Abstract
In this review paper, we give a historical perspective of the development of imaging modalities to visualize platelet biogenesis and how this contributed to our current understanding of megakaryopoiesis and thrombopoiesis. We provide some insight how distinct in vivo and in situ imaging methods, including ultramicrographs, have contributed to the current concepts of platelet formation. The onset of intravital microscopy into the mouse bone marrow has markedly modified and challenged our thinking of platelet biogenesis during the last decade. Finally, we discuss ongoing work, which was presented at the recent International Society on Thrombosis and Haemostasis (ISTH) meeting.
Keywords: bone marrow, imaging, megakaryocyte, platelet biogenesis, proplatelets
Essentials.
Imaging platelet biogenesis has tremendously advanced our understanding of thrombopoiesis.
Platelets originate from proplatelets derived from megakaryocytes predominantly situated at bone marrow sinusoids.
The combination of complementary imaging modalities will further enhance our knowledge of thrombopoiesis.
1. INTRODUCTION
All cells circulating in the peripheral blood are terminally differentiated cells that have developed in the bone marrow and finally crossed the endothelial barrier for their final life span. Blood platelets are the only exception to this, as these anuclear corpuscular fragments are derived from a progenitor cell, the megakaryocyte that disintegrates into cytoplasmic fragments while crossing the endothelial barrier. Platelet biogenesis, or thrombopoiesis, is thus the terminal process to generate a population of hemostatic active particles, with virtually the same size. The subcellular and molecular mechanisms underlying platelet production remain poorly characterized, especially as their progenitor cells are scarce within the bone marrow and not easily accessible. A better understanding of this process would be instrumental to decipher defects in inherited defects of platelet production or function, as well as in promoting the generation of platelets in vitro from progenitor cells including hematopoietic stem cells or induced pluripotent stem cells, to finally reduce massive costs for the high number of platelet transfusions each year.
The largest cell in the bone marrow was originally identified by Bizzozero as early as 1869. However, it was seminal work by Howell in 1890 who coined these cells “megakaryocytes” (MKs) and finally revealed that MKs are the precursors of the anucleated blood platelets.1 He performed elegant studies with the camera lucida to study cells in the bone marrow of cats, although similar observations were made in other mammals. Subsequent studies corroborated the relationship between MKs and platelets by Wright,2 followed by Duke’s seminal study,3 who recognized that a prolonged bleeding time was a consequence of a low peripheral platelet count.
2. CURRENT MODELS OF THROMBOPOIESIS
While MKs are clearly identified as immediate progenitors of platelets, the way how platelet biogenesis occurs, has remained a matter of debate during the last five decades, and even current observations challenge our thinking about platelet production across the endothelial barrier.
Platelets have an overall short life span of about 8‐10 days in humans and 5‐7 days in mice. Old platelets are sequestered by the liver and spleen and the population of platelets is permanently replenished. In addition, blood loss or episodes of bleeding might induce an additional production to compensate for the loss. The concentration of circulating platelets is rather tightly regulated and controlled by the thrombopoietin (THPO) axis.4 THPO is typically produced constitutively by hepatocytes in the liver (and too a much lesser extent in the kidney or bone marrow stroma cells) and released into the circulation. The thrombopoietin receptor c‐Mpl is expressed on early hematopoietic stem cells and among the megakaryocytic lineage, up to platelets. The compound pool of c‐Mpl on MKs and platelets leads to clearance of THPO from the circulation and transduces signals (mainly mediated by Jak2) to proliferative, anti‐apoptotic signaling cascades that induce differentiation into the megakaryocytic lineage. Low platelet counts lead to an increased plasma level and drive the proliferation and differentiation of progenitor cells into MKs and platelets. Older platelets lose the terminal sialic acid on their sugar moieties on receptors and these desialylated platelets are recognized by the Ashwell‐Morell receptor in the liver where they modulate THPO production.5
Our current understanding of hematopoiesis in the bone marrow is based on findings that a pool of hematopoietic stem cells (HSCs) resides in a specific, protected microenvironment, typically under hypoxic conditions. Under steady state conditions HSCs are typically quiescent, in the G0 phase. A low rate of both symmetric cell divisions, which allow to maintain and replenish this stem cell pool, as well as asymmetric cell divisions that will lead into the generation of more committed progenitor cells. These precursors will finally give rise to cells of all blood lineages. All mature, circulating blood cells are thus generated from these progenitor cells within the bone marrow compartment, while the endothelial layer of the sinusoidal vessels builds the critical interface and is considered the blood–bone marrow barrier. This concept, however, requires that the HSCs migrate out of the “stem cell niche,” start to differentiate (and proliferate), and steadily migrate toward the vascular niche at the bone marrow sinusoids, where finally mature MKs shed platelets into the circulation. This model is typically referred to as the “flow model,” while alternative models with a “protoplatelet hypothesis” have been suggested. The resulting “cytoplasmic fragmentation model” addresses how “platelet territories,”6 despite the lack of detectable polymerized microtubule filaments, remain true future platelets.7, 8, 9 These conflicting models are based on distinct experimental models and imaging approaches that finally need to translate into a coherent model of platelet biogenesis.
The concept that megakaryocytic progenitor cells mature while migrating toward the vessel, where the final thrombopoiesis occurs and new blood platelets are released, is based on a combination of in vitro and in vivo studies. The seminal study by Avecilla et al. shows that FGF‐4 and CXCL12 (SDF‐1) play complementary roles to increase platelet production, even in the absence of the TPO/c‐Mpl axis.10 While TPO is required for the expansion of megakaryocytic progenitors, FGF‐4 supports the adhesion of MKs to the endothelial cells and CXCL12 enhances the transmigration across the endothelial barrier. The expression of the CXCR4 receptor on MKs has also led to migration studies using Dunn chambers and transwell assays.11, 12, 13, 14, 15 Of note, clear‐cut evidence based on in vivo real‐time analyses proving that MKs actually migrate within the bone marrow is missing.
3. CAUGHT IN STILL LIFE: ELECTRON MICROSCOPY
Thin layer transmission electron microscopy (TEM) has been the imaging approach of choice for decades and high‐quality images of megakaryocytes and platelets have shaped our thinking about the megakaryocytes as precursors of platelets.16 The internal membrane system has first been considered to delineate single platelet territories6, 17 and designated the “demarcation membrane system” (DMS). Work by Radley has realized that this term is a misnomer,18, 19 but alternative names like “invagination membrane system” have not really been used in the field, including rather novel approaches to further characterize this membrane reservoir as required for platelet production.20, 21, 22, 23, 24 While classic TEM allows the required ultraresolution to capture the process of platelet biogenesis, it harbors several disadvantages: the total field is rather limited and the sample preparation is overall tedious. Moreover, while platelets and MKs in suspension are readily pelleted prior to subjection to TEM, the interaction of MK with the vessel wall in situ is additionally hampered by the mineralized bone, which typically requires decalcification for optimal sample processing. Nevertheless, Eckly et al. presented at the recent ISTH congress their preliminary study using focused ion beam–scanning electron microscopy (FIB‐SEM) on cellular contacts between MKs and endothelial cells in the bone marrow.25 They report four types of MK/EC interactions: (i) planar contacts, (ii) short MK extensions, (iii) long MK protrusions that penetrate into endothelial invaginations, and (iv) transmigrating MK fragments. Using FIB‐SEM 3D imaging the authors demonstrated that these transmigrating MK fragments are much thicker than classical proplatelets and might indicate transendothelial passage.25
4. CINEMICROGRAPHY: IMAGING THE ELUSIVE
The primary challenge was to study the dynamic process of platelet biogenesis at a virtually inaccessible site within the bone marrow by static microscopy and image processing. Haller and Radley describe the efforts to study platelet production from MKs of various species, including rats, mice, dogs, rabbits, or humans, and different organs (bone marrow, spleen). They conclude the “presence of blebs or small protrusions of the megakaryocyte surface to represent the development of platelets which are destined to be released by a budding mechanism.”26 Both investigators adopted an approach to implement a time‐resolved “cinemicrography” and scanning electron microscopy. These are among the first images of long cytoplasmic protrusions as an intermediate state of platelet formation in vitro. The mouse MKs were cultured in the presence of 2% fetal bovine serum, as a specific growth factor was only identified in 1994. The availability of this cytokine to the scientific community boosted in vitro (and in vivo) studies and led to the concept of proplatelets,27 showing that platelet‐like particles can be produced in the cell culture dish that share all properties of platelets isolated out of the blood stream. The systematic time‐lapse approach by Italiano and coworkers together with the approach to use fetal liver‐derived MKs as the source for studies has then led to a model of proplatelet budding, branching and elongation to increase the number of free tips, where mature and nascent platelets are shed.28 This model has become the paradigm of platelet biogenesis and has been reviewed extensively. Its elegant approach and comprehensive model system allowed to use transgenic mouse models to identify the key transcription factors (like p45NF‐E2), cytoskeletal proteins (like beta1‐tubulin) or regulatory proteins (like SLPI) and many to follow.29, 30, 31 However, proplatelet formation appeared to be undirected, even when the starting point of proplatelet formation seemed to be at one pole of the MK.
By serendipity, the combination of cranial intravital two‐photon microscopy (2PM) to study processes in the bone marrow of living mice, with a suitable mouse model where a significant fraction of MKs were intrinsically fluorescent, led to the first description of platelet biogenesis in the living mouse.32 The fluorescent protein eYFP was heterozygously expressed under the GPIIb promoter in the CD41 wt/eYFP mouse line,33 which is among the earliest marker proteins of the megakaryocytic lineage. The artificial myristyl anchor site at the C‐terminus leads to posttranslational modification and incorporation into the internal DMS membrane pool.22 Orientation within the bone marrow had to be provided by two additional colors, of which one was detected by second‐harmonic generation of fibrillar collagen. High molecular FITC‐dextran was intravenously injected to visualize the blood vessels and sinusoids. This combination allowed to identify that most MKs were found in direct contact with the vasculature. The technical parameters used at that time allowed to image a single animal up to about three hours. During this time, some of the high molecular dextran had already leaked out of the vessels. The authors found that those cytoplasmic fragments that are released into the blood stream, are much larger (7 μm) than the platelet‐sized particles (2 μm) found by the fetal liver‐cultures. In one case part of the cytoplasm has translocated into the vessel leading to partial obstruction of the vessel (movie S9 in Junt et al.32; see also Figure 1). Their findings concluded on previous findings that the terminal “platelet morphogenesis takes place in the bloodstream” as titled in a paper by Behnke and Forer in 1998.34 The release or shedding of particles larger than platelets, raises the question of how and where this maturation in the blood stream occurs. The free circulating particles that have been designated preplatelets are larger than platelets and defined as an intermediate platelet precursor, in distinction (and addition) to proplatelets, which have platelet‐sized structures at each end of an interconnected beaded structure in vivo).35 Tubulin immunofluroescene stainings and images from transmission electron microscopy of these preplatelets have identified microtubule coils like in platelets that are still interconnected by long filaments. These barbell‐like structures can perform the final platelet maturation and sizing by further fission into single blood platelets. Beyond that, studies from the Weyrich lab could show, by fluorescence widefield microscopy of living platelets in vitro (and complementary electron microscopy) that even single platelets from the circulation are capable to divide and form progeny.36
Figure 1.
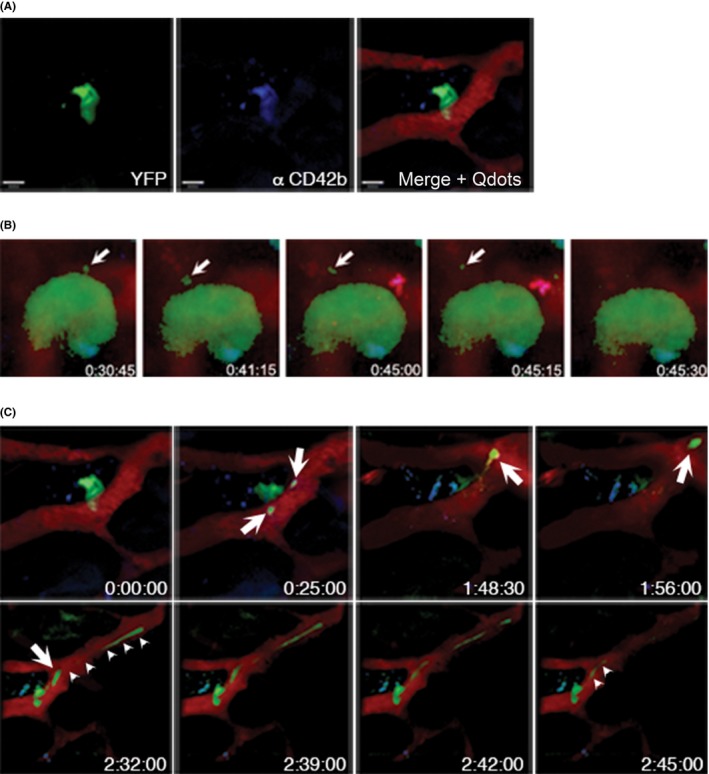
Platelet biogenesis in vivo. (A) eYFP+‐MKs are detected in MPM (left panel) and are additionally labeled by anti‐CD42b‐antibodies (middle panel). Vessels and sinusoids are visualized by intravenous injection of Qdots‐655 (right panel). Scale bars indicate 50 μm. (B) Still images from time‐lapse microscopy video 1 hour after antibody injection. Antibody decorated MKs are able to form proplatelets (arrows). (C) Still images from a video showing that antibody‐decorated MKs are able to form platelet‐sized particles as well as long, extended protrusions (proplatelets, arrows), which grow substantially within the overall observation period (arrow‐heads, lower panel)
Two‐photon microscopy has afterwards been used by a few groups interested in MKs to study the effect of drugs (like dasatinib) on thrombopoiesis in vivo15 or to investigate knock‐out mice for platelet production.37, 38, 39, 40 MKs were visualized using either reporter strains that express fluorescent proteins specifically in MKs and platelets,15, 37, 41 or using fluorophore‐conjugated antibody derivatives against the von Willebrand receptor subunit GPIX prior to imaging. This antibody is widely used in in vivo platelet studies and is described not to interfere with platelets or megakaryocytes in these settings.42 The vessel lumen is usually stained with fluorophore‐conjugated high‐molecular weight dextrans, quantum dots (see Figure 1) or fluorophore‐conjugated BSA, while we do use anti‐CD105 (anti‐endoglin) antibodies to label the endothelial lining, in addition to the vessel lumen.38, 39, 40, 42, 43, 44 The optimization of labeling techniques as well as technical improvements of two‐photon microscopes have increased the spatial resolution compared to the initial study.32 Taking advantage of this, Nishimura et al. could show that interleukin 1α is a marker for acute platelet need and leads to a different mechanism for megakaryocytic fragmentation that has been designated “megakaryocyte rupture.”41 However, it is unclear whether this fragmentation is a general acute response upon increased platelet demand, or whether additional inflammatory stimuli are required to trigger MK rupture.37, 41, 42
5. RECENT INSIGHTS INTO PLATELET BIOGENESIS
The novel applications of intravital microscopy provide many possibilities to study platelet biogenesis in the living mouse. However, the cranial window, which is typically used, has also some limitations. Mostly, young mice (not older than 6‐12 weeks) are imaged, in order to allow sufficient penetration depth. Imaging of older mice is in principle possible, but requires that the skull bone is somewhat reduced by mechanical means. Analysis of more inner vessel systems can be addressed by implantation of a cranial window, as described for stroke analysis. We and others have tried to use the tibia bone for imaging, which is readily accessible and provides an extended field of view. The original fixation procedure at the Greater Trochanter muscle has led to minor bleedings that might be acceptable for wildtype mice, but not suitable for any mouse model or condition with a potential platelet or bleeding phenotype. A recent study provides an improved approach to use the long bones for imaging.45
Nevertheless, two‐photon microscopy of cranial bone marrow has become the method of choice to study platelet biogenesis in vivo. At the ISTH conference in Berlin several studies were presented that utilized 2PM to assess the effects of gene deficiencies on platelet biogenesis.40, 42, 43, 44, 46, 47, 48
Münzer et al. analyzed mice lacking the casein kinase 2β, a serine threonine kinase expressed in MKs and platelets, and observed an abnormal microtubule structure in vitro and enhanced proportion of premature platelets that were released into the BM cavity as observed by 2PM.46, 47 resembling the defective MK morphology of mice lacking the small GTPases Cdc42 and Rac1.49 The ineffective platelet production in CK2β‐deficient animals resulted in a macrothrombocytopenia, which was further caused by a slightly reduced platelet lifespan.47 Reduced MK stability and elevated ectopic platelet release was also observed in mice lacking the adhesion and degranulation promoting adaptor protein ADAP, suggesting a critical role of this adaptor in the final stages of platelet production.48 Also in Adap −/− mice the impaired MK stability seemed to be caused by a defective cytoskeleton, as F‐actin organization was defective in ADAP‐deficient MKs.
Notably, Spindler et al. made use of a recently developed clearing procedure42 allowing them to image the uncut bone by confocal microscopy. We have developed this clearing procedure for mouse femur, sternum, and skull42, 44 and combined it with a relatively young microscopic method, light sheet fluorescence microscopy (LSFM). The authors report a highly dense network of vessels in each of the studied bone, while vessel‐distant niches are very limited. The intravascular space was quite densely packed with MKs. This is predominantly due to their extremely large size, merely by number, their frequency remains approximately 1% of nucleated cells. While there is not much space for migration of mature MKs, there might be a distinct pool of HSCs or megakaryocytic progenitor cells directly at the sinusoids that might differentiate and mature in permanent contact with the endothelial cells. This is in line with data from intravital imaging that MKs are overall sessile cells compared to lymphocytes.32, 42 The impact of cytokines or lipid gradients (like CXCL12 produced by CAR‐cells at the vessels or sphingosin‐1‐phosphate has been described37, 50 to guide MK migration, or at least the transmigration of proplatelet‐forming protrusions, but some questions remain.
With regard to the final step of MK polarization and proplatelet release Pleines presented a recent study40, 43 that the two small GTPases Cdc42 and RhoA act as central regulators coordinating these processes. Functional deficiency of Cdc42, or the von Willebrand receptor GPIbα impairs transendothelial proplatelet formation as observed using 2PM. In contrast, lack of RhoA results in increased Cdc42 activity and the transmigration of entire MKs into the vessel lumen.40, 43
6. PLATELET BIOGENESIS AT THE RIGHT SITE?
The current state of knowledge prompts us to study platelet biogenesis at the obvious site. The bone marrow is the site where all cells (and cellular fragments) circulating in the blood stream (or which reside in secondary lymphatic organs) are derived from. Bone marrow transplantation allows to reconstitute a complete blood system in malignant as well as in non‐malignant conditions, even when the transplantation of purified hematopoietic stem cells is now often the preferred choice. Nevertheless, it is well known that under disease conditions (and in mice also under physiological conditions) other organs can contribute to blood cell formation. This extramedullary hematopoiesis has not yet been investigated intensively by intravital approaches in typical organs including liver, spleen, or the lung. The mouse spleen is well known to have functional MKs that contribute to the overall platelet mass. The lung provides the first capillary bed where most bone marrow veins drain into. Single MKs, parts of it or naked nuclei are readily found in lung sections. Indeed, Aschoff reported MKs as cells with a “very large nucleus” (megakaryo) as early as 189351 and assumed that these cells would migrate into the lung out of the bone marrow by a chemotactic gradient. There has been a discussion starting in the thirties of the last century about whether MKs are formed in the lung or whether they immigrate. William Henry Howell suggested the lung as the primary source of platelets.52 Contrary experimental evidence was provided in 1965 by Kaufman using shunt experiments of dogs. He concluded that the MK origin is clearly in the bone marrow.53, 54 The lung capillary bed might act as a filter55 and thus indirectly contribute to platelet production, while circulating MKs in distinct vessels remain rare.56 Imaging of the lung in mice has been technically challenging. A recent study has solved this technical problem and provides evidence for MKs in the lung and platelet production by ectopic lung transplantation.57 However, their mathematical extrapolation that MKs in the lung contribute to 50% to the circulating platelet population remains disputable, as we and others do not detect this amount of MKs using light sheet fluorescence microscopy or classical histological approaches, even in mice with increased MK egress out of the bone marrow.40, 42, 43 On the other hand, it is clear that in vitro–derived MKs transfused into mice end up in the lungs and predominantly release platelets into the pulmonary vasculature.58
7. A SNEAK PEEK INTO THE FUTURE
Several limits of optical microscopy have been challenged during the last 10 years and Abbe’s diffraction resolution limit has been elegantly bypassed by stochastic illumination approaches—highlighted by the 2014 Nobel Prize in Chemistry to three of the pioneers (Eric Betzig, Stefan W. Hell, and William E. Moerner) of localization microscopy. While it is currently unclear, which of these improvements will finally translate into microscopy approaches in our field, we can be assured that even some of the current innovations will radically change the way how we are able to image samples, both in vitro and in vivo (Table 1). A recent review provides a comprehensive overview of next generation microscopy apporaches for imaging biological specimens.59 One of several outstanding and promising approaches is the “correlative light and electron microscopy” (CLEM), which allows to identify the region of interest with the help of fluorescence microscopy. Subsequently, the ultrastructure can be analyzed by serial sectioning tomography and aligned electron microscopy. While this approach is still mainly restricted to fixed samples, it can be applied in intravital approaches and might in the foreseeable future allow to visualize certain aspects of polarization in MKs in vitro.
Table 1.
Advantages and disadvantages of methods for imaging platelet biogenesis
| Method | Advantages | Disadvantages and technical limitations | References for application in MKs |
|---|---|---|---|
| Transmission electron microscopy (TEM) | High spatial resolution in the range of nanometers | Fixed specimen requiredTiny visual field | 34 |
| Epifluorescence microscopy: confocal laser scanning microscopy (CLSM). spinning disk confocal microscopy (SDCM) | Well established and versatile method, also applicable in vivo; costaining with multiple fluorophores allows colocalization studies | High phototoxicity, large, voluminous samples require sectioning; limited scanning speed has been improved by resonance scanners; most of visible light is absorbed; limited spatial resolution of less than 200 nm (x/y) and 600 nm (z) | 61 |
| Two‐Photon Microscopy (2PM) | Intra‐vital imaging, penetration depth of about 1 mm,Dynamic imaging with several frames per second; can be combined with non‐linear microscopy approaches | Small visual field, limited observation time of few hours; requires pulsed lasers | 15, 32, 41, 57 |
Light‐Sheet Fluorescence Microscopy (LSFM):
|
Unprecedented volumes can be imaged which allows to image whole mouse organs (femur, lung, etc.); low phototoxicity and photobleaching | Transparent or optically cleared specimen required; analysis generates large datasets for subsequent image processing | 42 |
| Localization (or super‐resolution) microscopy: direct Stochastic Optical Localization Microscopy (dSTORM),Single Molecule Light Microscopy (SMLM), Stimulated emission depleted (STED),Photo‐Activated Localization Microscopy (PALM) | High spatial resolution (up to the visualization of single molecules) | Small visual fieldLimited temporal resolution. To date still with limited z‐resolution | 60, 62 and references within 59 |
| Correlative light and electron microscopy (CLEM) | Combination of fluorescence microscopy and TEM results in best resolution; intravital fluroescence and subsequent TEM is possible | Small visual field, low speed; technical alignment of region of interest in light microscopy with TEM |
Another step forward is the combination of single molecule localization microscopy (SMLM) imaging with transgenic tags that can be brought into cells with the help of the CRISPR‐Cas9‐system as recently demonstrated with cell lines.60 In the long run this system can be expanded to transiently transfecting MKs in vitro, or even used to generate transgenic animals thereby allowing single molecule imaging of platelet biogenesis.
8. CONCLUSIONS
The transformation of megakaryocytes to circulating platelets is a fascinating cell biological phenomenon and its understanding clinically highly relevant. In order to modulate this process, we need to identify the key players at the cellular and molecular level and characterize their interactions. Intravital microscopy has opened a huge door that allows us to study suitable mouse models by advanced imaging technologies. Our current thinking, however, how (and where) platelet biogenesis occurs under physiological or pathophysiological conditions, affects the way how experiments are performed and into which organs we look in the first place. Imaging through the skull into the bone marrow has led to a better understanding of cellular behavior for many cell types and has refined our thinking of platelet biogenesis, at least in the mouse. Several approaches will address the technical obstacle of a limited visual field. Other organs like liver, spleen, or the lung have already been adopted for intravital microscopy and will provide new insights—and by this—also produce many more questions.
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft (DFG, SFB 688 TP A21, B07). We would like to thank Imke Meyer for the intravital images and Daniela Semeniak for critical comments on the manuscript.
RELATIONSHIP DISCLOSURE
None of the authors have any disclosures relevant to this paper.
AUTHOR CONTRIBUTIONS
D. Stegner reviewed ISTH abstracts and literature for this manuscript and edited this manuscript. H. Schulze reviewed ISTH abstracts and literature, drafted and edited the manuscript.
Schulze H, Stegner D. Imaging platelet biogenesis in vivo. Res Pract Thromb Haemost. 2018;2:461–468. 10.1002/rth2.12112
REFERENCES
- 1. Howell WH. Observations upon the occurrence, structure, and function of the giant cells of the marrow. J Morphol. 1890;4:117–130. [Google Scholar]
- 2. Wright J. The origin and nature of blood platelets. Boston Med Surg J. 1906;154:643–645. [Google Scholar]
- 3. Duke WW. The relation of blood platelets to hemorrhagic disease. JAMA. 1910;55:1185–1192. [PubMed] [Google Scholar]
- 4. Kuter DJ, Rosenberg RD. The reciprocal relationship of thrombopoietin (c‐Mpl ligand) to changes in the platelet mass during busulfan‐induced thrombocytopenia in the rabbit. Blood. 1995;85:2720–2730. [PubMed] [Google Scholar]
- 5. Grozovsky R, Begonja AJ, Liu K, et al. The Ashwell‐Morell receptor regulates hepatic thrombopoietin production via JAK2‐STAT3 signaling. Nat Med. 2015;21:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamada E. The fine structure of the megakaryocyte in the mouse spleen. Acta Anat (Basel). 1957;29:267–290. [DOI] [PubMed] [Google Scholar]
- 7. Kosaki G. In vivo platelet production from mature megakaryocytes: does platelet release occur via proplatelets? Int J Hematol. 2005;81:208–219. [DOI] [PubMed] [Google Scholar]
- 8. Kosaki G. Platelet production by megakaryocytes: protoplatelet theory justifies cytoplasmic fragmentation model. Int J Hematol. 2008;88:255–267. [DOI] [PubMed] [Google Scholar]
- 9. Kosaki G, Kambayashi J. Thrombocytogenesis by megakaryocyte; Interpretation by protoplatelet hypothesis. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:254–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Avecilla ST, Hattori K, Heissig B, et al. Chemokine‐mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. [DOI] [PubMed] [Google Scholar]
- 11. Drayer AL, Sibinga CT, Blom NR, De Wolf JT, Vellenga E. The in vitro effects of cytokines on expansion and migration of megakaryocyte progenitors. Br J Haematol. 2000;109:776–784. [DOI] [PubMed] [Google Scholar]
- 12. Abbonante V, Gruppi C, Rubel D, Gross O, Moratti R, Balduini A. Discoidin domain receptor 1 protein is a novel modulator of megakaryocyte‐collagen interactions. J Biol Chem. 2013;288:16738–17746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Valet C, Levade M, Chicanne G, et al. A dual role for the class III PI3K, Vps34, in platelet production and thrombus growth. Blood. 2017;130:2032–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mazharian A, Thomas SG, Dhanjal TS, Buckley CD, Watson SP. Critical role of Src‐Syk‐PLC{gamma}2 signaling in megakaryocyte migration and thrombopoiesis. Blood. 2010;116:793–800. [DOI] [PubMed] [Google Scholar]
- 15. Mazharian A, Ghevaert C, Zhang L, Massberg S, Watson SP. Dasatinib enhances megakaryocyte differentiation but inhibits platelet formation. Blood. 2011;117:5198–5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zucker‐Franklin D. (ed). Atlas of Blood Cells: Function and Pathology, 2nd edn Philadelphia: Lea & Febiger; 1988. [Google Scholar]
- 17. Behnke O. An electron microscope study of the megacaryocyte of the rat bone marrow. I. The development of the demarcation membrane system and the platelet surface coat. J Ultrastruct Res. 1968;24:412–433. [DOI] [PubMed] [Google Scholar]
- 18. Radley JM, Scurfield G. The mechanism of platelet release. Blood. 1980;56:996–999. [PubMed] [Google Scholar]
- 19. Radley JM, Haller CJ. The demarcation membrane system of the megakaryocyte: a misnomer? Blood. 1982;60:213–219. [PubMed] [Google Scholar]
- 20. Kakuta S, Ishikawa Y, Hashimoto T. Morphological studies on the forming processes and patterns of the platelet demarcation membrane system in the megakaryocytic series of embryonic rat livers. Arch Histol Jpn. 1986;49:255–265. [DOI] [PubMed] [Google Scholar]
- 21. Mahaut‐Smith MP, Thomas D, Higham AB, et al. Properties of the demarcation membrane system in living rat megakaryocytes. Biophys J. 2003;84:2646–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schulze H, Korpal M, Hurov J, et al. Characterization of the megakaryocyte demarcation membrane system and its role in thrombopoiesis. Blood. 2006;107:3868–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eckly A, Heijnen H, Pertuy F, et al. Biogenesis of the demarcation membrane system (DMS) in megakaryocytes. Blood. 2014;123:921–930. [DOI] [PubMed] [Google Scholar]
- 24. Antkowiak A, Viaud J, Severin S, et al. Cdc42‐dependent F‐actin dynamics drive structuration of the demarcation membrane system in megakaryocytes. J Thromb Haemost. 2016;14:1268–1284. [DOI] [PubMed] [Google Scholar]
- 25. Eckly A, Rinckel JY, Proamer F, Lanza F, de la Salle H, Gachet C. Ultrastructural characterization of the cellular contacts between megakaryocytes and endothelial cells in the bone marrow. Res Pract Thromb Haemost. 2017;1(Suppl 1):224. [Google Scholar]
- 26. Haller CJ, Radley JM. Time‐lapse cinemicrography and scanning electron microscopy of platelet formation by megakaryocytes. Blood Cells. 1983;9:407–418. [PubMed] [Google Scholar]
- 27. Choi ES, Nichol JL, Hokom MM, Hornkohl AC, Hunt P. Platelets generated in vitro from proplatelet‐displaying human megakaryocytes are functional. Blood. 1995;85:402–413. [PubMed] [Google Scholar]
- 28. Italiano JE Jr, Lecine P, Shivdasani RA, Hartwig JH. Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. J Cell Biol. 1999;147:1299–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shivdasani RA, Rosenblatt MF, Zucker‐Franklin D, et al. Transcription factor NF‐E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell. 1995;81:695–704. [DOI] [PubMed] [Google Scholar]
- 30. Schwer HD, Lecine P, Tiwari S, Italiano JE Jr, Hartwig JH, Shivdasani RA. A lineage‐restricted and divergent beta‐tubulin isoform is essential for the biogenesis, structure and function of blood platelets. Curr Biol. 2001;11:579–586. [DOI] [PubMed] [Google Scholar]
- 31. Schulze H, Korpal M, Bergmeier W, Italiano JE Jr, Wahl SM, Shivdasani RA. Interactions between the megakaryocyte/platelet‐specific beta1 tubulin and the secretory leukocyte protease inhibitor SLPI suggest a role for regulated proteolysis in platelet functions. Blood. 2004;104:3949–3957. [DOI] [PubMed] [Google Scholar]
- 32. Junt T, Schulze H, Chen Z, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317:1767–1770. [DOI] [PubMed] [Google Scholar]
- 33. Zhang J, Varas F, Stadtfeld M, Heck S, Faust N, Graf T. CD41‐YFP mice allow in vivo labeling of megakaryocytic cells and reveal a subset of platelets hyperreactive to thrombin stimulation. Exp Hematol. 2007;35:490–499. [DOI] [PubMed] [Google Scholar]
- 34. Behnke O, Forer A. From megakaryocytes to platelets: platelet morphogenesis takes place in the bloodstream. Eur J Haematol. 1998;61:3–23. [DOI] [PubMed] [Google Scholar]
- 35. Thon JN, Montalvo A, Patel‐Hett S, et al. Cytoskeletal mechanics of proplatelet maturation and platelet release. J Cell Biol. 2010;191:861–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwertz H, Koster S, Kahr WH, et al. Anucleate platelets generate progeny. Blood. 2010;115:3801–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang L, Orban M, Lorenz M, et al. A novel role of sphingosine 1‐phosphate receptor S1pr1 in mouse thrombopoiesis. J Exp Med. 2012;209:2165–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bender M, Stritt S, Nurden P, et al. Megakaryocyte‐specific Profilin1‐deficiency alters microtubule stability and causes a Wiskott‐Aldrich syndrome‐like platelet defect. Nat Commun. 2014;5:4746. [DOI] [PubMed] [Google Scholar]
- 39. Stritt S, Nurden P, Favier R, et al. Defects in TRPM7 channel function deregulate thrombopoiesis through altered cellular Mg(2+) homeostasis and cytoskeletal architecture. Nat Commun. 2016;7:11097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dütting S, Gaits‐Iacovoni F, Stegner D, et al. A Cdc42/RhoA regulatory circuit downstream of glycoprotein Ib guides transendothelial platelet biogenesis. Nat Commun. 2017;8:15838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nishimura S, Nagasaki M, Kunishima S, et al. IL‐1alpha induces thrombopoiesis through megakaryocyte rupture in response to acute platelet needs. J Cell Biol. 2015;209:453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stegner D, van Eeuwijk JMM, Angay O, et al. Thrombopoiesis is spatially regulated by the bone marrow vasculature. Nat Commun. 2017;8:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dütting S, Pleines I, Popp M, et al. A Cdc42/RhoA regulatory circuit downstream of glycoprotein ib guides transendothelial platelet biogenesis. Res Pract Thromb Haemost. 2017;1(Suppl 1):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stegner D, van Eeuwijk JMM, Angay O, et al. Thrombopoiesis is spatially regulated by the bone marrow vasculature. Res Pract Thromb Haemost. 2017;1(Suppl. 1):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reismann D, Stefanowski J, Gunther R, et al. Longitudinal intravital imaging of the femoral bone marrow reveals plasticity within marrow vasculature. Nat Commun. 2017;8:2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Münzer P, Walker‐Allgaier B, Geue S, et al. CK2β deficiency results in severe macrothrombocytopenia due to premature megakaryocyte fragmentation. Res Pract Thromb Haemost. 2017;1(Suppl. 1):240. [Google Scholar]
- 47. Munzer P, Walker‐Allgaier B, Geue S, et al. CK2beta regulates thrombopoiesis and Ca(2+)‐triggered platelet activation in arterial thrombosis. Blood. 2017;130:2774–2785. [DOI] [PubMed] [Google Scholar]
- 48. Spindler M, van Eeuwijk JMM, et al. Loss of the hematopoietic adaptor protein ADAP impairs megakaryocyte polarization and induces ectopic platelet release. Res Pract Thromb Haemost. 2017;1(Suppl. 1):224. [Google Scholar]
- 49. Pleines I, Dutting S, Cherpokova D, et al. Defective tubulin organization and proplatelet formation in murine megakaryocytes lacking Rac1 and Cdc42. Blood. 2013;122:3178–3187. [DOI] [PubMed] [Google Scholar]
- 50. Kunisaki Y, Bruns I, Scheiermann C, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Aschoff L. Über capilläre Embolie riesenkernhaltiger Zellen. Arch Path Anat Physiol. 1893;134:11. [Google Scholar]
- 52. Howell WH, Donahue DD. The production of blood platelets in the lung. J Exp Med. 1937;65:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kaufman RM, Airo R, Pollack S, Crosby WH. Circulating megakaryocytes and platelet release in the lung. Blood. 1965;26:720–731. [PubMed] [Google Scholar]
- 54. Kaufman RM, Airo R, Pollack S, Crosby WH, Doberneck R. Origin of pulmonary megakaryocytes. Blood. 1965;25:767–775. [PubMed] [Google Scholar]
- 55. Pedersen NT. The pulmonary vessels as a filter for circulating megakaryocytes in rats. Scand J Haematol. 1974;13:225–231. [DOI] [PubMed] [Google Scholar]
- 56. Pedersen NT. Occurrence of megakaryocytes in various vessels and their retention in the pulmonary capillaries in man. Scand J Haematol. 1978;21:369–375. [DOI] [PubMed] [Google Scholar]
- 57. Lefrancais E, Ortiz‐Munoz G, Caudrillier A, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544:105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Johnston I, Jarocha D, Hayes V, Rauova L, Poncz M. Platelet release from infused megakaryocytes is largely limited to the pulmonary vasculature. Res Pract Thromb Haemost. 2017;1(Suppl. 1):226. [Google Scholar]
- 59. Follain G, Mercier L, Osmani N, Harlepp S, Goetz JG. Seeing is believing–multi‐scale spatio‐temporal imaging towards in vivo cell biology. J Cell Sci. 2017;130:23–38. [DOI] [PubMed] [Google Scholar]
- 60. Khan AO, Simms VA, Pike JA, Thomas SG, Morgan NV. CRISPR‐Cas9 mediated labelling allows for single molecule imaging and resolution. Sci Rep. 2017;7:8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jenne CN, Wong CH, Petri B, Kubes P. The use of spinning‐disk confocal microscopy for the intravital analysis of platelet dynamics in response to systemic and local inflammation. PLoS ONE. 2011;6:e25109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Poulter NS, Pollitt AY, Davies A, et al. Platelet actin nodules are podosome‐like structures dependent on Wiskott‐Aldrich syndrome protein and ARP2/3 complex. Nat Commun. 2015;6:7254. [DOI] [PMC free article] [PubMed] [Google Scholar]


