Abstract
Background
Venous thromboembolism prophylaxis remains underutilized in hospitalized medical patients at high risk for venous thromboembolism. We previously reported that a multifaceted intervention was associated with a sustained increase in appropriate thromboprophylaxis and reduced symptomatic venous thromboembolism among medical patients hospitalized in two urban teaching hospitals. The effectiveness of this intervention in community hospitals is unknown.
Methods
We performed a prospective multicenter cohort study in three community hospitals. All medical patients admitted from February 1, 2011 to January 31, 2014 were eligible. Consecutive eligible patients were enrolled into the 12‐month “control,” 12‐month “intervention,” or 12‐month “maintenance” group. We provided electronic alerts, physician performance feedback, and targeted medical education for the intervention group. Only the alert component of the intervention continued in the maintenance group. The primary outcome was the rate of appropriate thromboprophylaxis among patients at high risk for venous thromboembolism defined as the prescription of guideline recommended chemoprophylaxis, or identification of a chemoprophylaxis contraindication. Secondary outcomes included rates of symptomatic venous thromboembolism, major bleeding, all‐cause mortality, heparin‐induced thrombocytopenia, physician satisfaction, and alert fatigue.
Results
Appropriate thromboprophylaxis when compared to the control group rate of 67% was higher for the intervention group (85%) and for the maintenance group (77%; P < .001 for each comparison). A reduction of 90‐day symptomatic venous thromboembolism accompanied the intervention (control 4.5%, intervention 3.4%, maintenance 3.0%, P = .04).
Conclusions
This multifaceted intervention was associated with an overall increase in appropriate thromboprophylaxis of medical patients compared with the control period. Hospital‐associated venous thrombosis rates decreased.
Keywords: prevention, quality improvement, thromboprophylaxis medical patient, venous thromboembolism
Essentials.
Multidisciplinary VTE Reduction initiative was conducted at three community hospitals over 3 years.
All hospitalists at three Intermountain Healthcare community hospitals participated.
Compared with the control year, appropriate thromboprophylaxis improved over the two subsequent years.
Providing just‐in‐time alerts and education re: thrombosis risk may protect patients from VTE.
1. INTRODUCTION
Historical studies employing phlebography surveillance suggest that as many as 15% of hospitalized medical patients will develop venous thromboembolism (VTE) during hospitalization.1, 2, 3 Contemporary evidence suggests that clinically overt thrombosis rates approximate 0.3% to 9.7% in hospitalized medical patients.4, 5 In spite of these data, only about 40% of hospitalized medical patients at high risk for VTE receive appropriate thromboprophylaxis defined as chemoprophylaxis with low‐molecular‐weight heparin, unfractionated heparin, or fondaparinux.6, 7, 8
Guideline authors have recommended adoption of formalized VTE risk assessment models8, 9, 10, 11, 12, 13 that have been variably validated and compared,14, 15 however, they have not been uniformly adopted.16, 17 Selective application of venous thrombosis chemoprophylaxis reduces the number of adverse events associated with thromboprophylaxis such as bleeding and heparin‐induced thrombocytopenia (HIT)18, 19 by limiting chemoprophylaxis to only those patients likely to benefit.20, 21 We along with others have reported that interventions to inform physicians of thrombosis risk and to provide guidance regarding appropriate thromboprophylaxis improve outcomes.5, 22, 23 Electronic alerts have been described as one mechanism to positively impact appropriate thromboprophylaxis rates among some4, 24, 25, 26 but not all27 patient populations. The importance of a reliable methodology to identify patients at high risk for hospital‐associated VTE and reduce that risk is highlighted by a recent Centers for Disease Control Hospital‐Associated Venous Thromboembolism (HA‐VTE) Reduction Challenge.28
We previously reported a multifaceted intervention that was associated with improved thromboprophylaxis, improved chemoprophylaxis, a reduction of VTE, and was well‐received by hospitalists in tertiary care metropolitan teaching hospitals.5 We wished to assess the performance of the intervention in community hospitals. Our primary objective was to report the rate of appropriate thromboprophylaxis among hospitalized medical patients at high risk for symptomatic VTE defined as the prescription of chemoprophylaxis or documenting a contraindication thereof following implementation of a multifaceted intervention including (i) targeted electronic alerts for high‐risk patients, (ii) provision of comparative prophylaxis metrics to practitioners, and (iii) practitioner‐specific continuing medical education. Eligible patients included those adults (≥18 years of age) admitted to the hospitalist service at the participating community hospitals for greater than 24 hours. Appropriate thromboprophylaxis rates were compared over a 3‐year period. Secondarily we report 30‐ and 90‐day rates of symptomatic VTE, in‐hospital major bleeding, in‐hospital HIT, in‐hospital and 90‐day all‐cause mortality, practitioner response to electronic messaging, alert fatigue, and practitioner satisfaction with the intervention. The Intermountain Healthcare Institutional Review Board approved this study (Institutional Review Board # 1019819).
2. METHODS
The multifaceted healthcare quality improvement initiative entitled the Venous Thromboembolism Reduction Initiative II (VRI II), was presented to the hospitalists at each hospital’s monthly meeting and each hospitalist provided voluntary signed informed consent to participate in this initiative that was recognized as a value‐based incentive project for each hospitalist group. Three community hospitals participated in VRI II (Hospital 1, Hospital 2, and Hospital 3). As we formerly reported5 the VRI consisted of three interventions. The first intervention was delivery of an electronic alert. To generate this, we developed an electronic VTE risk assessment model26, 29 which interrogated the electronic medical record daily and generated a VTE risk score classifying each patient as being either high risk for VTE (a VTE risk score of ≥4 as defined by Kucher et al)26 or not (a VTE risk score <4). Then, another electronic tool interrogated the medication administration record for appropriate thromboprophylaxis as recommended by the American College of Chest Physicians18; or therapeutic anticoagulation.5 Because in this study we defined appropriate thromboprophylaxis as the administration of recommended doses of the aggregate of unfractionated heparin, low‐molecular‐weight heparin, or fondaparinux, the specific details on the type of prophylaxis were not recorded. If a high‐risk patient not receiving prophylaxis was detected, then an electronic alert was generated reminding the responsible hospitalist to consider prophylaxis. Second, an audit‐and‐feedback assessment of each hospitalist’s VTE prophylaxis rates generated a monthly report of each hospitalist’s performance in comparison with their de‐identified peers. Third, a proprietary targeted online continuing medical education activity was completed by each hospitalist. We assessed the effect of all three of these during the “intervention” phase of the trial. During the “maintenance” phase, only the alert intervention was continued.
The VRI II began on February 1, 2011 at Hospital 1 (March 1, 2011 at Hospital 2 and Hospital 3) with the prospective collection of data during the control period of 12 months followed by consecutive 12 month periods for the intervention period and the maintenance period. An electronic interface with the hospitalist billing program identified the attending hospitalist of record for each patient every day. In the intervention period and maintenance period the alert message was sent through electronic medical record system. This alert permitted the hospitalist to interface with the electronic system to document any reasons that prophylaxis was being withheld (e.g, active bleeding, hospice, etc.). By doing so, the daily alert would be turned off for 5 days, and the hospitalist would be credited with having appropriately dispensed VTE prophylaxis. At the end of the 5 days, if thromboprophylaxis had not yet been ordered, the alert would be resent. Patients were excluded from analysis if they had VTE as an admit diagnosis, if the alert timestamp occurred after patient discharge or if their stay overlapped the transition between study periods.
During the intervention phase, each hospitalist was provided a monthly email link to a secure website where individual thromboprophylaxis performance metrics were presented along with the performance of the hospitalist’s de‐identified peers. Coincident with this calculation, proprietary software (Twine Clinical Consulting, LLC & Medical Impact Ventures, LLC) identified the characteristics of those patients cared for by the hospitalist that did not receive appropriate thromboprophylaxis, and then customized an educational offering. For example, if a given hospitalist’s rate of thromboprophylaxis was 85% overall but only 35% among patients with cancer, then that hospitalist was invited to complete the continuing medical education activity entitled “Mitigating thrombosis risk among patients with cancer.” By the end of the intervention period, as part of an annual Hospitalist Group incentive project, and with 100% of hospitalists participating (to avoid selection bias), each hospitalist completed a total of 6 continuing medical education offerings surrounding the importance of VTE thromboprophylaxis. These can be found at http://www.vte.physicianimprovement.com. During the maintenance period the electronic alert continued, but no provider metrics or continuing education materials were provided. All outcomes were measured in the same fashion.
The primary outcome was prescription of appropriate VTE thromboprophylaxis defined as the prescription of guideline recommended chemoprophylaxis, or notation of a chemoprophylaxis contraindication during the intervention and maintenance periods among medical patients identified as being at high risk for venous thrombosis. We also report chemoprophylaxis. This was measured for each patient each day.
Venous thrombosis was identified using natural language processing interrogation of the electronic medical record, using methods we have previously described.30 For a patient to be considered at high risk for VTE, they must have spent greater than 50% of the hospitalization classified as high risk.
Hospital‐associated major bleeding was identified by electronic medical record interrogation as we have previously performed.25, 31 We defined major bleeding by International Classification of Diseases, Ninth Revision code as bleeding into a critical space including the spinal cord, brain, eye, retroperitoneum, or pericardium, or clinically overt bleeding that was associated with the transfusion of ≥2 units of packed red blood cells. We reported major bleeding rates stratified for patients that received ≥1 dose of VTE chemoprophylaxis compared with those who did not after first excluding all patients with an admission diagnosis for major bleeding defined by the presence of any International Classification of Diseases, Ninth Revision code. Death was identified upon interrogation of the electronic medical record for a flag that denoted death, or in the Intermountain Healthcare mortality database that incorporates an interface with state‐wide mortality data. HIT was considered present if the International Classification of Diseases, Ninth Revision code of 289.84 was associated with the hospitalization. Heparin‐induced thrombocytopenia was stratified for patients that received ≥1 dose of VTE chemoprophylaxis compared with those who did not. Ninety‐day electronic follow‐up was completed for 100% of patients.
Alert fatigue is described as the observation that interruptive alerts, if they occur too frequently or are felt to be clinically irrelevant in some instances, are associated with physicians ignoring the alert.32, 33 In an attempt to measure if the hospitalists’ experienced alert fatigue over the course of the study, the hospitalist response to the alert was captured. To report hospitalists’ response to alerts we calculated the percent of patients for whom an alert was generated that subsequently had prophylaxis ordered, or contraindication for prophylaxis entered, within 24 hours.
2.1. Statistical analysis
Demographic information was summarized overall as well as for the high‐ and non–high‐risk groups (Table 1). Demographic information was also summarized by period and found to be substantively stable across all 3 years of the study. The rates for all primary and secondary outcomes from the control period, intervention period, and maintenance period were formally compared using Chi‐squared tests for proportions, or Fisher’s exact test when appropriate. A significance level of 0.05 was used for all comparisons and multiple comparisons were controlled for using a false discovery rate of 5%.34 95% exact confidence intervals were also calculated for all primary and secondary outcomes. All analyses were conducted using the R Statistical Package.35
Table 1.
Patient demographics
| Characteristic | Overall (N = 27 778) | High riska (N = 9374) | Non‐high risk (N = 18 404) |
|---|---|---|---|
| Demographic | |||
| Age in yearsa; median (IQR) | 66 (50‐79) | 73 (60‐81) | 61 (44‐76) |
| Female; n (%) | 12 698 (46%) | 4207 (45%) | 8491 (46%) |
| Comorbidities; n (%) | |||
| Cancerb | 3476 (13%) | 2974 (32%) | 502 (3%) |
| Obesityb | 7098 (26%) | 4807 (51%) | 2291 (12%) |
| Hypercoagulabilityb | 1881 (7%) | 1759 (19%) | 122 (1%) |
| Prior VTEb | 4766 (17%) | 4641 (50%) | 125 (1%) |
| Hormone replacement therapyb | 864 (3%) | 584 (6%) | 280 (2%) |
| Congestive heart failure | 8215 (30%) | 4044 (43%) | 4171 (23%) |
| Diabetes | 6629 (24%) | 2680 (29%) | 3949 (21%) |
| Current tobacco use | 6984 (25%) | 1860 (20%) | 5124 (28%) |
| Hospital detail; n (%) | |||
| Bed resta | 10 042 (36%) | 6774 (72%) | 3268 (18%) |
| Surgery (in the past month)b | 3621 (13%) | 3071 (33%) | 550 (3%) |
| Central venous catheter | 2995 (11%) | 1773 (19%) | 1222 (7%) |
| Infection | 7076 (25%) | 2710 (29%) | 4366 (24%) |
| PICC line | 1903 (7%) | 856 (9%) | 1047 (6%) |
| Sepsis | 4566 (16%) | 1760 (19%) | 2806 (15%) |
| ICU admission | 11 529 (42%) | 3910 (42%) | 7619 (41%) |
| Length of stay (days); median (IQR) | 2.9 (1.7‐4.8) | 3.5 (2.1‐5.7) | 2.7 (1.6‐4.1) |
ICU, intensive care unit; PICC, peripherally inserted central catheter; VTE, venous thromboembolism.
Patients at high risk for at least 50% of their hospital stay were classified as high risk overall.
Component of the risk stratification score.
3. RESULTS
3.1. Appropriate thromboprophylaxis
During the study, 95 236 patient‐days occurred, of which 35 620 (37%) were scored high risk for venous thrombosis. There were 35 702 patient‐days in the control period (38.1% high risk), 31 682 patient days in the intervention period (36.7% high risk), and 27 852 patient days (37.2% high risk) during the maintenance period. The primary outcome, rate of appropriate thromboprophylaxis (defined as both application of appropriate chemoprophylaxis and the identification by the Hospitalist of a contraindication to chemoprophylaxis) among patients at high risk for VTE increased significantly comparing the control period (67%; 95% CI 66%‐68%) with the intervention period (85%; 95% CI 84%‐86%) and the maintenance period (77%; 95% CI 76%‐78%); P < .001 for all comparisons. The decrease in appropriate thromboprophylaxis comparing the intervention period (85%) to the maintenance period (77%) was significant; P < .001 (Figure 1). Hospitalists indicated a contraindication to thromboprophylaxis for 637 of 3267 (19%) of high‐risk patient encounters in the intervention period and 341 of 3037 (11%) high‐risk patient encounters during the maintenance period. The rate of chemoprophylaxis ordered comparing the control (67%) intervention (69%) and maintenance (67%) periods was unchanged. Figure 2 presents the rate of prescription of appropriate thromboprophylaxis by each individual hospitalist. All secondary outcomes are reported in Table 2.
Figure 1.
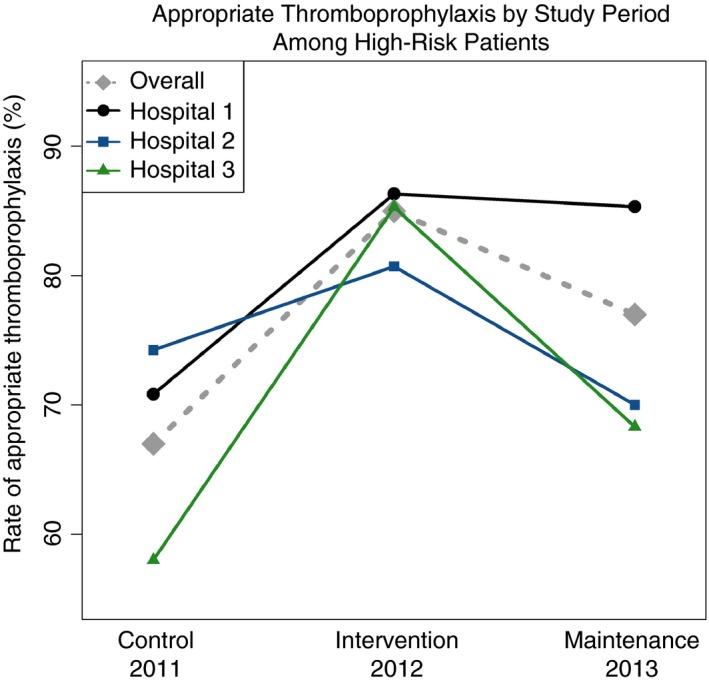
The grey dashed line represents the rate of appropriate thromboprophylaxis of all hospitals. The line with an embedded black dot, blue square, and green triangle represent the rate of appropriate thromboprophylaxis hospitals 1, 2, and 3, respectively
Figure 2.
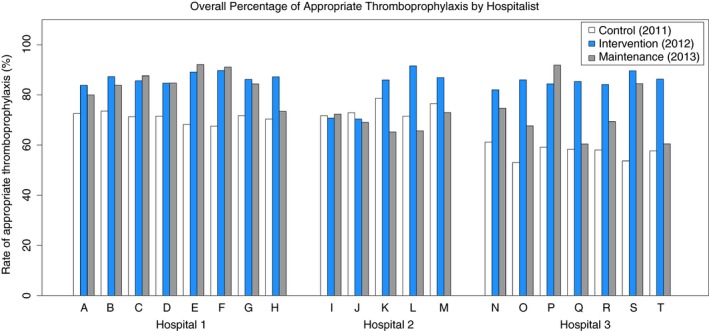
Each bar represents the annual rate of appropriate thromboprophylaxis ordered by each hospitalist (A‐T) for the control (white), intervention (blue), and maintenance (grey) years
Table 2.
Rate of VTE, mortality, major bleeding, and heparin induced thrombocytopenia for high‐risk patients
| Outcome by study period (95% CI) | Control | Intervention | Maintenance | P valuea |
|---|---|---|---|---|
| 90‐day VTEb % | 4.5 (3.8‐5.2) | 3.4 (2.8‐4.1) | 3.0 (2.5‐3.7) | .039 |
| 30‐day VTEc % | 3.5 (2.9‐4.1) | 2.5 (2.0‐3.1) | 2.3 (1.8‐2.9) | .046 |
| 90‐day all‐cause mortality % | 16.3 (15.1‐17.7) | 14.5 (13.3‐15.8) | 16.0 (14.7‐17.4) | .15 |
| In‐hospital mortality % | 4.6 (3.9‐5.4) | 3.6 (3.0‐4.3) | 4.4 (3.7‐5.2) | .15 |
| Alerted % | 47d (45‐49) | 41 (39‐42) | 41 (39‐42) | <.001 |
| Yes: 90‐day VTE % | 4.4e (3.5‐5.6) | 3.4 (2.4‐4.5) | 3.5 (2.5‐4.6) | .29 |
| No: 90‐day VTE % | 4.5 (3.6‐5.6) | 3.4 (2.7‐4.4) | 2.8 (2.0‐3.7) | .05 |
| Thromboprophylaxisf | ||||
| Yes: in‐hospital HIT % | 0.38 (0.18‐0.73) | 0.21 (0.07‐0.49) | 0.09 (0.01‐0.33) | .15 |
| Yes: Major bleeding % | 0.72 (0.42‐1.15) | 0.84 (0.51‐1.29) | 0.59 (0.31‐1.00) | .61 |
| No: Major bleeding % | 1.74 (1.00‐2.81) | 0.67 (0.22‐1.56) | 2.09 (1.18‐3.43) | .10 |
CI, confidence interval; HIT, heparin‐induced thrombocytopenia; VTE, venous thromboembolism.
Controlled for multiple comparisons using a false discovery rate of 5%.
Pairwise tests: Control period is significantly different from the intervention period (P = .03), but the intervention period is not significantly different than the follow up period (P = .47).
Pairwise tests: Control period is significantly different from the intervention period (P = .03), but the intervention period is not significantly different than the follow up period (P = .69).
In the control period no alert was sent, however it would have been given the criteria applied during the intervention period and follow‐up year.
In the control period no alert was sent, however it would have been given the criteria applied during the intervention period and follow up year.
Thromboprophylaxis is defined as ever receiving a dose of chemoprophylaxis.
Bold values represents the statistically significant value (P ≤ .05).
3.2. Symptomatic VTE
The 90‐day rate of symptomatic VTE among high‐risk patients during the control period, the intervention period, and the maintenance period was 4.5%, 3.4%, and 3.0% respectively, and decreased significantly (P = .039; Figure 3). The 30‐day rate of symptomatic VTE among high‐risk patients during the control period, the intervention period, and the maintenance period was 3.5%, 2.5%, and 2.3% respectively, and decreased significantly (P = .046).
Figure 3.
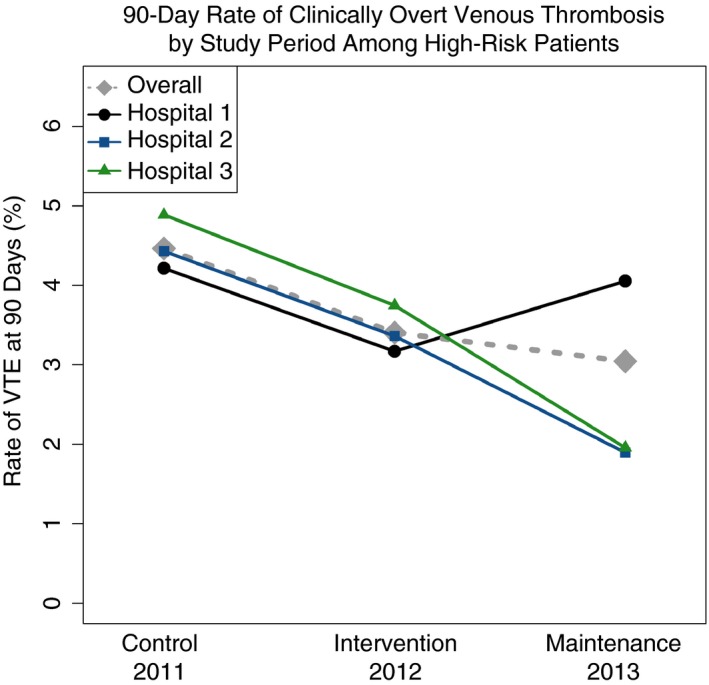
The grey dashed line represents the rate of 90‐day VTE of all hospitals. The line with an embedded black dot, blue square, and green triangle represent the rate of rate of 90‐day VTE of hospitals 1, 2, and 3, respectively. VTE, venous thromboembolism
3.3. Major bleeding
Major bleeding among patients at high risk for venous thrombosis that received ≥1 dose of chemoprophylaxis compared with those that did not receive any chemoprophylaxis was lower or no different during sequential years (control: 0.72% vs 1.74%; intervention: 0.84% vs 0.67%; maintenance period: 0.59% vs 2.09%). Major bleeding among patients at high risk for venous thrombosis that received thromboprophylaxis or that did not receive thromboprophylaxis did not differ significantly across years (Table 2).
3.4. HIT
Among high‐risk patients that received ≥1 dose of chemoprophylaxis, in‐hospital heparin‐induced thrombocytopenia was rare and occurred at a rate of 0.38% in the control, 0.21% in the intervention, and 0.09% in the maintenance period. The rate of in‐hospital heparin‐induced thrombocytopenia was not significantly different from year to year (P = .15).
3.5. Mortality
Among high‐risk patients neither the rate of in‐hospital mortality (control: 4.6%; intervention: 3.6%; maintenance period: 4.4%; P = .15) nor the 90‐day mortality rate (control: 16.3%; intervention: 14.5%; maintenance period: 16.0%; P = .15) differed significantly.
3.6. Alert fatigue
During the intervention period, 1993 alerts were sent, while 2446 were sent during the maintenance period. Hospitalist behavior was considered changed if within 24 hours of an alert, appropriate thromboprophylaxis was ordered or if a contraindication to thromboprophylaxis was recorded. Of the 1993 alerts sent during the intervention period, 977 (49%) were associated with a behavioral change (340 instances of new prophylaxis orders; 637 entries of a contraindication for prophylaxis). Of the 2446 alerts sent during the maintenance period, 651 (27%) were associated with a behavioral change (310 prophylaxis orders; 341 contraindication entries). A significant reduction of 22.4% (95% CI: 19.6%‐25.3%, P < .001) in response to alerts sent was observed comparing the intervention period and the maintenance period.
4. DISCUSSION
Among community hospitals in our system we demonstrated an increase in the rate of appropriate thromboprophylaxis defined as the prescription of appropriate chemoprophylaxis (or documenting a contraindication) comparing the control period with the intervention period and the maintenance period. No change in the prescription of chemoprophylaxis was observed comparing the control, intervention, and maintenance periods. These results differ from our previous study that demonstrated chemoprophylaxis rates that improved and were sustained over time in academic metropolitan hospitals. We observed differences between the community hospitals and our prior intervention in teaching hospitals. These differences included that in community hospitals there was no change in the rate of chemoprophylaxis comparing the control period with the intervention period and the maintenance period, and the documentation of a chemoprophylaxis contraindication decreased from the intervention period to the maintenance period; while both rates increased over the analogous time interval at teaching hospitals. Our observations are important because the comparative effectiveness of interventions such as ours in community hospitals vs academic centers is limited. Others have reported lower rates of appropriate chemoprophylaxis among community hospitals when compared with academic institutions.36 We conclude that the reduction in appropriate thromboprophylaxis rates comparing the intervention to maintenance periods reflected the community hospitalists no longer taking the time to document contraindications during the maintenance period. These observations suggest that the community hospitalists may have become less engaged with the electronic tool when moving from the intervention period to the maintenance period. We hypothesize that the audit‐and‐feedback and/or continuing medical education (CME) components provided to the hospitalists during the intervention period may have motivated hospitalist interaction with the alert system.
A reduced rate of symptomatic VTE among high‐risk medical inpatients was observed and sustained. We hypothesize that the VRI II engaged hospitalists and broadened their awareness of the importance of general thromboprophylaxis strategies among hospitalized patients (perhaps including unmeasured factors, such as emphasizing early and frequent ambulation and the use of sequential pneumatic compression devices). Likewise, we observed that the best chemoprophylaxis rates occurred during the intervention year, when the full package of interventions (alerting, audit‐and‐feedback and tailored CME) were active. This suggests that a multi‐component intervention may be more effective than a simple alert system. Finally, secular pressures to reduce HA‐VTE rates in an era of VTE reduction performance metrics may have also influenced our observed results.
The rate of major bleeding did not differ during the initiative; an observation that is consistent with reports of prior randomized controlled trials of VTE prophylaxis,1, 2, 3 our previous study,5 and previous studies assessing utility of electronic alerts to improve chemoprophylaxis.5, 11, 16, 26 We attribute the higher rate of major bleeding among those patients for whom chemoprophylaxis was withheld to the hospitalists (appropriately) refraining from prescribing chemoprophylaxis to patients that were at an increased risk for bleeding. The overall rate of HIT we observed was low and analogous to previously reported rates by others20, 37, 38, 39 and ourselves.5 While a nonsignificant decrease in HIT was observed, no organized program to mitigate HIT risk occurred during the study. In‐hospital and 90‐day all‐cause mortality did not differ between years.
More alerts (n = 2446) were sent during the maintenance period compared with the intervention period (n = 1993). We observed a significant 22.4% (95% CI: 19.6%‐25.3%, P < .001) reduction in the response to alerts sent during the maintenance period compared with the intervention period, and the rate of appropriate thromboprophylaxis did not increase. We cannot exclude the possibility that the hospitalists found the alerts of limited/no utility and therefore responded to fewer alerts over time, which could be indicative of alert fatigue. No change in chemoprophylaxis ordered comparing the control, intervention, and maintenance periods supports this hypothesis. Our ongoing research is assessing the variables that effect hospitalists’ prescriptive behavior and response to alerts in the hopes of improving the efficiency and utility of future alerting.
Strengths of our study included that we performed this intervention at three community hospitals with multiple hospitalist groups and with 100% of hospitalists participating. We reported 3 years of data captured and follow‐up and described in detail our initiative. Our initiative was accompanied by reduced rates of symptomatic VTE over time. We achieved 100% electronic follow‐up for the secondary outcomes reported.
Limitations of our study include those attributable to performing this prospective interventional study in the setting of routine clinical practice. These include the secular influences surrounding VTE prophylaxis and those attributable to clinical care. Because all hospitalists provided signed informed consent, we cannot refute the possibility that a Hawthorne effect led to a higher rate of chemoprophylaxis during the control period than would otherwise have existed. Because we reported chemoprophylaxis as the aggregate of guideline‐recommended dosing for unfractionated heparin, low‐molecular‐weight heparin, and fondaparinux, we cannot quantitatively explore whether there was a change in the type of prophylaxis being used. However, the practice of our hospitalists is to primarily (~80% of the time) prescribe low‐molecular‐weight heparin, prescribe unfractionated heparin ~20% of the time, and rarely prescribe fondaparinux. We were not able to report the prescription or utilization of mechanical prophylactic devices (not captured electronically at our hospitals) or institution‐specific initiatives to reduce the burden of thromboembolic disease. Additionally, our study was limited by the constraints of defining thrombosis outcomes using natural language processing and an inability to capture patient events that occurred outside our hospital system. However we have reported a high degree of accuracy in the utility of this approach to identify patients with thrombosis.30, 40
In conclusion, the VRI II was associated with a significant increase in appropriate thromboprophylaxis of medical inpatients driven by hospitalists engaging with VRI II to identify contraindications to chemoprophylaxis; although no increase in chemoprophylaxis prescription occurred. We cannot exclude the possibility of alert fatigue as evidenced by a reduction in hospitalist response to alerts generated over time. Optimal thromboprophylaxis was coincident with a targeted CME initiative. We report an overall reduction and sustained downward trend in hospital‐acquired symptomatic VTE. No change in major bleeding, HIT, or all‐cause mortality occurred. This study reports results of a VTE reduction initiative within community hospitals within Intermountain Healthcare. Intermountain Healthcare was awarded a 2015 Centers for Disease Control and Prevention HA‐VTE Reduction Champion award for this intervention within our metropolitan teaching institutions.41
RELATIONSHIP DISCLOSURE
S. Woller and S. Stevens report grant support from Bristol‐Meyers‐Squibb and Twine Clinical Consulting LLC paid to Intermountain Healthcare. G. Elliott reports personal fees from Janssen Research & Development. D. Wray and M. Wayne report financial support provided by GlaxoSmithKline, Bristol‐Myers Squibb, Janssen, Daiichi Sankyo, Eli Lilly, and Sanofi Aventis. J. Christensen, J. Lloyd, S Evans, V. Aston and E. Wilson report none.
AUTHOR CONTRIBUTIONS
All authors had access to the data and a role in writing the manuscript. I, Scott C. Woller, MD, take responsibility for the integrity of the data and the accuracy of the data analysis.
Woller SC, Stevens SM, Evans RS, et al. Electronic alerts, comparative practitioner metrics, and education improve thromboprophylaxis and reduce venous thrombosis in community hospitals. Res Pract Thromb Haemost. 2018;2:481–489. 10.1002/rth2.12119
Grant support from Twine Clinical Consulting LLC and the Intermountain Research and Medical Foundation (Grant #610).
REFERENCES
- 1. Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332:325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leizorovicz A, Cohen AT, Turpie AG, Olsson CG, Vaitkus PT, Goldhaber SZ. Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110:874–9. [DOI] [PubMed] [Google Scholar]
- 3. Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341:793–800. [DOI] [PubMed] [Google Scholar]
- 4. Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8:2450–7. [DOI] [PubMed] [Google Scholar]
- 5. Woller SC, Stevens SM, Evans RS, et al. Electronic alerts, comparative practitioner metrics, and education improves thromboprophylaxis and reduces thrombosis. Am J Med. 2016;129:1124.e1117–26. [DOI] [PubMed] [Google Scholar]
- 6. Amin AN, Stemkowski S, Lin J, Yang G. Inpatient thromboprophylaxis use in U.S. hospitals: adherence to the seventh American College of Chest Physician’s recommendations for at‐risk medical and surgical patients. J Hosp Med. 2009;4:E15–21. [DOI] [PubMed] [Google Scholar]
- 7. Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132:936–45. [DOI] [PubMed] [Google Scholar]
- 8. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients. Chest. 2012;141(2 Suppl):e195S–226S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caprini JA, Arcelus JI, Hasty JH, Tamhane AC, Fabrega F. Clinical assessment of venous thromboembolic risk in surgical patients. Semin Thromb Hemost. 1991;17(Suppl 3):304–12. [PubMed] [Google Scholar]
- 10. Cohen AT, Alikhan R, Arcelus JI, et al. Assessment of venous thromboembolism risk and the benefits of thromboprophylaxis in medical patients. Thromb Haemost. 2005;94:750–9. [PubMed] [Google Scholar]
- 11. Samama MM, Dahl OE, Mismetti P, et al. An electronic tool for venous thromboembolism prevention in medical and surgical patients. Haematologica. 2006;91:64–70. [PubMed] [Google Scholar]
- 12. Chopard P, Spirk D, Bounameaux H. Identifying acutely ill medical patients requiring thromboprophylaxis. J Thromb Haemost. 2006;4:915–6. [DOI] [PubMed] [Google Scholar]
- 13. Qaseem A, Chou R, Humphrey LL, Starkey M, Shekelle P. Venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline From the American College of Physicians. Ann Intern Med. 2011;155:625–32. [DOI] [PubMed] [Google Scholar]
- 14. Stuck AK, Spirk D, Schaudt J, Kucher N. Risk assessment models for venous thromboembolism in acutely ill medical patients. A systematic review. Thromb Haemost. 2017;117:801–8. [DOI] [PubMed] [Google Scholar]
- 15. Greene MT, Spyropoulos AC, Chopra V, et al. Validation of risk assessment models of venous thromboembolism in hospitalized medical patients. Am J Med. 2016;129:1001.e1009–18. [DOI] [PubMed] [Google Scholar]
- 16. Maynard G, Stein J. Designing and implementing effective venous thromboembolism prevention protocols: lessons from collaborative efforts. J Thromb Thrombolysis. 2010;29:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deheinzelin D, Braga AL, Martins LC, et al. Incorrect use of thromboprophylaxis for venous thromboembolism in medical and surgical patients: results of a multicentric, observational and cross‐sectional study in Brazil. J Thromb Haemost. 2006;4:1266–70. [DOI] [PubMed] [Google Scholar]
- 18. Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition). Chest. 2008;6(Suppl):381S–453S. [DOI] [PubMed] [Google Scholar]
- 19. Warkentin TE, Greinacher A, Koster A, Lincoff AM. Treatment and prevention of heparin‐induced thrombocytopenia: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition). Chest. 2008;6(Suppl):340S–80S. [DOI] [PubMed] [Google Scholar]
- 20. Creekmore FM, Oderda GM, Pendleton RC, Brixner DI. Incidence and economic implications of heparin‐induced thrombocytopenia in medical patients receiving prophylaxis for venous thromboembolism. Pharmacotherapy. 2006;26:1438–45. [DOI] [PubMed] [Google Scholar]
- 21. Guyatt GH, Eikelboom JW, Gould MK, et al. Approach to outcome measurement in the prevention of thrombosis in surgical and medical patients. Chest. 2012;141(2 suppl):e185S–94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jenkins IH, White RH, Amin AN, et al. Reducing the incidence of hospital‐associated venous thromboembolism within a network of academic hospitals: findings from five University of California medical centers. J Hosp Med. 2016;11(Suppl 2):S22–8. [DOI] [PubMed] [Google Scholar]
- 23. Mahan CE, Liu Y, Turpie AG, et al. External validation of a risk assessment model for venous thromboembolism in the hospitalised acutely‐ill medical patient (VTE‐VALOURR). Thromb Haemost. 2014;112:692–9. [DOI] [PubMed] [Google Scholar]
- 24. Lecumberri R, Marqués M, Díaz‐Navarlaz MT, et al. Maintained effectiveness of an electronic alert system to prevent venous thromboembolism among hospitalized patients. Thromb Haemost. 2008;100:699–704. [DOI] [PubMed] [Google Scholar]
- 25. Woller SC, Stevens SM, Towner S, et al. Computerized clinical decision support improves warfarin management and decreases recurrent venous thromboembolism. Clin Appl Thromb Hemost. 2015;21:197–203. [DOI] [PubMed] [Google Scholar]
- 26. Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352:969–77. [DOI] [PubMed] [Google Scholar]
- 27. Lecumberri R, Marques M, Panizo E, et al. High incidence of venous thromboembolism despite electronic alerts for thromboprophylaxis in hospitalised cancer patients. Thromb Haemost. 2013;110:184–90. [DOI] [PubMed] [Google Scholar]
- 28. CDC’s HA‐VTE Prevention Challenge. 2015. [cited 2015 Dec 6]. Available from http://www.cdc.gov/features/ha-vte-challenge/index.html.
- 29. Piazza G, Rosenbaum EJ, Pendergast W, et al. Physician alerts to prevent symptomatic venous thromboembolism in hospitalized patients. Circulation. 2009;119:2196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Evans RS, Lloyd JF, Aston VT, et al. Computer surveillance of patients at high risk for and with venous thromboembolism. AMIA Annu Symp Proc. 2010;2010:217–21. [PMC free article] [PubMed] [Google Scholar]
- 31. Intermountain Joint Replacement Center Writing . C. A prospective comparison of warfarin to aspirin for thromboprophylaxis in total hip and total knee arthroplasty. J Arthroplasty. 2012;27:1–9.e2. [DOI] [PubMed] [Google Scholar]
- 32. Isaac T, Weissman JS, Davis RB, et al. Overrides of medication alerts in ambulatory care. Arch Intern Med. 2009;169:305–11. [DOI] [PubMed] [Google Scholar]
- 33. Lo HG, Matheny ME, Seger DL, Bates DW, Gandhi TK. Impact of non‐interruptive medication laboratory monitoring alerts in ambulatory care. J Am Med Inform Assoc. 2009;16:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc. 1995;57:289–300. [Google Scholar]
- 35. R Development Core Team . R: A Language and Environment for Statistical Computing. Computing RFfS , editor. Vienna, Austria, 2011. [cited 2011 Apr 6]. Available from http://www.R-project.org/. [Google Scholar]
- 36. Kahn SR, Panju A, Geerts W, et al. Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res. 2007;119:145–55. [DOI] [PubMed] [Google Scholar]
- 37. Kato S, Takahashi K, Ayabe K, et al. Heparin‐induced thrombocytopenia: analysis of risk factors in medical inpatients. Br J Haematol. 2011;154:373–7. [DOI] [PubMed] [Google Scholar]
- 38. Jenkins I, Helmons PJ, Martin‐Armstrong LM, Montazeri ME, Renvall M. High rates of venous thromboembolism prophylaxis did not increase the incidence of heparin‐induced thrombocytopenia. Jt Comm J Qual Patient Saf. 2011;37:163–9. [DOI] [PubMed] [Google Scholar]
- 39. Beeler PE, Eschmann E, Schumacher A, Studt JD, Amann‐Vesti B, Blaser J. Impact of electronic reminders on venous thromboprophylaxis after admissions and transfers. J Am Med Inform Assoc. 2014;21(e2):e297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Evans RS, Linford LH, Sharp JH, White G, Lloyd JF, Weaver LK. Computer identification of symptomatic deep venous thrombosis associated with peripherally inserted central catheters. AMIA Annu Symp Proc. 2007;1:236–40. [PMC free article] [PubMed] [Google Scholar]
- 41. Prevention TUSCfDCa. The 2015 Healthcare‐Associated Venous Thromboembolism Prevention Challenge Champions. 2016. [cited 2016 Mar 29]. Available from: http://www.cdc.gov/ncbddd/dvt/ha-vte-challenge.html.


