Abstract
After completing anticoagulation therapy for acute venous thromboembolism (VTE), patients with unprovoked VTE are at increased risk of recurrent thrombotic events. Recent studies suggest a risk of nearly 10% in the first year after stopping anticoagulants and 30% at 8 years. Therefore, it is important to consider extended anticoagulation for secondary prevention in these high‐risk patients. While several oral anticoagulants are available for this purpose, there is limited information available regarding the optimal agent to minimize bleeding risks and maximize efficacy at VTE prevention. This review article summarizes the evidence available for Vitamin‐K antagonists (VKAs) and direct oral anticoagulants (DOACs) for extended treatment of VTE. We also introduce the COVET trial, the first head‐to‐head comparison of VKAs to DOACs, rivaroxaban and apixaban, for extended management of unprovoked VTE.
Keywords: anticoagulation, bleeding, recurrent, secondary prevention, venous thromboembolism, venous thrombosis
Essentials.
Patients with unprovoked venous thromboembolism (VTE) are at increased risk for recurrent VTE.
Oral anticoagulants reduce this risk substantially but carry a risk of bleeding.
Limited data exists regarding the safest and most effective oral anticoagulant.
Additional direct comparison trials of oral agents for extended treatment are needed.
1. INTRODUCTION
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common, potentially fatal, yet treatable condition and is the third leading cause of mortality by cardiovascular disease.1, 2, 3 Up to 5% of the population will experience VTE in their lifetime and this incidence increases with age for both men and women.1, 2, 3 Anticoagulation is the mainstay of acute VTE therapy.4
After completing 3‐6 months of anticoagulants for acute VTE, the risk of recurrent VTE varies depending on the presence or absence of transient factors contributing to the index event. In those with provoked VTE, i.e, a VTE that is attributed to reversible or temporary factors such as recent surgery or major trauma, the risk of recurrence at 1 year is 1% and patients can safely discontinue anticoagulants.5 However, more than half of patients have unprovoked VTE events and the risk for recurrent VTE is substantial, reaching 10% in the first year after stopping anticoagulants, 5% in the following year,6 and 30% at 8 years.7 The associated case‐fatality rate of recurrent VTE is 3.6%.8
The International Society on Thrombosis and Haemostasis (ISTH) suggests continuing anticoagulation in those whose risk of recurrent VTE 1 year after stopping treatment is >5%9 and oral anticoagulants effectively reduce the risk of recurrent VTE by 80% to 90%.10 The 2016 American College of Chest Physicians (ACCP) guidelines suggest extended oral anticoagulation in patients with unprovoked VTE and low‐moderate bleeding risk.4 It is important to note that the ACCP guidelines make a stronger recommendation to avoid prolonged anticoagulants in high bleeding risk patients, defined as an annual major bleeding risk of >6%.4 Patients deemed to be high bleeding risk are those with at least two of several risk factors such as age >65, prior bleeding, (metastatic) cancer, liver or renal failure, thrombocytopenia, prior stroke, diabetes, and anemia. This categorization strata has not been validated, but is based on available evidence on the risks of anticoagulation‐associated bleeding.4 With continuing anticoagulation comes the potential harm of major bleeding which is fatal in around 11% of cases.8 In recent years, the relative safety of DOACs over VKAs has led to a consideration for extended, rather than limited, duration anticoagulation therapies for VTE.11 The next important question to consider is which anticoagulant to choose to balance the benefit of preventing recurrent VTE and minimize the harms of bleeding? This review article summarizes the evidence available for extended antithrombotic treatment of VTE and introduces the COVET trial, a direct comparison of VKAs to rivaroxaban and apixaban, for extended treatment of unprovoked VTE.
2. ORAL ANTICOAGULANTS
Vitamin‐K antagonists (VKAs) were the only class of oral anticoagulants for many decades, and were therefore the standard of care for extended treatment of VTE. In the last decade, direct oral anticoagulants (DOACs) have been studied and approved for use in the secondary prevention of recurrent VTE. These include the direct thrombin inhibitor dabigatran and direct Factor Xa inhibitors rivaroxaban and apixaban. Acetylsalicylic acid (ASA) is the only antiplatelet agent to be evaluated in secondary prevention of VTE. Randomized controlled trials (RCTs) have compared combinations of ASA, full dose and reduced dose DOACs, standard and low‐intensity VKAs, and placebo for prevention of recurrent VTE. Figure 1 summarizes antithrombotic therapies available during the phases of VTE treatment.
Figure 1.
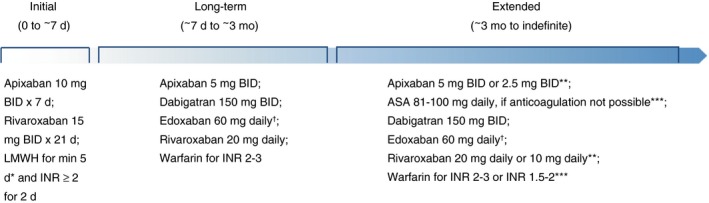
Antithrombotic dosing during phases of VTE treatment. ASA, acetylsalicylic acid; BID, bis in die, twice daily; INR, international normalized ratio; LMWH, low molecular weight heparin; VTE, venous thromboembolism.*LMWH is required for 5‐10 days prior to starting dabigatran or edoxaban; †30 mg daily if CrCl 30–50 mL/min or weight < 60 kg; **Dose reduction may be considered after 6 months of therapy; ***Less effective than alternative options available.
2.1. VKAs
VKAs have been the mainstay of oral anticoagulation for extended therapy. Low‐intensity VKAs do not reduce recurrent VTE rates as effectively as standard international normalized ratio (INR) range 2‐3 and do not lead to lower bleeding risk.12 A recent RCT of patients with unprovoked PE treated with VKAs for 6 months and then randomized to either an additional 18 months of VKAs or placebo13 confirmed previous trial findings6, 14, 15, 16: extended VKA therapy reduces rates of recurrent VTE during treatment only; once anticoagulation is stopped, rates of recurrence return to the same as those seen in patients who are treated for shorter duration.
Treatment with VKA presents many challenges including drug and food interactions, and a narrow therapeutic window requiring regular laboratory monitoring. These can be burdensome for the elderly and those with comorbidities who require intensive monitoring and dosing changes. While VKAs are inexpensive, safer monitoring practices are needed given their association with potentially fatal bleeding.17 DOACs offer efficient anticoagulation with predictable pharmacokinetics without the need for routine laboratory monitoring. In VTE patients without active cancer, guidelines suggest use of DOACs over VKAs.4, 18 Dabigatran, rivaroxaban, and apixaban have all been evaluated in secondary prevention trials showing reduced or similar VTE recurrence rates vs comparators. Edoxaban has not been evaluated in this context. Baseline characteristics and summary findings of DOAC extension trials are in Tables 1 and 2, respectively.
Table 1.
Characteristics of DOAC extension trials
| Study | Comparator 1 (Treatment and n) | Comparator 2 (Treatment and n) | Comparator 3 (Treatment and n) | Duration of OAC for Acute VTE (months) | Mean Age (years)a | Unprovoked VTE (%)a | Recurrent VTE eventsa | Major bleeding eventsa |
|---|---|---|---|---|---|---|---|---|
| RE‐MEDY | Dabigatran 150 mg BID (n = 1430) | VKA, INR 2‐3 (n = 1426) | — | 3‐12 | 54/55/‐ | Not reported | 26/18/‐ | 13/25/‐ |
| RE‐SONATE | Dabigatran 150 mg BID (n = 681) | Placebo (n = 662) | — | 6‐18 | 56/56/‐ | Not reported | 3/37/‐ | 2/0/‐ |
| EINSTEIN‐EXT | Rivaroxaban 20 mg daily (n = 602) | Placebo (n = 594) | — | 6‐12 | 58/58/‐ | 73/74/‐ | 8/42/‐ | 4/0/‐ |
| EINSTEIN‐CHOICE | Rivaroxaban 20 mg daily (n = 1107) | Rivaroxaban 10 mg daily (n = 1127) | ASA 100 mg (n = 1131) | 6‐12 | 58/59/59 | 40/43/41 | 17/13/50 | 6/5/3 |
| AMPLIFY‐EXT | Apixaban 5 mg BID (n = 813) | Apixaban 2.5 mg BID (n = 840) | Placebo (n = 829) | 6‐12 | 56/57/57 | 91/93/91 | 14/14/73 | 1/2/4 |
Comparator 1/Comparator 2/Comparator 3 (where applicable).
BID, bis in die, twice daily; DOAC, direct oral anticoagulant; INR, international normalized ratio; VKA, vitamin‐K antagonists; VTE, venous thromboembolism.
Table 2.
Summary of findings for DOAC extension trials
| Study | Comparator 1 | Comparator 2 | Recurrent VTEa HR (95% CI) | MB HR (95% CI) | MB/CRNMBHR (95% CI) |
|---|---|---|---|---|---|
| RE‐MEDY | Dabigatran 150 mg BID | VKA (INR 2‐3) | 1.44 (0.78‐2.64) | 0.52 (0.27‐1.02) | 0.54 (0.41‐0.71) |
| RE‐SONATE | Dabigatran 150 mg BID | Placebo | 0.08 (0.02‐0.25) | Not estimable | 2.92 (1.52‐5.60) |
| EINSTEIN‐EXT | Rivaroxaban 20 mg daily | Placebo | 0.18 (0.09‐0.39) | Not estimable | 5.19 (2.3‐11.7) |
| EINSTEIN‐CHOICE | Rivaroxaban 20 mg daily | ASA 100 mg | 0.34 (0.20‐0.59) | 2.01 (0.50‐8.04) | 1.59 (0.94‐2.69) |
| EINSTEIN‐CHOICE | Rivaroxaban 10 mg daily | ASA 100 mg | 0.26 (0.14‐0.47) | 1.64 (0.39‐6.84) | 1.16 (0.67‐2.03) |
| AMPLIFY‐EXT | Apixaban 5 mg BID | Placebo | Relative risk: 0.20 (0.11‐0.34) | Relative risk: 0.25 (0.03‐2.24) | Relative risk: 1.62 (0.96‐2.73) |
| AMPLIFY‐EXT | Apixaban 2.5 mg BID | Placebo | Relative risk: 0.19 (0.11‐0.33) | Relative risk: 0.49 (0.09‐2.64) | Relative risk: 1.20 (0.69‐2.10) |
ASA, acetylsalicylic acid; BID, bis in die, twice daily; CI, confidence interval; CRNMB, clinically relevant non‐major bleeding; DOAC, direct oral anticoagulant; HR, hazard ratio; INR, international normalized ratio; MB, major bleeding; VKA, vitamin‐K antagonist; VTE, venous thromboembolism.
Primary outcome was recurrent VTE and fatal PE except for RE‐SONATE (recurrent VTE or unexplained death) and AMPLIFY‐EXT (recurrent VTE and death from any cause).
2.2. Dabigatran
Dabigatran is the only DOAC to be compared to VKA for extended treatment of VTE. In the RE‐MEDY trial,19 patients deemed to be high risk for recurrent VTE were randomized to dabigatran 150 mg BID vs VKA target INR 2‐3. Rates of recurrent VTE were similar between the dabigatran and VKA groups. Safety outcomes such as major bleeding (MB) and MB or clinically relevant non‐major bleeding (CRNMB) were twice as low in the dabigatran group as in the VKA group (Table 2). Although dabigatran was similarly effective and had few bleeding events compared to VKA, acute coronary syndrome was more common in the dabigatran group than VKA group (0.9% and 0.2%, respectively; P = .02).
Simultaneous to RE‐MEDY was the RE‐SONATE trial, a placebo‐controlled comparison to dabigatran for extended treatment of VTE.19 Not surprisingly, anticoagulation with dabigatran 150 mg BID significantly reduced the rate of recurrence compared to placebo by >90%. Although MB events were similar between the groups, MB/CRNMB events were significantly higher in the anticoagulant arm (Table 2). There were no differences in acute coronary syndrome with one event occurring in each group.
2.3. Rivaroxaban
The EINSTEIN‐EXT Study was the first to compare a DOAC (rivaroxaban) to placebo for extended VTE therapy.20 Standard dose rivaroxaban 20 mg daily was compared to placebo for a median duration of 265 days. As in the RE‐SONATE trial, anticoagulation was superior to placebo for reducing the primary efficacy outcome of recurrent VTE events, with one recurrent event being a fatal PE in the placebo group. MB was the primary safety outcome which occurred only in patients on rivaroxaban and all events were non‐fatal. MB/CRNMB events were significantly higher in the rivaroxaban group than placebo (Table 2).
To determine whether a reduced dose of rivaroxaban 10 mg daily is as effective at lowering risk of recurrent VTE as rivaroxaban 20 mg daily, the EINSTEIN Investigators conducted the EINSTEIN CHOICE trial randomizing patients to rivaroxaban 20 mg daily, 10 mg daily, or ASA 100 mg daily.21 Unlike the EINSTEIN‐EXT study, only a minority of patients in EINSTEIN CHOICE had index VTE that was definitely unprovoked (75% vs 40%, respectively) (Table 1). The primary efficacy outcome, symptomatic recurrent VTE (fatal or non‐fatal PE, and DVT), was significantly lower in both rivaroxaban groups (Table 2). MB was the principal safety outcome and rates were similar between all groups (Table 2). The composite of MB/CRNMB rates were also similar and not statistically significantly different (Table 2). While these results offer reassurance that either standard dose or reduced dose rivaroxaban are more effective and safer than ASA, transferring this data to the clinical realm requires careful patient selection. This trial was not powered to evaluate direct comparison of rivaroxaban 20 mg vs 10 mg. Furthermore, patients with provoked VTE generally have lower risk of recurrent VTE; thus it is unclear if rivaroxaban 10 mg is as effective as rivaroxaban 20 mg in unselected high‐risk patients with unprovoked index VTE.
2.4. Apixaban
AMPLIFY‐EXT randomized patients to three groups: standard‐dose apixaban 5 mg twice daily, reduced‐dose apixaban 2.5 mg twice daily, or placebo22 for 12 months of extended treatment. Although the primary efficacy outcome was a composite of symptomatic recurrent VTE and all‐cause mortality, the secondary outcome of symptomatic recurrent VTE (fatal and non‐fatal PE, and DVT) is reported, for ease of comparison to other DOAC trials. Use of both standard and low‐dose apixaban was statistically significant at reducing rates of recurrent VTE compared to placebo (Table 2). The evaluation of safety with MB and MB/CRNMB rates were similar and not statistically significantly different between treatment groups (Table 2). The AMPLIFY‐EXT trial provides reassurance that low dose and standard dose apixaban reduce the risk of recurrent VTE in high risk patients, without an increased risk of MB; however, it was not powered to directly compare the two doses of apixaban to each other.
2.5. Aspirin
Two trials have evaluated the efficacy of ASA 100 mg daily for secondary prevention of recurrent VTE. The WARFASA23 and ASPIRE24 trials were both placebo‐controlled trials in patients with unprovoked index VTE who had completed anticoagulation treatment. The primary efficacy outcome was symptomatic recurrent VTE (fatal or non‐fatal PE, and DVT) and MB the safety outcome in WARFASA, while ASPIRE used MB/CRNMB composite. In WARFASA the rate of recurrent VTE in the ASA group was nearly half that in the placebo group (Table 2) and MB rates were the same in both groups.
The ASPIRE trial24 was unable to meet its original sample size due to slow recruitment and was reduced, with plans to pool final results with those of WARFASA trial. ASPIRE alone was unable to show a statistically significant benefit to ASA compared to placebo for recurrent VTE reduction. The rate of MB/CRNMB events was similar between groups (Table 2). Pooled data from ASPIRE and WARFASA showed a significant reduction in recurrent VTE24 for ASA use. Pooled estimates for MB/CRNMB showed no increased bleeding risk (Table 2).
While pooled proportions for ASA demonstrated a reduction in VTE recurrence compared to placebo, the EINSTEIN CHOICE trial clearly demonstrated an advantage of standard and low dose anticoagulant over ASA, within limitations of the predominantly provoked VTE population in EINSTEIN CHOICE.
2.6. Edoxaban
Edoxaban is the third direct Factor Xa inhibitor and the last agent approved for treatment of VTE. Like dabigatran, the acute treatment of VTE requires a 5‐day lead in with heparins prior to starting the oral agent.25 Edoxaban has not been evaluated for extended therapy for secondary prevention of VTE. A review of clinicaltrials.gov shows no such pending trials for this DOAC.
3. CONSIDERATIONS FOR CHOOSING ANTICOAGULANTS
The prescription of DOACs continues to increase in North America.26, 27 DOAC use is expected to grow further because, despite the lack of direct comparison trials for extended treatment, the 2016 Chest guidelines suggests use of DOACs for treatment of acute VTE and continuation of the same anticoagulant for prolonged therapy.4 This is a practical suggestion, yet additional factors may need to be considered when deciding which anticoagulant to use.
The DOACs are expensive and this cost can be prohibitive for extended use. These agents will not be available in generic form for several years. Some patients may have private drug insurance plans that reduce the price absorbed by the individual; however, co‐pay fees may remain considerable. In Canada, some provinces provide coverage for DOAC use for qualifying patients during acute VTE treatment up to 6 months. For extended anticoagulation, economic evaluation has not shown benefit of DOACs over VKAs,28 resulting in frequent transition to VKAs for extended prevention.
Patient comorbidities must also be considered when prescribing extended anticoagulation.29 Patients using medications that are strong P‐glycoprotein or CYP3A4 inducers/inhibitors should avoid DOACs due to altered levels of systemic anticoagulant that may impact thrombotic and bleeding risks. Organ dysfunction may also impact anticoagulant choice, in particular for DOACs markedly dependent on renal function for drug clearance such as dabigatran. All DOACs should be avoided in moderate to severe hepatic dysfunction. Gastrointestinal (GI) illness and prior history of GI bleeding may necessitate use of VKAs or apixaban for extended anticoagulation due to the higher associated bleeding risk with dabigatran and rivaroxaban. There is also limited data on the use of DOACs in patients with obesity and body weight >120 kg, or after gastric bypass surgery.30
Lastly, in the patient who agrees to remain on indefinite anticoagulants, patient preference for a DOAC needs to be measured in the correct clinical and financial contexts that weigh value of the risks of recurrent VTE vs bleeding events. Patients who display nonadherence to VKAs are not ideal candidates for DOACs because missed doses of these shorter acting agents lead to greater risk of recurrent thrombosis, compared to a missed dose of the longer half‐life VKAs. Dosing frequency may be of concern for some clinicians and patients, so for those who are poorly compliant with twice‐daily medications, once‐daily rivaroxaban may be the right choice. Education and engagement of patients in their treatment plan will facilitate regular follow‐up visits and medication use. Availability of reversal agents is also expected to allay concerns of bleeding and extended DOAC use.31
4. LIMITATIONS OF DOAC TRIALS FOR EXTENDED VTE TREATMENT
There are several limitations to these trials that do not permit ease of data transfer to clinical practice when determining which patients are high risk and may benefit from secondary prevention. These include heterogeneous patient populations of both provoked and unprovoked index thromboses, selection bias, variable treatment durations, and most importantly, lack of direct comparisons between DOACs. Indirect comparisons via network meta‐analysis show that dabigatran, rivaroxban, and apixaban reduce risk of recurrent VTE by more than 80% and are associated with mild‐moderate bleeding risk.10 These gaps in evidence highlight the decisional dilemmas faced by patients, clinicians, and policy makers.
We recently initiated a clinical trial that is a head‐to‐head comparison of anticoagulants for extended treatment: the COVET Trial (Comparison of Oral Anticoagulants for Extended VEnous Thromboembolism; NCT03196349). This pragmatic trial is a multicenter collaboration between Canadian and U.S. investigators and is sponsored by Patient‐Centered Outcomes Research Institute. COVET will randomize patients with unprovoked index VTE who have completed 3‐12 months of oral anticoagulation to rivaroxaban, apixaban, or warfarin for 12 months of extended treatment (Figure 2). The dosing of anticoagulants is based on currently approved dosages by Health Canada and the Federal Drug Administration for the extended treatment of VTE. Patients are recruited from the outpatient clinic setting and eligibility criteria are less stringent than Phase III RCTs of DOACs (see online appendix). The primary safety outcome is clinically relevant bleeding events and the primary efficacy outcome is recurrent VTE. We anticipate this trial will demonstrate that rivaroxaban and apixaban are safer than and as effective as warfarin.
Figure 2.
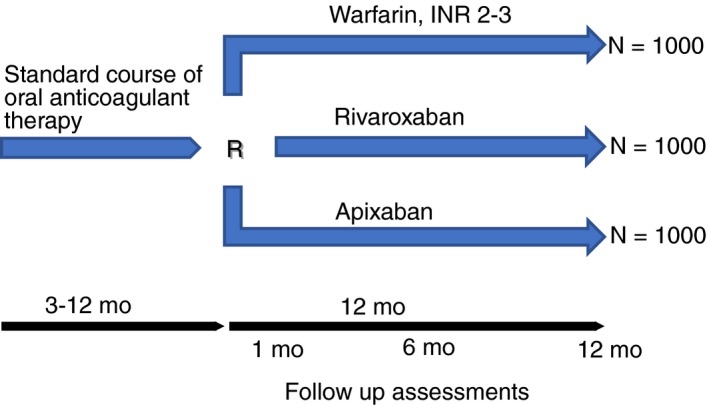
COVET trial study design. INR, international normalized ratio
In summary, while awaiting the results of the head‐to‐head COVET trial for extended treatment of unprovoked VTE, it is essential to remember that anticoagulants are more effective than aspirin, and compared to no treatment, apixaban and rivaroxaban will substantially reduce the risk of recurrent VTE. Future studies, including the COVET trial, will determine whether simplicity and safety of DOACs make them the strongly preferred choice for extended VTE management.
RELATIONSHIP DISCLOSURE
G Le Gal holds a CP Has Heart Cardiovascular Award from the Heart and Stroke Foundation of Ontario, the Facuty of Medicine Department of Medicine Chair on the Diagnosis of Venous Thromboembolism from the Department of Medicine, and an Early Researcher Award from the Province of Ontario. K de Wit is funded by Hamilton Health Sciences Early Career Award and the Physician Services Ontario Knowledge Translation Fellowship. LA Castellucci, D Garcia, and TL Ortel are each partially supported by an award from the Patient‐Centered Outcomes Research Institute (PCORI) (NOACs1511‐33599). The statements in this article are solely the responsibility of the authors and do not necessarily represent the views of the Patient‐Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee.
Supporting information
Castellucci LA, de Wit K, Garcia D, Ortel TL, Le Gal G. Extended anticoagulation for unprovoked venous thromboembolism. Res Pract Thromb Haemost. 2018;2:529–534. 10.1002/rth2.12121
REFERENCES
- 1. White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107:I‐4–8. [DOI] [PubMed] [Google Scholar]
- 2. Heit J. The epidemiology of venous thromboembolism in the community: implications for prevention and management. J Thromb Thrombolysis. 2006;21:23–9. [DOI] [PubMed] [Google Scholar]
- 3. Kyrle PA, Minar E, Bialonczyk C, Hirschl M, Weltermann A, Eichinger S. The risk of recurrent venous thromboembolism in men and women. N Engl J Med. 2004;350:2558–63. [DOI] [PubMed] [Google Scholar]
- 4. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- 5. Baglin T, Luddington R, Brown K, Baglin C. Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet. 2003;362:523–6. [DOI] [PubMed] [Google Scholar]
- 6. Agnelli G, Prandoni P, Santamaria MG, et al.; Warfarin Optimal Duration Italian Trial Investigators . Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. N Engl J Med. 2001;345:165–9. [DOI] [PubMed] [Google Scholar]
- 7. Rodger MA, Scarvelis D, Kahn SR, et al. Long‐term risk of venous thrombosis after stopping anticoagulants for a first unprovoked event: a multi‐national cohort. Thromb Res. 2016;143:152–8. [DOI] [PubMed] [Google Scholar]
- 8. Carrier M, Le Gal G, Wells PS, Rodger MA. Systematic review: case‐fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med. 2010;152:578–89. [DOI] [PubMed] [Google Scholar]
- 9. Kearon C, Iorio A, Palareti G. Risk of recurrent venous thromboembolism after stopping treatment in cohort studies: recommendation for acceptable rates and standardized reporting. J Thromb Haemost. 2010;8:2313–5. [DOI] [PubMed] [Google Scholar]
- 10. Castellucci LA, Cameron C, Le Gal G, et al. Efficacy and safety outcomes of oral anticoagulants and antiplatelet drugs in the secondary prevention of venous thromboembolism: systematic review and network meta‐analysis. BMJ. 2013;347:f5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chai‐Adisaksopha C, Hillis C, Isayama W, Lim W, Iorio A, Crowther M. Mortality outcomes in patients receiving direct oral anticoagulants: a systematic review and meta‐analysis of randomized controlled trials. J Thromb Haemost. 2015;13:2012–20. [DOI] [PubMed] [Google Scholar]
- 12. Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low‐intensity warfarin therapy with conventional‐intensity warfarin therapy for long‐term prevention of recurrent venous thromboembolism. N Engl J Med. 2003;349:631–9. [DOI] [PubMed] [Google Scholar]
- 13. Couturaud F, Sanchez O, Pernod G, et al. Six months vs extended oral anticoagulation after a first episode of pulmonary embolism: the PADIS‐PE Randomized Clinical Trial. JAMA. 2015;314:31–40. [DOI] [PubMed] [Google Scholar]
- 14. Schulman S, Granqvist S, Holmström M, et al.; Duration of Anticoagulation Trial Study Group . The duration of oral anticoagulant therapy after a second episode of venous thromboembolism. N Engl J Med. 1997;336:393–8. [DOI] [PubMed] [Google Scholar]
- 15. Agnelli G, Prandoni P, Becattini C, et al.; Warfarin Optimal Duration Italian Trial Investigators . Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med. 2003;139:19–25. [DOI] [PubMed] [Google Scholar]
- 16. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med. 1999;340:901–7. [DOI] [PubMed] [Google Scholar]
- 17. Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy ‐ Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141:e44S–88S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–73. [DOI] [PubMed] [Google Scholar]
- 19. Schulman S, Kearon C, Kakkar AK, et al.; RE‐MEDY and the RE‐SONATE Trials Investigators . Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368:709–18. [DOI] [PubMed] [Google Scholar]
- 20. Bauersachs R, Berkowitz SD, Brenner B, et al.; EINSTEIN Investigators . Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510. [DOI] [PubMed] [Google Scholar]
- 21. Weitz JI, Lensing AWA, Prins MH, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376:1211–22. [DOI] [PubMed] [Google Scholar]
- 22. Agnelli G, Buller HR, Cohen A, et al.; AMPLIFY‐EXT Investigators . Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368:699–708. [DOI] [PubMed] [Google Scholar]
- 23. Becattini C, Agnelli G, Schenone A, et al.; WARFASA Investigators . Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366:1959–67. [DOI] [PubMed] [Google Scholar]
- 24. Brighton TA, Eikelboom JW, Mann K, et al.; ASPIRE Investigators . Low‐dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367:1979–87. [DOI] [PubMed] [Google Scholar]
- 25. Büller HR, Décousus H, Grosso MA, et al.; Hokusai‐VTE Investigators . Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406–15. [DOI] [PubMed] [Google Scholar]
- 26. Weitz JI, Semchuk W, Turpie AGG, et al. Trends in prescribing oral anticoagulants in Canada, 2008‐2014. Clin Ther. 2015;37:2506–14. [DOI] [PubMed] [Google Scholar]
- 27. Barnes GD, Lucas E, Alexander GC, Goldberger Z. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klarenbach S, Lee K, Boucher M, So H, Manns B, Tonelli M. Direct Oral Anticoagulants for the Treatment of Venous Thromboembolic Events: Economic Evaluation. CADTH Technology Review Ottawa: Canadian Agency for Drugs and Technologies in Health; 2016. [PubMed] [Google Scholar]
- 29. Burnett AE, Mahan CE, Vazquez SR, Oertel LB, Garcia DA, Ansell J. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martin K, Beyer‐Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:1308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yeh CH, Hogg K, Weitz JI. Overview of the new oral anticoagulants: opportunities and challenges. Arterioscler Thromb Vasc Biol. 2015;35:1056–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


