Abstract
Antiplatelet therapy is a cornerstone in the secondary prophylaxis of adverse cardiovascular events such as myocardial infarction and stroke. The cyclooxygenase inhibitor aspirin remains the most frequently prescribed antiplatelet drug, followed by adenosine diphosphate P2Y12 receptor blockers. Glycoprotein IIb‐IIIa antagonists are intravenously available antiplatelet agents preventing platelet‐to‐platelet aggregation via the fibrinogen receptor. The thrombin receptor inhibitor vorapaxar allows the targeting of yet a third pathway of platelet activation. Despite the advent of novel agents and major advances in antiplatelet treatment over the last decade, atherothrombotic events still impair the prognosis of many patients with cardiovascular disease. Consequently, antiplatelet therapy remains a field of intense research and a large number of studies on its various aspects are published each year. This review article summarizes recent developments in antiplatelet therapy in cardiovascular disease focusing particularly on the duration of dual antiplatelet therapy, new treatment regimens, the role of platelet function testing, and potential future targets of antiplatelet agents.
Keywords: antiplatelet therapy, cardiovascular disease, combination, duration, targets
Essentials.
Two risk scores may be used to individualize the duration of dual antiplatelet therapy.
Dual antithrombotic therapy is a new treatment option in AF patients undergoing PCI.
Low‐dose rivaroxaban on top of aspirin offers a new strategy to prevent thrombotic events.
Novel antiplatelet agents have yielded promising results in preclinical trials.
1. INTRODUCTION
Ischemic events such as myocardial infarction (MI) and stroke are the main cause of morbidity and mortality in high income countries, and account for 15 million deaths per year worldwide. Undesired intravascular platelet activation at the site of endothelial injury plays a key role in the processes ultimately resulting in atherothrombosis with subsequent end organ damage.1, 2, 3 Accordingly, antiplatelet therapy became a cornerstone in the secondary prophylaxis of adverse cardiovascular outcomes (Figure 1).4
Figure 1.
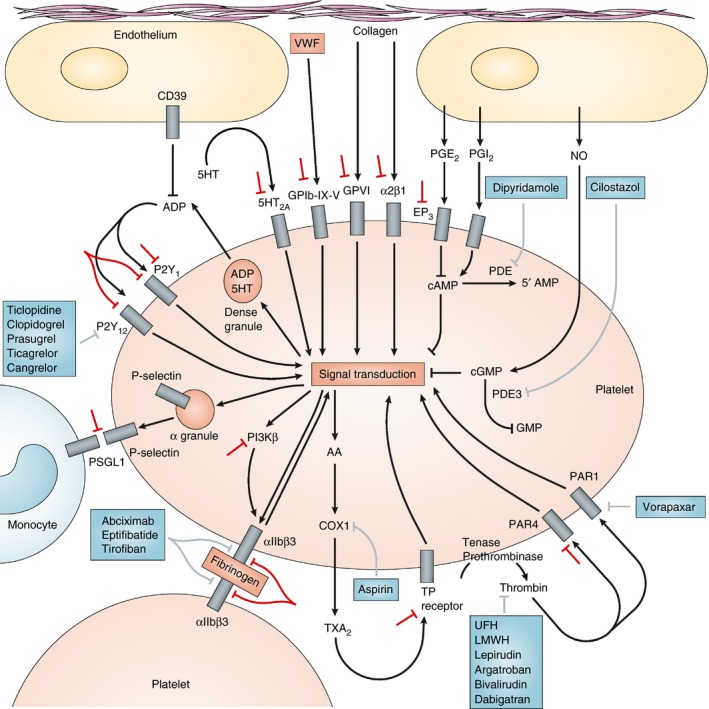
Platelet function and molecular targets of antiplatelet agents. Initial platelet adhesion to damaged vessel walls is mediated by the binding of exposed collagen to platelet surface glycoprotein (GP) VI and integrin α2β1 and by the binding of von Willebrand factor (VWF) to the platelet surface GPIb‐IX‐V complex. This complex is also a receptor for other platelet ligands (thrombospondin, collagen and P‐selectin), leukocyte integrin αMβ2, and procoagulant factors (thrombin, kininogen, factor XI and factor XII). Thrombin, generated by the coagulation cascade, is a potent activator of human platelets through two platelet surface receptors: protease‐activated receptor (PAR)‐1 and PAR‐4. Three groups of platelet surface receptors provide important positive feedback loops for platelet activation: P2Y1 and P2Y12 are stimulated by ADP released from platelet dense granules; 5‐hydroxytryptamine 2A receptors (5HT2A) are stimulated by 5‐hydroxytryptamine (5‐HT; also known as serotonin) released from platelet dense granules; and the thromboxane prostanoid (TP) receptor is stimulated by thromboxane A2 (TXA2) generated by the platelet cyclooxygenase (COX)‐1‐dependent signaling pathway. Platelet‐to‐platelet aggregation is mediated by fibrinogen and, at high shear flow, by VWF binding to activated integrin αIIbβ3. Perpetuation of platelet‐to‐platelet aggregation is augmented by other receptors, including junctional adhesion molecule A (JAMA) and JAMC, growth‐arrest specific gene 6 receptor, and ephrin. Platelet‐monocyte adhesion is initially mediated by the binding of platelet surface P‐selectin to its constitutively expressed cognate receptor, P‐selectin glycoprotein ligand‐1 (PSGL‐1), on the monocyte surface. Activated platelets, monocytes and microparticles bind coagulation factors and provide a surface for the generation of a fibrin clot. Approved antiplatelet agents and their molecular targets are shown in boxes. Indirect inhibitors (unfractionated heparin [UFH], low‐molecular‐weight heparin [LMWH]) and direct inhibitors (lepirudin, argatroban, bivalirudin and dabigatran) of thrombin, unlike PAR‐1 antagonists, are anticoagulants rather than specific antiplatelet drugs. However, their inhibition of thrombin results in reduced platelet activation. Investigational strategies for novel antiplatelet agents are shown by the symbols adjacent to: GPIb‐IX‐V, GPVI, α2β1, EP3, 5HT2A, PAR‐4, P2Y1, P2Y12, PSGL1, PI3Kβ, αIIbβ3 and the TP receptor. AA, arachidonic acid; EP3, prostaglandin E2 receptor EP3 subtype; NO, nitric oxide; PDE, phosphodiesterase; PG, prostaglandin; PI3Kβ, phosphoinositide 3‐kinase β‐isoform. Modified from Michelson AD. Nat Rev Drug Discov. 2010 with permission4
Aspirin is the most popular antiplatelet agent in acute and long‐term secondary prevention of ischemic events (Table 1).5 It exerts its inhibitory effect by irreversible acetylation of a serine residue of cyclooxygenase‐1 and ‐2, thereby blocking synthesis of prostaglandin G2 and H2, and consequently thromboxane A2 generation (Figure 1).4 Large meta‐analyses revealed a 20% reduction of atherothrombotic outcomes by aspirin in high‐risk patients.6, 7 Five adenosine diphosphate (ADP) P2Y12 receptor antagonists have been approved for the clinical use in patients (Figure 1)8: ticlopidine, clopidogrel, and prasugrel belong to the thienopyridine family and need to be metabolized by the hepatic cytochrome P450 enzyme system in order to become pharmacologically active and inhibit ADP‐mediated platelet aggregation (Table 1).4, 9 Ticlopidine is not recommended by the current guidelines due to its many side effects.10, 11 In contrast, clopidogrel plus aspirin is the state‐of‐the‐art dual antiplatelet therapy (DAPT) following elective percutaneous coronary intervention (PCI) or peripheral angioplasty with stenting,12 and prasugrel together with aspirin can be prescribed in acute coronary syndrome (ACS) patients undergoing PCI (Table 1).10, 11, 13 The triazolopyrimidine ticagrelor acts as a direct and reversible inhibitor at the ADP P2Y12 receptor,4 and has been approved in combination with aspirin for ACS patients without and with PCI (Table 1).10, 11 Cangrelor, an adenosine triphosphate analog, is an intravenous P2Y12 antagonist directly and reversibly blocking the ADP receptor with a short half‐life of 3 to 5 minutes.4 Its administration together with aspirin is approved for PCI patients without prior P2Y12 inhibitor treatment (Table 1).10, 11, 14 The group of glycoprotein (GP) IIb‐IIIa antagonists comprises 3 agents (Figure 1)4: abciximab, tirofiban, and eptifibatide are given intravenously in the peri‐interventional setting to block platelet‐to‐platelet aggregation via the fibrinogen receptor on human platelets,15 eg, in ACS patients with a high thrombotic burden or in case of a no reflow syndrome after PCI (Table 1).16 Finally, the thrombin receptor inhibitor vorapaxar prevents platelet activation by thrombin via protease‐activated receptor (PAR)‐1 on human platelets (Figure 1).4 Based on the results of the TRA 2P‐TIMI 50 (Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events – Thrombolysis in Myocardial Infarction) trial in patients with stable atherosclerotic disease,17 vorapaxar may be used in addition to standard antiplatelet therapy in the secondary prophylaxis of ischemic events in patients with a history of MI or symptomatic peripheral artery disease (PAD; Table 1).
Table 1.
Approved antiplatelet agents in cardiovascular disease
| Agent | Structure | Administration | Mechanism | Indication |
|---|---|---|---|---|
| Aspirin | Acetylsalicylic acid | Oral | COX‐1 inhibition | CAD, PAD, CVD, CABG, CEA, coronary and peripheral stents |
| Ticlopidine | Thienopyridine | Oral | P2Y12 inhibition | CVD, coronary stents |
| Clopidogrel | Thienopyridine | Oral | P2Y12 inhibition | Prior MI, stroke or symptomatic PAD, as monotherapy; ACS or coronary stenting, in combination with aspirin |
| Prasugrel | Thienopyridine | Oral | P2Y12 inhibition | ACS patients undergoing PCI with stenting, in combination with aspirin |
| Ticagrelor | Triazolopyrimidine | Oral | P2Y12 inhibition | ACS patients, in combination with aspirin |
| Cangrelor | Adenosine triphosphate analog | Intravenous | P2Y12 inhibition | P2Y12 inhibitor naïve PCI patients |
| Abciximab | Fab fragment of mouse human chimeric antibody 7E3 | Intravenous | GPIIb‐IIIa inhibition | PCI |
| Tirofiban | Non‐peptide mimetic based on RGD | Intravenous | GPIIb‐IIIa inhibition | ACS, PCI |
| Eptifibatide | KGD‐containing cyclic heptapeptide | Intravenous | GPIIb‐IIIa inhibition | ACS, PCI |
| Vorapaxar | Tricyclic himbacine derivative | Oral | PAR‐1 inhibition | Prior MI, PAD |
ACS, acute coronary syndrome; CABG, coronary artery bypass graft; CEA, carotid endarterectomy; CAD, coronary artery disease; COX‐1, cyclooxygenase‐1; CVD, cerebrovascular disease; GPIIb‐IIIa, glycoprotein IIb‐IIIa; KGD, Lys‐Gly‐Asp; MI, myocardial infarction; PAD, peripheral artery disease; PAR‐1, protease‐activated receptor‐1; PCI, percutaneous coronary intervention; RGD, Arg‐Gly‐Asp.
Despite the advent of novel agents and major advances in antiplatelet treatment over the last decade, atherothrombotic events still impair the prognosis of many patients with cardiovascular disease. Consequently, antiplatelet therapy remains a field of intense research and a large number of studies on its various aspects are published each year. This review article summarizes recent developments in antiplatelet therapy in cardiovascular disease focusing particularly on the duration of DAPT, new treatment regimens, the role of platelet function testing, and potential future targets of antiplatelet agents.
2. DURATION OF DAPT
While the need for lifelong therapy with one antiplatelet agent, ie, aspirin or clopidogrel, is commonly agreed upon in patients who suffered an ischemic cardiovascular event,6, 7 the optimal duration of DAPT following an ACS and/or PCI with stent implantation is less well‐established. Current guidelines recommend DAPT with aspirin and clopidogrel for 6 months following elective PCI with stent implantation, and DAPT with aspirin and prasugrel or ticagrelor for 12 months in ACS patients undergoing PCI and stenting.10, 11 In the latter, clopidogrel should be prescribed instead of prasugrel or ticagrelor if the patient cannot be treated with one of the two newer ADP P2Y12 inhibitors. Medically managed ACS patients should receive DAPT with aspirin and ticagrelor or—in case of an increased bleeding risk—clopidogrel for 12 months following the acute event.10, 11
However, the duration of DAPT in the above‐mentioned patient populations has recently been challenged by numerous clinical trials. In the DAPT study, 9961 patients who underwent PCI with drug‐eluting stent implantation and were subsequently treated with aspirin plus clopidogrel or prasugrel for 12 months were randomly assigned to continue thienopyridine treatment or to receive placebo in combination with aspirin for another 18 months.18 The coprimary efficacy end points were stent thrombosis and the composite of MI, stroke, or death during the period from 12 to 30 months. The primary safety end point was moderate or severe bleeding. The authors found that continued thienopyridine treatment significantly reduced both coprimary efficacy end points at the cost of a higher risk of moderate or severe bleeding in their patient population.18 The benefits of longer‐term DAPT were more evident in the ACS subgroup.19 In the PEGASUS‐TIMI (Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction) 54 trial which was based on the promising subgroups in the prior CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management and Avoidance) trial of clopidogrel plus aspirin, 21 162 aspirin‐treated patients who had suffered an MI 1 to 3 years earlier were randomized in a double‐blind 1:1:1 fashion to ticagrelor at a dose of 90 mg twice daily, ticagrelor at a dose of 60 mg twice daily, or placebo, and followed for a median of 33 months.20 The primary efficacy end point was the composite of MI, stroke, or cardiovascular death, and the primary safety end point was TIMI major bleeding. Both ticagrelor regimens significantly reduced the primary efficacy end point but increased the rates of TIMI major bleeding (without increasing the risk of intracranial hemorrhage or fatal bleeding).20
In contrast to the DAPT study and the PEGASUS‐TIMI 54 trial, several other studies investigated shorter DAPT regimens yielding promising results regarding the reduction of bleeding complications.10 Prompted by the results from these previous trials, the DAPT score and the PRECISE‐DAPT score were designed,21, 22 and introduced in the 2017 Update on DAPT by the European Society of Cardiology (ESC) as risk stratification tools for ischemia and bleeding to determine the optimal duration of DAPT.10 The DAPT score is calculated 12 months after uneventful DAPT and is comprised of 9 factors (age, heart failure/low left ventricular ejection fraction, vein graft stenting, MI at presentation, prior MI or PCI, diabetes, smoking, stent diameter <3 mm, paclitaxel‐eluting stent) resulting in −2 to +10 points.21 It was developed from patient data in the DAPT study and validated in 8163 patients from the PROTECT (Patient Related Outcomes with Endeavor vs Cypher Stenting) trial.18, 21, 23 Within the DAPT study, patients with a DAPT score ≥2 experienced fewer ischemic outcomes (number needed to treat [NNT] 34) and only a moderate increase in bleeding events (number needed to harm [NNH] 272) when they received 30 months of DAPT.21 On the other hand, patients with a DAPT score <2 showed no reduction of ischemic events, but a higher bleeding risk (NNH 64) with prolonged DAPT.
The PRECISE‐DAPT (Predicting Bleeding Complications in Patients undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy) score was generated from data of 14 963 patients with elective, urgent or emergent PCI enrolled in 8 multicenter randomized clinical trials,22 and validated in 8595 and 6172 patients undergoing PCI from the PLATO (Platelet Inhibition and Patient Outcomes) study and the BERN PCI registry,22, 24 respectively. The PRECISE‐DAPT score is calculated at the time of coronary stenting by a 5‐item algorithm (age, creatinine clearance, white blood cell count, hemoglobin, and prior spontaneous bleeding) resulting in 0 to 100 points, and allows an estimate of the bleeding risk in patients receiving DAPT. In the validation cohorts, patients with a PRECISE‐DAPT score ≥25 were exposed to a high bleeding risk by prolonged DAPT (NNH: 38) while gaining no ischemic benefit.22 The guidelines therefore suggest limiting DAPT to 3 to 6 months in these patients, while patients with a score of ≤25 may benefit from 12 months or even further prolongation (up to 24 months) of DAPT.10
In summary, the DAPT and PRECISE‐DAPT risk scores represent a promising opportunity for a more individualized DAPT strategy to optimize protection from ischemia and minimize bleeding risk. However, both scores need to be evaluated prospectively in large populations of patients with different clinical manifestations of ischemic heart disease in order to draw definitive conclusions. As a cautionary note in ACS patients, the SMART‐DATE (Safety of 6‐month Duration of Dual Antiplatelet Therapy After Acute Coronary Syndromes) trial recently found a much higher rate of MI in ACS patients treated with 6 months of DAPT vs 12 or more months of DAPT.25, 26
3. NEW TREATMENT REGIMENS
3.1. Dual vs triple antithrombotic therapy in patients with AF undergoing percutaneous coronary intervention
Oral anticoagulation (OAC) with direct oral anticoagulants (DOACs) or vitamin K antagonists (VKAs) is the treatment of choice to avoid stroke and systemic embolism in patients with AF and a CHA2DS2‐VASc score ≥2.27, 28 Likewise, DAPT is mandatory in the initial phase after PCI to prevent stent thrombosis and ischemic outcomes.10, 11 Thus, until recently patients with AF undergoing coronary stenting have been treated with triple therapy consisting of OAC, aspirin and clopidogrel for up to 6 months, thereby being exposed to a pronounced risk of bleeding complications. In 2013, the open‐label, randomized, controlled WOEST (What is the Optimal Antiplatelet and Anticoagulant Therapy in Patients with Oral Anticoagulation and Coronary Stenting) trial was the first to challenge this paradigm by randomizing 573 patients on VKAs to aspirin plus clopidogrel vs clopidogrel alone post PCI.29 The primary outcome of the WOEST trial was any bleeding episode within 1 year after coronary stenting, which occurred significantly less often in patients on VKA plus clopidogrel compared with triple therapy while no increase in thrombotic events was observed. However, the WOEST trial was not adequately powered to reveal differences of ischemic events between both treatment groups.29 Recently, two large randomized clinical trials were published comparing dual‐ or low‐dose triple antithrombotic therapy with DOACs to standard triple therapy with VKAs in patients with nonvalvular AF undergoing PCI.30, 31 The PIONEER AF‐PCI (Open‐Label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose‐Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects with Atrial Fibrillation who Undergo Percutaneous Coronary Intervention) trial randomly assigned 2124 AF patients in a 1:1:1 ratio to 15 mg rivaroxaban once daily plus a P2Y12 inhibitor for 12 months (group 1), 2.5 mg rivaroxaban twice daily plus DAPT for 1, 6, or 12 months (group 2), or standard therapy with dose‐adjusted warfarin plus DAPT for 1, 6, or 12 months (group 3).30 The intended duration of DAPT and the type of P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor) were based on physician′s choice and had to be prespecified by the investigator before randomization. At 1 year, the primary end point of clinically significant bleeding had occurred significantly less frequently in dual and low‐dose triple therapy with rivaroxaban as compared to conventional triple therapy with warfarin. The rates of cardiovascular death, MI, or stroke were similar in the 3 treatment groups. However, as with the WOEST trial, PIONEER AF‐PCI was underpowered to detect differences in ischemic outcomes.30 In the RE‐DUAL PCI (Randomized Evaluation of Dual Antithrombotic Therapy with Dabigatran vs Triple Therapy with Warfarin in Patients with Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention) trial, 2725 patients with non‐valvular AF who had undergone PCI were randomized to triple therapy with warfarin plus a P2Y12 inhibitor (clopidogrel or ticagrelor) and aspirin for 1 to 3 months or dual therapy with dabigatran (110 mg or 150 mg twice daily) plus a P2Y12 inhibitor (clopidogrel or ticagrelor).31 The duration of DAPT in the triple therapy group was 1 month in patients with bare metal stent implantation and 3 months in those receiving drug eluting stents, and therefore shorter than in PIONEER AF‐PCI in which 22% of patients received 1 year of triple therapy.30, 31 The primary end point of major or clinically relevant non‐major bleeding was significantly reduced by both dual antithrombotic regimens compared with triple therapy over a mean follow‐up of 14 months. Analyzing both doses of dabigatran together, dual therapy with dabigatran was non‐inferior to triple therapy regarding the composite efficacy end point of death, MI, stroke, systemic embolism, or unplanned revascularization (power of 83.6%).31 However, the RE‐DUAL PCI study was not adequately powered to prove noninferiority regarding the efficacy end point for the higher‐ or lower‐dose dabigatran regimen alone vs triple therapy. Furthermore, the rate of stent thrombosis was numerically higher in patients randomized to dabigatran 110 mg twice daily plus ADP receptor antagonist compared with triple therapy, suggesting that the lower dose of dabigatran may only be used as part of a dual antithrombotic regimen in patients fulfilling dose reduction criteria and in those at very high bleeding risk. Of note, in both trials mainly clopidogrel was used as the ADP receptor antagonist in combined antithrombotic therapy: in PIONEER AF‐PCI only 4% to 7% of the patients in the three treatment groups received prasugrel or ticagrelor as the P2Y12 inhibitor,30 and in RE‐DUAL PCI only 12% of patients were treated with ticagrelor.31 Thus, clopidogrel remains the ADP receptor antagonist of choice in patients requiring a combination of OAC and antiplatelet therapy.
The growing evidence of the superior safety profile of dual antithrombotic therapy compared with triple therapy is reflected by the latest update on DAPT of the ESC. Specifically, the ESC now recommends dual antithrombotic therapy consisting of OAC and clopidogrel after coronary stenting in AF patients in whom the bleeding risk outweighs the ischemic risk (class IIa, level of evidence A).10 The 2018 European Heart Rhythm Association Practical Guide on the use of DOACs in patients with AF states that in the absence of randomized data on dual therapy with apixaban and edoxaban, dabigatran 150 mg twice daily dual therapy appears to be the preferred choice over triple therapy for most patients based on the results from RE‐LY (Randomized Evaluation of Long‐Term Anticoagulation Therapy) and RE‐DUAL PCI.31, 32, 33 Dual therapy with 110 mg dabigatran twice daily or rivaroxaban 15 mg once daily (10 mg in patients with moderate renal impairment) are suggested as viable alternatives for patients at high bleeding risk.32 Since large clinical trials of combined antithrombotic therapy with the two remaining DOACs, ie, apixaban and edoxaban, are currently ongoing, more data on bleeding and ischemic outcomes with dual vs triple therapy in AF patients undergoing PCI will be available in the near future.
3.2. Combination of aspirin and rivaroxaban in stable atherosclerosis
The recently published COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial compared standard antiplatelet therapy with low‐dose aspirin (100 mg/day) vs rivaroxaban 2.5 mg twice daily plus aspirin or rivaroxaban 5 mg twice daily in 27 395 patients with stable coronary artery disease (CAD) and/or PAD.34 Patients with CAD who were younger than 65 years of age were also required to have documentation of atherosclerosis involving at least two vascular beds or at least two additional risk factors (smoking, diabetes, chronic kidney disease with an estimated glomerular filtration rate <60 mL/min, heart failure, or nonlacunar ischemic stroke ≥1 month earlier).34 The COMPASS trial was halted early after a mean follow‐up of 23 months due to the superiority of the rivaroxaban‐plus‐aspirin regimen, including a lower rate of all‐cause mortality. The primary composite outcome of MI, stroke, or cardiovascular death was significantly reduced by the combination of 2.5 mg rivaroxaban twice daily with low‐dose aspirin compared with aspirin alone. The end point reduction was mainly driven by a decrease in the rate of ischemic stroke and stroke of uncertain type as well as cardiovascular mortality in the overall study population, as well as in patients with CAD and PAD.34, 35, 36 Furthermore, the rate of acute limb ischemia was significantly lower in both the rivaroxaban plus aspirin and the rivaroxaban only group in patients with PAD.35 As with ischemic stroke, acute limb ischemia often has an embolic cause. Previous studies revealed an increased risk of AF in patients with atherosclerotic disease, in particular in PAD.37, 38 One may, therefore, speculate that the beneficial effects of low‐dose rivaroxaban on top of aspirin in the COMPASS trial may—at least in part—be mediated by the prevention of intracardiac thrombus formation by rivaroxaban in patients with silent AF. Alternatively, the combination of submaximal simultaneous inhibition of primary and secondary hemostasis may account for the superiority of the rivaroxaban‐plus‐aspirin regimen compared with platelet inhibition alone by aspirin. Even dual antiplatelet therapy in high risk coronary populations has been shown to reduce ischemic stroke in the CHARISMA and PEGASUS trials.20, 39, 40 Since the main safety outcome of major bleeding occurred more frequently in the rivaroxaban‐plus‐aspirin group of the COMPASS trial, it remains to be established which patient populations benefit most from the combination of rivaroxaban with aspirin. Besides patient selection based on clinical risk stratification, eg, patients with multiple prior ischemic events or those with more than one affected vascular bed, the development of a new score from the COMPASS trial or the application of already existing risk prediction models may allow the identification of patients with an optimal benefit/risk ratio for the combined rivaroxaban‐plus‐aspirin regimen out of the large number of potentially eligible patients with stable atherosclerosis. An analysis from the REACH registry suggests that at least half to two‐thirds of patients with stable CAD or PAD might have been eligible for enrollment in COMPASS.41 Moreover, strategies to minimize bleeding risk with the combined treatment approach need to be evaluated. Since the main safety end point in the COMPASS trial was driven by gastrointestinal bleeding events, the concomitant prescription of proton pump inhibitors could be a promising option to prevent upper gastrointestinal bleeding. Furthermore, it is possible that the combination of low‐dose rivaroxaban with clopidogrel instead of aspirin may be safer with regard to gastrointestinal bleeding events. However, details on the exact location of gastrointestinal bleeding in the COMPASS trial and on the effect of proton pump inhibitors on the occurrence of bleeding events have not been published to date.
4. ROLE OF PLATELET FUNCTION TESTING
The response to antiplatelet therapy differs from one patient to the next,42, 43 and in particular clopidogrel‐mediated platelet inhibition is subject to considerable interindividual variations.44, 45 Moreover, patients with high on‐treatment residual platelet reactivity (HRPR) as assessed by platelet function tests are at an increased risk of ischemic outcomes post PCI.46, 47 Increasing clopidogrel dosage as well as switching to prasugrel or ticagrelor decreases on‐treatment platelet reactivity in patients with poor response to clopidogrel.48, 49, 50, 51 However, the intensification of antiplatelet therapy based on platelet function testing did not result in improved outcomes in patients with insufficient clopidogrel‐mediated platelet inhibition in large clinical trials.51, 52, 53, 54 Accordingly, routine laboratory monitoring of antiplatelet therapy is not recommended by the current guidelines on DAPT of the ESC and the American College of Cardiology/American Heart Association (ACC/AHA).10, 11 While HRPR enhances the risk of ischemic events, low‐on treatment residual platelet reactivity (LRPR), which is predominantly seen in patients receiving prasugrel or ticagrelor,55, 56, 57 may increase bleeding complications following PCI.47, 55, 56, 58, 59, 60 Therefore, two recent studies investigated the feasibility and outcomes of de‐escalating P2Y12 inhibition based on the results of platelet function testing. TROPICAL‐ACS (Testing Responsiveness to Platelet Inhibition on Chronic Antiplatelet Treatment for Acute Coronary Syndromes) was a randomized, open‐label trial investigating a monitoring‐guided de‐escalation strategy of thienopyridine therapy in 2610 ACS patients following PCI.61 Patients were assigned to two treatment groups: the guided de‐escalation group (n = 1304) received 10 mg or 5 mg prasugrel per day (according to the label and the current guideline recommendation) for 1 week followed by 1 week of 75 mg clopidogrel daily. At day 14, on‐treatment platelet reactivity was measured by multiple electrode aggregometry. Patients with HRPR were immediately switched back to prasugrel, while those without HRPR continued on clopidogrel. In contrast, the control group (n = 1306) received standard antiplatelet treatment with prasugrel for 1 year. The composite primary end point comprised cardiovascular death, MI, stroke, or bleeding grade 2 or higher according to BARC criteria at 12 months after randomization, and occurred in 7% and 9% of the patients in the guided de‐escalation group and control group, respectively.61 The authors concluded that guided de‐escalation of antiplatelet therapy based on the results of multiple electrode aggregometry was noninferior to standard treatment with prasugrel at 1 year after PCI.61 In another study, Cuisset et al. randomized 645 ACS patients receiving DAPT with aspirin plus prasugrel or ticagrelor 1 month after PCI to unchanged DAPT (n = 323) vs switched DAPT with aspirin plus 75 mg clopidogrel (n = 322).62 Over 12 months, they found a significant reduction of BARC ≥2 bleeding complications in the switched DAPT group compared with patients on unchanged DAPT (4% vs 14.9%), whereas no significant differences regarding the occurrence of ischemic end points were observed. In a subsequent analysis, the same group of authors revealed that the switched DAPT regimen was particularly beneficial with respect to the reduction of bleeding events in patients with LRPR at 1 month.63
In summary, guided early de‐escalation of P2Y12 inhibition may become an alternative treatment strategy in ACS patients managed with PCI, especially in those at a high risk of bleeding. First, however, more clinical data reinforcing the noninferiority of de‐escalated P2Y12 inhibition regarding the occurrence of ischemic events, and its superiority regarding the prevention of bleeding complications, are needed.
5. FUTURE TARGETS OF ANTIPLATELET AGENTS
5.1. P2Y1 receptor
Currently available ADP receptor antagonists target only P2Y12, but not the second ADP receptor on human platelets, P2Y1 (Figure 1).4, 64 Activation of P2Y1 initiates ADP‐induced platelet aggregation, and is responsible for platelet shape change,65 while P2Y12 activation leads to amplification and stabilization of the aggregation response.66 A complex interplay between P2Y1 and P2Y12 has been described previously,67 and coactivation of both seems essential for full platelet aggregation.66, 68 However, ADP may activate platelets of patients receiving P2Y12 inhibitors via P2Y1 to some extent, resulting in HRPR and an increased risk of ischemic outcomes despite antiplatelet therapy.46, 47 Recently, we synthesized a series of new diadenosine tetraphosphate (Ap4A) analogs and evaluated these Ap4A derivatives as platelet aggregation inhibitors, and with respect to their effects on platelet P2Y1, P2Y12, and P2X1 receptors.66, 69 Based on the results of these experiments, 1 compound (GLS‐409) that synergistically inhibited P2Y1 and P2Y12 without affecting P2X1 was selected for studies on antiplatelet efficacy (Table 2).66 GLS‐409 significantly inhibited ADP‐ and collagen‐induced platelet aggregation in rats and immediately attenuated platelet‐mediated thrombosis in a canine model of ACS without affecting rat or canine hemodynamics.66 Finally, GLS‐409 effectively blocked agonist‐stimulated platelet aggregation irrespective of concomitant aspirin therapy.66 Thus, the dual antagonist of P2Y1 and P2Y12 (Figure 1) may be a promising antiplatelet drug candidate, in particular for the initial phase of ACS.66
Table 2.
Potential future antiplatelet agents in cardiovascular disease
| Agent | Structure | Administration | Mechanism | Possible field of application |
|---|---|---|---|---|
| GLS‐409 | Diadenosine tetraphosphate derivative | Intravenous | Synergistic inhibition of P2Y1 and P2Y12 | ACS, PCI |
| PZ‐128 | Cell‐penetrating lipopeptide | Intravenous | PAR‐1 inhibition | ACS, PCI |
| BMS‐986120 | 2‐methoxy‐6‐[6‐methoxy‐4‐[[5‐methyl‐2‐(4‐morpholinyl)‐4‐thiazolyl]methoxy]‐2‐benzofuranyl]‐imidazo[2,1‐b]‐1,3,4‐thiadiazole | Oral | PAR‐4 inhibition | CAD, PAD, CVD |
| Troα6, Troα10 | Hexa‐ and deca‐peptides derived from the C‐terminal region of trowaglerix | Intravenous | Glycoprotein VI inhibition | ACS, PCI |
| BI1002494 | (R)‐4‐{(R)‐1‐[7‐(3,4,5‐trimethoxy‐phenyl)‐[1,6]naphthyridin‐5‐yloxy]‐ethyl}pyrrolidin‐2‐one | Oral | Spleen tyrosine kinase inhibition | CAD, PAD, CVD |
| ML‐355 | N‐(benzo[d]thiazol‐2‐yl)‐4‐((2‐hydroxy‐3‐methoxybenzyl)amino) benzenesulfonamide | Oral | 12‐lipoxygenase inhibition | CAD, PAD, CVD |
ACS, acute coronary syndrome; CAD, coronary artery disease; CVD, cerebrovascular disease; PAD, peripheral artery disease; PAR, protease‐activated receptor; PCI, percutaneous coronary intervention.
5.2. Protease‐activated receptors
The long half‐life and slow off‐rate are downsides of the approved PAR‐1 antagonist vorapaxar.70 Despite promising Phase II data, development of the PAR‐1 antagonist atopaxar was halted.71, 72 PZ‐128 is a new cell‐penetrating peptide‐based inhibitor of PAR‐1 which may overcome these limitations (Table 2).73 PZ‐128 targets the receptor‐G‐protein interface on the inside surface of platelets thereby blocking downstream G protein signaling. PZ‐128 has recently been shown to inhibit platelet activation rapidly and reversibly in animal models and in patients with CAD or with multiple risk factors for CAD,73, 74 and may become useful for short‐term inhibition of platelet aggregation, in particular in patients undergoing PCI.
Besides PAR‐1, PAR‐4 as second thrombin receptor on human platelets (Figure 1) may serve as a target of new antiplatelet agents.4, 70 Indeed, an orally active, selective, and reversible PAR‐4 inhibitor named BMS‐986120 yielded promising results in a nonhuman primate model as it exerted pronounced antithrombotic activity with only a minor prolongation of bleeding time (Table 2).75 In a subsequent phase I trial, Wilson et al. found BMS‐986120 to be well‐tolerated and to reduce substantially platelet‐rich thrombus formation ex vivo.76
5.3. Glycoprotein VI
Glycoprotein VI (Figure 1) is the major signaling receptor for collagen on human platelets with important functions in thrombosis and other platelet‐mediated processes.2 GPVI inhibitors that have previously been developed as potential antiplatelet drugs include the soluble GPVI‐Fc fusion protein Revacept and inhibitory anti‐GPVI antibodies.70, 77, 78 More recently, hexa‐ and deca‐peptides (Troα6 and Troα10) derived from the C‐terminal region of the GPVI‐specific agonist Tro (trowaglerix), which was purified from Tropidolaemus wagleri venom,79 were shown to potently inhibit collagen‐induced platelet aggregation without prolonging the bleeding time (Table 2).70, 80 The antiplatelet effect may be achieved by targeting of immunoglobulin‐like domains of GPVI by Troα6 and Troα10. These small‐mass hexa‐/deca‐peptide GPVI antagonists have therapeutic potential in patients with cardiovascular disease.80
Since activation of spleen tyrosine kinase (Syk) downstream of GPVI is crucial for platelet activation,70 Syk inhibitors have also been investigated as possible antiplatelet agents. Van Eeuwijk et al. reported that the orally available selective Syk inhibitor BI1002494 prevented arterial thrombosis and resulted in smaller infarct sizes and a significantly better neurological outcome 24 hours after transient middle cerebral artery occlusion in a mouse model (Table 2).81
5.4. Platelet oxidases
Lipoxygenases (LOXs) are enzymes catalyzing the oxygenation of polyunsaturated fatty acids which leads to the synthesis of various signaling molecules.70 12‐LOX is expressed in megakaryocytes and platelets, and oxidizes arachidonic acid at carbon 12.82 Growing evidence suggests that 12‐LOX is involved in platelet activation.83, 84, 85, 86 Recently, Adili et al. studied the impact of the selective 12‐LOX inhibitor ML355 on thrombosis and hemostasis (Table 2).87 They found a dose‐dependent decrease of human platelet aggregation by ML355, an effect that was reversed after exposure to high concentrations of thrombin in vitro. Moreover, oral administration of ML355 in mice reduced thrombus formation and vessel occlusion in FeCl3‐induced mesenteric and laser‐induced cremaster arteriole thrombosis models with only minimal effects on hemostasis.87
6. CONCLUSIONS
Recent data on abbreviated and prolonged DAPT challenged the current dogma on the optimal duration of combined therapy with aspirin and a P2Y12 inhibitor after coronary stenting,18, 20 and resulted in two new risk scores which may be used to individualize the duration of DAPT post PCI in patients at high risk of bleeding and ischemic events, respectively.10, 21, 22 Dual antithrombotic therapy with OAC and clopidogrel can be prescribed instead of triple therapy to minimize bleeding complications in AF patients undergoing PCI,10, 30, 31, 32 while low‐dose rivaroxaban on top of aspirin offers a new strategy to prevent thrombotic events more effectively in patients with stable atherosclerosis.34 Routine laboratory monitoring of antiplatelet therapy is not currently recommended.10, 11 However, early switching from prasugrel or ticagrelor to clopidogrel based on the results of platelet function testing may become an alternative option to reduce bleeding risk while maintaining adequate platelet inhibition following ACS, though more study is needed.61 Finally, new antiplatelet agents have yielded promising results in preclinical trials and may in the future become meaningful additions to the current pharmacological armamentarium in cardiovascular disease.66, 73, 74, 75, 76, 80, 81, 87
RELATIONSHIP DISCLOSURES
Dr. Thomas Gremmel: Lecture and consulting fees: AstraZeneca, Bayer, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Daiichi‐Sankyo, and Pfizer. Dr. Alan D. Michelson: Scientific advisory committees: AstraZeneca, Instrumentation Laboratory, Janssen; Research funding: Eisai, GLSynthesis, Ionis, Ironwood, Medtronic, Pfizer and Sysmex. Dr. Andrew L. Frelinger: Research funding: Eisai, GLSynthesis, Ionis, Ironwood, Medtronic, Pfizer and Sysmex. Dr. Deepak L. Bhatt: Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute), Cleveland Clinic, Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice‐Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; clinical trial steering committee), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR‐ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Amarin, Amgen, AstraZeneca, Bristol‐Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co‐Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, PLx Pharma, Takeda.
AUTHOR CONTRIBUTIONS
Dr. Thomas Gremmel: writing the article, Dr. Alan D. Michelson: critical revision and final approval, Dr. Andrew L. Frelinger: critical revision and final approval, Dr. Deepak L. Bhatt: critical revision and final approval.
Gremmel T, Michelson AD, Frelinger AL III, Bhatt DL. Novel aspects of antiplatelet therapy in cardiovascular disease. Res Pract Thromb Haemost. 2018;2:439–449. 10.1002/rth2.12115
REFERENCES
- 1. Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–94. [DOI] [PubMed] [Google Scholar]
- 2. Gremmel T, Frelinger AL 3rd, Michelson AD. Platelet physiology. Semin Thromb Hemost. 2016;42:191–204. [DOI] [PubMed] [Google Scholar]
- 3. Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8:1227–34. [DOI] [PubMed] [Google Scholar]
- 4. Michelson AD. Antiplatelet therapies for the treatment of cardiovascular disease. Nat Rev Drug Discov. 2010;9:154–69. [DOI] [PubMed] [Google Scholar]
- 5. Patrono C, Rocca B. Aspirin, 110 years later. J Thromb Haemost. 2009;7(Suppl 1):258–61. [DOI] [PubMed] [Google Scholar]
- 6. Antithrombotic Trialists’ Collabtoration . Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta‐analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cattaneo M. New P2Y12 blockers. J Thromb Haemost. 2009;7(Suppl 1):262–5. [DOI] [PubMed] [Google Scholar]
- 9. Floyd CN, Passacquale G, Ferro A. Comparative pharmacokinetics and pharmacodynamics of platelet adenosine diphosphate receptor antagonists and their clinical implications. Clin Pharmacokinet. 2012;51:429–42. [DOI] [PubMed] [Google Scholar]
- 10. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213–60. [DOI] [PubMed] [Google Scholar]
- 11. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1082–115. [DOI] [PubMed] [Google Scholar]
- 12. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation. N Engl J Med. 2001;345:494–502. [DOI] [PubMed] [Google Scholar]
- 13. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. [DOI] [PubMed] [Google Scholar]
- 14. Bhatt DL, Stone GW, Mahaffey KW, et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368:1303–13. [DOI] [PubMed] [Google Scholar]
- 15. Schror K, Weber AA. Comparative pharmacology of GP IIb/IIIa antagonists. J Thromb Thrombolysis. 2003;15:71–80. [DOI] [PubMed] [Google Scholar]
- 16. Kristensen SD, Wurtz M, Grove EL, et al. Contemporary use of glycoprotein IIb/IIIa inhibitors. Thromb Haemost. 2012;107:215–24. [DOI] [PubMed] [Google Scholar]
- 17. Morrow DA, Braunwald E, Bonaca MP, et al. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366:1404–13. [DOI] [PubMed] [Google Scholar]
- 18. Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 months of dual antiplatelet therapy after drug‐eluting stents. N Engl J Med. 2014;371:2155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yeh RW, Kereiakes DJ, Steg PG, et al. Benefits and risks of extended duration dual antiplatelet therapy after PCI in patients with and without acute myocardial infarction. J Am Coll Cardiol. 2015;65:2211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bonaca MP, Bhatt DL, Cohen M, et al. Long‐term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–800. [DOI] [PubMed] [Google Scholar]
- 21. Yeh RW, Secemsky EA, Kereiakes DJ, et al. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315:1735–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE‐DAPT) score: a pooled analysis of individual‐patient datasets from clinical trials. Lancet. 2017;389:1025–34. [DOI] [PubMed] [Google Scholar]
- 23. Camenzind E, Wijns W, Mauri L, et al. Stent thrombosis and major clinical events at 3 years after zotarolimus‐eluting or sirolimus‐eluting coronary stent implantation: a randomised, multicentre, open‐label, controlled trial. Lancet. 2012;380:1396–405. [DOI] [PubMed] [Google Scholar]
- 24. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–57. [DOI] [PubMed] [Google Scholar]
- 25. Hahn JY, Song YB, Oh JH, et al. 6‐month versus 12‐month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART‐DATE): a randomised, open‐label, non‐inferiority trial. Lancet. 2018;391:1274–84. [DOI] [PubMed] [Google Scholar]
- 26. Motovska Z, Bhatt DL. 12 months of DAPT after acute coronary syndrome still beats 6 months. Lancet. 2018;391:1240–2. [DOI] [PubMed] [Google Scholar]
- 27. van Walraven C, Hart RG, Singer DE, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta‐analysis. JAMA. 2002;288:2441–8. [DOI] [PubMed] [Google Scholar]
- 28. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–962. [DOI] [PubMed] [Google Scholar]
- 29. Dewilde WJ, Oirbans T, Verheugt FW, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open‐label, randomised, controlled trial. Lancet. 2013;381:1107–15. [DOI] [PubMed] [Google Scholar]
- 30. Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423–34. [DOI] [PubMed] [Google Scholar]
- 31. Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513–24. [DOI] [PubMed] [Google Scholar]
- 32. Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330–93. [DOI] [PubMed] [Google Scholar]
- 33. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 34. Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–30. [DOI] [PubMed] [Google Scholar]
- 35. Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double‐blind, placebo‐controlled trial. Lancet. 2017; 10.1016/S0140-6736(17)32409-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36. Connolly SJ, Eikelboom JW, Bosch J, et al. Rivaroxaban with or without aspirin in patients with stable coronary artery disease: an international, randomised, double‐blind, placebo‐controlled trial. Lancet. 2017; 10.1016/S0140-6736(17)32458-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37. Griffin WF, Salahuddin T, O’Neal WT, Soliman EZ. Peripheral arterial disease is associated with an increased risk of atrial fibrillation in the elderly. Europace. 2016;18:794–8. [DOI] [PubMed] [Google Scholar]
- 38. O’Neal WT, Efird JT, Nazarian S, Alonso A, Heckbert SR, Soliman EZ. Peripheral arterial disease and risk of atrial fibrillation and stroke: the Multi‐Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2014;3:e001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–17. [DOI] [PubMed] [Google Scholar]
- 40. Bhatt DL, Flather MD, Hacke W, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49:1982–8. [DOI] [PubMed] [Google Scholar]
- 41. Darmon A, Bhatt DL, Elbez Y, et al. External applicability of the COMPASS trial: an analysis of the reduction of atherothrombosis for continued health (REACH) registry. Eur Heart J. 2018;39:750–757a. [DOI] [PubMed] [Google Scholar]
- 42. Gremmel T, Steiner S, Seidinger D, Koppensteiner R, Panzer S, Kopp CW. Comparison of methods to evaluate clopidogrel‐mediated platelet inhibition after percutaneous intervention with stent implantation. Thromb Haemost. 2009;101:333–9. [PubMed] [Google Scholar]
- 43. Gremmel T, Steiner S, Seidinger D, Koppensteiner R, Panzer S, Kopp CW. Comparison of methods to evaluate aspirin‐mediated platelet inhibition after percutaneous intervention with stent implantation. Platelets. 2011;22:188–95. [DOI] [PubMed] [Google Scholar]
- 44. Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol. 2005;45:246–51. [DOI] [PubMed] [Google Scholar]
- 45. Gremmel T, Panzer S. Clinical, genetic and confounding factors determine the dynamics of the in vitro response/non response to clopidogrel. Thromb Haemost. 2011;106:211–8. [DOI] [PubMed] [Google Scholar]
- 46. Bonello L, Tantry US, Marcucci R, et al. Consensus and future directions on the definition of high on‐treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56:919–33. [DOI] [PubMed] [Google Scholar]
- 47. Tantry US, Bonello L, Aradi D, et al. Consensus and update on the definition of on‐treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62:2261–73. [DOI] [PubMed] [Google Scholar]
- 48. Gremmel T, Steiner S, Seidinger D, Koppensteiner R, Panzer S, Kopp CW. A high maintenance dose increases the inhibitory response to clopidogrel in patients with high on‐treatment residual platelet reactivity. Int J Cardiol. 2012;160:109–13. [DOI] [PubMed] [Google Scholar]
- 49. Gurbel PA, Bliden KP, Butler K, et al. Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies: the RESPOND study. Circulation. 2010;121:1188–99. [DOI] [PubMed] [Google Scholar]
- 50. Gremmel T, Eslam RB, Koppensteiner R, Lang IM, Panzer S. Prasugrel reduces agonists’ inducible platelet activation and leukocyte‐platelet interaction more efficiently than clopidogrel. Cardiovasc Ther. 2013;31:e40–5. [DOI] [PubMed] [Google Scholar]
- 51. Trenk D, Stone GW, Gawaz M, et al. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug‐eluting stents: results of the TRIGGER‐PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) study. J Am Coll Cardiol. 2012;59:2159–64. [DOI] [PubMed] [Google Scholar]
- 52. Price MJ, Berger PB, Teirstein PS, et al. Standard‐ vs high‐dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305:1097–105. [DOI] [PubMed] [Google Scholar]
- 53. Montalescot G, Vicaut E, Collet JP. Bedside monitoring of antiplatelet therapy for coronary stenting. N Engl J Med. 2013;368:871–2. [DOI] [PubMed] [Google Scholar]
- 54. Cayla G, Cuisset T, Silvain J, et al. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open‐label, blinded‐endpoint, randomised controlled superiority trial. Lancet. 2016;388:2015–22. [DOI] [PubMed] [Google Scholar]
- 55. Cuisset T, Grosdidier C, Loundou AD, et al. Clinical implications of very low on‐treatment platelet reactivity in patients treated with thienopyridine: the POBA study (predictor of bleedings with antiplatelet drugs). JACC Cardiovasc Interv. 2013;6:854–63. [DOI] [PubMed] [Google Scholar]
- 56. Bonello L, Mancini J, Pansieri M, et al. Relationship between post‐treatment platelet reactivity and ischemic and bleeding events at 1‐year follow‐up in patients receiving prasugrel. J Thromb Haemost. 2012;10:1999–2005. [DOI] [PubMed] [Google Scholar]
- 57. Alexopoulos D, Stavrou K, Koniari I, et al. Ticagrelor vs prasugrel one‐month maintenance therapy: impact on platelet reactivity and bleeding events. Thromb Haemost. 2014;112:551–7. [DOI] [PubMed] [Google Scholar]
- 58. Gurbel PA, Bliden KP, Navickas IA, et al. Adenosine diphosphate‐induced platelet‐fibrin clot strength: a new thrombelastographic indicator of long‐term poststenting ischemic events. Am Heart J. 2010;160:346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sibbing D, Schulz S, Braun S, et al. Antiplatelet effects of clopidogrel and bleeding in patients undergoing coronary stent placement. J Thromb Haemost. 2010;8:250–6. [DOI] [PubMed] [Google Scholar]
- 60. Patti G, Pasceri V, Vizzi V, Ricottini E, Di Sciascio G. Usefulness of platelet response to clopidogrel by point‐of‐care testing to predict bleeding outcomes in patients undergoing percutaneous coronary intervention (from the Antiplatelet Therapy for Reduction of Myocardial Damage During Angioplasty‐Bleeding Study). Am J Cardiol. 2011;107:995–1000. [DOI] [PubMed] [Google Scholar]
- 61. Sibbing D, Aradi D, Jacobshagen C, et al. Guided de‐escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL‐ACS): a randomised, open‐label, multicentre trial. Lancet. 2017;390:1747–57. [DOI] [PubMed] [Google Scholar]
- 62. Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38:3070–8. [DOI] [PubMed] [Google Scholar]
- 63. Deharo P, Quilici J, Camoin‐Jau L, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome according to on‐treatment platelet reactivity: the TOPIC‐VASP pre‐specified analysis of the TOPIC randomized study. JACC Cardiovasc Interv. 2017;10:2560–70. [DOI] [PubMed] [Google Scholar]
- 64. Cattaneo M. P2Y12 receptors: structure and function. J Thromb Haemost. 2015;13(Suppl 1):S10–6. [DOI] [PubMed] [Google Scholar]
- 65. Jin J, Daniel JL, Kunapuli SP. Molecular basis for ADP‐induced platelet activation. II. The P2Y1 receptor mediates ADP‐induced intracellular calcium mobilization and shape change in platelets. J Biol Chem. 1998;273:2030–4. [DOI] [PubMed] [Google Scholar]
- 66. Gremmel T, Yanachkov IB, Yanachkova MI, et al. Synergistic inhibition of both P2Y1 and P2Y12 adenosine diphosphate receptors as novel approach to rapidly attenuate platelet‐mediated thrombosis. Arterioscler Thromb Vasc Biol. 2016;36:501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hardy AR, Jones ML, Mundell SJ, Poole AW. Reciprocal cross‐talk between P2Y1 and P2Y12 receptors at the level of calcium signaling in human platelets. Blood. 2004;104:1745–52. [DOI] [PubMed] [Google Scholar]
- 68. Jin J, Kunapuli SP. Coactivation of two different G protein‐coupled receptors is essential for ADP‐induced platelet aggregation. Proc Natl Acad Sci USA. 1998;95:8070–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yanachkov IB, Chang H, Yanachkova MI, et al. New highly active antiplatelet agents with dual specificity for platelet P2Y and P2Y adenosine diphosphate receptors. Eur J Med Chem. 2015;107:204–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Grover SP, Bergmeier W, Mackman N. Platelet signaling pathways and new inhibitors. Arterioscler Thromb Vasc Biol. 2018;38:e28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. O’Donoghue ML, Bhatt DL, Wiviott SD, et al. Safety and tolerability of atopaxar in the treatment of patients with acute coronary syndromes: the lessons from antagonizing the cellular effects of Thrombin‐Acute Coronary Syndromes Trial. Circulation. 2011;123:1843–53. [DOI] [PubMed] [Google Scholar]
- 72. Wiviott SD, Flather MD, O’Donoghue ML, et al. Randomized trial of atopaxar in the treatment of patients with coronary artery disease: the lessons from antagonizing the cellular effect of Thrombin‐Coronary Artery Disease Trial. Circulation. 2011;123:1854–63. [DOI] [PubMed] [Google Scholar]
- 73. Zhang P, Gruber A, Kasuda S, et al. Suppression of arterial thrombosis without affecting hemostatic parameters with a cell‐penetrating PAR1 pepducin. Circulation. 2012;126:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gurbel PA, Bliden KP, Turner SE, et al. Cell‐penetrating pepducin therapy targeting PAR1 in subjects with coronary artery disease. Arterioscler Thromb Vasc Biol. 2016;36:189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wong PC, Seiffert D, Bird JE, et al. Blockade of protease‐activated receptor‐4 (PAR4) provides robust antithrombotic activity with low bleeding. Sci Transl Med 2017;9:pii:eaaf5294. [DOI] [PubMed] [Google Scholar]
- 76. Wilson SJ, Ismat FA, Wang Z, et al. PAR4 (Protease‐Activated Receptor 4) antagonism with BMS‐986120 inhibits human ex vivo thrombus formation. Arterioscler Thromb Vasc Biol. 2018;38:448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Andrews RK, Arthur JF, Gardiner EE. Targeting GPVI as a novel antithrombotic strategy. J Blood Med. 2014;5:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ungerer M, Rosport K, Bultmann A, et al. Novel antiplatelet drug revacept (Dimeric Glycoprotein VI‐Fc) specifically and efficiently inhibited collagen‐induced platelet aggregation without affecting general hemostasis in humans. Circulation. 2011;123:1891–9. [DOI] [PubMed] [Google Scholar]
- 79. Herr AB. Charming the Snake: venom‐derived peptides show surprising efficacy as glycoprotein VI‐targeting antithrombotic agents. Arterioscler Thromb Vasc Biol. 2017;37:1266–8. [DOI] [PubMed] [Google Scholar]
- 80. Chang CH, Chung CH, Tu YS, et al. Trowaglerix venom polypeptides as a novel antithrombotic agent by targeting immunoglobulin‐like domains of glycoprotein VI in platelet. Arterioscler Thromb Vasc Biol. 2017;37:1307–14. [DOI] [PubMed] [Google Scholar]
- 81. van Eeuwijk JM, Stegner D, Lamb DJ, et al. The Novel Oral Syk Inhibitor, Bl1002494, protects mice from arterial thrombosis and thromboinflammatory brain infarction. Arterioscler Thromb Vasc Biol. 2016;36:1247–53. [DOI] [PubMed] [Google Scholar]
- 82. Tourdot BE, Holinstat M. Targeting 12‐lipoxygenase as a potential novel antiplatelet therapy. Trends Pharmacol Sci. 2017;38:1006–15. [DOI] [PubMed] [Google Scholar]
- 83. Yeung J, Apopa PL, Vesci J, et al. 12‐lipoxygenase activity plays an important role in PAR4 and GPVI‐mediated platelet reactivity. Thromb Haemost. 2013;110:569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yeung J, Tourdot BE, Adili R, et al. 12(S)‐HETrE, a 12‐lipoxygenase oxylipin of dihomo‐gamma‐linolenic acid, inhibits thrombosis via galphas signaling in platelets. Arterioscler Thromb Vasc Biol. 2016;36:2068–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yeung J, Tourdot BE, Fernandez‐Perez P, et al. Platelet 12‐LOX is essential for FcgammaRIIa‐mediated platelet activation. Blood. 2014;124:2271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tourdot BE, Adili R, Isingizwe ZR, et al. 12‐HETrE inhibits platelet reactivity and thrombosis in part through the prostacyclin receptor. Blood Adv. 2017;1:1124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Adili R, Tourdot BE, Mast K, et al. First selective 12‐LOX inhibitor, ML355, impairs thrombus formation and vessel occlusion in vivo with minimal effects on hemostasis. Arterioscler Thromb Vasc Biol. 2017;37:1828–39. [DOI] [PMC free article] [PubMed] [Google Scholar]


