Abstract
Background
The impact on health‐care costs and utilization of a single out‐of‐range (OOR) INR value not associated with bleeding or thromboembolic complication among chronic warfarin‐treated patients is not well described.
Methods
At four large phone‐based anticoagulation clinics (total 14 948 patients), warfarin‐treated patients with atrial fibrillation (AF) or venous thromboembolism were retrospectively propensity matched into an OOR INR group (n = 116) and a control group (n = 58). Types and frequency of contacts (eg, phone, voicemail, facsimile) and personnel involved were identified. A prospective time study analysis of 59 OOR and 92 control patients was performed over 8.5 days to record the time required to care for these patients. 2016 USD cost estimates were generated from average salaries.
Results
OOR and in‐range INR patients experienced an average of 4.2 and 3.2 (P < .001) INR lab draws until two sequential tests were in range. OOR INR patients required an average of 5.3 interactions with the anticoagulation clinic vs 3.7 for in‐range INR patients (P < .001). OOR INR patients more often required phone calls, fewer mailed letters, and more often required multiple types of contact than in‐range INR patients. In the prospective analysis, total median time involved for each OOR INR value was 5.1 minutes (IQR 3.7‐9.5) vs 2.9 minutes (IQR 1.8‐5.8) for control INR values (P < .001). At the clinic level, OOR INR values were associated with a yearly staff cost of $17 938 (IQR $8969‐$31 391).
Conclusions
We quantified the amount of extra anticoagulation staff effort required to manage warfarin‐treated patients who experience a single OOR INR value without bleeding or thromboembolic complications, which leads to higher healthcare utilization costs.
Keywords: costs and cost analysis, delivery of health care, health services, pharmacist, warfarin
Essentials.
Out‐of‐range INR values are common for warfarin‐treated patients.
Phone‐based anticoagulation clinics commonly manage a large number of warfarin‐treated patients.
Out‐of‐range INR values requires an average of 2.2 minutes more staff time than in‐range INRs.
Out‐of‐range INRs required an average of nearly $18 000 additional staff costs annually.
1. INTRODUCTION
Warfarin is commonly used to prevent stroke among patients with atrial fibrillation and to prevent recurrent venous thromboembolism. However, due to complex pharmacokinetic properties and multiple drug‐drug and drug‐food interactions, warfarin’s in vivo effect is notoriously variable. Coupled with a narrow therapeutic window, patients on chronic warfarin require close laboratory monitoring of their international normalized ratio (INR) level along with frequent dose adjustment.
Patients on chronic warfarin therapy are typically managed either by a dedicated anticoagulation management service or by individual providers.1, 2 While patients with in‐range INR values (typically 2‐3 for patients with atrial fibrillation or venous thromboembolism) require minimal staff time to manage, it is unclear how much time and staff resources are required to manage patients with out‐of‐range (OOR) INR values. This additional workload is a somewhat unrecognized burden on the health‐care system, when the OOR INR value is not associated with any bleeding or thromboembolic complications.
To better understand the health‐care resource utilization for single OOR INR values without bleeding or thromboembolic complications, we identified eligible cases and matched controls from four large anticoagulation clinics in Michigan. We examined the methods by which anticoagulation clinic staff contacted patients and the level of training for each staff member involved. We hypothesized that patients with single OOR INR values, not associated with any clinical complications, would experience a higher number of contacts by the anticoagulation clinic than patients with no OOR values.
2. METHODS
At four large telephone‐based anticoagulation management services in the state of Michigan, patients newly starting warfarin therapy were randomly selected and followed as part of a Blue Cross Blue Shield of Michigan quality improvement project, the Michigan Anticoagulation Quality Improvement Initiative (MAQI2).3 Each INR value, contact with the anticoagulation clinic, and any adverse events (e.g, emergency department visit, diagnosed thromboembolic or bleeding event) are captured in a database.
2.1. Phase 1. Retrospective data collection
Patients treated with chronic warfarin for either stroke prevention in atrial fibrillation or venous thromboembolism were eligible for this noninterventional study. We identified as “cases” the patients who experienced a single OOR INR value (<1.8 or >3.2) which was not caused by a temporary hold for procedures, and patients must have had two previous in‐range INR values (1.8‐3.2) at regular intervals of 3‐5 weeks without any bleeding events. Control patients were identified using the same criteria except the index INR had to be in range (1.8‐3.2) with no bleeding events occurring during and after the interaction. Cases and controls were matched with propensity scores based on demographics and comorbidities using the nearest neighbor method to generate two closely matched groups. From the two groups, patients were randomly selected without replacement with a 2:1 ratio to obtain a final set of 116 cases and 58 control patients.
Trained data abstractors identified and recorded the number and type of attempted and successful contacts between the anticoagulation clinic staff and the patients as documented in the medical chart. All attempted contacts and INR values were tracked until the patients had two consecutive in‐range INR values (1.8‐3.2), not including the index INR value. The data abstractors also identified the anticoagulation staff member who initiated contact based on their level of training.
2.2. Phase 2. Prospective data collection
Trained research assistants spent a total of 8.5 full work days (68 hours) monitoring anticoagulation staff at the four participating anticoagulation clinics. The observers used a stop watch to record the time required to complete each phase of management for all OOR INR values and randomly selected in‐range (control) INR values. INR management was divided into a pre‐interaction, interaction, and post‐interaction phase based on the time related to when a patient was being contacted (e.g, by phone, voicemail, email, mailed letter). Direct observations were made of nurses, pharmacists, and administrative assistants and total time required for each INR value was recorded for both in‐range and OOR INRs.
2.3. Statistical analysis
The frequencies of contacts for each group were summarized using descriptive statistics (adjusted mean, median, and interquartile range [IQR]) as well as compared using Poisson regression.
2.4. Cost estimates
Using 2016 US Dollars, we acquired the mean salaries for pharmacists, registered nurses, licensed practical nurses, and administrative assistants at the four participating anticoagulation clinics. We then multiplied the average salary for each staff member type at each center by the number of minutes spent on INR management by that specific staff member type at that center to generate total staff costs. With the knowledge of average staff time for an OOR INR and for an in‐range INR from prospective data collection, we were able to estimate the additional cost of managing an OOR INR as opposed to managing an in‐range INR.
After acquiring the volume of patients managed by the four clinics annually and using the MAQI2 registry to obtain the average number of OOR INRs per patient in each year, we calculated the total number of OOR INRs being managed by the four clinics each year. Adding in the additional cost of one OOR INR, we then were able to get the additional costs spent on managing all the OOR INRs across the four clinics each year. Based on 260 workdays a year, we were able to derive the extra daily staff cost for managing OOR INRs.
Funding for this project was provided by Pfizer and Bristol‐Myers Squibb. Blue Cross Blue Shield of Michigan had no role in this study. The study was approved by the University of Michigan Institutional Review Board.
3. RESULTS
3.1. Phase 1: Retrospective data collection
Between 2014 and 2015, 4029 patients with atrial fibrillation or venous thromboembolism in the MAQI2 registry were managed by the four, large‐volume, phone‐based anticoagulation clinics. Of those patients, 716 had 975 OOR INRs that met the out‐of‐range INR inclusion criteria and 1908 patients had 20 378 in‐range INRs meeting in‐range INR inclusion criteria. The demographics for the final set of 116 cases and 58 controls, randomly selected from the propensity matched groups, were statistically similar (Table 1). There were higher proportions of patients with atrial fibrillation and prior stroke or transient ischemic attack in the OOR INR group.
Table 1.
Demographics and Comorbidities of Retrospective Patient Review (Stage 1)
| Patients with OOR INR (n = 116) | Patients with in‐range INR (n = 58) | P‐value | |
|---|---|---|---|
| Age (mean ± SD) | 72.1 ± 13.0 | 74.3 ± 11.3 | .26 |
| Male | 58 (50.0%) | 38 (65.5%) | .05 |
| White race | 92/110 (83.6%) | 48/54 (88.9%) | .37 |
| Married or living with partner | 70/115 (60.9%) | 40/57 (70.2%) | .23 |
| Medicare or Medicaid | 56/114 (49.1%) | 35/58 (60.3%) | .16 |
| Indication | |||
| Atrial fibrillation | 67 (57.8%) | 42 (72.4%) | .06 |
| Venous thromboembolism | 49 (42.2%) | 16 (27.6%) | |
| Comorbidities | |||
| Diabetes | 28 (24.1%) | 17 (29.3%) | .46 |
| Hypertension | 88 (75.9%) | 45 (77.6%) | .8 |
| Congestive heart failure | 16 (13.8%) | 5 (8.6%) | .46 |
| Prior stroke or transient ischemic attack | 13 (11.2%) | 2 (3.4%) | .09 |
| Chronic kidney disease | 10 (8.6%) | 7 (12.1%) | .47 |
| Malignancy | 27 (23.3%) | 16 (27.6%) | .53 |
| Prior venous thromboembolism | 34 (29.3%) | 13 (22.4%) | .33 |
| CHA2DS2‐VASc for atrial fibrillation only (mean ± SD) | 3.9 ± 1.5 | 3.7 ± 1.4 | .43 |
| HAS‐BLED for atrial fibrillation only (mean ± SD) | 3.3 ± 1.4 | 2.9 ± 1.1 | .09 |
INR, international normalized ratio; OOR, out‐of‐range; SD, standard deviation.
Patients in the OOR INR group (cases) experienced more subsequent INR lab draws than control patients with consistently in‐range INR (adjusted means 4.2 vs 3.2 INR values, P < .001; Figure 1). Patients with a single OOR INR value were also associated with more attempted contacts by the anticoagulation clinic staff than consistently in‐range INR value patients (adjusted means 5.3 vs 3.7, P < .001; Table 2). These included more frequent telephone calls and voicemail messages, with fewer mailed letters. Overall, OOR INR patients were more often associated with multiple methods of contact than consistently in‐range INR patients (83.6% vs 55.2%, P < .001).
Figure 1.
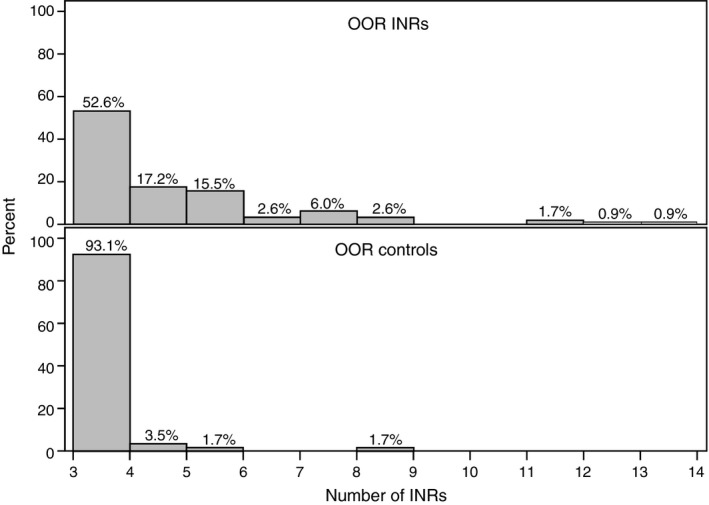
Number of INR values Until Steady State Achieved in Retrospective Patients. Distibution of the number of INR values until patients returned to a steady state of in‐range INR values. OOR INR—Median (IQR) 3 (3‐5); Control INR—Median (IQR) 3 (3‐3). INR, international normalized ratio; IQR, interquartile range; OOR, out‐of‐range
Table 2.
Frequency of Staff Contact for Retrospective Patients
| Patients with OOR INR (n = 116) | Patients with in‐range INR (n = 58) | P‐value | |||
|---|---|---|---|---|---|
| Median (IQR) | Adjusted mean | Median (IQR) | Adjusted mean | ||
| Total contacts | 5 (3‐6) | 5.3 | 3 (3‐4) | 3.7 | <.001 |
| Phone call | 2 (1‐4) | 2.9 | 1 (0‐1) | 0.9 | <.001 |
| Letter | 1 (0‐2) | 1.3 | 3 (2‐3) | 2.3 | <.001 |
| Voicemail | 1 (0‐1) | 0.9 | 0 (0‐0) | 0.5 | .03 |
| Multiple modes of contact | 97 (83.6%) | 32 (55.2%) | <.001 | ||
| RN | 3 (2‐5) | 3.4 | 3 (3‐3) | 2.9 | .059 |
| LPN | 0 (0‐0) | 0.3 | 0 (0‐0) | 0.2 | .43 |
| Pharmacist | 0 (0‐0) | 0.3 | 0 (0‐0) | 0.1 | .05 |
| Administrative assistant | 0 (0‐2.5) | 1.3 | 0 (0‐1) | 0.5 | <.001 |
| Multiple provider types | 51 (44.0%) | 18 (31.0%) | .10 | ||
INR, international normalized ratio; IQR, interquartile range; LPN, licensed practical nurse; OOR, out‐of‐range; RN, registered nurse.
From the retrospective patient data collection phase, this table summarizes the median (interquartile range) and adjusted mean number of contacts made between anticoagulation staff and patients for OOR INR values and in‐range (control) INR values. These are presented for both the type of contact (e.g, phone call, letter, voicemail) as well as the type of staff (e.g, nursing, pharmacist, administrative assistant) performing the contact.
The average number of contacts by nursing and pharmacist staff did not differ between the OOR and in‐range INR patients (Table 2). However, there were more contacts by administrative assistants for the OOR patients as compared to in‐range patients (adjusted means 1.3 vs 0.5, P < .001). A similar percentage of patients in both groups experienced contact from multiple types of providers.
The mean number of non‐INR laboratory tests ordered during the follow‐up period was similar between the out‐of‐range and in‐range groups (adjusted means 0.4 vs 0.4, P = .74).
3.2. Phase 2: Prospective data collection
Interactions between patients and anticoagulation clinic staff related to managing 59 OOR INR patients and 92 control patients were observed during the prospective study period. Length of warfarin treatment was similar for the two groups (mean ± SD 3.2 ± 3.4 years and 3.5 ± 4.2 years, respectively). Total median time involved in management of INR values was 5.1 minutes (IQR 3.7‐9.5 minutes) for OOR INR values vs 2.9 minutes (IQR 1.8‐5.8 minutes) for control INR values (P < .001; Table 3). Time spent on pre‐interaction preparation was similar for out‐of‐range INR patients (1.2 min [0.8‐1.8]) and control INR patients (1.0 min [0.7‐1.4], P = .20). Time spent interacting with patients (2.3 min [1.4‐4.5] vs 1.2 min [0.7‐2.3], P < .001) and post‐interaction documentation (1.5 min [10.8‐3.2] vs 0.7 min [0.2‐2.0], P = .002) were longer for out‐of‐range INR patients vs control INR patients.
Table 3.
Work Time by Phase of Interaction and Activity for Prospective Patients (Phase 2)
| Pre‐Interaction | Interaction | Post‐Interaction | Total | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OOR INR | Control INR | OOR INR | Control INR | OOR INR | Control INR | OOR INR | Control INR | |||||||||
| N | Time (Minutes) | N | Time (Minutes) | N | Time (Minutes) | N | Time (Minutes) | N | Time (Minutes) | N | Time (Minutes) | N | Time (Minutes) | N | Time (Minutes) | |
| Total | 57 | 1.2 (0.8‐1.8) | 92 | 1.0 (0.7‐1.4) | 56 | 2.3 (1.4‐4.5) | 84 | 1.2 (0.7‐2.3) | 55 | 1.5 (0.8‐3.2) | 84 | 0.7 (0.2‐2.0) | 59 | 5.1 (3.7‐9.5) | 92 | 2.9 (1.8‐5.8) |
| Phone call | 34 | 0.9 (0.6‐1.5) | 58 | 1.0 (0.7‐1.4) | 36 | 4.0 (2.3‐5.5) | 58 | 1.8 (0.9‐3.3) | 35 | 1.9 (0.9‐4.8) | 58 | 0.9 (0.3‐2.5) | 36 | 7.4 (4.7‐10.5) | 58 | 4.3 (2.3‐7.9) |
| Voicmail | 19 | 1.4 (1.1‐2.0) | 18 | 1.2 (0.5‐1.6) | 19 | 1.1 (0.5‐1.7) | 18 | 1.2 (0.9‐1.2) | 19 | 1.1 (0.5‐1.8) | 18 | 0.7 (0.2‐1.4) | 19 | 4.0 (2.6‐4.3) | 18 | 2.6 (1.8‐3.4) |
| 1 | 1.5 | 5 | 0.7 (0.5‐1.1) | 1 | 0.1 | 5 | 0.1 (0.1‐0.1) | 1 | 0.4 | 5 | 0.2 (0.1‐0.2) | 1 | 2.0 | 5 | 1. 0 (1.0‐1.2) | |
| Letter | 0 | ‐ | 3 | 0.6 (0.4‐1.0) | 0 | ‐ | 3 | 0.2 (0.2‐0.3) | 0 | ‐ | 3 | 0.1 (0.1‐0.6) | 0 | ‐ | 3 | 1.0 (0.7‐1.8) |
| Other | 3 | 3.0 (1.8‐3.1) | 8 | 1.8 (1.1‐3.2) | 0 | ‐ | 0 | ‐ | 0 | ‐ | 0 | ‐ | 3 | 3.0 (1.8‐3.1) | 8 | 1.8 (1.1‐3.2) |
From the prospective patient data collection phase, this table summarizes the median (interquartile range) of time staff spent with each type of anticoagulation clinic‐patient interaction (e.g, phone call, voicemail email) for patients with OOR INR or in‐range (control) INR values are presented. These are divided based on the phase of care (pre‐interaction, interaction, post‐interaction). INR, international normalized ratio; OOR, out‐of‐range.
3.3. Cost estimates
The mean daily number of OOR INR values being managed by the staff within each anticoagulation clinic was 58 (IQR 29‐101). Compared to in‐range INR patients, the OOR INR patients required an additional 2.2 minutes of total staff time. Across the four participating centers, the average hourly salary ranged from $49.28 to $62.50 for pharmacists, $27.50‐$39.15 for registered nurses, $19.00‐$19.71 for licensed practical nurses, and $14.04‐$18.22 for administrative assistants. Based on the minutes spent by each staff member type, the median (IQR) cost for managing an in‐range INR value was $1.25 ($0.74‐$3.38) and an OOR INR was $2.45 ($1.66‐$4.40). When these numbers are extrapolated across the 14 948 actively managed patients on warfarin at the four participating centers, patients with OOR INR values are associated with an additional annual personnel cost of $71 750 (IQR $35 875‐$125 563), or $17 938 (IQR $8969‐$31 391) per clinic.
4. DISCUSSION
Patients on chronic warfarin therapy who experience a single out‐of‐range INR value without any thromboembolic or bleeding complication utilize increased resources from their anticoagulation providers. Specifically, these patients require more INR blood draws and more contact between their anticoagulation provider via phone than patients with consistently in‐range INR values. They also required more anticoagulation clinic staff time (5.1 minutes vs 2.9 minutes) to complete management than patients with in‐range INR values.
Prior studies have assessed the cost of managing warfarin‐treated patients in anticoagulation clinics.4 They assessed the average time and cost required for routine, intermediate, and extended “visits”, noting an INR costs between $3 and $42 (measured in 2003 US dollars). They also discussed that staff labor contributed to 40% to 50% of overall costs. However, they did not assess the impact of OOR INRs on the overall health‐care utilization burden.
In our study, an OOR INR was associated with more subsequent INR lab draws, more attempted contacts by anticoagulation clinic staff, and more staff time dedicated to managing those INR values. In fact, for every 30 OOR INR values, an extra hour of staff work is required each day. This has significant health‐care cost implications, especially for large anticoagulation clinics. Our four centers averaged 230 (IQR 115‐402) daily OOR INR values at an incremental cost of $276 (IQR $138‐$482). When these numbers are extrapolated across the nearly 15 000 actively managed patients on warfarin at the four participating centers, an additional $18 000 per clinic in staff time is required. Notably, these estimates only include salaries and do not include other fringe benefits, which may increase the estimates.
Other studies have explored the impact of cost from societal, payer, and patient perspectives as well as comparing various methods of INR management.5, 6 Another study compared the costs of warfarin management in patients with and without bleeding events, noting increased costs associated with bleeding.7 However, none of these studies has explicitly outlined the health‐care utilization burden in terms of work performed and staff training level associated with OOR INR values that are not associated with bleeding or thromboembolic events.
Our study provides important insights into the challenging and important work performed by thousands of anticoagulation care providers across the United States and worldwide. While some may interpret these data to imply benefit of direct oral anticoagulants (DOAC) over warfarin, we have a more nuanced interpretation. It is true that the patients in this study would likely have required less contact with the health‐care system if they had been treated with a DOAC (e.g, dabigatran, rivaroxaban, apixaban, or edoxaban) instead of warfarin. However, reduced contact between the healthcare system and patients may not be an ideal goal. First, some patients prefer to have regular contact with their health‐care providers. Second, clinical trial protocols of the four available DOACs each mandated frequent clinic visits and/or phone contacts with patients. However, these practices have not been routinely adopted in “real world” practice for patients taking a DOAC.8, 9, 10, 11, 12, 13, 14, 15, 16 To help address this concern, the European Heart Rhythm Association recommends clinical follow up of DOAC‐treated patients at least every 6 months, but suggests that every 1‐3 months may be appropriate for many patients.17 This important contact between the patient and the health‐care system can be performed by primary care providers, specialists, or an anticoagulation clinic. In fact, the anticoagulation clinic may be ideally structured to provide this ongoing monitoring and support for patients, regardless of the oral anticoagulant they are currently prescribed.18
Our study has important strengths, including the use of trained chart abstractors to detail any and all documented contact between patients and the anticoagulation clinic. This study is also strengthened by the multi‐center design. Finally, the salaries used reflect real world data, but also are very similar to national salary figures published by the Bureau of Labor Statistics.19 However, important limitations must be considered. These include limited geographic range of the four involved healthcare centers as well as the focus on anticoagulation clinic care as compared to care provided by individual physician offices and their staff. Additionally, while dosing protocols exist at each center, the individual nurse or pharmacist is able to determine the most appropriate management strategy based on their knowledge of the patients, their clinical experience, and the guidance of the clinic protocols. Finally, the anticoagulation staff were aware that the study team was monitoring and measuring their practice, which could have influenced how quickly they delivered care.
In summary, we have quantified the additional staff effort required to manage patients on chronic warfarin therapy who experience a single OOR INR value without associated bleeding or thromboembolic complications, which leads to increased health‐care system utilization. Optimizing anticoagulation clinic resource utilization with patient expectations and clinical outcomes remains an important goal for further study.
5. RELATIONSHIP DISCLOSURE
This research was funded by Pfizer and Bristol‐Myers Squibb. GDB, XG, EKR, CG, EP, and JBF are employees of the University of Michigan which received financial support from Pfizer and Bristol‐Myers Squibb in connection with the research presented in this manuscript.
AUTHOR CONTRIBUTIONS
GDB, EKR, and JBF conceived of the project idea and supervised all data collection and analysis. EKR, CG, and EP performed data collection. XG performed statistical analysis. GDB drafted the manuscript. XG, EKR, CG, EP, KT, EM, TC, and JBF provided critical feedback and revisions to the manuscript.
Barnes GD, Gu X, Kline‐Rogers E, et al. Out‐of‐range INR results lead to increased health‐care utilization in four large anticoagulation clinics. Res Pract Thromb Haemost. 2018;2:490–496. 10.1002/rth2.12110
REFERENCES
- 1. Entezari‐Maleki T, Dousti S, Hamishehkar H, Gholami K. A systematic review on comparing 2 common models for management of warfarin therapy; pharmacist‐led service versus usual medical care. J Clin Pharmacol. 2016;56:24–38. [DOI] [PubMed] [Google Scholar]
- 2. Garcia DA, Witt DM, Hylek E, et al. Delivery of optimized anticoagulant therapy: consensus statement from the Anticoagulation Forum. Ann Pharmacother. 2008;42:979–88. [DOI] [PubMed] [Google Scholar]
- 3. Barnes GD, Kaatz S, Golgotiu V, et al. Use of warfarin for venous thromboembolism prophylaxis following knee and hip arthroplasty: results of the Michigan Anticoagulation Quality Improvement Initiative (MAQI(2)). J Thromb Thrombolysis. 2013;35:10–4. [DOI] [PubMed] [Google Scholar]
- 4. Menzin J, Boulanger L, Hauch O, et al. Quality of anticoagulation control and costs of monitoring warfarin therapy among patients with atrial fibrillation in clinic settings: a multi‐site managed‐care study. Ann Pharmacother. 2005;39:446–51. [DOI] [PubMed] [Google Scholar]
- 5. Gaw JR, Crowley S, Monagle P, Jones S, Newall F. The economic costs of routine INR monitoring in infants and children—examining point‐of‐care devices used within the home setting compared to traditional anticoagulation clinic monitoring. Thromb Res. 2013;132:26–31. [DOI] [PubMed] [Google Scholar]
- 6. Schulman S, Anderson DR, Bungard TJ, et al. Direct and indirect costs of management of long‐term warfarin therapy in Canada. J Thromb Haemost. 2010;8:2192–200. [DOI] [PubMed] [Google Scholar]
- 7. Abdelhafiz AH, Wheeldon NM. Use of resources and cost implications of stroke prophylaxis with warfarin for patients with nonvalvular atrial fibrillation. Am J Geriatr Pharmacother. 2003;1:53–60. [DOI] [PubMed] [Google Scholar]
- 8. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Committee RE‐LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 9. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–52. [DOI] [PubMed] [Google Scholar]
- 10. Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510. [DOI] [PubMed] [Google Scholar]
- 11. Granger CB, Alexander JH, McMurray JJ, et al. ARISTOTLE Committee and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 12. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 13. Buller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–97. [DOI] [PubMed] [Google Scholar]
- 14. Agnelli G, Buller HR, Cohen A, et al. Oral Apixaban for the Treatment of Acute Venous Thromboembolism. N Engl J Med. 2013;369:799–808. [DOI] [PubMed] [Google Scholar]
- 15. Giugliano RP, Ruff CT, Braunwald E, et al. ENGAGE AF‐TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104. [DOI] [PubMed] [Google Scholar]
- 16. Hokusai VTEI, Buller HR, Decousus H, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406–15. [DOI] [PubMed] [Google Scholar]
- 17. Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non‐vitamin K antagonist anticoagulants in patients with non‐valvular atrial fibrillation. Europace. 2015;17:1467–507. [DOI] [PubMed] [Google Scholar]
- 18. Barnes GD, Nallamothu BK, Sales AE, Froehlich JB. Reimagining anticoagulation clinics in the era of direct oral anticoagulants. Circ Cardiovasc Qual Outcomes. 2016;9:182–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bureau of Labor Statistics . May 2016 National Occupational Employment and Wage Estimates. [Accessed November 9, 2017]. Available from https://www.bls.gov/oes/current/oes_nat.htm-29-0000.


