Abstract
Background
A mechanism involved in high on‐aspirin treatment residual platelet reactivity is platelet multidrug resistance protein 4 (MRP4) overexpression. Aspirin enhances platelet MRP4 expression with a PPARα‐dependent mechanism and reduces miR‐21 expression that, in turn, downregulates PPARα expression.
Objective
The aim of our study was to verify the relationship between miR‐21 and MRP4‐PPARα levels induced by aspirin treatment.
Methods
We evaluated the changes in MRP4‐PPARα, mRNA, MRP4 protein, and miR‐21 expression induced by aspirin in: (i) in vitro–treated megakaryoblastic cell line (DAMI), (ii) primary megakaryocytes cultures and derived platelets, (iii) healthy volunteers’ platelets treated with aspirin, and (iv) aspirinated patients (aspirin‐treated patients) and in a control population (control).
Results
We observed an aspirin‐induced reverse relationship between the expression of miR‐21 and PPARα‐MRP4. In DAMI cells the miR‐21 mimic transfection reduces PPARα and MRP4 expression, even if cells were treated with aspirin after transfection. MiR‐21 inhibitor transfection induces PPARα and MRP4 expression that are not enhanced by aspirin treatment. In human megakaryocytes, aspirin treatment lead to a miR‐21 downregulation and a MRP4 upregulation and this trend is confirmed in derived platelets. In aspirin‐treated volunteers, an inverse relationship between miR‐21 and MRP4 platelet expression was found after aspirin treatment. A similar negative relationship was found in aspirin‐treated patients vs the control population.
Conclusion
The results reported in this study provide information that aspirin induces the modulation of platelet miR‐21 expression levels and this modulation can be responsible for MRP4 enhancement in circulating platelets.
Keywords: aspirin, biomarkers, microrna, platelets, thrombosis
Essentials.
Aspirin reduces miR‐21 expression that downregulates PPARα expression.
To evaluate multidrug resistance protein 4 (MRP4), PPARα, and miR‐21 expression, after aspirin treatment.
Inverse relationship between miR‐21 and MRP4 expression in aspirin‐treated platelets.
Platelet miR‐21 may be a useful biomarker to identify patients less sensitive to aspirin action.
1. INTRODUCTION
Multidrug resistance protein (MRP) 4 is a member of the MRP/ATP Binding Cassette subfamily C (ABCC) subfamily of ATP‐binding cassette transporters, which are capable of pumping several molecules including drugs out to the cell.1 MRP4 expression and function have been associated with drug resistance.2 Interestingly, MRP4 plays an important role in platelet function, an impaired cAMP homeostasis and platelet activation were reported in MRP4‐deficent mice.3, 4
MRP4 inhibition showed a reduced platelet function and thrombus formation.5 Recently, we demonstrated that aspirin modulates MRP4 expression through a PPARα‐dependent mechanism6 and that its upregulation is an adaptive response to limit intracellular toxicity.7 Aspirin MRP4‐induced over‐expression correlates with high on‐aspirin treatment residual platelet reactivity, that is due in part to incomplete COX‐1 inhibition, and in part to COX‐1–independent mechanisms.8
The PLATO trial group recently suggested a phenomenon called platelet adaptome, that originating in megakaryocytes given their diversity and ability to respond at the molecular level to pharmacological inhibition.9
Furthermore, Lan et al. demonstrated that aspirin treatment resulted in a reduction of miR‐21 transcription in colorectal cancer cells.10
MicroRNAs (miRNAs) are endogenous ~22‐nucleotide noncoding RNAs that play important roles in the regulation of target genes by recognizing the complementary region in mainly the 3′‐untranslated region and causing transcriptional repression or mRNA degradation. Recently, Kida et al. found that the nuclear receptor PPARα is regulated by miR‐21 in human liver cells.11
As miRNAs are key regulators of gene expression in both healthy and pathological conditions,12, 13, 14 these data prompt us to investigate whether miRNAs play an important role in platelet function.
In this study, we verified if aspirin treatment, both in vitro and in vivo, is involved to regulate PPARα and MRP4 expression.
2. METHODS
2.1. Cell culture
DAMI cell line (human megakaryoblastic culture) was maintained in Roswell Park Memorial Institute (RPMI) supplemented with 10% heat‐inactivated fetal bovine serum, 100 U/mL of penicillin G sodium, 100 μg/mL streptomycin sulphate, and 20 mmol/L l‐glutamine in a humidified atmosphere with 5% CO2 at 37°C.15 The membrane phenotype (CD61+, CD41+, CD42+) of DAMI cell was monitored by fluorescence‐activated cell sorting analysis (data not shown).
Cells were treated with aspirin (50 μmol/L) (SIGMA Chemicals Company, St. Louis, MO) for 48 hours.
2.2. miRNA Transfection in DAMI cells
Lipofectamine RNAiMAX Transfection Reagent (ThermoFisher Scientific, Waltham, MA) was used to transfect mimic hsa‐miR‐21 (miR‐21 analogue), scrambled miRs, antisense locked nucleic acid (LNA) oligonucleotide against miR‐21 and negative control oligonucleotide, in DAMI cells.
Briefly, cells were plated into each well of a 12‐well plate shortly before transfection. Mimic (Qiagen, Valencia, CA) and antisense LNA oligonucleotide were dissolved according to the instructions protocol to achieve a final concentration of 10 μmol/L. Briefly, miRNA analogue and knockdown oligos were used at a final concentration of 40 nmol/L and 3 μL HiPerFect Reagent was added.
A FAM‐labeled negative control LNA oligonucleotide (AM 17012; ThermoFisher Scientific) was transfected in the same conditions to measure transfection efficiency that was estimated as 90%.
Cells, after 24 hours of transfection, were treated with or without aspirin (50 μmol/L) for 48 hours with the same procedure.
2.3. Purification of human hematopoietic progenitor cell
Peripheral blood (PB) was obtained from male donors after written informed consent. Low density mononuclear cells (MNCs) (in average 0.1%) were isolated by Ficoll‐Hypaque (Lympholyte CL5020; Cederlane Lab, Burlington, Canada) density gradient (1.077 g/mL) centrifugation at 600 g for 30 minutes, RT. MiniMACS isolation system (Milteny, Bergisch, Gladbach, Germany) was used to purify CD34+ cells according to the manufacturer's instructions. Purified cells were more than 90% CD34+ (as evaluated by cytofluorimeter, Epics Profile) and were cultured in serum free (FCS−) liquid culture with addition of 100 ng/mL thrombopoietin (TPO) to induce selective megakaryocytic cell differentiation,16 alone or in combination with 50 μmol/L aspirin. Aspirin 50 μmol/L was daily added from day 6 of culture for the following 4 days.6
Cultures were maintained for 14 days at 37°C in a fully humidified atmosphere of 5% CO2, 5% O2, and 90% N2. megakaryocytes were collected, counted, and analyzed for viability, morphology, and phenotype at different days of culture (6, 9, 12, and 14) in order to control the differentiation stage.16 At day 14 megakaryocytes collected were processed for RNA extraction. Platelet‐like particles were obtained by centrifugation at 800 g for 10 minutes at room temperature and processed for RNA extraction.
2.4. Patients
To evaluated platelet miR‐21 expression after aspirin treatment, we enrolled:
Ten healthy Caucasian volunteers taking aspirin 300 mg/d for 15 days and we performed analysis after 1 day of pill treatment (T1), and subsequently after 7 (T7) and 15 days (T15). These volunteers were previously analyzed for MRP4 expression.6
Ten healthy Caucasian volunteers taking aspirin 100 mg/d for 8 weeks and we performed analysis after 1 day of pill treatment with aspirin (T1) and after 4 and 8 weeks of treatment (respectively T4 and T8). These volunteers were previously analyzed for MRP4 expression.8
Volunteers are subjects obtained from our transfusion center who had no risk factors and had no taken any medications.
To evaluate platelet MRP4 and miR‐21 expression after chronic aspirin treatment, we enrolled:
Twenty‐five aspirin free patients (control population);
Twenty‐five aspirin chronic treated patients (100 mg/d), aspirin exposure >2 months (aspirin‐treated patients >2 months).
In patients under chronic treatment, the compliance was assessed both by interview and as arachidonic acid‐induced platelet aggregation (0.75 mmol/L) < 10%.17
In our study, were excluded all those patients with cardiovascular diseases including: Ischemic Heart Disease (IHD), cerebrovascular disease or transient ischemic attack, established peripheral vascular disease, and class III to IV heart failure, in the previous 15 days.
Informed written consent was obtained from each healthy volunteer and patient.
All clinical parameters of the study population are presented in Table 1. The study was approved by the Hospital Ethics Committee.
Table 1.
Demographics and clinical characteristics of patients treated with aspirin more than 2 months (aspirin‐treated patients >2 months) and aspirin‐free control population (control)
| Characteristics | Baseline characteristics of patients | P value: aspirin‐treated patients >2 months vs control | |
|---|---|---|---|
| Aspirin‐treated patients >2 months (N = 25) | Control (N = 25) | ||
| Age (mean±SD, years) | 69 ± 10 | 65 ± 13 | .56 |
| Males, n (%) | 10 (40) | 9 (36) | .77 |
| Clinical conditions, n (%) | |||
| Hypertension | 10 (40) | 4 (15) | .06 |
| Hypercholesterolaemia | 6 (24) | 5 (20) | .73 |
| Diabetes | 0 (0) | 0 (0) | >.9 |
| Smoke | 4 (15) | 5 (20) | .71 |
| Previous MI | 0 (0) | 0 (0) | >.9 |
| Previous stroke | 4 (15) | 0 (0) | .04 |
| Previous TIA | 4 (15) | 3 (12) | .68 |
| Medications, n (%) | |||
| Beta‐blocking agents | 5 (20) | 3 (12) | .44 |
| Calcium antagonist | 3 (12) | 2 (8) | .63 |
| ACE‐inhibitors | 5 (20) | 2 (8) | .22 |
| ARBs/angiotensin 2 | 5 (20) | 2 (8) | .22 |
| Nitrates | 2 (8) | 1 (3) | .55 |
| Statin | 5 (20) | 1 (3) | .08 |
| Diuretics | 1 (3) | 1 (3) | >.9 |
| Insulin/Metformin | 0 (0) | 0 (0) | >.9 |
MI, myocardial infarction; TIA, transient ischemic attack.
2.5. Platelets preparation and isolation
Platelet preparation was performed according to Pulcinelli et al.18 Platelet pellet was resuspended with Tyrode buffer (containing 134 mmol/L NaCl, 12 mmol/L NaHCO3, 2.9 mmol/L KCl, 5 mmol/L Glucose, 20 mmol/L Hepes, 1 mmol/L MgCl2, 0.34 mmol/L Na2HPO4, pH 7.3) with addition of EDTA (5 mmol/L), and then filtered on a 5 m syringe‐adaptable filter to remove contamination of white blood cell.6 After centrifugation at 800 g for 15 minutes a platelet pellet without contaminants was obtained, that was stored at −80°C.
To show that leukocytes contamination was not significantly detectable, we analyzed the mature transcript for CXCL8, a leukocyte‐specific gene product.19
2.6. RNA preparation and RT‐Q‐PCR analysis for MRP4 and PPARα evaluation
Total RNA, from human cells and platelets, was extracted by Eurogold RNA Pure reagent (EuroClone, Milan, Italy). To detect mRNA, total RNA (1 μg) was transcribed by Gene Amp Gold RNA PCR Reagent Kit pAW109 (ThermoFisher Scientific) according to the manufacturer's suggestion.
The analysis of gene expression was performed according to Massimi et al.6 MRP4, PPARα, CXCL8, and ACTIN mRNA amounts were quantified by using ΔΔCt method using SDS software version 2.3 (Life Technologies, Warrington, UK)
2.7. RT‐Q‐PCR for miRNA analysis
For the detection of mature miR‐21, it has been made a reverse‐transcriptase reaction in which 10 ng of total RNA were reversely transcribed using the High Capacity cDNA Archive Kit (ThermoFisher Scientific).
Real‐Time PCR was performed using a miRNA‐specific Taq‐Man Assay (ThermoFisher Scientific) in an Applied Biosystems 7900HT Sequence Detection System (Life Technologies). U6 snRNA was used for normalization. The program of amplification used was the same as for MRP4 analysis.
2.8. Protein extraction and Western blot
To analyze the MRP4 protein, platelets were washed twice with platelet washing buffer, collected and centrifuged at 7000 g for 3 minutes.
Platelet and cell pellets were then resuspended in lysis buffer and protein extracts were analyzed by Western Blot.6
The National Institutes of Health ImageG analyzer program was performed for densitometric analysis.
2.9. Statistics
Data are presented as mean ± SD. The level of significance was determined by paired, 2‐tailed Student's t test and, for differences among groups, by using one‐way ANOVA and Bonferroni post‐hoc test (Software 3.6 of KaleidaGraph, Synergy Software, Reading, PA, USA).
Results were considered statistically significant when P value was less than .05.
3. RESULTS
3.1. Aspirin‐dependent mir‐21 downregulation is associated with MRP4 and PPARα upregulation in DAMI culture
Aspirin dependent miR‐21 expression was investigated in in vitro experiments. To verify if miR‐21 was regulated after aspirin treatment, we treated DAMI cells with aspirin 50 μmol/L for 48 hours, a concentration and a time required to upregulate MRP4 expression through a mechanism PPARα dependent, as previously reported.6
Real‐time PCR analysis showed that, in cells treated with aspirin, the expression of miR‐21 was inhibited by 46% compared to control cells (Figure 1A). This miR‐21 downregulation was associated with a MRP4 and PPARα mRNA upregulation (Figure 1B,C).
Figure 1.
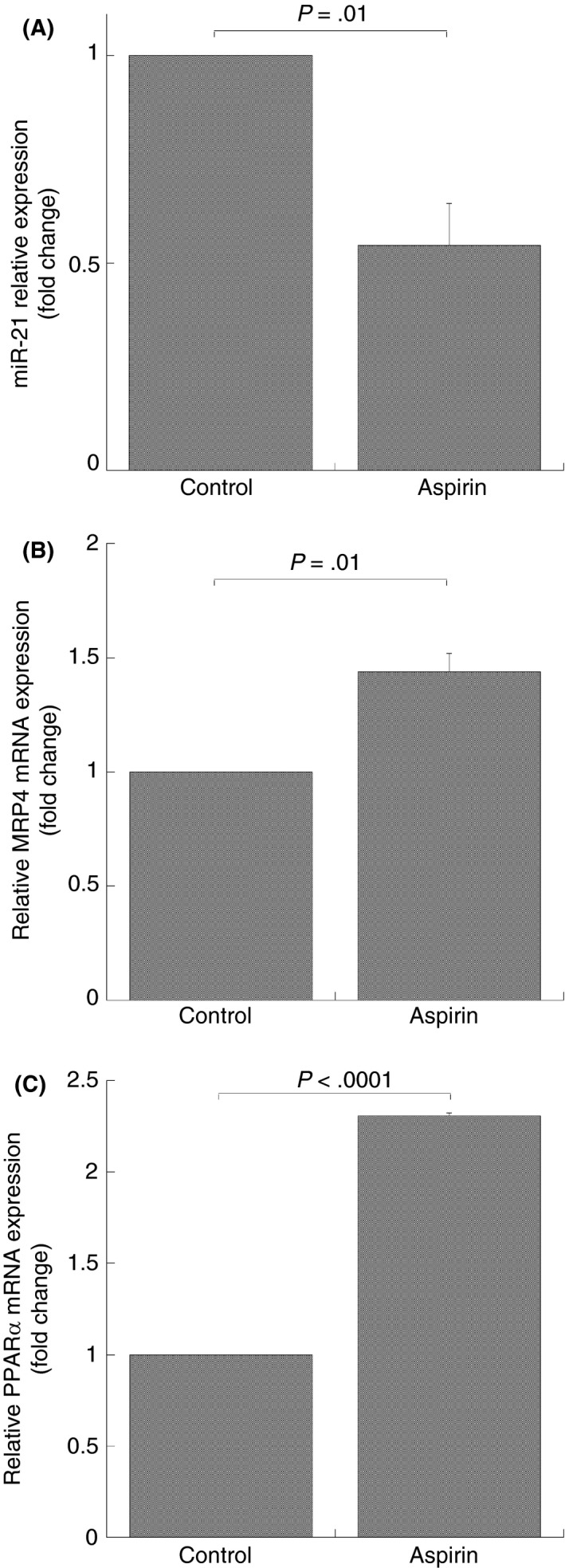
MiR‐21 and mRNA expression in aspirin‐treated DAMI cells. Q‐RT‐PCR analysis, miR‐21 (A), MRP4 (B) and PPARα (C) mRNA expression after 50 mol/L aspirin treatment for 48 hours. MiRNA and mRNA levels were quantified by using the ∆∆Ct method. MiR‐21 and MRP4‐PPARα are normalized with U6b small nuclear RNA (snRNA) and ACTIN expression, respectively. Histograms represent the mean values of fold increase or decrease for three independent experiments (±SD); statistical differences were evaluated by Student's t test for paired samples
3.2. MiR‐21 downregulates PPARα and MRP4 in DAMI cells
In order to establish if miR‐21 downregulates PPARα and MRP4 expression levels, we performed miR‐21 analogue transfection in the DAMI cell line. After verifying whether miR‐21 expression was upregulated in transfected cells compared to cells transfected with scrambled miRs (Figure 2A), we analyzed MRP4 and PPARα mRNA expression. Cells transfected with miR‐21 analogue exhibited a significant downregulation of MRP4 and PPARα mRNA expression compared to cells transfected with scrambled miRs (inhibition: 39 ± 7% and 56 ± 20%, respectively) (Figure 2B,C). Overall, these data suggest a transcriptional inhibition of PPARα and MRP4 mRNA by miR‐21.
Figure 2.
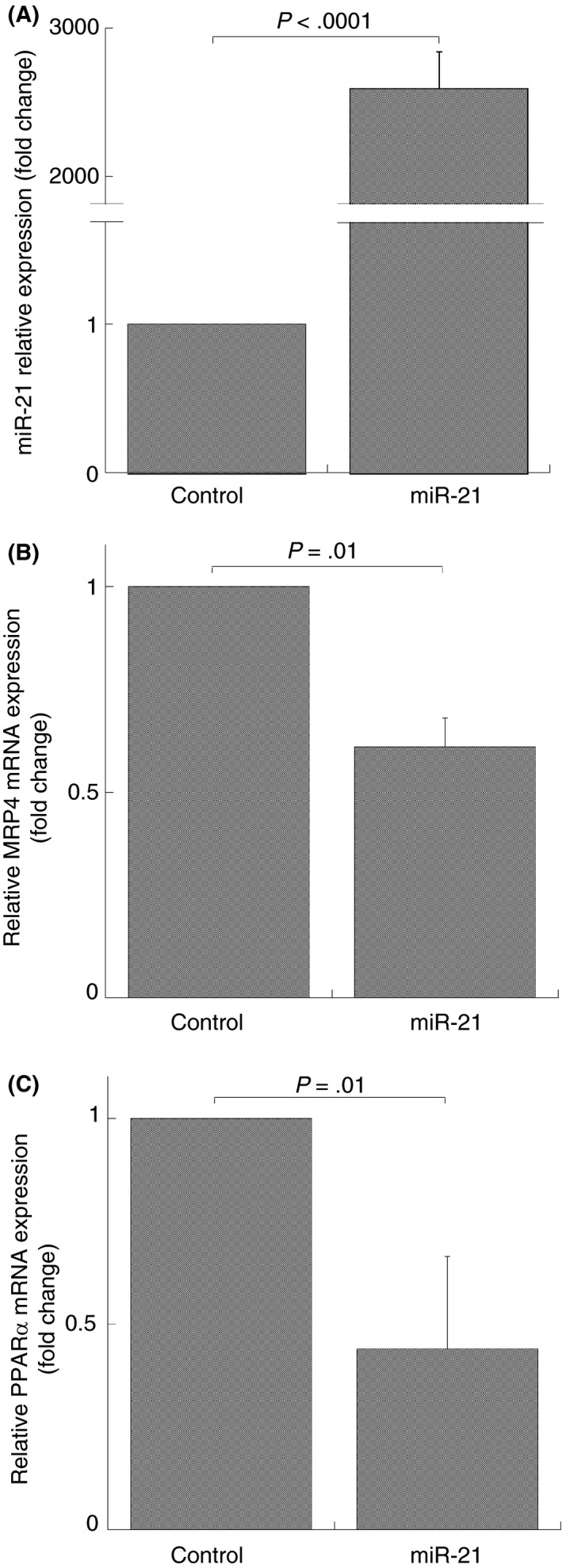
MiR‐21 and mRNA expression in DAMI cells transfected by miR‐21 analogue. Q‐RT‐PCR analysis, miR‐21 (A), MRP4 (B) and PPARα (C) mRNA expression after 40 nmol/L mimic hsa‐miR‐21 transfection (miR‐21) for 48 hours. MiRNA and mRNA levels were quantified by using the ∆∆Ct method. MiR‐21 and MRP4‐PPARα are normalized with U6b small nuclear RNA (snRNA) and ACTIN expression, respectively. Histograms represent the mean values of fold increase or decrease for three independent experiments (±SD); statistical differences were evaluated by Student's t test for paired samples
In order to establish whether miR‐21 over‐expression downregulates aspirin‐dependent PPARα and MRP4 overexpression in DAMI cells, we performed miR‐21 analogue transfection in DAMI cells for 72 hours. After 24 hours from the transfection, cells were treated with aspirin (50 μmol/L) for 48 hours.
As reported in Figure 3A, cells transfected with miR‐21 analogue showed miR‐21 over‐expression compared to those transfected with scrambled miRs and untransfected cells (data not shown).
Figure 3.
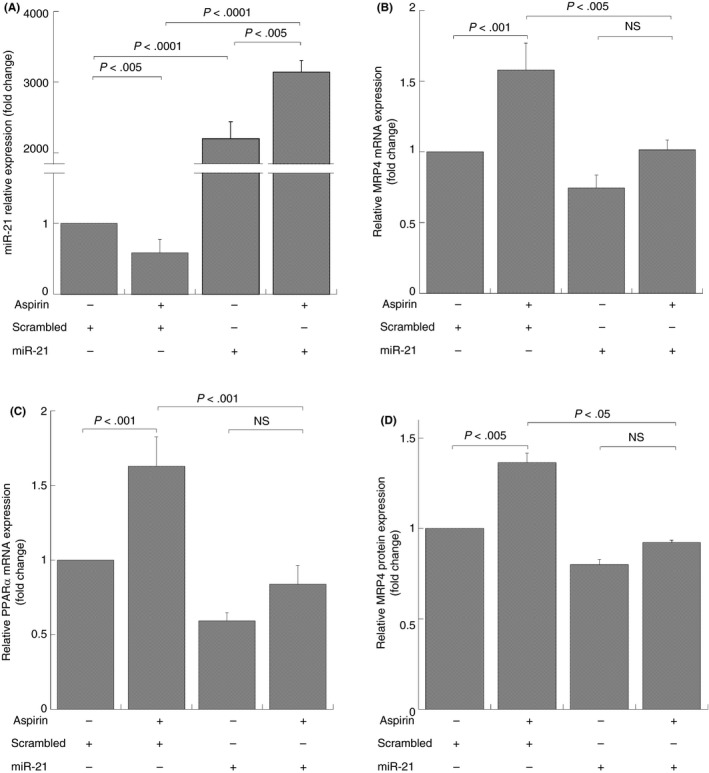
MiR‐21 and MRP4/PPARα expression in DAMI cells transfected by miR‐21 analogue and treated with aspirin. Q‐RT‐PCR and Western Blot analysis, miR‐21 (A), MRP4 (B), and PPARα (C) mRNA and MRP4 protein (D) expression after 40 nmol/L mimic hsa‐miR‐21 transfection (miR‐21) and scrambled miRs (scrambled) for 72 hours and simultaneously treated with aspirin for 48 hours. MiRNA and mRNA levels were quantified by using the ∆∆Ct method. MiR‐21 and MRP4‐PPARα are normalized with U6b small nuclear RNA (snRNA) and ACTIN expression, respectively. Histograms represent the mean values of fold increase or decrease for three independent experiments (±SD). Statistical differences were evaluated by one‐way ANOVA test and Bonferroni post‐hoc test (NS: not significant)
MiR‐21 transfection failed the enhance of aspirin dependent PPARα and MRP4 overexpression, compared to cells transfected with scrambled miRs that show an increase of MRP4 and PPARα mRNA expression after aspirin treatment (Figure 3B,C).
To further confirm such correlation we also analyzed MRP4 protein expression through Western blot. Figure 3D show densitometric analysis of platelet MRP4 and point out a MRP4 protein reduction in cells transfected with miR‐21 analogue compared to cells transfected with scrambled miRs. Furthermore, in cells transfected with scrambled miRs, aspirin induced MRP4 protein overexpression, whereas in cells transfected with miR‐21 analogue aspirin failed to increase expression.
Overall, these data strongly suggest a transcriptional inhibition of MRP4 and PPARα mRNA by miR‐21, and confirme the role of miR‐21 in aspirin dependent genes expression.
To confirm these data, we transfected cells with a miR‐21 inhibitor. After verifying that miR‐21 expression is significantly downregulated in cells transfected with antisense LNA oligonucleotide against miR‐21 compared to those transfected with negative control (neg control) (Figure 4A) and untransfected cells (data not shown), we analyzed MRP4 and PPARα mRNA expression. We have shown that, in cells transfected with miR‐21 inhibitor, MRP4 and PPARα, mRNAs expression were induced, and this expression was not enhanced by aspirin treatment (Figure 4B,C), providing an indication that the inhibition of miR‐21 and aspirin treatment don't result in a synergic effect for the gene expression, but miR‐21 exerts a specific transcriptional suppression of these genes.
Figure 4.
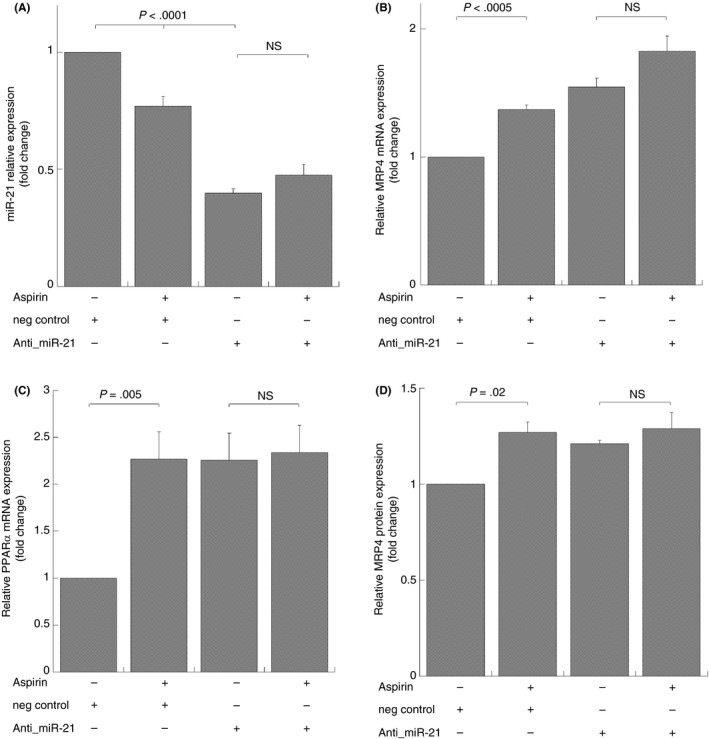
MiR‐21 and MRP4/PPARα expression in DAMI cells transfected by antisense LNA oligonucleotide against miR‐21 and treated with aspirin. Q‐RT‐PCR and Western blot analysis, miR‐21 (A), MRP4 (B), and PPARα (C), mRNA expression and MRP4 protein (D) after 40 nmol/L antisense LNA oligonucleotide against miR‐21 (anti‐miR‐21) and negative control oligonucleotide (neg control) for 72 hours and simultaneously treated with aspirin for 48 hours. MiR‐21 and MRP4‐PPARα are normalized with U6b small nuclear RNA (snRNA) and ACTIN expression, respectively. Histograms represent the mean values of fold increase or decrease for three independent experiments (±SD). Statistical differences were evaluated by one‐way ANOVA test and Bonferroni post‐hoc test (NS: not significant)
To further confirm such correlation we also studied MRP4 protein expression, Figure 4D shows densitometric analysis of platelet MRP4 and confirms data previously shown. MiR‐21 inhibition is sufficient to induce PPARα and, consequently, MRP4 expression.
3.3. MiR‐21 is modulated by aspirin in human megakaryocytes
In order to demonstrate that aspirin modulates miR‐21 expression in human megakaryocytes and that there is a correspondence with MRP4 upregulation, we studied the aspirin effect in human peripheral blood progenitor cell cultures during maturation along the megakaryocytes lineage. In our previous studies we optimized an in vitro unlineage serum‐free liquid culture system for a virtually “pure” and complete megakaryocytes differentiation, including pro‐platelets and platelet‐like particles’ formation.16 Aspirin (50 mol/L) was added at day 6 to differentiating megakaryocytes (when most cells are precursors, see Guerriero et al.16) and this treatment was continued for 4 days.6 As shown in Figure 5 a statistically significant reduction of miR‐21 expression was evident both in differentiating megakaryocytes treated with aspirin and collected at day 12 (inhibition: 76 ± 5%) (Figure 5A) and in derived platelet‐like particles on day 14 of differentiation (inhibition: 30 ± 3%) (Figure 5C).
Figure 5.
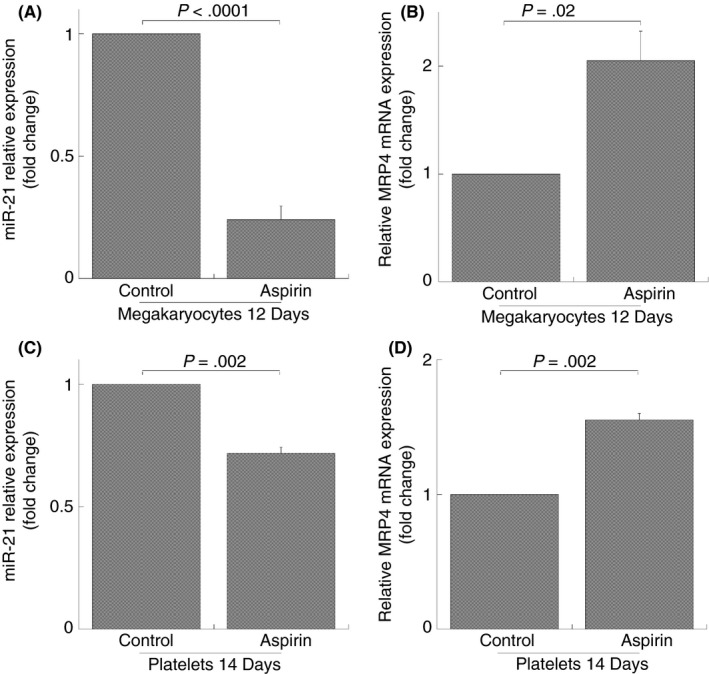
MiR‐21 and MRP4 expression in megakaryocytes obtained from peripheral HPCs and in derived platelets (platelet‐like particles). Q‐RT‐PCR analysis, miR‐21 (A, C), MRP4 mRNA (B, D) expression after 50 mol/L aspirin treatment. MiR‐21 and MRP4 are normalized with U6b small nuclear RNA (snRNA) and ACTIN expression, respectively. Histograms represent the mean values of fold increase or decrease for three independent experiments (±SD). Student's t test for paired samples were evaluated for statistical differences (NS: not significant)
Interestingly MRP4 mRNA is increasingly expressed in both aspirin‐treated megakaryocytes during days 12 of maturation (2.0 ± 0.3 fold increase) (Figure 5B), and in the newly formed platelets (1.6 ± 0.05 fold increase) (Figure 5D) as compared with control cultures.
These data confirm that miR‐21 enhancement and MRP4 decrease aspirin dependent is evident in human megakaryocytes and in derived platelets obtained at the end of megakaryocytes culture.
3.4. MiR‐21 downregulates MRP4 in human platelets
To investigate whether aspirin could regulate miR‐21 expression even after in vivo administration and to verify the correlation between MRP4 and miR‐21, we explored miR‐21 expression in platelets obtained from volunteers after aspirin treatment which have aspirin induced MRP4 over‐expression, as previously shown.6, 8
In 300 mg/d aspirin‐treated volunteers that show MRP4 upregulation after 15 days of treatment, miR‐21 expression is downregulated in a statistically significant manner after 7 and 15 days (inhibition: 45 ± 17% and 47 ± 19%, respectively) (Figure 6A).
Figure 6.
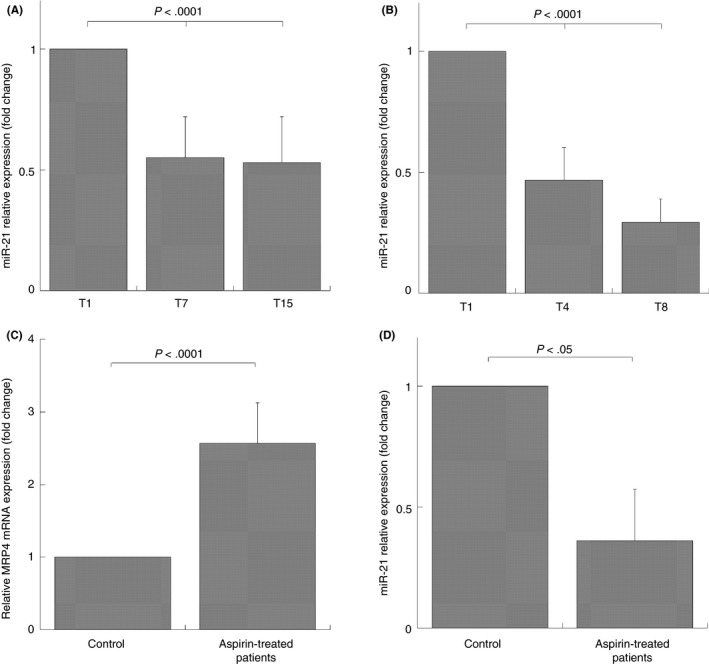
MiR‐21 expression in platelets of healthy volunteers, aspirin‐free control population, and patients under chronic aspirin treatment. Q‐RT‐PCR analysis, miR‐21 expression in platelets of healthy volunteers (N = 10) taking aspirin 300 mg/d (A) for 1 (T1), for 7 (T7), and 15 (T15) days and taking aspirin 100 mg/d (B) for 1 day (T1), for 4 (T4), and 8 (T8) weeks. MRP4 (C) and miR‐21 expression (D) in aspirin free control population (control; N = 25), patients under chronic treatment (aspirin‐treated patients; N = 25). Data were normalized with U6b small nuclear RNA (snRNA) and ACTIN expression for miR‐21 and MRP4, respectively. Data are reported as mean of fold increase or decrease ± SD; statistical differences were evaluated by one‐way ANOVA test and Bonferroni post‐hoc test (A, B) and evaluated by Student's t test for paired samples (C, D)
This reverse relationship between miR‐21 and MRP4 expression was also found in platelets of volunteers after 100 mg/d aspirin administration who exhibited a significant MRP4 upregulation after 8 weeks and miR‐21 downregulation (inhibition: 71 ± 23%), that is statistically significant also after 4 weeks from the start of treatment (inhibition: 54 ± 25%) (Figure 6B).
A similar reverse relationship was found in aspirin‐treated patients, since they had a significant MRP4 increase (2.07 ± 0.22 fold increase) and a significant miR‐21 decrease (inhibition: 49 ± 18%), compared to the control population (Figure 6C,D).
4. CONCLUSION
Previously, we demonstrated that platelet MRP4 overexpression reduces aspirin‐induced COX‐1 inhibition for its ability to extrude this drug from platelets.20 We also demonstrated that both in vitro and in vivo aspirin treatment induces MRP4 upregulation through the activation of nuclear receptor PPARα.6
In this work, we demonstrate that the reduction of miR‐21 expression is involved in aspirin‐dependent PPARα and MRP4 regulation in megakaryoblastic cell line (DAMI) and in human cultured megakaryocytes.
In fact, we show that miR‐21 is dysregulated in DAMI cells after aspirin treatment. By transfecting DAMI cells with a synthetic miRNA, with the same sequence of miR‐21, we found a significant reduction of PPARα and MRP4 expression. Furthermore, miR‐21 transfection fully blocked aspirin‐induced upregulation of PPARα and MRP4.
In vitro regulation of miR‐21 by aspirin was previously demonstrated in epithelial cancer cells.10 It was demonstrated that the nuclear receptors PPARα is downregulated by miR‐21, in in vitro cells of human liver.11
For the first time, we demonstrated that a reduction of miR‐21 expression levels results in an aspirin dependent increased expression of PPARα and MRP4.
We observed the same effect of aspirin on miR‐21 decrease and MRP4 increase in megakaryocytes cultures; in fact, in vitro aspirin treatment induced MRP4 expression and decreased miR‐21 expression in megakaryocytes and the same regulation was found in platelet‐like particles recovered at the end of the cultures. These data suggest that miR‐21 and MRP4 expression were affected by aspirin in megakaryocytes and transferred into the derived platelet‐like particles.
The transcriptional effect on megakaryocytes, responsible for platelet gene expression adaptome, was previously showed, in fact transcriptional change that can be transmitted to circulating platelets is evident for PPARα activation in human bone marrow megakaryocytes.6, 21
The ability of aspirin to modulate miR‐21 and MRP4 expression in megakaryocytes and to transfer this modulation to platelets was confirmed in our in vivo studies, in fact in platelets obtained from aspirin‐treated volunteers, we found an inverse correlation between miR‐21 and MRP4 expression. This correlation was evident after 7 and 15 days from the start of treatment with 300 mg/d aspirin and after 8 weeks with a lower aspirin dosage (100 mg/d). This work is a further confirm that MRP4 expression is regulated by a PPARα‐dependent mechanism.
Moreover, we report that patients under chronic aspirin treatment express low miR‐21 levels compared to a control population. It is now established that mRNA transcripts expression are the results of a transcriptional and posttranscriptional regulation by miRNAs.22, 23 Therefore, multiple evidences show that megakaryocytes transfer mRNA and miRNA to circulating platelets and that this transferred genetic code changes in different diseases.24
On the basis of our study, we can strongly suggest that the reduction of miR‐21 in platelets is dependent on aspirin action in megakaryocytes and subsequently transferred to the platelets, as previously demonstrated.6
Platelets from patients under chronic aspirin treatment with high levels of MRP4 have increased rates of high on‐aspirin treatment residual platelet reactivity. Moreover, platelets from patients with osteoarthritis, who are regular NSAID users, have an increased platelet reactivity due to an higher expression of MRP4.25
Importantly, emerging studies support an association between high on‐aspirin treatment residual platelet reactivity and adverse clinical outcomes in patients with cardiovascular and cerebrovascular disease.26, 27, 28, 29
A recent study has shown that targeted inhibition of miR‐21 maturation with designed RNA‐binding proteins is very important useful in the oncogenic treatment of tumors,30 suggesting that this treatment can also be translated in other situations.
In conclusion, in our study, we have demonstrated that miR‐21 downregulation plays an important role in the modulation of MRP4 over‐expression and that its enhancement can abolish aspirin‐induced MRP4 over‐expression.
Evidence showed in this study, provides information that aspirin induces the modulation of platelet miR‐21 expression levels and this modulation can be responsible of the MRP4 enhancement in circulating platelets. This may open a new finding to identify patients with high on‐aspirin treatment residual platelet reactivity, with high thrombotic risks.
RELATIONSHIP DISCLOSURES
Isabella Massimi, Laura Alemanno, Maria Luisa Guarino, Raffaella Guerriero, Luigi Frati, Luigi Biasucci, and Fabio M. Pulcinelli declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
I. Massimi designed the study, performed experiments, analyzed results, and wrote the manuscript. L. Alemanno and M.L. Guarino contributed to the study design, data collection, writing, and patient enrollment, R. Guerriero contributed to the study design, data collection, writing, and performed experiments, L. Biasucci and L. Frati contributed to the study design, interpreted data and revision of the intellectual content of the manuscript. F.M. Pulcinelli was responsible for the concept of the study, designed the study, analyzed results, and wrote the manuscript.
Massimi I, Alemanno L, Guarino ML, et al. MiR‐21 role in aspirin‐dependent PPARα and multidrug resistance protein 4 upregulation. Res Pract Thromb Haemost. 2018;2:596–606. 10.1002/rth2.12104
REFERENCES
- 1. Russel FG, Koenderink JB, Masereeuw R. Multidrug resistance protein 4 (MRP4/ABCC4): a versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol Sci. 2008;29:200–7. [DOI] [PubMed] [Google Scholar]
- 2. Schuetz JD, Connelly MC, Sun D, et al. MRP4: a previously unidentified factor in resistance to nucleoside‐based antiviral drugs. Nat Med. 1999;5:1048–51. [DOI] [PubMed] [Google Scholar]
- 3. Decouture B, Dreano E, Belleville‐Rolland T, et al. Impaired platelet activation and cAMP homeostasis in MRP4‐deficient mice. Blood. 2015;126:1823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheepala SB, Pitre A, Fukuda Y, et al. The ABCC4 membrane transporter modulates platelet aggregation. Blood. 2015;126:2307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lien LM, Chen ZC, Chung CL, et al. Multidrug resistance protein 4 (MRP4/ABCC4) regulates thrombus formation in vitro and in vivo. Eur J Pharmacol. 2014;737:159–67. [DOI] [PubMed] [Google Scholar]
- 6. Massimi I, Guerriero R, Lotti LV, et al. Aspirin influences megakaryocytic gene expression leading to upregulation of multidrug resistance protein‐4 in human platelets. Br J Clin Pharmacol. 2014;78:1343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Massimi I, Ciuffetta A, Temperilli F, et al. Multidrug resistance protein‐4 influences aspirin toxicity in human cell line. Mediators Inflamm. 2015;2015:607957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Massimi I, Lotti LV, Temperilli F, et al. Enhanced platelet MRP4 expression and correlation with platelet function in patients under chronic aspirin treatment. Thromb Haemost. 2016;116:1100–10. [DOI] [PubMed] [Google Scholar]
- 9. Lowenstern A, Storey RF, Neely M, et al.; PLATO Investigators . Platelet‐related biomarkers and their response to inhibition with aspirin and p2y12‐receptor antagonists in patients with acute coronary syndrome. J Thromb Thrombolysis. 2017;44:145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lan F, Yue X, Han L, et al. Genome‐wide identification of TCF7L2/TCF4 target miRNAs reveals a role for miR‐21 in Wnt‐driven epithelial cancer. Int J Oncol. 2012;40:519–626. [DOI] [PubMed] [Google Scholar]
- 11. Kida K, Nakajima M, Mohri T, et al. PPARalpha is regulated by miR‐21 and miR‐27b in human liver. Pharm Res. 2011;28:2467–76. [DOI] [PubMed] [Google Scholar]
- 12. Ozsolak F, Poling LL, Wang Z, et al. Chromatin structure analyses identify miRNA promoters. Genes Dev. 2008;22:3172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. [DOI] [PubMed] [Google Scholar]
- 14. Cui Q, Yu Z, Purisima EO, Wang E. MicroRNA regulation and interspecific variation of gene expression. Trends Genet. 2007;23:372–5. [DOI] [PubMed] [Google Scholar]
- 15. Greenberg SM, Rosenthal DS, Greeley TA, Tantravahi R, Handin RI. Characterization of a new megakaryocytic cell line: the Dami cell. Blood. 1988;72:1968–77. [PubMed] [Google Scholar]
- 16. Guerriero R, Testa U, Gabbianelli M, et al. Unilineage megakaryocytic proliferation and differentiation of purified hematopoietic progenitors in serum‐free liquid culture. Blood. 1995;86:3725–36. [PubMed] [Google Scholar]
- 17. Temperilli F, Rina A, Massimi I, et al. Arachidonic acid‐stimulated platelet tests: identification of patients less sensitive to aspirin treatment. Platelets. 2015;26:783–7. [DOI] [PubMed] [Google Scholar]
- 18. Pulcinelli FM, Gresele P, Bonuglia M, Gazzaniga PP. Evidence for separate effects of U73122 on phospholipase C and calcium channels in human platelets. Biochem Pharmacol. 1998;56:1481–4. [DOI] [PubMed] [Google Scholar]
- 19. Lindemann SW, Yost CC, Denis MM, McIntyre TM, Weyrich AS, Zimmerman GA. Neutrophils alter the inflammatory milieu by signal‐dependent translation of constitutive messenger RNAs. Proc Natl Acad Sci USA. 2004;101:7076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mattiello T, Guerriero R, Lotti LV, et al. Aspirin extrusion from human platelets through multidrug resistance protein‐4‐mediated transport: evidence of a reduced drug action in patients after coronary artery bypass grafting. J Am Coll Cardiol. 2011;58:752–61. [DOI] [PubMed] [Google Scholar]
- 21. Hashizume S, Akaike M, Azuma H, et al. Activation of peroxisome proliferator‐activated receptor alpha in megakaryocytes reduces platelet‐derived growth factor‐BB in platelets. J Atheroscler Thromb. 2011;18:138–47. [DOI] [PubMed] [Google Scholar]
- 22. Krek A, Grun D, Poy MN, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. [DOI] [PubMed] [Google Scholar]
- 23. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. [DOI] [PubMed] [Google Scholar]
- 24. Rondina MT, Weyrich AS. Regulation of the genetic code in megakaryocytes and platelets. J Thromb Haemost. 2015;13(Suppl 1):S26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Temperilli F, Di Franco M, Massimi I, et al. Nonsteroidal anti‐inflammatory drugs in‐vitro and in‐vivo treatment and multidrug resistance protein 4 expression in human platelets. Vascul Pharmacol. 2016;76:11–7. [DOI] [PubMed] [Google Scholar]
- 26. Frelinger AL 3rd, Li Y, Linden MD, et al. Association of cyclooxygenase‐1‐dependent and ‐independent platelet function assays with adverse clinical outcomes in aspirin‐treated patients presenting for cardiac catheterization. Circulation. 2009;120:2586–96. [DOI] [PubMed] [Google Scholar]
- 27. Mayer K, Bernlochner I, Braun S, et al. Aspirin treatment and outcomes after percutaneous coronary intervention: results of the ISAR‐ASPI registry. J Am Coll Cardiol. 2014;64:863–71. [DOI] [PubMed] [Google Scholar]
- 28. Gori AM, Grifoni E, Valenti R, et al. High on‐aspirin platelet reactivity predicts cardiac death in acute coronary syndrome patients undergoing PCI. Eur J Intern Med. 2016;30:49–54. [DOI] [PubMed] [Google Scholar]
- 29. Fiolaki A, Katsanos AH, Kyritsis AP, et al. High on treatment platelet reactivity to aspirin and clopidogrel in ischemic stroke: a systematic review and meta‐analysis. J Neurol Sci. 2017;376:112–6. [DOI] [PubMed] [Google Scholar]
- 30. Chen Y, Yang F, Zubovic L, et al. Targeted inhibition of oncogenic miR‐21 maturation with designed RNA‐binding proteins. Nat Chem Biol. 2016;12:717–23. [DOI] [PMC free article] [PubMed] [Google Scholar]


