Abstract
Amyotrophic lateral sclerosis (ALS) can be associated with a spectrum of cognitive and behavioural symptoms, but the related patterns of focal cortical atrophy in non-demented ALS patients remain largely unknown. We enrolled 48 non-demented ALS patients and 26 healthy controls for a comprehensive neuropsychological assessment and a magnetic resonance exam. Behavioural and cognitive impairment was defined on the basis of a data-driven multi-domain approach in 21 ALS patients. Averaged cortical thickness of 74 bilateral brain regions was used as a measure of cortical atrophy. Cortical thinning in a fronto-parietal network, suggesting a disease-specific pattern of neurodegeneration, was present in all patients, independent of cognitive and behavioural status. Between-group and correlational analyses revealed that inferior frontal, temporal, cingular and insular thinning are markers for cognitive and behavioural deficits, with language impairment mainly related to left temporal pole and insular involvement. These specific correlates support the concept of a spectrum of deficits, with an overlap between the ALS cognitive phenotypes and the syndromes of frontotemporal dementia.
Abbreviations: ALS, amyotrophic lateral sclerosis; ALSbi, ALS with mild behavioural impairment; ALSci, ALS with mild cognitive impairment; ALScn, cognitively-normal ALS; ALSimp, ALS with cognitive and/or behavioural impairment; C9+ ALS, ALS harbouring C9orf72 repeat expansion; C9– ALS, ALS without C9orf repeat expansion; CT, cortical thickness; GM, grey matter; FTD, frontotemporal dementia; HC, healthy control; MD, multi-domain
Keywords: Amyotrophic lateral sclerosis, Cognitive impairment, Cortical thickness, Temporal lobe, Cognitive profiles
Highlights
-
•
Language, social cognition and executive dysfunctions are frequent symptoms in ALS.
-
•
Fronto-parietal cortical thinning is present in non-demented ALS patients.
-
•
Temporal, cingular and insular thinning are markers for cognitive impairment in ALS.
-
•
Left temporal pole and insular thinning is linked to language impairment in ALS.
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by the degeneration of motor neurons of the cortex, brain stem and spinal cord, leading to progressive muscle weakness. Motor symptoms may be accompanied by a spectrum of cognitive and behavioural symptoms, ranging from mild impairment to a full-blown dementia syndrome fulfilling the criteria for frontotemporal dementia (FTD) (Strong et al., 2017). The neuropsychological profile of non-demented ALS patients is extremely heterogeneous (Montuschi et al., 2015; Phukan et al., 2012; Strong et al., 2017; Taylor et al., 2013). Behavioural changes (ALSbi) have been reported in ALS patients not fulfilling the criteria for FTD (Consonni et al., 2013; Gibbons et al., 2008; Lillo et al., 2011; Strong et al., 2017). Apathy is the most frequently identified behaviour symptom in ALS (Strong et al., 2017), but ALS patients may also present disinhibition, loss of sympathy, perseverations and change in dietary habits (Burke et al., 2017; Consonni et al., 2013; Gibbons et al., 2008). From a cognitive prospective, deficits in executive functions, language, social cognition and memory have been observed (Beeldman et al., 2016; Boschi et al., 2017; Carluer et al., 2015; Taylor et al., 2013). In a previous study based on data-driven principal component analysis, we reported in non-demented ALS patients a separate cognitive phenotype involving language, social cognition and memory domains, besides dysexecutive and behavioural impairment (Consonni et al., 2016).
The heterogeneity of cognitive profiles can be predicted to reflect the presence of distinct patterns of involvement of cortical and subcortical structures subserving behavioural and cognitive changes. Structural magnetic resonance imaging (MRI) studies associated with advanced automated imaging analysis have indicated a gradient of increasing cortical atrophy across the whole ALS-FTD spectrum (Agosta et al., 2016; Leslie et al., 2015; Mioshi et al., 2013; Schuster et al., 2014). Specifically, they documented a common pattern of atrophy of motor and premotor cortices in all patients with ALS, extending to frontotemporal lobes and insula when dementia is present. The brain imaging evidence in patients with cognitive deficits, not fulfilling the criteria for dementia, is however limited. In some cases, ALSci patients were classified only on the basis of executive dysfunction (Machts et al., 2015; Mioshi et al., 2013). Only a few studies stratified ALS patients considering also non-executive cognitive domains; the relation between cortical atrophy and neuropsychological profiles was however not investigated (Christidi et al., 2012; Schuster et al., 2014). Studies addressing the correlation between cognitive and behavioural deficits and the pattern of cortical involvement have focused on specific domains. They indicated that inferior frontal and dorsolateral prefrontal areas are mainly related to executive dysfunction (Libon et al., 2012; Menke et al., 2014), action naming impaired abilities (Grossman et al., 2008) and apathy (Tsujimoto et al., 2011). Left inferior frontal and temporal regions are related to syntactic processing (Ash et al., 2015), whereas the anterior temporal lobe involvement reflects the severity of semantic impairment (Leslie et al., 2015). Right fronto-insular and anterior cingulate cortices are linked to affective empathy (Cerami et al., 2014). Hippocampal volume reduction reflects the severity of episodic memory deficits (Abdulla et al., 2014; Bede et al., 2013; Machts et al., 2015; Raaphorst et al., 2015). Overall, the heterogeneity of cognitive profiles might reflect individual cortical and subcortical signatures, a hypothesis that we tested in a single cohort of unselected ALS patients assessed with a comprehensive test battery.
The current study aims revealing distinctive patterns of focal cortical atrophy of non-demented ALS patients and their relation to cognitive phenotypes. To this aim, ALS patients were submitted to a comprehensive neuropsychological battery for the assessment of behavioural changes and cognitive impairment, not restricted to executive dysfunction but extending to language, memory, social cognition and visuo-spatial abilities. Behavioural and cognitive profiles were defined on the basis of a multi-domain approach (Consonni et al., 2016). Data of ALS patients with cognitive and/or behavioural impairment were compared to a cohort of age-, sex- and education-matched healthy volunteers and a cohort of ALS patients with normal cognitive and behavioural profile. Between-group analyses revealed brain regions sensitive to ALS degeneration processes. Their relation to non-motor ALS clinical profiles was then assessed by correlation analyses.
2. Methods
2.1. Participants
Fifty-two consecutive hospitalized patients diagnosed with probable or definite ALS, according to El Escorial revised criteria (Brooks et al., 2000) and 26 healthy control (HC) subjects were recruited at the Motor Neuron Diseases Centre of the “Carlo Besta” Neurological Institute of Milan, Italy. Exclusion criteria were comorbid frontotemporal dementia (Rascovsky et al., 2011), Alzheimer's disease (NIA-AA), evidence of another neurologic condition affecting cognition (e.g. head trauma, hydrocephalus, vascular disease), drug or alcohol abuse, neurocognitive developmental disorder, primary psychiatric disorders (e.g. bipolar disorder, major depression), other severe medical conditions and mother tongue other than Italian. Four patients satisfying inclusion criteria were subsequently excluded by the study because of claustrophobia during MRI scan (1) and excessive movement artefacts revealed by MRI (3).
2.2. Materials
The revised ALS Functional Rating Scale (ALSFRS-R) was used to assess disability. Disease duration was calculated from symptom onset to scan date in months. Patients were assigned to ALS-cognitively impaired (ALSimp) versus ALS-cognitively normal (ALScn) subgroups on the basis of a cognitive screening protocol assessing multiple cognitive and behavioural domains (Consonni et al., 2016). Specifically, all participants underwent a broad battery of standardized neuropsychological tests, with published norms for the Italian population, assessing executive functions, language, memory, social cognition and visuo-spatial abilities (see Consonni et al., 2016, for details). At the time of evaluation, none of the patients had dysarthria severe enough to affect naming or verbal memory recall tasks. The internal ethical review board approved the study and each subject was enrolled after giving written informed consent.
2.3. Patient classification
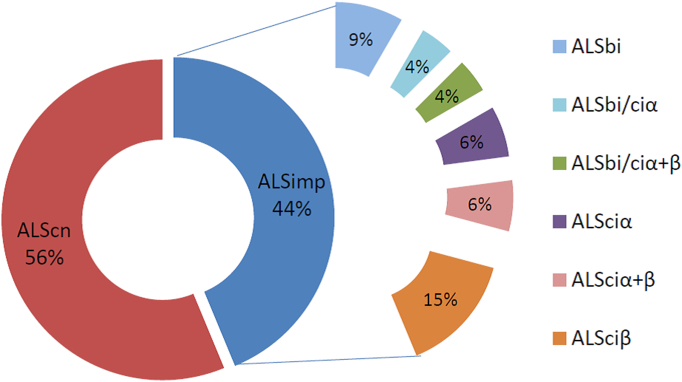
Patients were classified following a multi-domain (MD) data-driven analysis, based on three dimensions of non-motor manifestations in ALS patients 1) behavioural (ALSbi), 2) dysexecutive (ALSciα), and 3) non-executive profile (ALSciβ), including language, social cognition and episodic memory deficits (Consonni et al., 2016). Briefly, ALSbi characterized ALS patients who did not meet full criteria for the frontal variant of FTD, but were rated by caregivers as having at least two non-overlapping behavioural disturbances. ALSciα patients were those performing below cut-off on at least two of the tests tapping executive functions. ALSciβ patients had at least two neuropsychological performances below the normal range on non-executive measures, i.e.: language and/or social cognition and/or episodic memory. Notably, ALSbi, ALSciα and ALSciβ might coexist in ALS patients (Fig. 1). Our cohort was composed of 27 patients with normal cognitive profile (ALScn) and 21 with cognitive and/or behavioural impairment (ALSimp).
Fig. 1.
Neuropsychological profiles of ALS patients according to the MD classification.
ALScn = cognitively-normal ALS patients; ALSbi = ALS patients mild behavioural impairment; ALSciα = ALS patients with mild dysexecutive impairment (with ≥2 performances below cut-off on tests addressing executive cognitive functions); ALSciβ = ALS patients with ≥2 performances below cut-off on tests addressing non-executive cognitive functions (social cognition, language and memory); ALSciα + β = ALS patients with a mixed cognitive profile; ALSbi-ciβ = ALSbi patients with non-executive cognitive impairment; ALSbi-ciα + β = ALSbi patients with the ALSci α + β profile.
2.4. MRI data acquisition and MR image processing
All patients and healthy subjects underwent structural scanning, within a week of neuropsychological assessment, on a Philips Achieva 3.0 T scanner using a 3D T1-weighted sequence (FFE, 240 sagittal slices, TR = 9.9 ms, TE = 4.6 ms, matrix 240 × 240, voxel size = 1 × 1 × 1 mm3, flip angle = 8°). Cortical reconstruction and parcellation was performed with the Freesurfer image analysis suite (version 5.3), documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). The technical details of these procedures are described in prior publications. Briefly, this processing includes removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Segonne et al., 2007) automated Talairach transformation, intensity normalization (Sled et al., 1998), tessellation of the grey matter (GM)/white matter (WM) boundary, automated topology correction (Segonne et al., 2007) and surface deformation following intensity gradients to optimally place the GM/WM and GM/cerebrospinal fluid (CSF) borders at the location where the greatest shift in intensity defines the transition to the other tissue class (Fischl and Dale, 2000). This method uses both intensity and continuity information from the entire three dimensional MR volumes in segmentation and deformation procedures to produce representations of CT, calculated as the closest distance from the GM/WM boundary to the GM/CSF boundary at each vertex on the tessellated GM/WM boundary surface (Fischl and Dale, 2000). When skull stripping was leaving some dural signal (especially in the occipital regions) in the brain mask, it was removed manually and automatic process was recalled. For each subject, CT values averaged on each gyral and sulcal structure were calculated after registration to a spherical atlas which utilized individual cortical folding patterns to match cortical geometry across subjects (Fischl et al., 1999) and automatic parcellation of the cerebral cortex into 74 bilateral brain regions (Destrieux et al., 2010). This approach has been shown to be a sensitive tool to detect the extra-motor cortical involvement in ALS patients (Agosta et al., 2012).
2.5. Statistical analyses
The Kolmogorov-Smirnov test was used to test the normality of the distribution of demographic, clinical, neuropsychological and CT data separately for each group (ALSimp, ALScn and HC). Accordingly, parametric and non-parametrical analyses were performed. Chi-Square, One-way Anova and Kruskal-Wallis tests were used with demographic, clinical and neuropsychological data. Bonferroni post hoc and Mann-Whitney U tests were then used to test specific group comparisons: “ALSimp vs. HC”, “ALScn vs. HC” and “ALSimp vs. ALScn”. CT variability between groups was analysed by ANCOVA, with age and total intra-cranial volume as covariates. The effect size (partial eta squared) was also reported. Bonferroni post hoc tests were then performed for the aforementioned group comparisons. Regions with a p value of 0.05 or lower were considered as significant and included in correlation analyses, with the aim to identify their relationship with ALS neuropsychological and clinical profiles.
We computed partial correlations (correcting for age, education and total intra-cranial volume) between CT measures of brain regions resulting significantly thinner in ALS patients than controls, and neuropsychological measures showing significant differences between ALS and HC groups. For this purpose, standardized z scores for each CT and neuropsychological measure were calculated by subtracting the mean score of HC subjects from the patient's score, and then dividing the difference by the HC group standard deviation. The same procedure was applied to clinical data (correcting for age and total intra-cranial volume). Significant correlations were then bias corrected and accelerated bootstrap 95% confidence intervals (CIs) were computed with 1000 bootstrap equally-sized samples obtained by randomly resampling replacement from the original data. To control for multiple testing across cognitive measures (12), each p value underwent Bonferrroni correction. Partial correlations with p < 0.05 and CIs not crossing “0” surviving Bonferroni correction were reported.
3. Results
3.1. Clinical and demographic data
Descriptive statistics are summarized in Table 1. ALScn, ALSimp and HC groups did not differ in to age (F2,73 = 0.252, p = 0.252), gender (X2 = 4.233, p = 0.809) and education (F2,73 = 1.346, p = 0.267). Disease duration (T45 = 0.149, p = 0.882), functional disability assessed with ALSFRS-R (T45 = 0.344, p = 0.733), type of onset (X2 = 1.382, p = 0.239) and Riluzole therapy (X2 = 2.116, p = 0.146) were similar across ALS subgroups.
Table 1.
Demographic and clinical data.
| HC (N = 26) | ALScn (N = 27) | ALSimp (N = 21) | |
|---|---|---|---|
| Age (years) | 56.8 ± 9.96 | 58.3 ± 10.44 | 58.8 ± 10.76 |
| Education (years) | 11.8 ± 3.58 | 11.0 ± 3.84 | 9.6 ± 3.87 |
| Sex (male/female) | 10/16 | 11/16 | 10/11 |
| Disease duration (months) | – | 21.92 ± 15.78 | 19.95 ± 15.28 |
| Bulbar onset (yes/no) | – | 5/22 | 7/14 |
| Bulbar symptoms (yes/no) | – | 13/14 | 9/12 |
| Riluzole therapy (yes/no) | – | 16/11 | 8/13 |
| ALSFRS-R (range 0–48) | – | 38.6 ± 6.87 | 37.7 ± 5.68 |
| # C9ORF72 mutations | n.a. | 4 (2 n.a.) | 2 (4 n.a.) |
ALScn = ALS patients with a normal cognitive profile; ALSimp = ALS patients with behavioural and/or cognitive impairment; HC = healthy controls; n.a. = not available data.
3.2. Neuropsychological data
HC and ALScn groups had similar neuropsychological performances. The ALSimp group compared with HC and ALScn groups had lower performances in all cognitive domains, with the only exception of visuo-spatial performance (Table 2). Both ALS subgroups were more depressed (higher scores) than HC subjects. Additional Spearman correlations were performed between the GDS scores of ALS subgroups and the neuropsychological performances significantly reduced in the comparison with the HC group. The lack of significant correlation suggested that defective cognitive performances were not related to depression. In contrast, in the ALScn group, behavioural changes measured with the dysexecutive questionnaire were related to depressive mood (r = 0.429, p = 0.041, CIs = 0.04–0.74).
Table 2.
Neuropsychological performances and between-group comparisons.
| Neuropsychological measures | HC | ALScn | ALSimp | F/X (p value); post-hoc |
|---|---|---|---|---|
| M.M.S.E | 29.3 ± 0.93 | 28.6 ± 1.32 | 27.4 ± 2.03 | 13.543 (0.001); a*** |
| Geriatric depression scale | 1.6 ± 2.73 | 3.8 ± 2.70 | 4.6 ± 3.29 | 17.964 (<0.001); a***, c*** |
| Behaviour | ||||
| Frontal behavioural inventory (part A) | – | 1.3 ± 1.89 | 3.4 ± 4.54 | Ns |
| Frontal behavioural inventory (part B) | – | 0.7 ± 1.24 | 2.6 ± 4.60 | Ns |
| Dysexecutive questionnaire (DEX) | – | 5.7 ± 7.08 | 9.6 ± 8.42 | Ns |
| Social cognition | ||||
| SET intention attribution | 5.07 ± 1.05 | 4.7 ± 1.12 | 4.2 ± 1.32 | Ns |
| SET emotion attribution | 4.9 ± 1.05 | 4.8 ± 1.10 | 3.8 ± 1.79 | 6.013 (0.049); a*, b* |
| SET causal inference | 4.9 ± 0.89 | 4.6 ± 1.73 | 3.7 ± 1.58 | 8.341 (0.015); a***, b* |
| Emotion recognition (Ekman test) | 46.2 ± 6.15 | 47.9 ± 4.23 | 42.0 ± 6.86 | 6.110 (0.004); b* |
| Memory | ||||
| Verbal immediate recall (RAVLT) | 48.8 ± 8.4 | 46.2 ± 8.30 | 37.4 ± 10.14 | 10.277 (<0.001); a***, b*** |
| Verbal delayed memory (RAVLT) | 10.5 ± 2.43 | 9.6 ± 2.51 | 7.4 ± 2.96 | 8.029 (0.001); a***, b* |
| Non-verbal recognition memory | 24.5 ± 3.87 | 23.5 ± 4.3 | 21.3 ± 4.85 | 3.196 (0.047); a* |
| Attention/executive functions | ||||
| Digit span forward | 6.1 ± 1.37 | 5.8 ± 0.98 | 5.0 ± 1.51 | 8.556 (0.014);a**, b* |
| Digit span backward | 4.6 ± 1.29 | 4.7 ± 1.25 | 3.5 ± 1.20 | 6.255 (0.003); a**, b** |
| Stroop test (Stroop-effect - time) | 18.1 ± 7.00 | 19.6 ± 7.70 | 28.6 ± 18.71 | 5.252 (0.007); a*, b* |
| Phonemic fluency (F + P + L) | 38.1 ± 9.91 | 34.2 ± 8.7 | 25.3 ± 8.71 | 10.824 (<0.001); a***,b** |
| Phonemic fluency index (F) | 3.9 ± 1.44 | 4.7 ± 2.22 | 7.3 ± 4.38 | 8.434 (0.001); a***, b* |
| Brixton test | 19.1 ± 7.3 | 20.5 ± 9.03 | 21.9 ± 6.69 | Ns |
| Cognitive estimation (STEP) | 41.3 ± 5.96 | 44.4 ± 4.95 | 43.2 ± 4.27 | Ns |
| Language | ||||
| Object naming (BADA) | 28.5 ± 1.52 | 28.5 ± 1.12 | 25.7 ± 3.37 | 21.333 (<0.001); a***, b*** |
| Auditory sentence comprehension | 13.9 ± 0.40 | 13.7 ± 0.49 | 13.5 ± 0.82 | 7.274 (0.26); a* |
| Visuo-spatial abilities | ||||
| Position discrimination (VOSP) | 19.6 ± 0.69 | 19.7 ± 0.86 | 19.7 ± 0.71 | Ns |
BADA = Batteria per l'analisi del deficit afasico; RAVLT = Rey Auditory Verbal Learning test; SET = Story-based Empathy task; STEP = The Time and Weight Estimation Test; VOSP = Visual object and space perception battery. Ns = not significant difference; a = HC Vs. ALSimp; b = ALScn Vs. ALSimp; c = HC Vs. ALScn; * = p < 0.05; ** = p < 0.01; *** = p < 0.005.
3.3. CT region-wise data
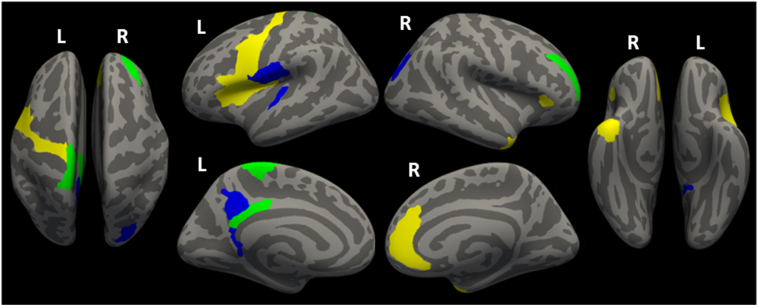
Both ALS patient groups had CT reduction in bilateral frontal, and left posterior cingular cortex (PCC) compared to HC individuals (Fig. 2). ALScn patients had reduced CT in posterior regions (PCC, parietal and occipital areas) and in the left transversal temporal gyrus. ALSimp patients showed a more widespread thinning in bilateral insula, left inferior frontal gyrus, right temporal pole, and right anterior cingulate cortex (AAC). Moreover, the direct comparisons between ALS subgroups revealed that ALSimp patients had a specific thinning of the right inferior temporal gyrus and sulcus compared with ALScn patients (Fig. 3).
Fig. 2.
Distribution of the cortical thinning in patients with ALS considering their cognitive status. Yellow indicates thinning in ALSimp patients compared to HC volunteers; blue indicates thinning in ALScn patients compared to HC volunteers; green indicates overlap between these comparisons. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.

Distribution of the cortical thinning (in red) on the pial surface of the right hemisphere (lateral and inferior views) in ALSimp patients compared to ALScn patients. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
We did not find any significant CT reduction in HC individuals compared with ALS subgroups. Table 3 displays further details. When patients with a repeat expansion in the C9orf72 gene (C9+) were excluded from the analyses (i.e.: 2 ALSimp and 4 ALScn), thickness differences for anterior and posterior cingular, subcentral, parietal and occipital areas were no more significant.
Table 3.
CT measures and between-group comparisons.
| HC (N = 26) | ALScn (N = 27) | ALSimp (N = 21) | F (partial eta squared) | Bonferroni corrected contrasts | |
|---|---|---|---|---|---|
| Frontal lobe | |||||
| Right middle frontal sulcus | 2.171 ± 0.12 | 2.056 ± 0.13 | 2.029 ± 0.12 | 9.225⁎⁎⁎ (0.211) | a⁎⁎⁎, c⁎⁎⁎ |
| Left precentral gyrus | 2.657 ± 0.17 | 2.563 ± 0.15 | 2.483 ± 0.15 | 3.925⁎⁎⁎ (0.159) | a⁎⁎⁎ |
| Left paracentral lobule and sulcus | 2.246 ± 0.13 | 2.089 ± 0.20 | 2.118 ± 0.14 | 6.221⁎⁎⁎ (0.153) | a⁎, c⁎⁎⁎ |
| Left pars opercularis of the IFG | 2.673 ± 0.13 | 2.622 ± 0.12 | 2.545 ± 0.16 | 4.469⁎ (0.115) | a⁎ |
| Left precentral sulcus (inferior) | 2.347 ± 0.11 | 2.296 ± 0.15 | 2.223 ± 0.20 | 3.732⁎ (0.098) | a⁎⁎ |
| Left subcentral lobule and sulcus§ | 2.589 ± 0.23 | 2.431 ± 0.21 | 2.465 ± 0.20 | 3.248⁎ (0.087) | c⁎ |
| Temporal lobe | |||||
| Right inferior temporal sulcus | 2.321 ± 0.24 | 2.364 ± 0.19 | 2.187 ± 0.19 | 4.317⁎ (0.111) | b⁎ |
| Right temporal pole | 3.387 ± 0.20 | 3.369 ± 0.22 | 3.205 ± 0.29 | 3.986⁎ (0.104) | a⁎ |
| Right inferior temporal gyrus | 2.724 ± 0.19 | 2.797 ± 0.12 | 2.668 ± 0.17 | 3.751⁎ (0.098) | b⁎ |
| Left transverse temporal gyrus | 2.380 ± 0.27 | 2.198 ± 0.24 | 2.193 ± 0.23 | 4.044⁎ (0.105) | c⁎ |
| Left temporal pole | 3.292 ± 0.23 | 3.273 ± 0.21 | 3.123 ± 0.26 | 3.294⁎ (0.087) | a^ |
| Limbic lobe | |||||
| Right anterior cingulate gyrus§ | 2.624 ± 0.10 | 2.578 ± 0.13 | 2.519 ± 0.18 | 3.162⁎ (0.082) | a⁎ |
| Left post. dorsal cingulate gyrus§ | 2.834 ± 0.16 | 2.692 ± 0.19 | 2.687 ± 0.22 | 4.651⁎ (0.119) | a⁎, c⁎ |
| Left post. ventral cingulate gyrus | 2.232 ± 0.24 | 2.062 ± 0.27 | 2.073 ± 0.23 | 3.573⁎ (0.094) | c⁎ |
| Insula | |||||
| Left superior circular sulcus | 2.517 ± 0.11 | 2.434 ± 0.14 | 2.393 ± 0.15 | 4.960⁎⁎ (0.126) | a⁎ |
| Left lateral sulcus (vertical ramus) | 2.297 ± 0.26 | 2.255 ± 0.26 | 2.110 ± 0.29 | 3.406⁎ (0.090) | a⁎ |
| Right lateral sulcus (horizont. ramus) | 2.210 ± 0.24 | 2.107 ± 0.19 | 2.066 ± 0.20 | 4.402⁎ (0.113) | a⁎ |
| Parietal lobe | |||||
| Left subparietal sulcus§ | 2.282 ± 0.17 | 2.169 ± 0.15 | 2.173 ± 0.18 | 3.526⁎ (0.093) | c⁎ |
| Occipital lobe | |||||
| Right superior - transverse sulci§ | 1.995 ± 0.14 | 1.870 ± 0.15 | 1.911 ± 0.18 | 4.201⁎ (0.109) | c⁎ |
Ns = not significant difference; a = HC Vs. ALSimp; b = ALScn Vs. ALSimp; c = HC Vs. ALScn.
= p < 0.05.
= p < 0.01.
= p < 0.005.
= uncorrected p < 0.05.
= not significant between group differences when excluding ALS cases with a repeat expansion in the C9orf72 gene.
3.4. Correlations
In the ALScn group, longer disease duration was related to CT reduction in the left precentral gyrus (r = −0.414, p = 0.049, CIs: −0.69 to −0.13), whereas increased motor disability (lower ALSFRS-R scores) was related to the thinning of the left superior circular sulcus (r = 0.486, p = 0.019, CIs: 0.16–0.70). There were no significant correlations among CT measures and clinical variables in ALSimp patients.
Significant correlations were found between cognitive performances and CT values in ALS patients (Table 4). Specifically, in ALScn patients abnormal results at episodic memory were related to thinning of left paracentral lobule and subparietal sulcus. ALSimp patients showed strong association between impaired performances in naming and verbal episodic memory and thinning of left temporal pole, insula and IFG.
Table 4.
Significant partial correlations (and bootstrapped 95% CIs) between neuropsychological performances and CT measures.
| HC | ALScn | ALSimp | |
|---|---|---|---|
| Frontal lobes | |||
| L paracentral lobule and sulcus | – | RM 0.586 (0.07; 0.85); p = 0.003 | – |
| L pars opercularis of the IFG | – | – | DR 0.710 (0.48; 0.89); p = 0.001 |
| Temporal lobe | |||
| L temporal pole | – | – | N 0.682 (0.25; 0.91); p = 0.002 DR 0.752 (0.50; 0.88); p < 0.001 |
| insula | |||
| L superior circular sulcus | – | – | N 0.644 (0.26; 0.87); p = 0.004 DR 0.659 (0.27; 0.88); p = 0.003 |
| Parietal lobe | |||
| L subparietal sulcus | – | DR 0.632(0.23; 0.82); p = 0.001 | – |
DR = Delayed Recall of the RAVLT; N = Naming; RM = Recognition Memory.
4. Discussion
We adopted a data-driven approach to identify the cognitive involvement in ALS to investigate the anatomical correlates of cognitive and behavioural profiles of non-demented patients (Consonni et al., 2016). This approach differs from the recently revised Strong et al. criteria (2017) in the separation of social cognition deficits from executive dysfunction, and in considering memory deficits. We identified a subgroup of 21 ALS patients, called ALSimp, showing a heterogeneous set of deficits encompassing behavioural (ALSbi), executive (ALSciα) and non-executive (ALSciβ) dysfunction related to language, social cognition and memory. In agreement with other studies (Agosta et al., 2016), ALSimp patients showed thinning of several specific cerebral areas besides the motor cortex. These included insula, AAC, temporal cortex and left inferior frontal gyrus (IFG). These cortical signatures were related to specific cognitive dysfunctions.
The thinning of the left insular cortex in the ALSimp group was related to poor performance in non-executive cognitive measures. Specifically, CT of the left vertical ramus of the lateral sulcus and the left superior circular sulcus was reduced compared to the HC group. The left vertical ramus is a perisylvian region connected to the superior segment of the circular sulcus of the insula in two thirds of the hemispheres (Destrieux et al., 2010). Because of its proximity to the pars triangularis of the Brodmann area 45 and the insula, it may be a candidate region to underpin language function. In our ALSimp cohort, the cortical thinning of the left superior circular sulcus was associated with defective naming and verbal recall performances. This association is in line with the pattern of neurodegeneration described in patients with the nonfluent/agrammatic variant of primary progressive aphasia (Gorno-Tempini et al., 2011; Nestor et al., 2003), suggesting that the left insula may be a cortical signature of language impairment in ALS.
AAC thinning has been associated with a wide range of extra-motor function impairment in ALS, including executive and socioemotional tasks (Cerami et al., 2014; Goldstein et al., 2011; Passamonti et al., 2013) and apathy (Woolley et al., 2011). Early diagnosed behavioural-variant FTD patients typically present impaired emotional and social processing, associated with selective atrophy of the ACC and frontal insular cortices (Piguet et al., 2011; Rascovsky et al., 2011). Similarly we demonstrated that the ACC was thinner in the ALSimp group compared to healthy volunteers. While these result may be biased by the inclusion of C9+ ALS patients (Cistaro et al., 2014), there is no consensus on the involvement of ACC in the C9+ pathology (Floeter et al., 2016; Westeneng et al., 2016).
The thinning of the IFG in the ALSimp group suggests that it could be a further early marker of cognitive decline. IFG thinning was associated with executive dysfunctions (Goldstein and Abrahams, 2013; Menke et al., 2014). In our cohort, the thinning of the left pars opercularis was not limited to C9+ ALS patients (Westeneng et al., 2016), being evident also in ALSimp patients, particularly those with lower performances at verbal recall tasks. These results suggest that the left IFG might be functionally related to different tasks assessing multiple cognitive domains, and support its role in the top-down cognitive control (Derrfuss et al., 2012), with the pars opercularis mainly involved in processing verbal information.
A further marker of cognitive impairment was found in the temporal lobes. This is one of most consistent finding in neuroimaging studies of grey matter atrophy in ALS (Chang et al., 2005). Yoshida (2004) reported marked cortical atrophy in the limbic system, including the temporal pole, in ALS cases with dementia defined at neuropathological exam. Therefore, the temporal lobe pathology may be a marker for the full-blown clinical picture of FTD spectrum (Lillo et al., 2012). The direct comparisons between ALSimp and ALScn groups showed a specific thinning of the right inferior temporal cortex, which has been previously associated with a more rapid clinical deterioration in ALSimp patients (Verstraete et al., 2012). On the other hand, the right temporal pole atrophy of ALSimp patients may reflect the presence and degree of semantic deficit, as in the case of the semantic variant of FTD (Leslie et al., 2015). The link between anterior temporal lobe atrophy and semantic impairment is supported by the strength of the correlations, showing a positive association between naming performances and CT in left temporal pole. These results are in line with the view that language dysfunction is an important component of the profile of cognitive impairment seen in ALS (Strong et al., 2017; Taylor et al., 2013), and that omitting language assessment in the evaluation of ALS cognitive changes may lead to underestimating specific cortical signatures. The inclusion of impaired naming performance as one of the requisites for cognitive impairment was sufficient to document temporal pole involvement in ALSimp patients. This finding strengthens the view that naming performance deserves major consideration, possibly like verbal fluency, in the assessment of cognitive impairment in ALS population.
The thinning of the temporal pole cortex was also linked to verbal episodic memory impairment in ALS patients. Correlation analyses controlled for age and educational level showed a significant direct relationship between naming and delayed recall performances at the Rey Auditory Verbal Learning Test (RAVLT) (r = 0.579, p < 0.001, bootstrapped CIs: 0.339–0.750). Lower naming performances corresponded to a reduced number of recalled words at RAVLT. In addition, 75% of ALS patients with defective performances at RAVLT delayed recall task had naming deficits, whereas only 23% of ALS patients with naming deficit had impaired performances at RAVLT delayed recall task (data not shown). These findings suggest that the association between thinning of the temporal pole and verbal episodic memory impairment could be partially biased by the underlying lexico-semantic deficit.
To summarize, the cortical signatures of cognitive and behavioural impairment in non-demented ALS patients overlap those typically observed in the main clinical presentations of FTD, i.e. the behavioural variant and progressive aphasias, supporting the concept of a frontotemporal spectrum disorder (FTSD).
We found cortical thinning common to both ALS subgroups. Specifically, the left paracentral lobule, considered as markers of central motor neuron degeneration (Agosta et al., 2012; Verstraete et al., 2012), was thinner in ALS subgroups. Surprisingly, in our ALScn sample, the thinning of the left paracentral lobule was related to poor visual recognition memory. The interpretation of this finding is unclear, as the primary motor cortex has been related mainly to motor learning (Richardson et al., 2006). ALS subgroups had also reduced CT in the right middle frontal sulcus. Some studies reported a negative correlation between ALS behavioural changes and GM volume in the right middle frontal gyrus (Terada et al., 2016). Our analyses and previous findings (Agosta et al., 2012) indicate instead that the reduced CT of this region is independent of the cognitive status and clinical features. A further cortical signature of both ALS subgroups is the thinning of the left PCC. Although PCC thinning was suggested to occur before the onset of ALS (Verstraete et al., 2012), its role in neurodegenerative process remains unclear. PCC is part of the default mode network (DMN), believed to integrate cognitive and emotional processing. Its increased activity has been associated with greater levels of disability and faster disease progression in ALS patients. This may reflect the loss of inhibitory neurons (Chenji et al., 2016), a known component of ALS pathology, or the increased functional recruitment of the DMN, maintaining an efficient cognitive performance in the presence of structural injury (Agosta et al., 2013).
The inclusion of C9+ ALS patients is a potential limitation of the study, even if the distribution of C9+ patients across ALS subgroups was relatively symmetrical (4 C9+ ALScn and 2 C9+ ALSimp patients). Our results are in line with the view of a more widespread cortical involvement in C9+ ALS (Westeneng et al., 2016), who showed cortical thinning of posterior (parietal, occipital, and dPCC), ACC and subcentral brain regions that we did not find in C9− ALS patients. Therefore, the presence of C9+ ALS individuals might underpin the CT reductions of the left subparietal sulcus, the right superior and transverse occipital sulci and the left subcentral cortex found in ALScn patients (Fig. 2).
Excluding the cortical regions associated with C9orf72 mutations, the left transverse temporal gyrus and the ventral portion of the PCC (vPCC) were thinner in ALScn patients than HC subjects. These regions have been already reported to be abnormal in ALS (Agosta et al., 2012; Devine et al., 2015; Westeneng et al., 2016). The significance of the thinning of the left transverse temporal gyrus, which contains the primary auditory cortex, is unclear. Subsequent analyses revealed that, at an uncorrected threshold, ALSimp group had reduced CT in both left vPCC and left transverse temporal Gyrus (data not shown) reducing the likelihood of a specific extra-motor involvement in C9− ALScn patients.
The CT measures were related to cognitive performances in ALS patients, including the ALScn group, but not in HC subjects. This may depend on the approach we used to identify the cognitive correlates of distinctive patterns of focal cortical atrophy of ALS, i.e. correlation analyses were performed only in brain regions that were significantly thinner in ALS patient groups than in the HC group. Therefore, ALSimp patients displayed extensive cortical thinning related to their cognitive deficits. ALScn patients, by definition, had HC-like cognitive performances but showed specific extra-motor involvement correlating with memory scores. The thickness reduction of the left subparietal sulcus was related to verbal episodic memory score only in ALScn patients, including the C9-ALScn subgroup (r = 0.582, p = 0.007, bootstrapped CIs: 0.134–0.783). This might represent an anatomical correlate of an ALS-related sub-threshold cognitive involvement. HC subjects did not show significant correlations surviving Bonferroni correction, probably due to the lower variability in CT and neuropsychological scores.
The relatively small size of our cohort may have prevented the identification of additional phenotypes. A larger cohort of cognitively impaired ALS patients, with a priori clustering based on anatomical patterns of involvement may allow the identification of specific cortical signatures for each neuropsychological profile. A comprehensive approach to cognition and behaviour in non-demented ALS subjects with extensive follow-up is warranted to further investigate these issues and correlate them with clinical course and specific phenotypes affecting behaviour, social cognition and different aspects of language performance.
In conclusion, our results demonstrate that distinctive patterns of focal cortical atrophy exist and differentiate non-motor clinical profiles of ALS patients, in agreement with the concept of FTSD, thus linking ALS to FTD.
Acknowledgments
Acknowledgements
We thank neurologists of the 3rd Neurology Unit of the IRCCS Foundation “Carlo Besta” Neurological Institute of Milan, Patrizia Dacci, Daniele Cazzato, and Susanna Usai, for referring some patients and Valentina C. Gessa for the neuropsychological assessment of some patients.
Funding
The study was financed by institutional funding (IRCCS Foundation “Carlo Besta” Neurological Institute, Ricerca Corrente, Italian Ministry of Health), Fondazione Regionale per la Ricerca Biomedica (FRRB) (20l5-0023) Regione Lombardia and Fondazione Italiana di Ricerca per la SLA (FGCR01/2013) - Sclerosi Laterale Amiotrofica (AriSLA).
Disclosure statement
M. Consonni, V.E. Contarino, E. Catricalà, E. Dalla Bella, V. Pensato, C. Gellera, G. Lauria, S.F. Cappa report no disclosures.
References
- Abdulla S., Machts J., Kaufmann J., Patrick K., Kollewe K., Dengler R., Heinze H.J., Petri S., Vielhaber S., Nestor P.J.L. Hippocampal degeneration in patients with amyotrophic lateral sclerosis. Neurobiol. Aging. 2014;35:2639–2645. doi: 10.1016/j.neurobiolaging.2014.05.035. [DOI] [PubMed] [Google Scholar]
- Agosta F., Valsasina P., Riva N., Copetti M., Messina M.J., Prelle A., Comi G., Filippi M. The cortical signature of amyotrophic lateral sclerosis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F., Canu E., Valsasina P., Riva N., Prelle A., Comi G., Filippi M. Divergent brain network connectivity in amyotrophic lateral sclerosis. Neurobiol. Aging. 2013;34:419–427. doi: 10.1016/j.neurobiolaging.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Agosta F., Ferraro P.M., Riva N., Spinelli E.G., Chiò A., Canu E., Valsasina P., Lunetta C., Iannaccone S., Copetti M., Prudente E., Comi G., Falini A., Filippi M. Structural brain correlates of cognitive and behavioral impairment in MND. Hum. Brain Mapp. 2016;37:1614–1626. doi: 10.1002/hbm.23124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S., Olm C., McMillan C.T., Boller A., Irwin D.J., McCluskey L., Elman L., Grossman M. Deficits in sentence expression in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal. Degener. 2015;16:31–39. doi: 10.3109/21678421.2014.974617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bede P., Elamin M., Byrne S., McLaughlin R.L., Kenna K., Vajda A., Pender N., Bradley D.G., Hardiman O. Basal ganglia involvement in amyotrophic lateral sclerosis. Neurology. 2013;81:2107–2115. doi: 10.1212/01.wnl.0000437313.80913.2c. [DOI] [PubMed] [Google Scholar]
- Beeldman E., Raaphorst J., Twennaar M.K., de Visser M., Schmand B.A., de Haan R.J. The cognitive profile of ALS: a systematic review and meta-analysis update. J. Neurol. Neurosurg. Psychiatry. 2016;87:611–619. doi: 10.1136/jnnp-2015-310734. [DOI] [PubMed] [Google Scholar]
- Boschi V., Catricalà E., Consonni M., Chesi C., Moro A., Cappa S.F. Connected speech in neurodegenerative language disorders: a review. Front. Psychol. 2017;8 doi: 10.3389/fpsyg.2017.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks B.R., Miller R.G., Swash M., Munsat T.L. World Federation of Neurology Research Group on motor neuron diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Burke T., Lonergan K., Pinto-Grau M., Elamin M., Bede P., Madden C., Hardiman O., Pender N. Visual encoding, consolidation, and retrieval in amyotrophic lateral sclerosis: executive function as a mediator, and predictor of performance. Amyotroph. Lateral Scler. Frontotemporal. Degener. 2017;13:1–9. doi: 10.1080/21678421.2016.1272615. [DOI] [PubMed] [Google Scholar]
- Carluer L., Mondou A., Buhour M.S., Laisney M., Pélerin A., Eustache F., Viader F., Desgranges B. Neural substrate of cognitive theory of mind impairment in amyotrophic lateral sclerosis. Cortex. 2015;65:19–30. doi: 10.1016/j.cortex.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Cerami C., Dodich A., Canessa N., Crespi C., Iannaccone S., Corbo M., Lunetta C., Consonni M., Scola E., Falini A., Cappa S.F. Emotional empathy in amyotrophic lateral sclerosis: a behavioural and voxel-based morphometry study. Amyotroph. Lateral. Scler. Frontotemporal. Degener. 2014;15:21–29. doi: 10.3109/21678421.2013.785568. [DOI] [PubMed] [Google Scholar]
- Chang J.L., Lomen-Hoerth C., Murphy J., Henry R.G., Kramer J.H., Miller B.L., Gorno-Tempini M.L. A voxel-based morphometry study of patter- of brain atrophy in ALS and ALS/FTLD. Neurology. 2005;65:75–80. doi: 10.1212/01.wnl.0000167602.38643.29. [DOI] [PubMed] [Google Scholar]
- Chenji S., Jha S., Lee D., Brown M., Seres P., Mah D., Kalra S. Investigating default mode and se-orimotor network connectivity in amyotrophic lateral sclerosis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christidi F., Zalonis I., Smyrnis N., Evdokimidis I. Selective attention and the three-process memory model for the interpretation of verbal free recall in amyotrophic lateral sclerosis. J. Int. Neuropsychol. Soc. 2012;18:809–818. doi: 10.1017/S1355617712000562. [DOI] [PubMed] [Google Scholar]
- Cistaro A., Pagani M., Montuschi A., Calvo A., Moglia C., Canosa A., Restagno G., Brunetti M., Traynor B.J., Nobili F., Carrara G., Fania P., Lopiano L., Valentini M.C., Chiò A. The metabolic signature of C9ORF72-related ALS: FDG PET comparison with nonmutated patients. Eur. J. Nucl. Med. Mol. Imaging. 2014;41:844–852. doi: 10.1007/s00259-013-2667-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consonni M., Iannaccone S., Cerami C., Frasson P., Lacerenza M., Lunetta C., Corbo M., Cappa S.F. The cognitive and behavioural profile of amyotrophic lateral sclerosis: application of the consensus criteria. Behav. Neurol. 2013;27:143–153. doi: 10.3233/BEN-2012-110202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consonni M., Catricalà E., Dalla Bella E., Gessa V.C., Lauria G., Cappa S.F. Beyond the consensus criteria: multiple cognitive profiles in amyotrophic lateral sclerosis? Cortex. 2016;81:162–167. doi: 10.1016/j.cortex.2016.04.014. (Degener 2014;15:21–9) [DOI] [PubMed] [Google Scholar]
- Derrfuss J., Vogtb V.L., Fiebacha C.J., von Cramonb D.Y., Tittgemeyerb M. Functional organization of the left inferior precentral sulcus: dissociating the inferior frontal eye field and the inferior frontal junction. NeuroImage. 2012;59:3829–3837. doi: 10.1016/j.neuroimage.2011.11.051. [DOI] [PubMed] [Google Scholar]
- Destrieux C., Fischl B., Dale A., Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage. 2010;53:1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine M.S., Pannek K., Coulthard A., McCombe P.A., Rose S.E., Henderson R.D. Exposing asymmetric gray matter vulnerability in amyotrophic lateral sclerosis. Neuroimage Clin. 2015;7:782–787. doi: 10.1016/j.nicl.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Tootell R.B., Dale A.M. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floeter M.K., Bageac D., Danielian L.E., Braun L.E., Traynor B.J., Kwan J.Y. Longitudinal imaging in C9orf72 mutation carriers: relationship to phenotype. Neuroimage Clin. 2016;22:1035–1043. doi: 10.1016/j.nicl.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons Z.C., Richardson A., Neary D., Snowden J.S. Behaviour in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2008;9:67–74. doi: 10.1080/17482960701642437. [DOI] [PubMed] [Google Scholar]
- Goldstein L.H., Abrahams S. Changes in cognition and behaviour in amyotrophic lateral sclerosis: nature of impairment and implications for assessment. Lancet Neurol. 2013;12:368–380. doi: 10.1016/S1474-4422(13)70026-7. [DOI] [PubMed] [Google Scholar]
- Goldstein L.H., Newsom-Davis I.C., Bryant V., Brammer M., Leigh P.N., Simmons A. Altered patterns of cortical activation in ALS patients during attention and cognitive response inhibition tasks. J. Neurol. 2011;258:2186–2198. doi: 10.1007/s00415-011-6088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Hillis A.E., Weintraub S., Kertesz A., Mendez M., Cappa S.F., Ogar J.M., Rohrer J.D., Black S., Boeve B.F., Manes F., Dronkers N.F., Vandenberghe R., Rascovsky K., Patterson K., Miller B.L., Knopman D.S., Hodges J.R., Mesulam M.M., Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M., Anderson C., Khan A., Avants B., Elman L., McCluskey L. Impaired action knowledge in amyotrophic lateral sclerosis. Neurology. 2008;71:1396–1401. doi: 10.1212/01.wnl.0000319701.50168.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie F.V., Hsieh S., Caga J., Savage S.A., Mioshi E., Hornberger M., Kiernan M.C., Hodges J.R., Burrell J.R. Semantic deficits in amyotrophic lateral sclerosis. Amyotroph. Lateral. Scler. Frontotemporal. Degener. 2015;16:46–53. doi: 10.3109/21678421.2014.987301. [DOI] [PubMed] [Google Scholar]
- Libon D.J., McMillan C., Avants B., Boller A., Morgan B., Burkholder L., Chandrasekaran K., Elman L., McCluskey L., Grossman M. Deficits in concept formation in amyotrophic lateral sclerosis. Neuropsychology. 2012;26:422–429. doi: 10.1037/a0028668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillo P., Mioshi E., Zoing M.C., Kiernan M.C., Hodges J.R. How common are behavioural changes in amyotrophic lateral sclerosis? Amyotroph. Lateral Scler. 2011;12:45–51. doi: 10.3109/17482968.2010.520718. [DOI] [PubMed] [Google Scholar]
- Lillo P., Mioshi E., Burrell J.R., Kiernan M.C., Hodges J.R., Hornberger M. Grey and white matter changes across the amyotrophic lateral sclerosis-frontotemporal dementia continuum. Plos-One. 2012;7 doi: 10.1371/journal.pone.0043993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machts J., Loewe K., Kaufmann J., Jakubiczka S., Abdulla S., Petri S., Dengler R., Heinze H.J., Vielhaber S., Schoenfeld M.A., Bede P. Basal ganglia pathology in ALS is associated with neuropsychological deficits. Neurology. 2015;85:1301–1309. doi: 10.1212/WNL.0000000000002017. [DOI] [PubMed] [Google Scholar]
- Menke R.A., Körner S., Filippini N., Douaud G., Knight S., Talbot K., Turner M.R. Widespread grey matter pathology dominates the longitudinal cerebral MRI and clinical landscape of amyotrophic lateral sclerosis. Brain. 2014;137:2546–2555. doi: 10.1093/brain/awu162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mioshi E., Lillo P., Yew B., Hsieh S., Savage S., Hodges J.R., Kiernan M.C., Hornberger M. Cortical atrophy in ALS is critically associated with neuropsychiatric and cognitive changes. Neurology. 2013;80:1117–1123. doi: 10.1212/WNL.0b013e31828869da. [DOI] [PubMed] [Google Scholar]
- Montuschi A., Iazzolino B., Calvo A., Moglia C., Lopiano L., Restagno G., Brunetti M., Ossola I., Lo Presti A., Cammarosano S., Canosa A., Chiò A. Cognitive correlates in amyotrophic lateral sclerosis: a population-based study in Italy. J. Neurol. Neurosurg. Psychiatry. 2015;86:168–173. doi: 10.1136/jnnp-2013-307223. [DOI] [PubMed] [Google Scholar]
- Nestor P.J., Graham N.L., Fryer T.D., Williams G.B., Patterson K., Hodges J.R. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain. 2003;126:2406–2418. doi: 10.1093/brain/awg240. [DOI] [PubMed] [Google Scholar]
- Passamonti L., Fera F., Tessitore A., Russo A., Cerasa A., Gioia C.M., Monsurrò M.R., Migliaccio R., Tedeschi G., Quattrone A. Dysfunctions within limbic-motor networks in amyotrophic lateral sclerosis. Neurobiol. Aging. 2013;34:2499–2509. doi: 10.1016/j.neurobiolaging.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Phukan J., Elamin M., Bede P., Jordan N., Gallagher L., Byrne S., Lynch C., Pender N., Hardiman O. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population-based study. J. Neurol. Neurosurg. Psychiatry. 2012;83:102–108. doi: 10.1136/jnnp-2011-300188. [DOI] [PubMed] [Google Scholar]
- Piguet O., Hornberger M., Mioshi E., Hodges J.R. Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurol. 2011;10:162–172. doi: 10.1016/S1474-4422(10)70299-4. [DOI] [PubMed] [Google Scholar]
- Raaphorst J., van Tol M.J., de Visser M., van der Kooi A.J., Majoie C.B., van den Berg L.H., Schmand B., Veltman D.J. Prose memory impairment in amyotrophic lateral sclerosis patients is related to hippocampus volume. Eur. J. Neurol. 2015;22:547–554. doi: 10.1111/ene.12615. [DOI] [PubMed] [Google Scholar]
- Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J., van Swieten J.C., Seelaar H., Dopper E.G., Onyike C.U., Hillis A.E., Josephs K.A., Boeve B.F., Kertesz A., Seeley W.W., Rankin K.P., Johnson J.K., Gorno-Tempini M.L., Rosen H., Prioleau-Latham C.E., Lee A., Kipps C.M., Lillo P., Piguet O., Rohrer J.D., Rossor M.N., Warren J.D., Fox N.C., Galasko D., Salmon D.P., Black S.E., Mesulam M., Weintraub S., Dickerson B.C., Diehl-Schmid J., Pasquier F., Deramecourt V., Lebert F., Pijnenburg Y., Chow T.W., Manes F., Grafman J., Cappa S.F., Freedman M., Grossman M., Miller B.L. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A.G., Overduin S.A., Valero-Cabré A., Padoa-Schioppa C., Pascual-Leone A., Bizzi E., Press D.Z. Disruption of primary motor cortex before learning impairs memory of movement dynamics. J. Neurosci. 2006;26:12466–12470. doi: 10.1523/JNEUROSCI.1139-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster C., Kasper E., Dyrba M., Machts J., Bittner D., Kaufmann J., Mitchell A.J., Benecke R., Teipel S., Vielhaber S., Prudlo J. Cortical thinning and its relation to cognition in amyotrophic lateral sclerosis. Neurobiol. Aging. 2014;35:240–246. doi: 10.1016/j.neurobiolaging.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Segonne F., Pacheco J., Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans. Med. Imaging. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Strong M.J., Abrahams S., Goldstein L.H., Woolley S., Mclaughlin P., Snowden J., Mioshi E., Roberts-South A., Benatar M., HortobáGyi T., Rosenfeld J., Silani V., Ince P.G., Turner M.R. Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph. Lateral. Scler. Frontotemporal. Degener. 2017;18:153–174. doi: 10.1080/21678421.2016.1267768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L.J., Brown R.G., Tsermentseli S., Al-Chalabi A., Shaw C.E., Ellis C.M., Leigh P.N., Goldstein L.H. Is language impairment more common than executive dysfunction in amyotrophic lateral sclerosis? J. Neurol. Neurosurg. Psychiatry. 2013;84:494–498. doi: 10.1136/jnnp-2012-303526. [DOI] [PubMed] [Google Scholar]
- Terada T., Obi T., Miyata J., Kubota M., Yoshizumi M., Murai T., Yamazaki K., Mizoguchi K. Correlation of frontal atrophy with behavioral changes in amyotrophic lateral sclerosis. Neurol. Clin. Neurosci. 2016;4:85–92. [Google Scholar]
- Tsujimoto M., Senda J., Ishihara T., Niimi Y., Kawai Y., Mizoguchi K. Behavioral changes in early ALS correlate with voxel-based morphometry and diffusion tensor imaging. J. Neurol. Sci. 2011;15:136–140. doi: 10.1016/j.jns.2011.05.025. [DOI] [PubMed] [Google Scholar]
- Verstraete E., Veldink J.H., Hendrikse J., Schelhaas H.J., van den Heuvel M.P., van den Berg L.H. Structural MRI reveals cortical thinning in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry. 2012;83:383–388. doi: 10.1136/jnnp-2011-300909. [DOI] [PubMed] [Google Scholar]
- Westeneng H.J., Walhout R., Straathof M., Schmidt R., Hendrikse J., Veldink J.H., van den Heuvel M.P., van den Berg L.H. Widespread structural brain involvement in ALS is not limited to the C9orf72 repeat expansion. J. Neurol. Neurosurg. Psychiatry. 2016;87:1354–1360. doi: 10.1136/jnnp-2016-313959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley S.C., Zhang Y., Schuff N., Weiner M.W., Katz J.S. Neuroanatomical correlates of apathy in ALS using 4 Tesla diffusion tensor MRI. Amyotroph. Lateral Scler. 2011;12:52–58. doi: 10.3109/17482968.2010.521842. [DOI] [PubMed] [Google Scholar]
- Yoshida M. Amyotrophic lateral sclerosis with dementia: the clinicopathological spectrum. Neuropathology. 2004;24:87–102. doi: 10.1111/j.1440-1789.2003.00544.x. [DOI] [PubMed] [Google Scholar]