Abstract
Objective. To evaluate the clinical effects of piribedil in adjuvant treatment of Parkinson’s Disease (PD) by pooling previously openly published studies. Methods. The related electronic databases of Medline (1960~2017.5), Cochrane central register of controlled trials (CENTRAL), EMBASE (1980~2017.5) and Wanfang (1986~20175.5) were searched by two reviewers (Lu Peihua and Wang Jianqian) independently for publications including the topic of prospective randomized controlled trials about clinical effects of piribedil in adjuvant treatment of PD. The data of each included study was extracted and pooled by Stata11.0 software (for meta-analysis). The statistical heterogeneity across the studies was evaluated by I2 test and the publication bias was calculated by begg’s funnel plot and Egger’s line regression test. Results. After searching the related electronic databases of Medline, CENTRAL, EMBSE and Wanfang databases, 11 clinical studies were included in this meta-analysis. The pooled RR (random effect model) of clinical efficacy was 1.29 (95%CI:1.18~1.41, P=4×10-3) indicating the clinical efficacy of piribedil group was signficat higher than those of control group. The standard mean difference (SMD) for UPDRS score changed before and after treatment was pooled by random effect model. The combined SMD was -0.41 (95%CI:-0.75~-0.06). For piribedil related side effects, the combined data indicated that there was no statistical difference for nausea and vomiting (RR=0.43, 95%CI:0.41~1.69, P=0.61), mental disorders (RR=0.85, 95%CI:0.45~1.59, P=0.61) and other toxicities (RR=0.32, 95%CI:0.09~1.16, P=0.08). Conclusion. Piribedil combined with Levodopa in adjuvant treatment of PD is more effective than Levodopa alone without increasing the drug related toxicity.
Keywords: Parkinson’s Disease, Meta-analysis, Clinical controlled trial, Adjuvant treatment, Piribedil, Levodopa
1. Introduction
Parkinson’s disease (PD) is one of the most common neurodegenerative diseases seen in clinic [1]. The main clinical features of PD are a group of clinical syndromes characterized mainly by static tremor, muscular rigidity, movement retardation and postural instability. The main pathological characteristics of PD are selective neuronal degeneration of dopamine neurons in striatum and characteristic eosinophilic inclusion bodies presenting in the remaining neurons [2, 3]. Therefore, the enzymes involved in the synthesis and metabolism of dopamine may become a drug target for the treatment of PD.
Piribedil also known as Pronoran, Trivastal Retard, Trastal, Trivastan and Clarium, is an antiparkinsonian agent and piperazine derivative which acts as a D2 and D3 receptor agonist. It also has α2-adrenergic antagonist properties [4, 5]. Piribedil is a direct dopamine agonist, in clinical use for treatment of dopaminergic system dysfunction. Recent work suggests that it is selective for the D3 subtype, for which it has 20 times higher affinity than for D2, and possesses no significant affinity for D1 receptors. Piribedil was used in treatment of PD, either as monotherapy (without levodopa) or in combination with levodopa therapy, in the early stages of the disease as well as in the advanced stages. Several published studies have evaluated the clinical efficacy and toxicities of piribedil combined with levodopa vs levodopa alone in the treatment of PD [6, 7]. However, the sample size of each individual study was small and the statistical power is weak. Therefore, we performed this meta-analysis by pooling the openly published data about piribedil combined with levodopa versus levodopa alone in the treatment of PD to further evaluate its clinical efficacy and providing more evidence for clinical practices.
2. Materials and methods
2.1. Publication searching
Prospective clinical studies about Piribedil combined with Levodopa vs Levodopa alone in the treatment of PD were electronicly searched in the electronic databases of Medline, the Cochrane central register of controlled trials (CENTRAL), EMBASE and Wanfang databases. The following search terms were used in searching each electronic database: “Parkinson’s Disease”, “PD”, “paralysis agitans”, “piribedil”, “Pronoran”, “Trivastal Retard”, “Trastal”, “Trivastan”, “Clarium”, “Levodopa”and “L-DOPA”. Searches were limited to human trials, with the language restriction of English and Chinese. The publication searches were performed by two reviewers (Lu Peihua and Wang Jianqian) independently. The references of the related articles were also reviewed to find any potential suitable publications.
2.2. Publication inclusion and exclusion criteria
The publication inclusion criteria were made according to the study type, patients, treatment methods, treatment regimen of experiment and control groups and the outcomes of the original publications:(1) The study type was prospective randomized controlled trials; (2) The patients were clinically diagnosed with PD; (3) Patients in the piribedil group were treated with Piribedil combined with Levodopa; (4) Patients in the control group were treated with Levodopa alone; (5)For the outcome, the original study should provided the treatment clinical efficacy, Unified Parkinson’s Disease Rating Scale (UPDRS) score changes and drug related toxicities. The title and abstract of the potential applicable studies were reviewed firstly to evaluate whether it was appropriate for inclusion criteria or not. Then all the potentially suitable studies were further evaluated as full-text papers.
2.3. Data extraction
Two reviewers (Lu Peihua and Wang Jianqian) independently reviewed the full-text papers and extracted the data from each of the included studies. The extracted data and information included: (1) Journal of the study published; (2) First and corresponding authors; (3) The paper publication year; (4) The country or area the study was performed; (5) The treatment regimen; (6) The sample size; and (7) outcomes such as clinical efficacy, UPDRS score changes and drug related toxicities. The results were cross-checked.
2.4. Statistical analysis
STATA/SE 11.0 software (for meta-analysis version) was used for the statistical analysis. Dichotomous data such as clinical efficacy and drug related side effects was demonstrated by risk ratio (RR). The measurement data such as UPDRS score changes was expressed by mean with its standard difference. The statistical heterogeneity of the included 11 publications was evaluated by I2 test [8]. The data were pooled by fixed or random effect model according to the statistical heterogeneity. The publication bias was assessed by Begg’s funnel plot and Egger’s line regression test. P<0.05 was deemed as statistically different [9].
3. Results
3.1. Publications searching
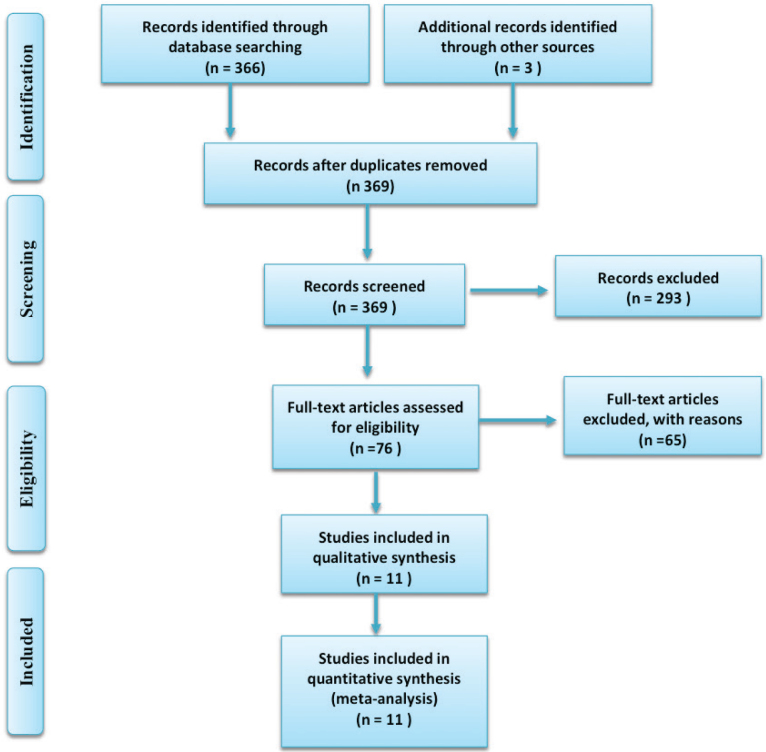
After searching the related electronic databases of Medline (1960~2017.5), CENTRAL, EMBASE (1980~2017.5) and CNKI (1986~20175.5), we firstly identified 369 relevant publications. After reading the titles and abstracts, 293 studies were excluded for not being suitable for inclusion. Seventy-six papers were reviewed as full text papers. Finally 11 clinical studies [6, 7, 10, 11, 12, 13, 14, 15, 16, 17, 18] were finally included in this meta-analysis. The publication inclusion process is shown in Figure 1. The general character of the included individual 11 publications are demonstrated in Table 1.
Figure 1.
The searching flow chart of this meta-analysis
Table 1.
The general features of the included RCTs
| First author | Country | Sample size | Age (year) | Regimen | Outcome | Course (moth) |
|---|---|---|---|---|---|---|
| Thobois2013 | France | 19 | 58.6±6.5 | Piribedil 300gm/d+Levodopa 75-300mg/d | Efficacy, UPDRS, HAMD, Side effects | 3 |
| Ziegler2003 | France | 61 | 63.4±7.3 | Piribedil 150gm/d+Levodopa 423 mg/d | Efficacy, UPDRS, Side effects | 6 |
| Tang GW2007 | China | 25 | 58.8±8.9 | Piribedil 150gm/d+Levodopa 125 mg/d | Efficacy, UPDRS | 3 |
| Peng X2014 | China | 29 | 60.6±7.1 | Piribedil 100-150gm/d+Levodopa 500-1000 mg/d | Efficacy, UPDRS, Side effects | 6 |
| Du DQ2013 | China | 21 | Na | Piribedil 100-150gm/d+Levodopa 250-750 mg/d | Efficacy, Side effects | 4 |
| Mao KS2014 | China | 38 | NA | Piribedil 150gm/d+Levodopa 125-1000 mg/d | Efficacy, UPDRS | 4 |
| Wang WF2010 | China | 36 | NA | Piribedil 100gm/d+Levodopa 125-500 mg/d | UPDRS | 6 |
| Cheng YB2007 | China | 20 | 62.8±5.2 | Piribedil 50-100gm/d+Levodopa 150 mg/d | UPDRS, HAMD | 6 |
| Zheng CM2010 | China | 29 | 64.3±7.1 | Piribedil 150-250gm/d+Levodopa 300-600 mg/d | Efficacy, UPDRS | 12 |
| Qian JJ2007 | China | 15 | 63.5±6.0 | Piribedil 50gm/d+Levodopa 250 mg/d | UPDRS | 6 |
| Gong XY2016 | China | 140 | 68.2±2.4 | Piribedil 100-150gm/d+Levodopa 375mg/d | Efficacy, UPDRS | 6 |
3.2. Clinical efficacy
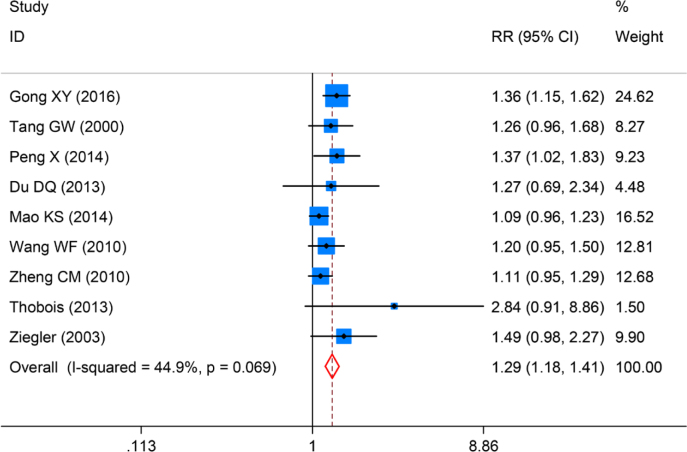
Nine studies evaluated the clinical efficacy between piribedil and control groups. We firstly evaluated the statistical heterogeneity of the 9 included studies by I2 test and found the statistical heterogeneity was not significant (I2=44.9%, P=0.07). The clinical efficacy was pooled by fixed effects model. The pooled RR was 1.29 (95%CI:1.18~1.41, P=4×10-3), Figure 2. This indicated that in patients with PD, treatment with piribedil combined with levodopa can significant improve the clinical efficacy.
Figure 2.
Forrest plot of clinical efficacy for piribedil in adjuvant treatment of Parkinson’s Disease
3.3. UPDRS score
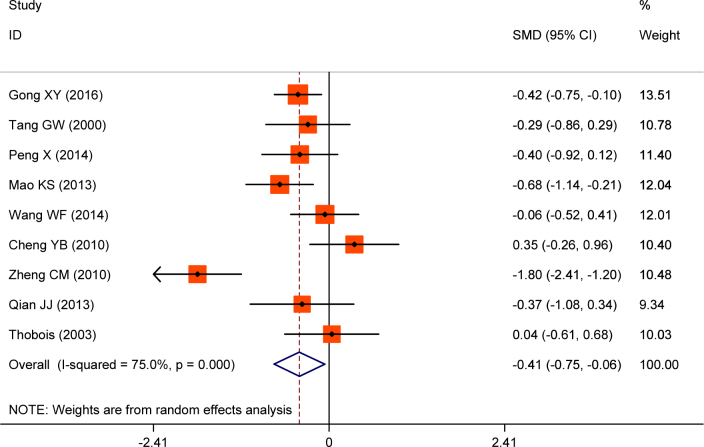
Nine studies reported the UPDRS score before and after treatment. Significant statistical heterogeneity was foud in the effect size of standard mean difference (SMD) for UPDRS score changes(I2=75.0%). The SMD was pooled by random effect model. The combined SMD was -0.41(95%CI:-0.75~-0.06), Figure 3. These results demonstrated that the UPDAR I score can be significantly improved in patients that receive combined treament.
Figure 3.
Forrest plot of UPDRS score changes for piribedil in adjuvant treatment of Parkinson’s Disease
3.4. Side effects
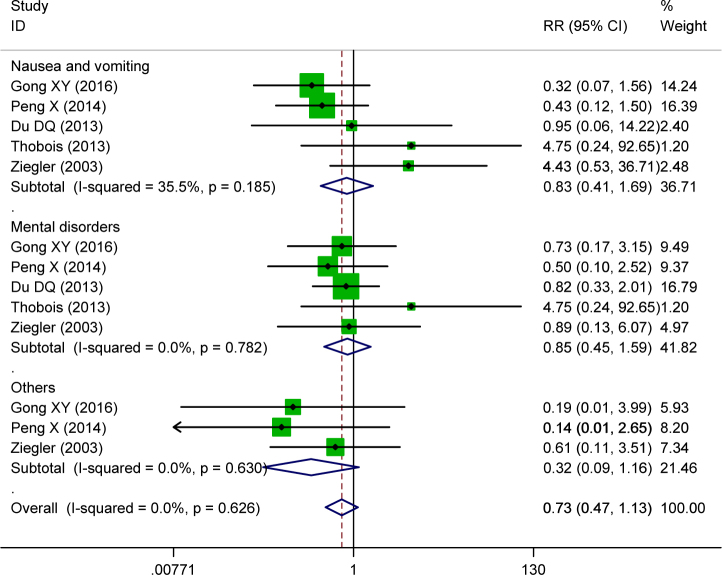
Five studies reported drug related nausea and vomiting side effects; 5 studies provided data on mental disorders of the two groups and 3 publications reported other side effects related to piribedil. There was no statistical heterogeneity for side effect evaluation parameters. The data was pooled by fixed effect model. The combined data indicated that there was no statistical difference for drug related side effects of nausea and vomiting (RR=0.43, 95%CI:0.41~1.69, P=0.61), mental disorders (RR=0.85, 95%CI:0.45~1.59, P=0.61) and other toxicities (RR=0.32, 95%CI:0.09~1.16, P=0.08), Figure 4. The pooled toxicity indicated the combined treatment didn’t elevate the drug related toxicity risk.
Figure 4.
Forrest plot of side effects of piribedil in adjuvant treatment of Parkinson’s Disease
3.5. Publication bias evaluation
We used Begg’s funnel plot and Egger’s line regression test to assess the publication bias for each effect size. There were no significant publication biases for clinical efficacy (t=0.41, P=0.69), Figure 5; UPDRS score changes (t=0.06, P=0.97), Figure 6; and side effects (t=-1.18, P=0.53), Figure 7. All of the funnel plots showed approximately left and right symmetry.
Figure 5.
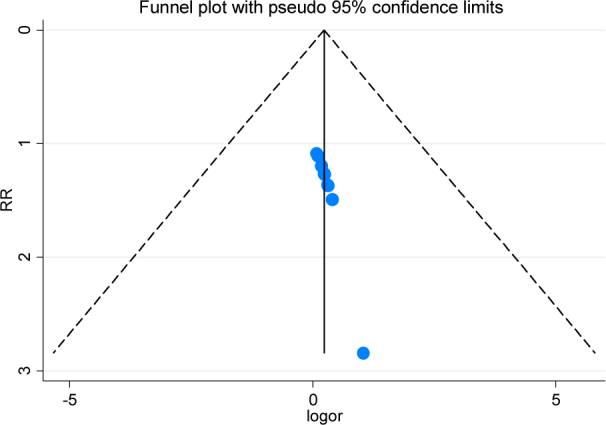
The Begg’s funnel plot for clinical efficacy of piribedil in adjuvant treatment of Parkinson’s Disease
Figure 6.
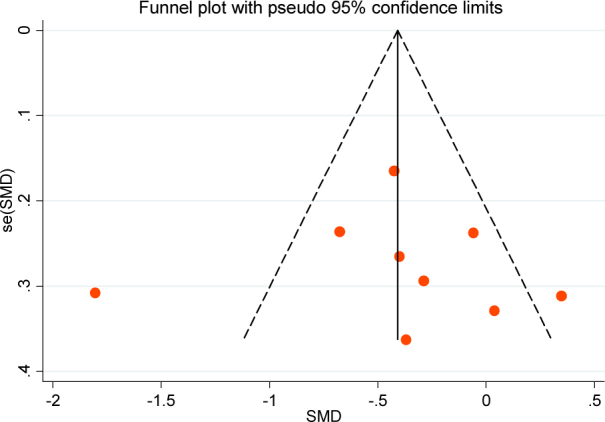
The Begg’s funnel plot for UPDRS score changes of piribedil in adjuvant treatment of Parkinson’s Disease
Figure 7.
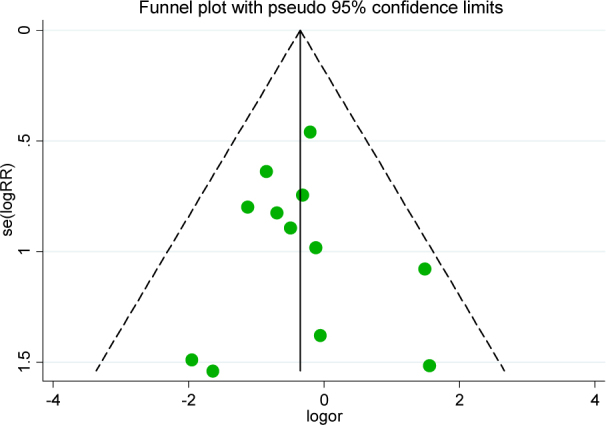
The Begg’s funnel plot for side effects of piribedil in adjuvant treatment of Parkinson’s Disease
4. Discussion
Although the clinical symptoms and characteristics of PD have been described in detail in the past two centuries, the exact etiology and pathogenesis remain incompletely understood [19, 20]. Previously published studies showed that the incidence of PD is associated with nigrostriatal degeneration and significant decrease of dopamine levels in the brain [21, 22, 23]. The main pathological changes of PD are selective neuronal degeneration of dopamine neurons in striatum and characteristic eosinophilic inclusion bodies present in the remaining neurons. At present, the most effective treatment for PD is levodopa combined with dopa decarboxylase inhibitors. However, long-term use of these drugs can lead to dyskinesia, “agent end effect” and “open - pass” phenomenon and other adverse reactions.
Piribedil is a synthetic non-ergot dopamine receptor agonist which selectively and directly stimulates the nigrostriatal pathway D1 and postsynaptic D2 receptor and limbic pathway D3 receptor. Previously studies indicated that Piribedil can increase dopamine receptor excitability, thereby improving motor symptoms in patients.
Clinical efficacy and drug related toxicity of Piribedil in adjuvant treatment of Parkinson’s Disease had been widely discussed according to the previously pubished studies. Zheng CM and his colleges [17] performed a prospective randomized clinical trial including 60 patients with PD and randomly divided them into control and experimental groups. Patients in the control group were given levodopa only and patients in the experimental group were administered levodopa and piribedil. After 12 months administration, the authors found that the clinical efficacy in the experimental group was significantly higher than those of control group and the UPDRS score was also significantly lower in the experimental group than that of control group after treatment. However, the improved clinical efficacy and UPDRS score were not found in another clinical trial performed by Du DQ and et al [13]. So, the clinical efficacy of Piribedil in adjuvant treatment of PD was not consistent. This may be due to the small sample size of each individual study with limited statistical power. Therefore, high quality meta-analysis was needed to further quantify the clinical efficacy of Piribedil in adjuvant treatment of PD by pooling data from previously openly published studies, which can increase the statistical power and provide more information for its clinical usage.
In our present meta-analysis, we systematically searched the electronic databases and finally included 11 clinical trials and evaluated the pooled clinical efficacy, UPDRS score changes and drug related toxicities. The pooled results indicated the clinical efficacy of piribedil group was signficantly higher than those of the control group (P<0.05). Clincial efficacy of patients with PD can be significantly improved by adding the piribedil. These results were in accordance with most of the previously published studies [4,8]. We also found that the combined treatment can signifcantly decrease the UPDRS I score compared to the control group. This indicated that the symptoms can be improved by adding piribedil. However, the combinded treatment did not increase drug related toxicities such as gastrointestinal reaction, mental disorders and other side effects. This meta-analysis indicated piribedil combined with levodopa in adjuvant treatment of PD is more effective than levodopa alone without increasing the drug related toxicity.
Our work found that piribedil combined with levodopa can significantly improve the clinical efficacy and UPDRS score in treatment of PD. However, there are two limitations for this meta-analysis. Firsly, we only inlcuded studies published in Chinese and English. Secondly, the treatment course of each individual study was not in accordance with the others; this may be a important clincial heterogeneity. In view of the above limitations, multi-center prospective randomized controlled trials with large samples are needed for further evaluation of the clinical efficacy of piribedil in adjuvant treatment of PD and provide more confident conclusions for clinical practices.
Footnotes
Conflict of interest
Conflict of interest statement: Authors state no conflict of interest.
References
- [1].Ellis JM, Fell MJ.. Current approaches to the treatment of Parkinson’s Disease. Bioorg Med Chem Lett. 2017;27:4247–4255. doi: 10.1016/j.bmcl.2017.07.075. [DOI] [PubMed] [Google Scholar]
- [2].Schneider SA, Obeso JA.. Clinical and pathological features of Parkinson’s disease. Curr Top Behav Neurosci. 2015;22:205–220. doi: 10.1007/7854_2014_317. [DOI] [PubMed] [Google Scholar]
- [3].Matsui H, Takahashi R.. [Pathological mechanisms of Parkinson’s disease] Brain Nerve. 2009;61:441–446. [PubMed] [Google Scholar]
- [4].Jenner P.. Parkinson’s disease: pathological mechanisms and actions of piribedil. J Neurol. 1992;239(1):S2–8. doi: 10.1007/BF00819559. [DOI] [PubMed] [Google Scholar]
- [5].Perez-Lloret S, Rascol O.. Piribedil for the Treatment of Motor and Non-motor Symptoms of Parkinson Disease. CNS Drugs. 2016;30:703–717. doi: 10.1007/s40263-016-0360-5. [DOI] [PubMed] [Google Scholar]
- [6].Thobois S, Lhommée E, Klinger H, Ardouin C, Schmitt E, Bichon A, Kistner A, Castrioto A, Xie J, Fraix V, Pelissier P, Chabardes S, Mertens P, Quesada JL, Bosson JL, Pollak P, Broussolle E, Krack P.. Parkinsonian apathy responds to dopaminergic stimulation of D2/D3 receptors with piribedil. Brain. 2013;136:1568–1577. doi: 10.1093/brain/awt067. [DOI] [PubMed] [Google Scholar]
- [7].Ziegler M, Castro-Caldas A, Del SS, Rascol O.. Efficacy of piribedil as early combination to levodopa in patients with stable Parkinson’s disease: a 6-month, randomized, placebo-controlled study. Mov Disord. 2003;18:418–425. doi: 10.1002/mds.10359. [DOI] [PubMed] [Google Scholar]
- [8].Higgins JP, Thompson SG, Deeks JJ, Altman DG.. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Song F, Gilbody S. Increase in studies of publication bias coincided with increasing use of meta-analysis. 7129. Vol. 316. BMJ (Clinical research ed.); 1998. Bias in meta-analysis detected by a simple, graphical test; p. 471. [PMC free article] [PubMed] [Google Scholar]
- [10].Xiao-ying G, Li Z, Ji-heng W, Hong-yan AN.. The efficacy of levodopa and benserazide combined piribedil in the treatment of Parkinson’s disease. Clinical Research and Practice. 2016;1:86–87. [Google Scholar]
- [11].Guowen T, Guofeng Z, Xiaowen W.. Clincial effect of levodopa combined piribedil Serta treatment of Parkinson’s disease. International Medicine & Health Guidance News. 2007;13:45–46. [Google Scholar]
- [12].Xiao P, Huifen H.. Effect of Piribedil Combined with Madopar in the Treatment of Parkinson’ s Disease. China Pharmacist. 2014:806–807. [Google Scholar]
- [13].Du Dengqing, Qiong Z, Xuefan C, Xinjiang L.. Clinical efficacy of Piribedil Combined with Madopar in the Treatment of Parkinson’ s Disease: Reported of 62 cases. China Practical Medical. 2013;8:130–131. [Google Scholar]
- [14].Keshi M.. Effect of Madopar and Piribedil combined treatment of Parkinson’s disease. China Foreign Medical Treatment. 2014:145–146. [Google Scholar]
- [15].Wenfeng W.. The curative effects of madopar and piribedil combined treatment on Parkinson disease. China Practical Medical. 2010;5:21–22. [Google Scholar]
- [16].Yan-Bo C, Jin-Jun Q, Cheng-Jie M, Kang-Yong L, Chun-Feng L.. Influence of Piribedil on Non-motor Symptoms in Early Parkinson’s Disease. Chinese Journal of Clinical Neurosciences. 2007;15:119–122. [Google Scholar]
- [17].Changmin Z, Guili S, Lei C.. The Therapeutic Effects of Madopa in Conjunction with Piribedil in Advanced Pakinson’s Disease. Guide of China Medicine. 2010;8:32–33. [Google Scholar]
- [18].Jinjun Q, Yanbo C, Chunfeng L, Chengjie M, Xuezhong L.. Impacts of piribedil and levodopa on 99mTc-TRODAT-1-SPECT imaging and motor function of early stage Parkinson’s disease. Jiangsu Medical Journal. 2007;33:447–449. [Google Scholar]
- [19].Ohta S.. [Study of Parkinson’s disease-causing mechanism and development of anti-Parkinson’s disease drugs using endogenous substances in brain] Nihon Shinkei Seishin Yakurigaku Zasshi. 2005;25:39–42. [PubMed] [Google Scholar]
- [20].Hirsch EC.. Mechanism and consequences of nerve cell death in Parkinson’s disease. J Neural Transm Suppl. 1999;56:127–137. doi: 10.1007/978-3-7091-6360-3_7. [DOI] [PubMed] [Google Scholar]
- [21].Melcangi RC, Caruso D, Levandis G, Abbiati F, Armentero MT, Blandini F.. Modifications of neuroactive steroid levels in an experimental model of nigrostriatal degeneration: potential relevance to the pathophysiology of Parkinson’s disease. J Mol Neurosci. 2012;46:177–183. doi: 10.1007/s12031-011-9570-y. [DOI] [PubMed] [Google Scholar]
- [22].Armentero MT, Levandis G, Bramanti P, Nappi G, Blandini F.. Dietary restriction does not prevent nigrostriatal degeneration in the 6-hydroxydopamine model of Parkinson’s disease. Exp Neurol. 2008;212:548–551. doi: 10.1016/j.expneurol.2008.04.006. [DOI] [PubMed] [Google Scholar]
- [23].Happe S, Baier PC, Helmschmied K, Meller J, Tatsch K, Paulus W.. Association of daytime sleepiness with nigrostriatal dopaminergic degeneration in early Parkinson’s disease. J Neurol. 2007;254:1037–1043. doi: 10.1007/s00415-006-0483-6. [DOI] [PubMed] [Google Scholar]