysis of the summary data from these studies using Review Manager (RevMan) [Computer program]. Version 5.3. (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen), and found that overall, compared with VKA, LMWH reduced the risks of recurrent VTE by 40% (relative risk [RR] 0.60, 95% CI 0.45‐0.80) (Figure 1A) with no difference in major bleeding (RR 1.07, 95% CI 0.65‐1.75) (Figure 1B). Several major clinical guidelines have recommended LMWH as the first line therapy for treatment of acute CAT.34, 35, 36
Figure 1.
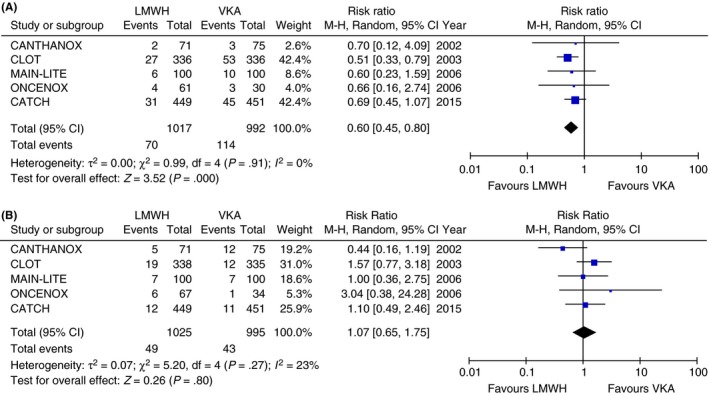
(A) Risk of recurrent venous thromboembolism in 6 months in randomized controlled trials of LMWH vs VKA (random effect model). (B) Risk of major bleeding in 6 months in randomized controlled trials of LMWH vs VKA (random effect model). CI, confidence interval; LMWH, low‐molecular‐weight heparin; M‐H, Mantel‐Haenszel; VKA, vitamin K antagonists
5.1.2. Direct oral anticoagulants
Four DOACs have been approved for the treatment of DVT and/or PE, including dabigatran, rivaroxaban, apixaban, and edoxaban. All have demonstrated a comparable efficacy and safety to VKA in the cancer subpopulation.37 However, the cancer subpopulation enrolled in these large studies was small and generally had lower risks of both recurrent VTE and hemorrhage when compared with patients enrolled in earlier studies specific to cancer patients (such as CLOT study).38 Meta‐analyses have suggested that DOACs and LMWH may have similar efficacy and safety in cancer patients, but these were based on indirect comparisons.38, 39 A few single‐center cohort studies of rivaroxaban for CAT have yielded encouraging results, but all had significant methodologic limitations.40, 41, 42, 43 A recent study explored the use of rivaroxaban in cancer patients with catheter‐related thrombosis also had promising results.44 Overall, these preliminary results are encouraging, but not definitive.
Randomized controlled trials are the gold standard to determine the efficacy and safety of DOACs compared with LMWH in cancer population. Recently, the results of the first study of this kind has been published. The Hokusai VTE Cancer study was an open‐label, randomized, noninferiority study, enrolling patients with a cancer diagnosis within the past 2 years, who developed an acute proximal DVT and/or PE.45 The primary outcome was the composite of the first recurrent VTE or major bleeding event within 12 months. Among the 1046 patients included in the modified intention‐to‐treat analysis, the primary endpoint occurred in 67 of 522 (12.8%) patients in the edoxaban group, compared with 71 of 524 (13.5%) patients in the dalteparin group (hazard ratio with edoxaban, 0.97, 95% CI 0.70‐1.36). Edoxaban was noninferior to dalteparin for the combined outcomes of recurrent VTE or major bleeding in this cancer population (P = .006 for noninferiority). Interestingly, patients randomized to edoxaban experienced numerically fewer recurrent VTE events (7.9% vs 11.3%, P = .09) but more major bleeding events (6.9% vs 4.0%, P = .04) than those in the dalteparin group. However, the frequency of severe (fatal or potentially fatal) major bleeding, adjudicated without knowledge of treatment assignment and according to criteria defined a priori, was not different between the edoxaban and dalteparin groups.46
The results of another study of this kind, the Select‐D pilot trial, were also recently reported as in the abstract format.47 A total of 406 cancer patients were randomized to rivaroxaban or dalteparin for the treatment of a proximal lower extremity DVT or PE. The primary endpoint—the 6‐month VTE recurrence rate—was 3.9% in patients on rivaroxaban, as compared with 8.9% in patients on dalteparin; major bleeding occurred numerically more frequently with rivaroxaban (5.4% vs 3.0%). CRNMB also occurred more in the patients treated with rivaroxaban (12.3% vs 3.0%). More detailed data are awaited in the final publication.
A combined analysis of the two randomized trials was perfomend and a forest plot generated by using Review Manager (RevMan) [Computer program]. Version 5.3. (The Nordic Cochrane Centre, The Cochrane Collaboration); the results are remarkably similar and suggest that DOACs are at least as effective as LMWH for the prevention of recurrent VTE (RR 0.65, 95% CI 0.42‐1.01, Figure 2A), but increase the risk of major bleeding (RR 1.74, 95% CI 1.05‐2.88, Figure 2B).48 The majority of bleeds was gastrointestinal in origin, and the bleeding risk difference was most evident in patients with gastrointestinal cancer. The Hokusai VTE Cancer study showed that the severity of bleeds was less in patients treated with DOACs, with the majority bleeding events treated by transfusion only. Because the absolute risk difference for major bleeding is modest and severeity is less, we expect that many clinicians and patients will chose a DOAC rather than LMWH because of the associated reduction in finanacial and lifestyle burden. At the time of this writing, the best available evidence supports edoxaban for the treatment of cancer‐associated VTE. However, more randomized controlled trials are under way to compare other DOACs to LMWH, including the CASTA‐DIVA study (NCT02746185) and the CONKO‐011 study (NCT02583191), using rivaroxaban compared with LMWH for the treatment of CAT; the CARAVAGGIO study (NCT03045406), using apixaban compared with dalteparin; and the CANVAS study, (NCT02744092) comparing DOAC class with LMWH class. Additional information (such as risk factors for bleeding) will undoubtedly emerge from these trials, along with those already published, that will help clinicians further optimize the risk–benefit tradeoffs of DOACs for cancer patients. For example, risk–benefit trade‐off discussion is particularly needed in patients with gastrointestinal malignancies, given the increased risk of bleeding seen in this subgroup.
Figure 2.
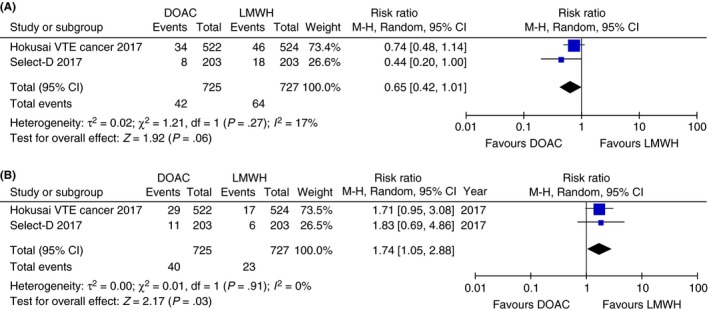
(A) Risk of recurrent VTE in 6 months in randomized controlled trials of DOAC vs LMWH (random effect model).48 (B) Risk of major bleeding in 6 months in randomized controlled trials of DOAC vs LMWH (random effect model).48 CI, confidence interval; DOAC, direct oral anticoagulants; LMWH, low‐molecular‐weight heparin; M‐H, Mantel‐Haenszel; VTE, venous thromboembolism
5.1.3. Vitamin K antagonists
Vitamin K antagonists was shown to be less effective than LMWH in the prevention of recurrent cancer‐associated VTE (Figure 1A). In addition, VKAs have disadvantages that include frequent laboratory monitoring and dose adjustment, multiple drug and diet interactions, and slow onset and offset. Therefore, VKA is not recommended as preferred treatment for CAT in major guidelines. However, it is still widely used in patients with CAT, given the relatively low cost, oral route, and high provider familiarity. A recent study using medical and pharmacy claims from a large insurance database showed that in 2940 cancer patients with a new VTE, the majority (47.7%) were initiated on warfarin as anticoagulation, while 25% on LWMH and 24.1% on rivaroxaban.49 Based on the aggregate of available evidence, warfarin and other VKA should be a “third choice” for cancer patients, used only in situations where netiher a DOAC nor LMWH is feasible.
5.2. Extended treatment (beyond 6 months)
The optimal duration and choice of anticoagulation beyond 6 months for CAT are not as well studied, as the duration of follow‐up and anticoagulation treatment in previously cited pivotal studies were limited to 3‐6 months. Outcomes beyond 6 months have been described in the DALTECAN study and in the TiCAT study, and both were single‐arm cohort studies treating CAT patients with dalteparin (DALTECAN) or tinzaparin (TiCAT) for 12 months.50, 51 Both studies showed that the risk of recurrent thrombosis or major bleeding is higher during the first 3‐6 months, with an ongoing risk of recurrent thrombosis beyond 6 months. Therefore, major guidelines recommend continuing anticoagulation as long as risk factors (ongoing active malignancy and/or cancer treatment) for VTE are present. The Hokusai VTE Cancer study followed enrolled patients for up to 12 months and demonstrated a continued risk of recurrent VTE beyond 6 months, supporting the practice of extended anticoagulation. The Select‐D study initially designed a secondary phase after the initial 6 months of anticoagulation: to stop anticoagulation if there was no residual DVT on repeat ultrasound, and to randomize patients to rivaroxaban or placebo in patients who did have residual DVT.47 However, this phase of the study was prematurally terminated because of slow recruitment.
As for the optimal type of anticoagulation for extended treatment of VTE in the cancer population, the recent Hokusai VTE Cancer study is the first large randomized study that followed anticoagulated cancer patients beyond 6 months, and showed that, based on a composite endpoint of recurrent VTE or major bleeding, edoxaban was not inferior to dalteparin during this timeframe. Therefore, edoxaban (and other DOACs, pending additional evidence) may be an attractive alternative to LMWH for extended treatment in patients with CAT. Future studies should test the hypothesis that lower doses of anticoagulation might be effective after some intial period of therapeutic anticoagulation. The STEP‐CAT study (NCT 027526047) is an ongoing multi‐center, open‐label, single‐arm study of enoxaparin 40 mg subcutaneously daily for patients with CAT who have completed 3‐6 months of therapeutic anticoagulation.
5.3. Treatment in special populations
5.3.1. Patients with recurrent VTE while on anticoagulation
Cancer patients often develop recurrent VTE despite anticoagulation. However, there is no high‐quality evidence to guide practice in this situation. Recommendations from major guidelines including changing oral anticoagulants to LMWH or escalating LMWH doses by 20%‐25% are based on limited retrospective studies.52, 53, 54 The results of a registry study addressing this issue were reported recently, including 212 cancer patients with recurrent VTE on anticoagulation.55 LMWH was associated with a reduced risk of recurrent VTE events when compared with vitamin K antagonists, but escalating the intensity of anticoagulation (such as dose increase) did not reduce the risk compared to lower intensity treatments. The validity of this small, observational study is limited by the possibility of confounding variables and selection bias. None of the patients in this registry received DOACs. The results of recent randomized studies utilizing DOACs may change the way we approach recurrent CAT despite LMWH, because both the Hokusai VTE Cancer study and the Select‐D study suggest that DOACs may be more effective than LMWH in preventing recurrent VTE. Better evidence is needed to guide the management of cancer patients who develop recurrent VTE despite anticoagulation.
5.3.2. Patients with thrombocytopenia
Cancer patients commonly have thrombocytopenia, either related to the underlying malignancy or to the cancer treatment.56 When they develop a CAT at the same time, the risk–benefit decisions are especially complex. Most of the studies in the literature addressing this problem are small and observational, with a great variety of treatment strategies, including different platelet transfusion thresholds and/or different LMWH dose‐adjustments for different levels of thrombocytopenia.57 LMWH has the most safety data in the setting of thrombocytopenia, most likely due to the frequency of this condition in cancer patients, for whom LMWH has been the anticoagulation of choice.
For example, in the setting of an acute VTE, the ISTH guideline, based mostly on expert consensus, recommends a therapeutic dose of anticoagulation for patients with platelet count ≥50 × 109/L.58 For patients with acute (recent) VTE and a platelet count <50 × 109/L, ISTH recommends platelet transfusion to allow for therapeutic anticoagulation, or alternatively, insertion of retrievable IVC filter until the platelet count is safe for anticoagulation.58 However, the platelet threshold of 50 × 109/L is not based on high‐quality evidence. In an observational study of 2747 cancer patients who had an IVC filter placed between 2005 and 2009, no reduction in short‐term mortality or PE prevention was demonstrated.59 Other than the possible exception of a patient with recent acute VTE AND a contraindication for anticoagulant therapy, we do not think the evidence of benefit from IVC filters justifies their risk.
For patients with subacute or chronic VTE, the ISTH guideline recommends the LMWH dose be reduced in patients with platelet count <50 × 109/L, and held altogether if the platelet count is <25 × 109/L. A recent cohort study investigated the optimal management of prior VTE during the period of thrombocytopenia associated with autologous stem cell transplantation.60 No differences in the 30‐day bleeding or VTE outcomes were found in patients who were continued on anticoagulation with platelet transfusion support, compared with anticoagulation interruption. No “safe” platelet threshold was found in the unadjusted analysis. Many other strategies involving different platelet threshold and anticoagulation dose have been published with various outcomes, making it difficult to know which approach is best. There is certainly a need for larger and higher quality studies to address this common problem.
5.3.3. Patients with brain tumors
Patients with brain tumors, either primary or metastatic, are at high risk of both thrombotic and bleeding complications, and therefore the optimal management is difficult and controversial. The risk of intracranial hemorrhage (ICH) has been shown to differ in metastatic disease compared to primary brain glioma. A matched cohort study in patients with metastatic brain tumor showed that anticoagulation was not associated with an increased risk of ICH.61 This result was confirmed by a subsequent meta‐analysis of nine retrospective cohort studies, which demonstrated that patients with brain metastases had no increased risk of ICH on anticoagulation (odds ratio [OR] 1.07, 95% CI 0.61‐1.88).62 When brain metastases from melanoma and renal cell carcinoma were analyzed separately (accounting for 35% of the population), the conclusion remained unchanged (OR 2.3, 95% CI 0.80‐6.59).
On the other hand, the same meta‐analysis showed that patients with glioma had a 3.75‐fold increased odds of intracranial hemorrhage associated with anticoagulation (OR 3.75, 95% CI 1.42‐9.95). This was confirmed by a subsequent matched retrospective cohort study that evaluated 133 patients with high‐grade glioma (50 patietns with VTE on enoxaparin and 83 patients without VTE).63 Enoxaparin was associated with a 3.37‐fold increased hazard of major ICH (anticoagulation vs no anticoagulation, 14.7% vs 2.5%, HR 3.37, 95% CI 1.02‐11.14). Despite the elevated risk of ICH, the lack of long‐term anticoagulation was a significant risk factor for recurrent VTE in patietns with primary brain tumors (HR 11.2, 95% CI 1.5‐86.3).64 Based on these results, the benefits of anticoagulation likely outweigh the risks for most patients with an acute VTE and metastatic brain tumors. However, in a patient with glioma, the risks and benefits of anticoagulation should be carefully evaluated with the knowledge that there is increased risk for both recurrent VTE and anticoagulant‐associated ICH in this subgroup of cancer patients.
5.3.4. Catheter‐related thrombosis
The incidence of catheter‐related thrombosis (CRT) in cancer patients varies widely, with more recent studies showing a rate of close to 20% when both symptomatic and asymptomatic events are included.65 Primary thromboprophylaxis has not been shown to be beneficial and therefore is not routinely recommended.66 At least one cohort study of cancer patients with catheter‐associated thrombosis suggests that many patients can be treated with anticoagulation and leaving the catheter in place.67 The optimal type and duration of anticoagulation are unclear. Guidelines usually recommend to continue anticoagulation as long as the catheter is in place and to use LMWH as preferred agents, extending from the standard of care for CAT as discussed above.66, 68 However, the optimal duration of anticoagulation after catheter removal remains unknown given the lack of literature. The CATHETER study enrolled 74 patients with CRT treated with dalteparin bridged to warfarin. At 3 months, the risk of major bleeding was 4% with no recurrent VTE, with a line preservation rate of 100%.67 The CATHETER 2 study further explored the use of DOAC in this condition. They treated 70 cancer patients and CRT with rivaroxaban, and found that at 12 weeks, the line preservation rate was 100%, with a rate of recurrent VTE of 1.43% and a major bleeding rate of 10%.44 Both studies were single arm intervention without controls, and cross trial comparisons cannot be made.
5.3.5. Incidental cancer‐related thrombosis
Cancer patients frequently receive staging scans, and it is estimated that approximately 3% of cancer patients can have incidental PE on routine imaging.69 Several retrospective or observational studies have indicated that the natural course of an incidental PE is comparable to a symptomatic one in terms of recurrence rate and survival.70, 71 Therefore, standard anticoagulation is generally recommended for incidental PE in cancer patients.72 To address this important issue, the Hokusai VTE Cancer study and Select‐D trial are the first two large prospective studies including patients with incidental VTE (32.5% and 53% of enrolled patients, respectively) (Table 1). Subgroup analyses of these patients will be forthcoming; however, for now, guidelines suggest that incidental PE in cancer patients be treated with therapeutic anticoagulation, with the possible exception of isolated subsegmental PE.62
6. CONCLUSION
Venous thromboembolism is the second leading cause of death in cancer patients. It can cause short‐ and long‐term morbidity and mortality, as well as impact a patient's cancer treatment. Therefore, the prevention and treatment of VTE are important priorities in the care of cancer patients. Recent randomized clinical trials indicate that DOACs are effective for the treatment of CAT, likely with a small increase in bleeding events compared with LMWH in selective cancer subgroups. More research is needed to answer remaining clinical questions about the best way to: acheive primary VTE prevention, manage acute VTE in the setting of thrombocytopenia, and treat cancer patients with “anticoagulation failure.”
RELATIONSHIP DISCLOSURE
T.‐F. Wang has received travel support and honorarium from Daiichi Sankyo. A. Li has declared no conflicts of interest. D. A. Garcia has received research grants from Bristol Meyers Squibb, Incyte, Bayer, Janssen, and Daiichi Sankyo, consulting honoraria and travel support from Genzyme Corporation, Pfizer, Bristol Meyers Squibb, Boehringer Ingelheim, Alexion, Incyte, Janssen, and other non‐financial support from Genzyme Corporation, Pfizer, Bristol Meyers Squibb, Boehringer Ingelheim, Alexion, Janssen.
AUTHOR CONTRIBUTIONS
D. A. Garcia contributed to the conception of the manuscript, and literature review, analysis, and interpretation. T.‐F. Wang and A. Li contributed to literature review, analysis and interpretation, and manuscript writing. All authors contributed to manuscript revision and approval of the final version.
ACKNOWLEDGMENTS
This work was supported by grant from the National Heart, Lung, and Blood Institute, National Institutes of Health under award number T32HL007093 (A. Li). The authors would also like to acknowledge the numerous cancer‐associated thrombosis researchers, both those cited here and those unable to be cited due to space constraints.
Wang T‐F, Li A, Garcia D. Managing thrombosis in cancer patients. Res Pract Thromb Haemost. 2018;2:429–438. 10.1002/rth2.12102
REFERENCES
- 1. Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer‐associated venous thrombosis. Blood. 2013;122:1712–23. [DOI] [PubMed] [Google Scholar]
- 2. Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–22. [DOI] [PubMed] [Google Scholar]
- 3. Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632–4. [DOI] [PubMed] [Google Scholar]
- 4. Lee AY, Levine MN, Baker RI, et al.; Randomized Comparison of Low‐Molecular‐Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators . Low‐molecular‐weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–53. [DOI] [PubMed] [Google Scholar]
- 5. Hisada Y, Mackman N. Cancer‐associated pathways and biomarkers of venous thrombosis. Blood. 2017;130:1499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lip GY, Chin BS, Blann AD. Cancer and the prothrombotic state. Lancet Oncol. 2002;3:27–34. [DOI] [PubMed] [Google Scholar]
- 7. Thomas GM, Panicot‐Dubois L, Lacroix R, Dignat‐George F, Lombardo D, Dubois C. Cancer cell‐derived microparticles bearing P‐selectin glycoprotein ligand 1 accelerate thrombus formation in vivo. J Exp Med. 2009;206:1913–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zwicker JI, Liebman HA, Neuberg D, et al. Tumor‐derived tissue factor‐bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res. 2009;15:6830–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thaler J, Ay C, Mackman N, et al. Microparticle‐associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J Thromb Haemost. 2012;10:1363–70. [DOI] [PubMed] [Google Scholar]
- 10. Geddings JE, Mackman N. Tumor‐derived tissue factor‐positive microparticles and venous thrombosis in cancer patients. Blood. 2013;122:1873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ay C, Simanek R, Vormittag R, et al. High plasma levels of soluble P‐selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). Blood. 2008;112:2703–8. [DOI] [PubMed] [Google Scholar]
- 12. Meier TR, Myers DD Jr, Wrobleski SK, et al. Prophylactic P‐selectin inhibition with PSI‐421 promotes resolution of venous thrombosis without anticoagulation. Thromb Haemost. 2008;99:343–51. [DOI] [PubMed] [Google Scholar]
- 13. Clark SR, Ma AC, Tavener SA, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–9. [DOI] [PubMed] [Google Scholar]
- 14. Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107:15880–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy‐associated thrombosis. Blood. 2008;111:4902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377–82. [DOI] [PubMed] [Google Scholar]
- 17. Pabinger I, Van Es N, Heinze G, et al. Development and external validation of a risk assessment model for cancerassociated venous thromboembolism. Res Pract Thromb Haemost. 2017;1:203. [Google Scholar]
- 18. Louzada ML, Carrier M, Lazo‐Langner A, et al. Development of a clinical prediction rule for risk stratification of recurrent venous thromboembolism in patients with cancer‐associated venous thromboembolism. Circulation. 2012;126:448–54. [DOI] [PubMed] [Google Scholar]
- 19. Menapace LA, McCrae KR, Khorana AA. Predictors of recurrent venous thromboembolism and bleeding on anticoagulation. Thromb Res. 2016;140(Suppl 1):S93–8. [DOI] [PubMed] [Google Scholar]
- 20. van Es N, Louzada M, Carrier M, et al. Predicting the risk of recurrent venous thromboembolism in patients with cancer: a prospective cohort study. Thromb Res. 2018;163:41–6. [DOI] [PubMed] [Google Scholar]
- 21. Chee CE, Ashrani AA, Marks RS, et al. Predictors of venous thromboembolism recurrence and bleeding among active cancer patients: a population‐based cohort study. Blood. 2014;123:3972–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khorana AA, Kamphuisen PW, Meyer G, et al. Tissue factor as a predictor of recurrent venous thromboembolism in malignancy: biomarker analyses of the CATCH Trial. J Clin Oncol. 2017;35:1078–85. [DOI] [PubMed] [Google Scholar]
- 23. Guo Q, Huang B, Zhao J, et al. Perioperative pharmacological thromboprophylaxis in patients with cancer: a systematic review and meta‐analysis. Ann Surg. 2017;265:1087–93. [DOI] [PubMed] [Google Scholar]
- 24. Fagarasanu A, Alotaibi GS, Hrimiuc R, Lee AY, Wu C. Role of extended thromboprophylaxis after abdominal and pelvic surgery in cancer patients: a systematic review and meta‐analysis. Ann Surg Oncol. 2016;23:1422–30. [DOI] [PubMed] [Google Scholar]
- 25. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuunemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel . Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141:7S–47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khorana AA, Carrier M, Garcia DA, Lee AY. Guidance for the prevention and treatment of cancer‐associated venous thromboembolism. J Thromb Thrombolysis. 2016;41:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Di Nisio M, Porreca E, Otten HM, Rutjes AW. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev. 2014;12:CD008500. [DOI] [PubMed] [Google Scholar]
- 28. Schünemann H, Ventresca M, Crowther M, et al. An individual participant data meta‐analysis of 13 randomized trials to evaluate the impact of prophylactic use of heparin in oncological patients. Blood. 2017;130:626. [Google Scholar]
- 29. Khorana AA, Francis CW, Kuderer NM, et al. Dalteparin thromboprophylaxis in cancer patients at high risk for venous thromboembolism: a randomized trial. Thromb Res. 2017;151:89–95. [DOI] [PubMed] [Google Scholar]
- 30. Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low‐molecular‐weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162:1729–35. [DOI] [PubMed] [Google Scholar]
- 31. Deitcher SR, Kessler CM, Merli G, Rigas JR, Lyons RM, Fareed J; ONCENOX Investigators . Secondary prevention of venous thromboembolic events in patients with active cancer: enoxaparin alone versus initial enoxaparin followed by warfarin for a 180‐day period. Clin Appl Thromb Hemost. 2006;12:389–96. [DOI] [PubMed] [Google Scholar]
- 32. Hull RD, Pineo GF, Brant RF, et al.; LITE Trial Investigators . Long‐term low‐molecular‐weight heparin versus usual care in proximal‐vein thrombosis patients with cancer. Am J Med. 2006;119:1062–72. [DOI] [PubMed] [Google Scholar]
- 33. Lee AY, Kamphuisen PW, Meyer G, et al.; CATCH Investigators . Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314:677–86. [DOI] [PubMed] [Google Scholar]
- 34. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- 35. Lyman GH, Bohlke K, Falanga A, et al.; American Society of Clinical Oncology . Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Oncol Pract. 2015;11:e442–4. [DOI] [PubMed] [Google Scholar]
- 36. Streiff MB, Holmstrom B, Ashrani A, et al. Cancer‐associated venous thromboembolic disease, version 1.2015. J Natl Compr Canc Netw. 2015;13:1079–95. [DOI] [PubMed] [Google Scholar]
- 37. Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta‐analysis. Chest. 2015;147:475–83. [DOI] [PubMed] [Google Scholar]
- 38. Carrier M, Cameron C, Delluc A, Castellucci L, Khorana AA, Lee AY. Efficacy and safety of anticoagulant therapy for the treatment of acute cancer‐associated thrombosis: a systematic review and meta‐analysis. Thromb Res. 2014;134:1214–9. [DOI] [PubMed] [Google Scholar]
- 39. Posch F, Konigsbrugge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: a network meta‐analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136:582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bott‐Kitslaar DM, Saadiq RA, McBane RD, et al. Efficacy and safety of rivaroxaban in patients with venous thromboembolism and active malignancy: a single‐center registry. Am J Med. 2016;129:615–9. [DOI] [PubMed] [Google Scholar]
- 41. Mantha S, Laube E, Miao Y, et al. Safe and effective use of rivaroxaban for treatment of cancer‐associated venous thromboembolic disease: a prospective cohort study. J Thromb Thrombolysis. 2017;43:166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Theberge I, Bowdridge J, Forgie MA, et al. Rivaroxaban shows promise as effective therapy for cancer patients with venous thromboembolic disease. Thromb Res. 2017;152:4–6. [DOI] [PubMed] [Google Scholar]
- 43. Pignataro BS, Nishinari K, Cavalcante RN, et al. Oral rivaroxaban for the treatment of symptomatic venous thromboembolism in 400 patients with active cancer. Clin Appl Thromb Hemost. 2017;23:883–7. [DOI] [PubMed] [Google Scholar]
- 44. Davies GA, Lazo‐Langner A, Gandara E, et al. A prospective study of rivaroxaban for central venous catheter associated upper extremity deep vein thrombosis in cancer patients (Catheter 2). Thromb Res. 2018;162:88–92. [DOI] [PubMed] [Google Scholar]
- 45. Raskob GE, van Es N, Verhamme P, et al.; Hokusai VTE Cancer Investigators . Edoxaban for the treatment of cancer‐associated venous thromboembolism. N Engl J Med. 2018;378:615–24. [DOI] [PubMed] [Google Scholar]
- 46. Bleker SM, Brekelmans MPA, Eerenberg ES, et al. Clinical impact of major bleeding in patients with venous thrombo‐embolism treated with factor Xa inhibitors or vitamin K antagonists. Thromb Haemost. 2017;117:1944–51. [DOI] [PubMed] [Google Scholar]
- 47. Young A, Marshall A, Thirlwall J, et al. Anticoagulation therapy in selected cancer patients at risk of recurrence of venous thromboembolism: results of the Select‐D Pilot Trial. Blood. 2017;130:625.28546143 [Google Scholar]
- 48. Li A, Garcia DA, Lyman GH, Carrier M. Direct oral anticoagulant (DOAC) versus low‐molecular‐weight heparin (LMWH) for treatmetn of cancer associatd thrombosis (CAT): a systemic review and meta‐analysis. Thromb Res. 2018. 10.1016/j.thromres.2018.02.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khorana AA, McCrae KR, Milentijevic D, et al. Cuurrent practice patterons and patient persistence with anticoagulant treatments for cancer‐associated thrombosis. Res Pract Thromb Haemost. 2017;1:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Francis CW, Kessler CM, Goldhaber SZ, et al. Treatment of venous thromboembolism in cancer patients with dalteparin for up to 12 months: the DALTECAN Study. J Thromb Haemost. 2015;13:1028–35. [DOI] [PubMed] [Google Scholar]
- 51. Jara‐Palomares L, Solier‐Lopez A, Elias‐Hernandez T, et al. Tinzaparin in cancer associated thrombosis beyond 6 months: TiCAT study. Thromb Res. 2017;157:90–6. [DOI] [PubMed] [Google Scholar]
- 52. Luk C, Wells PS, Anderson D, Kovacs MJ. Extended outpatient therapy with low molecular weight heparin for the treatment of recurrent venous thromboembolism despite warfarin therapy. Am J Med. 2001;111:270–3. [DOI] [PubMed] [Google Scholar]
- 53. Carrier M, Le Gal G, Cho R, Tierney S, Rodger M, Lee AY. Dose escalation of low molecular weight heparin to manage recurrent venous thromboembolic events despite systemic anticoagulation in cancer patients. J Thromb Haemost. 2009;7:760–5. [DOI] [PubMed] [Google Scholar]
- 54. Ihaddadene R, Le Gal G, Delluc A, Carrier M. Dose escalation of low molecular weight heparin in patients with recurrent cancer‐associated thrombosis. Thromb Res. 2014;134:93–5. [DOI] [PubMed] [Google Scholar]
- 55. Schulman S, Zondag M, Linkins L, et al. Recurrent venous thromboembolism in anticoagulated patients with cancer: management and short‐term prognosis. J Thromb Haemost. 2015;13:1010–8. [DOI] [PubMed] [Google Scholar]
- 56. Hitron A, Steinke D, Sutphin S, Lawson A, Talbert J, Adams V. Incidence and risk factors of clinically significant chemotherapy‐induced thrombocytopenia in patients with solid tumors. J Oncol Pharm Pract. 2011;17:312–9. [DOI] [PubMed] [Google Scholar]
- 57. Ibrahim RB, Skewes MD, Kuriakose P. ‘Sailing in troubled waters’: a review of the use of anticoagulation in adult cancer patients with thrombocytopenia. Blood Coagul Fibrinolysis. 2016;27:615–30. [DOI] [PubMed] [Google Scholar]
- 58. Carrier M, Khorana AA, Zwicker J, Noble S, Lee AY; Subcommittee on Haemostasis and Malignancy for the SSC of the ISTH . Management of challenging cases of patients with cancer‐associated thrombosis including recurrent thrombosis and bleeding: guidance from the SSC of the ISTH. J Thromb Haemost. 2013;11:1760–5. [DOI] [PubMed] [Google Scholar]
- 59. Brunson A, Ho G, White R, Wun T. Inferior vena cava filters in patients with cancer and venous thromboembolism (VTE): patterns of use and outcomes. Thromb Res. 2016;140(Suppl 1):S132–41. [DOI] [PubMed] [Google Scholar]
- 60. Li A, Davis C, Wu Q, et al. Management of venous thromboembolism during thrombocytopenia after autologous hematopoietic cell transplantation. Blood Adv. 2017;1:707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Donato J, Campigotto F, Uhlmann EJ, et al. Intracranial hemorrhage in patients with brain metastases treated with therapeutic enoxaparin: a matched cohort study. Blood. 2015;126:494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zwicker JI, Karp Leaf R, Carrier M. A meta‐analysis of intracranial hemorrhage in patients with brain tumors receiving therapeutic anticoagulation. J Thromb Haemost. 2016;14:1736–40. [DOI] [PubMed] [Google Scholar]
- 63. Mantia C, Uhlmann EJ, Puligandla M, Weber GM, Neuberg D, Zwicker JI. Predicting the higher rate of intracranial hemorrhage in glioma patients receiving therapeutic enoxaparin. Blood. 2017;129:3379–85. [DOI] [PubMed] [Google Scholar]
- 64. Edwin NC, Khoury MN, Sohal D, McCrae KR, Ahluwalia MS, Khorana AA. Recurrent venous thromboembolism in glioblastoma. Thromb Res. 2016;137:184–8. [DOI] [PubMed] [Google Scholar]
- 65. Lee AY, Kamphuisen PW. Epidemiology and prevention of catheter‐related thrombosis in patients with cancer. J Thromb Haemost. 2012;10:1491–9. [DOI] [PubMed] [Google Scholar]
- 66. Carrier M, Lazo‐Langner A, Shivakumar S, et al. Clinical challenges in patients with cancer‐associated thrombosis: Canadian expert consensus recommendations. Curr Oncol. 2015;22:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kovacs MJ, Kahn SR, Rodger M, et al. A pilot study of central venous catheter survival in cancer patients using low‐molecular‐weight heparin (dalteparin) and warfarin without catheter removal for the treatment of upper extremity deep vein thrombosis (The Catheter Study). J Thromb Haemost. 2007;5:1650–3. [DOI] [PubMed] [Google Scholar]
- 68. Debourdeau P, Farge D, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of thrombosis associated with central venous catheters in patients with cancer. J Thromb Haemost. 2013;11:71–80. [DOI] [PubMed] [Google Scholar]
- 69. Dentali F, Ageno W, Becattini C, et al. Prevalence and clinical history of incidental, asymptomatic pulmonary embolism: a meta‐analysis. Thromb Res. 2010;125:518–22. [DOI] [PubMed] [Google Scholar]
- 70. den Exter PL, Hooijer J, Dekkers OM, Huisman MV. Risk of recurrent venous thromboembolism and mortality in patients with cancer incidentally diagnosed with pulmonary embolism: a comparison with symptomatic patients. J Clin Oncol. 2011;29:2405–9. [DOI] [PubMed] [Google Scholar]
- 71. Dentali F, Ageno W, Giorgi Pierfranceschi M, et al. Prognostic relevance of an asymptomatic venous thromboembolism in patients with cancer. J Thromb Haemost. 2011;9:1081–3. [DOI] [PubMed] [Google Scholar]
- 72. Di Nisio M, Lee AY, Carrier M, Liebman HA, Khorana AA; Subcommittee on Haemostasis and Malignancy . Diagnosis and treatment of incidental venous thromboembolism in cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2015;13:880–3. [DOI] [PubMed] [Google Scholar]


