Abstract
Physical activity in community-living individuals after a stroke is usually scarce. This protocol describes a study that will evaluate a method to increase physical activity by performing a 3-month outdoor walking and muscle strengthening program and will examine the 3-month and 1-year effects of this program on individuals with acute stroke (AS) or transient ischemic attack (TIA). In a prospective randomized controlled trial in Uppsala, Sweden, 80 individuals with AS or TIA who maintained cognitive and motor function will be randomized into groups for continuous training for three months or for regular standard care. The training will be supervised by daily cellphone-delivered messages (short message services; SMS), and the intensity, duration and workload will be gradually increased. The primary outcome is a change in walking capacity according to the 6-Minute Walk Test and chair-rising at three months. Secondary outcomes include mobility, gait speed, handgrip strength, body composition (fat mass and muscle mass), biochemical risk-markers, health-related quality of life, and cardiovascular events. Adherence to the training program will be documented with a self-reported diary and step counts over two weeks. The major study started in November 2016, and results are expected in 2019. In a pilot study of 15 subjects post-stroke (mean-age 65 years), we observed improved walking capacity (increasing from 23 to 255 m) and chair-rising (decreasing 2.42 s) from baseline to three months. SMS-guided outdoor training will be tested as a potential therapeutic strategy to increase physical activity and thereby improve walking capacity and physical function following a stroke.
Keywords: Acute stroke, Transient ischemic attack, Physical activity, Walking capacity, Mobility, Body composition
Highlights
-
•
In contrast to many post-stroke exercise studies this study focus on the close post-stroke period.
-
•
The use of SMS and cellphones to communicate might improve the motivation to exercise.
-
•
Outdoor walking/strength training in the close post-stroke period might prove to be a therapeutic strategy.
1. Introduction
Acute stroke (AS) and transient ischemic attack (TIA) are diagnosed in approximately 26,500 and 8000–12,000 persons/year, respectively, in Sweden according to the Swedish Stroke Register (RIKSstroke, 2017). In the United States, approximately one person every 40 s experiences a stroke, and the prevalence is projected to increase by 25% until 2030 (Mozaffarian et al., 2016). Worldwide, older adults, black people and people with lower levels of education have a higher stroke prevalence (Mozaffarian et al., 2016). Approximately 75% of stroke victims are 70 years or older at stroke onset (RIKSstroke, 2017). However, in Sweden, the incidence of stroke is increasing in people aged 35–44 years despite a decrease in the overall incidence of stroke (RIKSstroke, 2017). Approximately two-thirds of the strokes occurring in Sweden are considered to be mild (RIKSstroke, 2017). An example of improved secondary prevention consists of smoking cessation and medical treatment with oral anti-coagulants, anti-hypertensive treatment and cholesterol-lowering treatment (Billinger et al., 2014). However, secondary prevention programs aiming at increased physical activity are scarce. Worldwide, physical inactivity is known to be the fourth leading risk factor for mortality, and >80% of adolescents worldwide are insufficiently physically active (WHO, 2016). Inactivity (i.e., no sessions of light/moderate or vigorous physical activity of 10 min or more) gradually increases with age from 24.8% (ages 18–44 years) to 51% (≥75 years of age) (Mozaffarian et al., 2016). Thus, hospital- and community-based studies consistently show even lower levels of physical activity among stroke survivors (Mozaffarian et al., 2016; Billinger et al., 2014); for example, one observational study indicated that people spent close to 11 h sitting per day following a stroke (English et al., 2016). Community-living individuals who suffer from a stroke have a 23% lower level of physical activity compared to individuals of the same age who have not suffered a stroke, as measured by the Physical Activity Scale for the elderly (Danielsson et al., 2014). In people suffering from a stroke, physical activity lowers the risk of stroke, coronary heart disease and premature mortality by improving risk-factors related to metabolic syndrome, i.e., hypertension, diabetes, hyperlipidemia and accumulation of body fat (Mozaffarian et al., 2016; Esenwa and Gutierrez, 2015). Outdoor walking with gradually increased intensity and strength training might be one way to achieve increased physical activity (Billinger et al., 2014).
In the guidelines for individuals with stroke, training should include low- to moderate-intensity aerobic activity and muscle-strengthening exercises (in bouts of 10 min or more); a reduction in sedentarism; and secondary risk management (Billinger et al., 2014). Studies evaluating walking capacity or aerobic training after stroke are usually performed in the subacute or chronic stage using a cycle ergometer or walking on a treadmill (Saunders et al., 2013). Outdoor walking and home-based strength training are cost-effective and easy to perform in most surroundings. Currently, more research is needed to establish the effect of outdoor walking interventions on physical function and health, in both the acute and more chronic stages after a stroke (Saunders et al., 2016).
The general trend is that hospital stays become shorter. Thus, it is important to find cost-effective and user-friendly methods for training after an acute stroke. The use of cellphones is a common way to communicate today and might improve the motivation to exercise. Cellphone-based rehabilitation, such as delivering an SMS for secondary prevention of cardiovascular diseases, seems to be effective (Gandhi et al., 2017; Varnfield et al., 2014). To date, no studies have been conducted to determine whether an outdoor community walking and muscle strength training program delivered via SMS following AS/TIA can improve physical functioning and metabolic profiles (Billinger et al., 2014; English et al., 2016; English et al., 2014).
1.1. Purpose and aims
The Strokewalk study was designed to evaluate if a method consisting of daily cellphone-delivered messages with training instructions for three months is better than the current standard of treatment to improve physical activity, physical functioning and biomarkers related to cardiovascular disease in people discharged from the hospital with AS or TIA for up to one year.
The primary outcome and hypothesis is that SMS-delivered training instructions will increase walking capacity compared with the current standard of treatment. Secondary outcomes and hypotheses are that the program will increase physical functioning (i.e., mobility, gait speed and hand-grip strength), improve body composition (lower fat mass and increased muscle mass), improve biochemical cardio-metabolic risk markers, decrease further cardiovascular incidents and improve the health-related quality of life (HRQoL)/self-perceived health.
2. Materials and methods
2.1. Study participants
Eligible participants are adults with verified AS or TIA, aged 18 years or older, scheduled to be discharged to an independent living situation and with a sufficient walking capacity, which is the ability to perform the 6-minute walking test (6 MWT) (Am. J. Respir. Crit. Care Med., 2002). Cognitive function, measured with the Montreal Cognitive Assessment scale (MoCA), must be ≥23 points (Nasreddine et al., 2005). Motor function, according to the Modified Rankin Scale (MRS), has to show a slight disability or less with a score of ≤2 (Banks and Marotta, 2007). Individuals with a MRS score of 3 with moderate disability are excluded since they requires some help and are more likely in need of hospital-based rehabilitation. The participants need to have access to a cellphone.
Exclusion criteria are subarachnoid hemorrhage, medical problems such as uncontrolled hypertension, untreated arrhythmias, significant valvular or coronary disease, unstable cardiovascular status, dementia, severe aphasia or cognitive impairment with difficulty understanding instructions.
2.2. Study design
This is a prospective, randomized, controlled two-armed trial, conducted in the stroke-unit at the Uppsala University Hospital, Sweden. Ethical approval for the present study, which complies with the Helsinki Declaration, was obtained from the Regional Ethical Review Board of Uppsala University Hospital; Dnr: 2015/550.
Ethical approval for the pilot study, which complies with the Helsinki Declaration, was obtained from the Regional Ethical Review Board of Uppsala University Hospital; Dnr: 2015/358. Data for the pilot study were collected from October 2015 to May 2016.
2.3. Informed consent
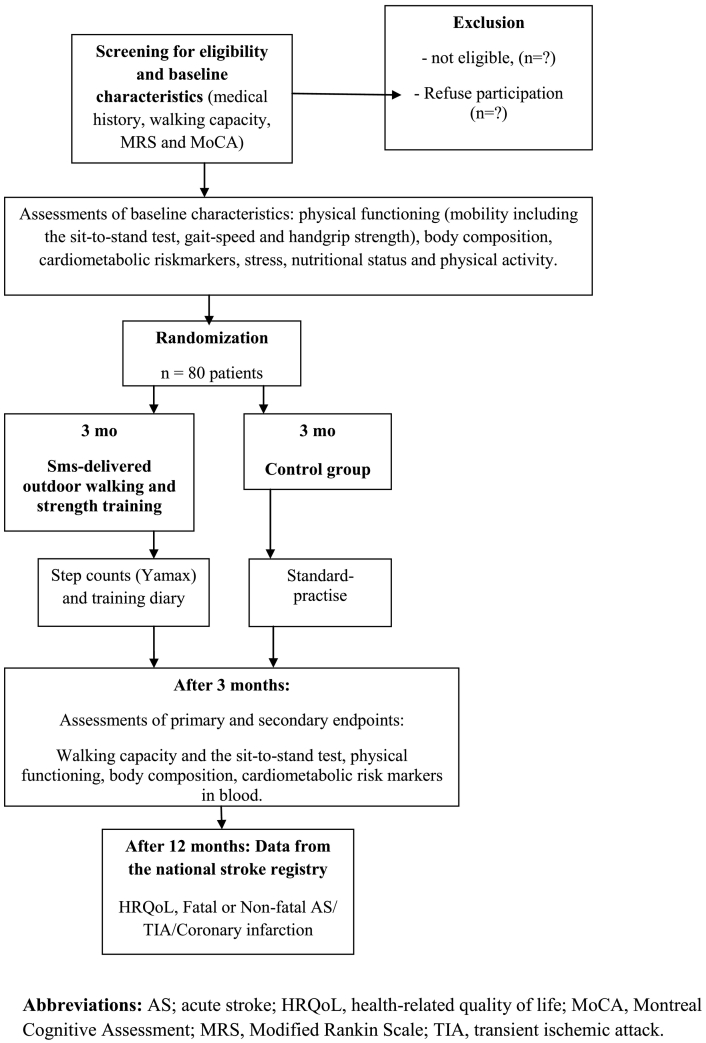
Written and verbal information about the study are provided simultaneously during the hospital stay, and all participants have to give their signed informed consent before screening for eligibility and enrollment. A flow-chart of the study design is shown in Fig. 1.
Fig. 1.
Flow chart for patient inclusion and follow-up in the randomized controlled trial.
Data were collected in Uppsala, Sweden.
2.4. Data collection – study protocol
Patients are screened for sufficient walking capacity, cognition and motor function before entering the study. All patients will be examined at baseline, after three months with intervention and one year after stroke/TIA. Cognition, motor function, nutritional status, stress and physical activity are measured as baseline characteristics. Changes in walking capacity, physical functioning (mobility, gait speed and hand-grip strength), body composition and blood levels of cardio-metabolic risk markers will be analyzed after three months of intervention. The assessments, except for those of body composition, will be performed by one of the authors (BV) before randomization, at the start of the intervention and at the end of the intervention at 3 months. BV will be blinded to the group allocations throughout the study. At one year, between group differences in register-based data on all-cause mortality, cardio-vascular incidences and health-related quality of life will be collected from the Swedish stroke register (RIKSstroke, 2017). A flow-chart of measurements at baseline, three months and one year is provided in Fig. 1.
Walking capacity and lower extremity performance are the primary outcomes of the present study. The 6 MWT (Am. J. Respir. Crit. Care Med., 2002) used in this study evaluates walking capacity. The maximal distance at a maximal pace over a 30 m course during 6 min is recorded (Am. J. Respir. Crit. Care Med., 2002). Heart rate is measured by an oximeter (Collinor®), and the perceived rate of exertion is rated on the Borg's rating of perceived exertion (RPE) scale® (Borg, 1982), ranging from 6 (nothing at all) to 20 (maximal).
The sit-to-stand test, one item of the SPPB, evaluates functional lower extremity strength, with the individual rising up from a seated position as quickly as possible 5 times (Guralnik et al., 1994; Mong et al., 2010).
The Montreal Cognitive Assessment scale (MoCA) (Nasreddine et al., 2005) (0–30 points) will be used to assess cognition. A sum score of <26 is often used as cut-off point for no cognitive problems (Nasreddine et al., 2005).
The Modified Rankin Scale (MRS) (Banks and Marotta, 2007) will be used to assess motor function. It is a measure of global disability. It is scored from 0 (no symptoms at all) to 6 (dead) with 3 indicating moderate disability: requiring some help but able to walk without assistance.
Since nutritional status can affect obesity, Type-II diabetes and LDL-cholesterol, nutrition will be assessed by the Mini Nutrition Assessment® tool (Guigoz, 2006) and by cardiometabolic risk markers. The test includes two screening sections, part 1 (14 points) and part 2 (16 points), with a total score of 30. Less than 17 points indicates malnutrition, 17–23.5 points indicates that the participant is at-risk for malnutrition, and 24–30 points indicates a normal nutritional status (Guigoz, 2006).
As a risk-factor for cardiovascular disease, stress will be assessed with a four-level self-reported scale with 20 claims with agreements from not at all (0 points) to fully agree (3 points), from the Swedish heart and lung foundation (The Heart and Lung Foundation, n.d.).
The 4-level Saltin Grimby Physical Activity Level Scale (SGPALS) (Rödjer et al., 2012) will be used to assess the self-reported level of physical activity (PA) before the stroke as the baseline measurements. The SGPALS assesses physical activity in four levels: physical inactivity, some light physical activity, regular moderate physical activity and training and hard physical training for competition sports (Rödjer et al., 2012). Compliance with the training/level of physical activity in the intervention group will be assessed with a self-reported training diary during three months and by using a movement sensor (the pedometer Yamax LS2000, Yamax Corporation, Japan) (Dohrn et al., 2016) for a 7-day period during waking hours at the beginning and end of the intervention.
The Short Physical Performance Battery test (SPPB) (Guralnik et al., 1994) will be used to assess lower-extremity performance and mobility. The test includes measures of balance, gait speed and chair rising and is scored from 0 to 4 for a maximum score of 12 points. A higher score reflects better mobility.
The 10-meter walking test (10 mWT) at self-selected pace will be used to measure gait speed. The time taken to walk 10 m is measured with a stop watch while the observer stands behind the participant. The test has acceptable psychometric properties in individuals who have suffered a stroke (Tyson and Connell, 2009).
Hand grip strength will be assessed using the Jamar hand dynamometer (Bellace et al., 2000). Subjects will be placed in a sitting position, and the better of two attempts will be recorded. Scores are recorded in kilograms.
Body composition, i.e., the fat-free mass index (FFMI, kg/m2), fat-mass index (FMI, kg/m2) and relative fat mass as a percent of body weight (FM %) will be estimated using a bioelectric impedance analysis (Tanita BC-558; Tanita Corporation, Tokyo, Japan), performed while lying down. Body weight and height will be measured to calculate the body mass index (BMI, kg/m2).
Non-fasting blood samples will be obtained, and biochemical analysis will be performed according to the methods of the Department of Clinical Chemistry at the Uppsala University Hospital. The following cardio-metabolic risk markers in serum or plasma will be analyzed: total cholesterol, HDL and LDL cholesterol. The following markers of inflammation, nutrition and glucose in serum or plasma will be analyzed: C-reactive protein (CRP), interleukin-6 (IL-6), albumin, insulin-like growth factor-1 (IGF-1) and HbA1c.
Data on the self-reported health/HRQoL, further non-fatal cardiovascular incidents (AS/TIA/coronary infarction) and all-cause mortality from the national Swedish stroke register will be analyzed at one year.
2.5. Randomization procedure
Allocation of participants to the intervention and control groups is performed after baseline measurements, informed consent and measurements are collected. Participants will be individually randomized to one of two parallel groups: intervention (outdoor walking and leg strength training) or a control group (standard practice) (Fig. 1). Randomization is based on a computerized random distribution that is stratified by sex and pre-arranged and performed with concealed closed envelopes. An independent research assistant will carry out the randomization procedure and inform the participants of their group affiliation.
2.6. Intervention: outdoor walking and strength training – study protocol
The training intervention lasts for three months. Participants receive a cost-free text message of short sentences with daily training instructions via SMS. The SMS texts are delivered through an internet service (www.intime.nu) every morning from a pre-designed training program. The intervention consists of outdoor walks and one functional strength exercise, performed during the three months of the study. One day per week consists of active resting, i.e., being active but not necessarily walking. The exercises will be gradually increased in intensity and frequency. During the first two weeks, every day begins with a 10-min walk. The intensity of the walk is estimated using the Borg exertion scale (Borg, 1982). Participants are asked to walk at a moderate intensity (Borg scale: 12–13) at the beginning of the training period and increase gradually to a strenuous intensity by the end of the training period (Borg scale: 15), and the transition is adapted to the individual's level of physical capacity (Borg, 1982). During the last four weeks, the outdoor walking training is performed in intervals or as a 30-minute outdoor walk. The interval training includes a warm-up (5 min) and quick walks for 4 min alternated with calmer walks for 3 min. This is repeated 3 times with a subsequent cooling off period of approximately 5 min. In addition to walking training, a functional leg strength exercise is added simultaneously. Study participants are asked to repeatedly rise from a sitting position, with an increasing number of repetitions. An example of an SMS text from week 1 is as follows: “Today, you shall walk for 15 minutes, Borg scale 12: (easy). Stand up from sitting 10 times, without support”.
2.6.1. Control group
Patients in the control group (CG) will be given the current standard of treatment, including a short conversation about their level of physical activity but with no specific advice. They have no restrictions with regards to physical activity or taking part in any rehabilitation service.
2.7. Data collection – pilot study
The pilot study was performed similar to the study protocol, but without measuring body composition or cardio-metabolic risk markers in blood. Outdoor walking and strength training were only recorded by a training diary. Moreover, the pilot study did not measure register-based all-cause mortality, cardio-vascular incidences and self-reported health/HRQoL at one year.
2.8. Planned statistical considerations
The null hypothesis of the present study is that all participants' means are equal for the primary outcome. It is estimated that 80 individuals will be required for 80% power with alpha = 0.05 to detect a 34-meter difference in the 6 MWT with a standard deviation of ±43 m, given an anticipated drop-out rate of 20% (Flansbjer et al., 2005). The power analysis performed prior to the study was based on variations previously reported on the effects of a progressive resistance exercise program in post-stroke individuals, as well as an earlier reported difference in the change in the 6 MWT (Flansbjer et al., 2005; Eng et al., 2004; Vahlberg et al., 2017) An intention-to-treat analysis will be applied to our statistical analyses. Per-protocol analyses will also be performed. Mean differences between the groups will be assessed using Student's t-test for continuous and normally distributed data. Non-parametric methods (Mann-Whitney U test) will be used to analyze non-normally distributed data. Survival at one year will be evaluated using Cox regression analyses. Participants will be compared before and after exercise in terms of the described endpoints and at one year by using the national stroke register. The level of significance is set at p < 0.05. All analyses will be done using SPSS version 25 (IBM, Armonk, NY, USA).
2.8.1. Statistical analyses – pilot study
Descriptive analyses were performed based on the type and distribution of variables. To check for normality, the Shapiro-Wilk W test was used. To analyze the within-group changes over time, a paired-samples two-tailed t-test was performed for continuous normally distributed data. Otherwise, the Wilcoxon sign rank test was used. Cohen's d was used as a measure of the within-group effect size for continuous variables and Wilcoxon sign rank test for ordinal data. The level of significance was set at p < 0.05. All analyses were performed using SPSS version 25 (IBM, Armonk, NY, USA).
3. Results
3.1. Study performance – pilot study
For the non-randomized pilot study with pre- and post-assessments, a total of 25 patients were screened. Eight women and seven men (mean age range: 26–82 years) with verified AS were accepted to participate. The individuals' characteristics are shown in Table 1. The 10 non-participants (3 women) (age 70.7 years) who did not sign up for the study declined for the following reasons: one of the screened individuals became ill and was no longer eligible for inclusion, and one did not want to participate since he was already very active. Six individuals did not want to participate, mostly because of a lack of time. Two included individuals did not complete the intervention because they were not able to perform the exercises due to chronic illness, i.e., kidney disease and rheumatoid arthritis. Two participating individuals were not able to perform the handgrip measurement due to weakness in both hands or paresis in one hand. Participants in all ages included in the study seemed to be suitable for the intervention.
Table 1.
Clinical characteristics of individual suffering from acute stroke. Data obtained from the pilot study in Uppsala, Sweden 2015–2016.
| Characteristics | Total (n = 15) |
|---|---|
| Age (years), mean (SD) | 65.0 (13.5) |
| Age (years), n (%) | |
| <50 | 1 (6.67) |
| 50–69 | 7 (46.67) |
| >70 | 7 (46.67) |
| Gender, female, n (%) | 8 (53.3) |
| Living status, married, n (%) | 13 (86.7) |
| Education, short, n (%) | 9 (60.0) |
| BMI (kg/m2), mean (SD) | 27.0 (3,7) |
| BMI, kg/m2, n (%) | |
| <22 (moderate underweight) | 1 (6,7) |
| 22–22,9 (normal/overweight) | 11 (73.3) |
| >30 (obese) | 3 (20.0) |
| Stress, yes | 7 (46.7) |
| Smoking, no | 15 (100) |
| MRS, (0–6) | 1 (1) |
| MoCAa (0–30 points), median (IQR) | 26 (3) |
| SGPALS, (1–3), n (%) | |
| 1 | 3 (20.0) |
| 2 | 11 (73.3) |
| 3 | 1 (6,7) |
| Type of stroke, n (%) | |
| Cerebral infarction | 15 (100) |
| Intra-cerebral hemorrhage | 0 |
Abbreviations: BMI, body mass index; MoCA, Montreal Cognitive Assessment; MRS, Modified Rankin Scale; SGPALS, Saltin Grimby Physical Activity Level Scale.
n = 12
The participants completed the three-month outdoor walking program together with a muscle strength exercise (chair-rising) as described above. Instructions were delivered daily via SMS texts to their cellphones. The 15 individuals had at least 68% attendance, which was considered an acceptable adherence rate, to the outdoor walking and strength training program. All participants who were able to fulfill the training program improved their 6 MWT from baseline to three months with a range of 23–255 m (Table 2). Within-group analyses at 12 weeks revealed that the following values were significantly improved (p < 0.05) from baseline: the 6 MWT (34%), 10 mWT (34%), SPPB (25%) and unsupported chair-rise (25%). The study protocol was slightly modified after the pilot study, as the participants were instructed to walk five more minutes in the beginning of the program. The participants were also asked to perform five more repetitions of the chair-stand test compared to the program in the pilot study. It became clear that most participants preferred SMS texts over video-links. No adverse events were reported.
Table 2.
Within-group differences of physical function at the intervention start and after three months (pilot study). Data were collected in Uppsala, Sweden 2015–2016.
| Baselinea | 3 monthsa | Mean/median change (95% CI) | % mean/median change | Effect size | pb | |
|---|---|---|---|---|---|---|
| Subjects, n | Male (n = 7), female (n = 8) | |||||
| 6 MWT (meter), mean (SD) | 437.5 (151.2) | 541.7 (115.2) | 104.20 (63 to 145) | 0.34 | 1.43 | <0.001 |
| SPPB (0–12 points), Md (IQR) | 9.3 (5) | 10.9 (2) | 1.0 (0.9 to 2.5) | 0.25 | 0.57 | 0.002 |
| Chair-rise (s), mean (SD) | 13.1 (5.6) | 10.7 (3.3) | −2.42 (−6.5 to 1.6) | −0.25 | 0.33 | 0.22 |
| 10 mWT (m/s), mean (SD) | 1.06 (0.34) | 1.34 (0.25) | 0.28 (0.14 to 0.42) | 0.34 | 1.10 | 0.001 |
| Jamar hö (kg), mean (SD) | 31.0 (12.3) | 31.7 (12.6) | 0.73 (−1.09 to 2.6) | 0.01 | 0.22 | 0.40 |
| Jamar vä (kg), mean (SD) | 26.4 (13.2) | 28.4 (13.7) | 1.93 (−0.37 to 4.2) | 0.08 | 0.46 | 0.09 |
Abbreviations: SPPB, Short Physical Performance Battery; 6 MWT, 6 Minute Walk Test; 10 mWT, 10 meter walking test. p < 0.05.
Assessed by performance-based measures.
Differences within the group using paired samples t-test and Wilcoxon's signed rank test.
4. Discussion
The Strokewalk study is the first study to evaluate an SMS-delivered outdoor walking and leg muscle strengthening program using cellphones to help individuals increase their physical activity level after AS or TIA. Information from the study will provide knowledge on whether mobile phones can be used to give short training instructions to improve a participant's level of physical activity and thereby improve physical function and reduce cardiovascular risk. An advantage of SMS-delivered training in addition to being cost-effective for both society and the individual, is the opportunity/possibility to perform the training close to one's own home and away from health-care facilities. Mobile health (mhealth) is increasing in popularity among health-care providers for the secondary prevention of cardiovascular diseases, and it has been shown to lead to adherence to medical therapy as well as the ability to reach blood pressure targets and exercise goals (Gandhi et al., 2017).
A predesigned single group pilot study with pre- and post-assessments in individuals with AS showed that both walking capacity and mobility, including the chair-rise and gait speed results, improved from baseline to three months after the intervention. The 6 MWT improved by 34%, and the smallest real difference (SRD%), representing the smallest change that indicates a real (clinical) improvement for one individual was shown to be 13% for the 6 MWT (Flansbjer et al., 2005; The EuroQol Group, 1990). The training program was in line with existing recommendations for exercising after stroke by training both lower leg muscle strength and aerobic capacity (Billinger et al., 2014). The overload principle was achieved to produce training effects by providing regular, individual increases in the frequency, intensity and duration of the outdoor walking and chair-rise exercises. However, suffering from a stroke might change the individual's life perspective, and by being part of a research study, certain risk factors may have been highlighted, especially the importance of physical activity. Age might influence compliance to SMS-delivered training as the ability to use cellphones might decrease with age. Furthermore, many older people have cellphones that are easier to use and less “smart.” Co-morbidity and balance disorders are more common in the oldest patients, which makes them less suitable for this intervention that requires unsupervised training.
In the previous pilot study, the mean age was 65 years, which is lower than the mean age of stroke onset in Sweden (about 75 years) (RIKSstroke, 2017). Approximately 13% of all strokes in Sweden in 2015 were caused by intracerebral bleeding (ICB), which is in contrast with the current study in which all analyzed patients had suffered from a cerebral infarction, thus affecting generalizability. The findings from the pilot study need to be further corroborated by the randomized controlled trial.
There are some limitations of the planned and ongoing study. It is a single-center study with subjects from one area. Additionally, it is a relatively small study, and the participants have suffered from mild AS or TIA, which might limit generalization to the entire stroke population. The data, compliance and adherence to the outdoor walking program will be self-reported or measured as step counts.
5. Conclusions
In conclusion, the results from the ongoing RCT study will provide insights into how modern technology and cellphones can be used in the rehabilitation of individuals with AS or TIA. The results from the study will provide further information on the impact of early rehabilitation with regular outdoor walking and strength training after AS or TIA on physical function, body composition and cardiovascular risk markers.
5.1. Implications for rehabilitation
SMS-guided outdoor training might be a potential therapeutic strategy to improve physical activity level, walking capacity and physical function after stroke.
Funding
This work was supported by STROKE-riksförbundet and the Uppsala County Council in Sweden.
Conflicts of interest
None.
Footnotes
Article category: Study protocol with pilot results of a prospective, randomized controlled trial
Trial registration: URL: http://www.clincaltrials.gov. Unique identifier: (NCT02902367)
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2018.05.016.
Appendix A. Supplementary data
Supplementary material
References
- ATS statement: guidelines for the six-minute walk testAm. J. Respir. Crit. Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- Banks J.L., Marotta C.A. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38(3):1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- Bellace J.V., Healy D., Besser M.P., Byron T., Hohman L. Validity of the Dexter Evaluation System's Jamar dynamometer attachment for assessment of hand grip strength in a normal population. J. Hand Ther. 2000;13(1):46–51. doi: 10.1016/s0894-1130(00)80052-6. [DOI] [PubMed] [Google Scholar]
- Billinger S.A., Arena R., Bernhardt J. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(8):2532–2553. doi: 10.1161/STR.0000000000000022. [DOI] [PubMed] [Google Scholar]
- Borg G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- Danielsson A., Meirelles C., Willen C., Sunnerhagen K.S. Physical activity in community-dwelling stroke survivors and a healthy population is not explained by motor function only. PM R. 2014;6(2):139–145. doi: 10.1016/j.pmrj.2013.08.593. [DOI] [PubMed] [Google Scholar]
- Dohrn I.M., Hagströmer M., Hellenius M.L., Ståhle A. Gait speed, quality of life, and sedentary time are associated with steps per day in community-dwelling older adults with osteoporosis. J. Aging Phys. Act. 2016;24(1):22–31. doi: 10.1123/japa.2014-0116. [DOI] [PubMed] [Google Scholar]
- Eng J.J., Dawson A.S., Chu K.S. Submaximal exercise in persons with stroke: test-retest reliability and concurrent validity with maximal oxygen consumption. Arch. Phys. Med. Rehabil. 2004;85(1):113–118. doi: 10.1016/s0003-9993(03)00436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English C., Manns P.J., Tucak C., Bernhardt J. Physical activity and sedentary behaviors in people with stroke living in the community: a systematic review. Phys. Ther. 2014;94(2):185–196. doi: 10.2522/ptj.20130175. [DOI] [PubMed] [Google Scholar]
- English C., Healy G.N., Coates A., Lewis L., Olds T., Bernhardt J. Sitting and activity time in people with stroke. Phys. Ther. 2016;96(2):193–201. doi: 10.2522/ptj.20140522. [DOI] [PubMed] [Google Scholar]
- Esenwa C., Gutierrez J. Secondary stroke prevention: challenges and solutions. Vasc. Health Risk Manag. 2015;11:437–450. doi: 10.2147/VHRM.S63791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flansbjer U.B., Holmbäck A.M., Downham D., Patten C., Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J. Rehabil. Med. 2005;37(2):75–82. doi: 10.1080/16501970410017215. [DOI] [PubMed] [Google Scholar]
- Gandhi S., Chen S., Hong L. Effect of mobile health interventions on the secondary prevention of cardiovascular disease: systematic review and meta-analysis. Can. J. Cardiol. 2017;33(2):219–231. doi: 10.1016/j.cjca.2016.08.017. [DOI] [PubMed] [Google Scholar]
- Guigoz Y. The Mini Nutritional Assessment (MNA) review of the literature—what does it tell us? J. Nutr. Health Aging. 2006;10(6):466–485. discussion 85-7. [PubMed] [Google Scholar]
- Guralnik J.M., Simonsick E.M., Ferrucci L. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Mong Y., Teo T.W., Ng S.S. 5-Repetition sit-to-stand test in subjects with chronic stroke: reliability and validity. Arch. Phys. Med. Rehabil. 2010;91(3):407–413. doi: 10.1016/j.apmr.2009.10.030. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D., Benjamin E.J., Go A.S. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Nasreddine Z.S., Phillips N.A., Bédirian V. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- RIKSstroke RIKSstroke, the Swedish Stroke Register. 2017. http://www.riksstroke.org Available from.
- Rödjer L., Jonsdottir I.H., Rosengren A. Self-reported leisure time physical activity: a useful assessment tool in everyday health care. BMC Public Health. 2012;12:693. doi: 10.1186/1471-2458-12-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders D.H., Sanderson M., Brazzelli M., Greig C.A., Mead G.E. Physical fitness training for stroke patients. Cochrane Database Syst. Rev. 2013;10 doi: 10.1002/14651858.CD003316.pub5. [DOI] [PubMed] [Google Scholar]
- Saunders D.H., Sanderson M., Hayes S. Physical fitness training for stroke patients. Cochrane Database Syst. Rev. 2016;3 doi: 10.1002/14651858.CD003316.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The EuroQol Group EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- The Heart and Lung Foundation Stress. [Internet] https://www.hjart-lungfonden.se/Documents/Skrifter/Skrift_stress_2011.pdf Available from. (Swedish) (cited April 2016)
- Tyson S., Connell L. The psychometric properties and clinical utility of measures of walking and mobility in neurological conditions: a systematic review. Clin. Rehabil. 2009;23(11):1018–1033. doi: 10.1177/0269215509339004. [DOI] [PubMed] [Google Scholar]
- Vahlberg B., Cederholm T., Lindmark B., Zetterberg L., Hellström K. Short-term and long-term effects of a progressive resistance and balance exercise program in individuals with chronic stroke: a randomized controlled trial. Disabil. Rehabil. 2017;39(16):1615–1622. doi: 10.1080/09638288.2016.1206631. [DOI] [PubMed] [Google Scholar]
- Varnfield M., Karunanithi M., Lee C.K. Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial. Heart. 2014;100(22):1770–1779. doi: 10.1136/heartjnl-2014-305783. [DOI] [PubMed] [Google Scholar]
- WHO . World Health Organization; 2016. Physical Activity Fact Sheet: World Health Organization.http://www.who.int/mediacentre/factsheets/fs385/en Available from. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material