Abstract
The practice of ovulation induction often falls to the reproductive endocrinology and infertility specialist. However, attitudes toward the evaluation and treatment of infertility has shifted among general obstetrician-gynecologists (OB-GYN). This review discusses the underlying scientific basis of anovulation and clinical guidelines regarding the use of different medications for the purpose of promoting follicular recruitment and ovulation for the general OB-GYN.
Keywords: Ovulation induction, Clomiphene citrate, Infertility
Background
Currently, 15.5% of American couples are affected by infertility [1]. This proportion is anticipated to increase to 1 in 7–8 by 2025, likely secondary to delayed childbearing [1]. Etiologies of infertility may include tubal factors (14–20%), male factors (30%), pelvic/uterine abnormalities (10–13%), and ovulatory dysfunction (21–25%) [2]. Ovulation induction (OI) is commonly used to treat infertility, particularly anovulation; however, ovulatory individuals with unexplained infertility may also benefit from OI [2].
OI medications encourage the production of a dominant follicle through various mechanisms, including preventing the conversion of androgens to estrogens, acting as an antagonist on estrogen receptors, insulin sensitization, or by direction stimulation of hypothalamus through gonadotropins. Surgical management may also be considered as second-line therapy via laparoscopic ovarian drilling (LOD) [3].
Traditionally, infertility treatment is managed by the REI specialist. However, attitudes toward evaluation and treatment of infertility have shifted among general obstetrician-gynecologists (OB-GYN). Though many clinicians held unfavorable opinions towards the treatment of reproductive difficulties in the 2000 s, more recent surveys suggest that the majority now consider the primary care setting appropriate for fertility management [4]. Nevertheless, many generalists report some degree of discomfort with administration of OI medications, citing lack of expertise concerning reproductive endocrinology, medication management, and monitoring ovarian response [4]. This review discusses the underlying scientific basis of anovulation and clinical guidelines regarding the use of different medications for the purpose of promoting follicular recruitment and ovulation.
General Physiology
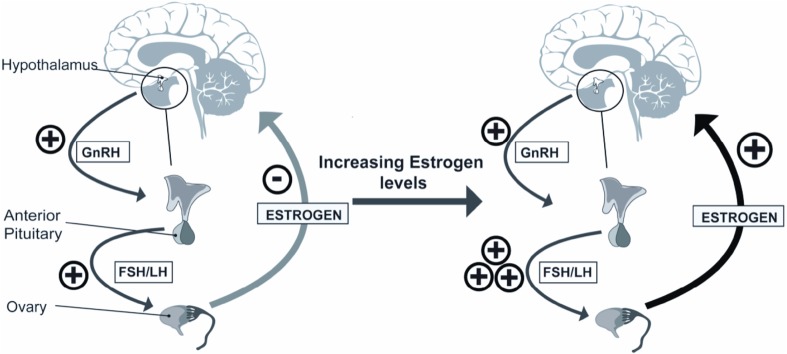
Ovulatory disorders account for approximately 25% of female infertility cases [5]. The creation of a mature oocyte is controlled by a complex feedback system that requires extensive coordination between the hypothalamus, anterior pituitary, and ovaries (HPO axis), as well as local factors produced by the ovary and external endocrine factors [6]. Dysregulation at any point in this pathway may result in various pathologies, culminating in ovulatory dysfunction, or even ovarian failure, and patients often present with infertility [5].
The production of the oocyte begins at 6–8 weeks of gestation, when primordial germ cells first undergo mitosis, then meiosis, to produce a primary oocyte that is housed in the primordial follicle within the ovarian cortex. These primordial follicles are surrounded by a single layer of granulosa cells that are arrested in the diplotene phase of Meiosis I until the onset of menses [6–8]. Though approximately one million oocytes are present in the ovary at birth, only 400–500 ovulate throughout the childbearing years [6]. The primordial follicle continues to develop into the primary follicle, then the secondary follicle, which is surrounded by granulosa cells that contain FSH receptors and theca cells. During these phases, the growth of the follicle is independent of gonadotropins. The majority of follicles at this point will undergo atresia [9].
The proliferation of the supporting granulosa and theca cells creates a fluid-filled space called the antrum. Once this is formed, the follicle becomes dependent on FSH for continued growth and development. During the latter half of this portion of the follicular phase, the follicles may be referred to as antral follicles, Graafian follicles, or preovulatory follicles. Although multiple follicles are recruited each month, it takes several menstrual cycles for them to mature enough to achieve preovulatory status. When a threshold level of FSH is reached, during a time period known as the “FSH window”, a set cohort of preovulatory follicles is recruited. These follicles are able to respond to the rising FSH/LH levels, secrete estrogen, and continue to grow (Fig. 1) [10, 11]. One follicle responds more robustly, creating high estrogen levels within the follicle’s microenvironment through aromatase activity and generating a higher concentration of FSH receptors which perpetuate this follicle’s growth. On a macroenvironment level, FSH levels are waning secondary to estrogen’s inhibitory effect and the remaining preovulatory follicles undergo apoptosis.
Fig. 1.
Hypothalamic–pituitary–ovarian axis
The theca cells surrounding the dominant follicle contain LH receptors. In response to LH stimulation, the theca cell produces steroids, the precursors necessary for the production of more estrogen via aromatization. As estrogen levels continue to rise, FSH activity switches to increasing the concentration of LH receptors in the granulosa cells. Once a peak level of estradiol is reached, the hypothalamus is stimulated, causing an LH surge and enabling ovulation [6]. The remaining follicle degrades to form the corpus luteum, which secretes progesterone and supports the pregnancy until the formation of the placenta [12].
Infertility Workup
Prior to initiating any infertility treatment, a complete workup should be performed to rule out underlying pathologies, such as endocrine dysfunction. Clinical workup should include a complete menstrual, obstetrical, medical and surgical history, as well as the assessment of current medications or drug use that could impact fertility. Laboratory workup involves evaluation of thyroid function, prolactin levels, day 3 FSH with estradiol, progesterone on day 21 or 1 week prior to onset of menses, any sexually transmitted infections, and consideration to anti-Mullerian hormone for ovarian reserve [2]. Imaging should include confirmation of tubal patency via hysterosalpingogram or saline infusion sonohysterogram [2]. As male factor is present in approximately 50% of infertile couples, it would be remiss not to include a complete history and physical of the male partner, in addition to a semen analysis [2].
Classification of Ovulatory Dysfunction
The World Health Organization (WHO) provides a classification system for ovulation dysfunction that guides infertility treatment. Ovarian insufficiency is divided by gonadotropin and estrogen levels (Table 1) [5].
Table 1.
WHO Classifications of ovulatory dysfunction
| Group | Gonadotropin levels | Estrogen secretion | Cause |
|---|---|---|---|
| I | Low | Low | Hypothalamic-pituitary failure |
| II | Normal | Normal | Hypothalamic-pituitary-ovarian axis failure |
| III | High | Low | Ovarian failure |
WHO Group I
WHO Group I describes 5–10% of anovulatory patients with dysfunction of the HPO axis, demonstrated by low or sometimes normal levels of gonadotropins (FSH/LH) and low estrogen. Without an intact HPO axis, WHO Group 1 patients are unable to respond to FSH/LH and, therefore, do not recruit any follicles or have appreciable increase in estradiol during the follicular phase [5]. As these individuals have underlying low levels of estrogen, a progesterone withdrawal will not induce menses since the endometrium has not been properly primed by estrogen. Numerous etiologies may be present, including disease, infection, or neoplasia of the hypothalamus or anterior pituitary. However, WHO Group I anovulation is often seen with negative energy balance, such as in anorexia nervosa or endurance athletes.
WHO Group II
Approximately, 85% of anovulatory patients fall under the WHO Group II classification [5]. They often have normal levels of FSH and normal to elevated LH levels, but the ovaries do not respond appropriately; thus, no dominant follicle is established. WHO Group II can be due to various etiologies including Polycystic Ovary Syndrome (PCOS), congenital adrenal hyperplasia, abnormal levels of FSH/LH receptors, and certain medications [5].
PCOS is the most commonly encountered condition in this group and is defined by excessive ovarian androgen production, oligo or amenorrhea, and polycystic ovaries [5]. The high level of androgens in PCOS facilitates the recruitment of multiple follicles, but never reaches the FSH window; thus, no dominant follicle is produced nor does estrogen reach the appropriate threshold to stimulate the LH surge [5].
WHO Group III
WHO Group III includes women with anovulation due to excessive gonadotropin production with low estrogen levels due to non-functioning ovaries. This is also known as premature ovarian failure or premature menopause, where FSH/LH levels are extremely high as the pituitary continues to secrete gonadotropins in an attempt to stimulate the non-functioning ovaries [5]. These individuals are often resistant to any type of OI treatment [5].
Medications Used for OI
Clomiphene Citrate
Mechanism and Clinical Outcomes
Clomiphene citrate (CC) is a selective estrogen receptor modulator (SERM) that has been first-line treatment of patients with anovulation or oligomenorrhea for more than 40 years. CC competes with endogenous estrogen at receptors in the hypothalamus and pituitary gland, interfering with the negative feedback signaling of natural estrogen. Compared to natural estrogen, CC binds in the hypothalamus for a duration of weeks rather than days, effectively blocking the replenishment of estrogen receptors. Due to this hypoestrogenic state, release of GnRH and FSH is uninhibited; therefore, CC administration requires an intact HPO axis to respond appropriately. The elevated levels of FSH cause hyperstimulation of the ovary and the potential for multiple follicles to develop [6].
CC has traditionally been a cornerstone of OI treatment because of its efficacy. The live birth rate (LBR) associated with CC monotherapy is 23.3% [13, 14].
Administration and Dosing
CC dosage varies with body weight; however, there is no reliable way to accurately predict what dose will be required in an individual woman. Therefore, a ‘stair-step’ protocol is often employed to enable optimal dosing without excessive stimulation. Ovulation is expected to occur 5–10 days after the last dose of CC [15]. Once ovulation has occurred, there should be no increase in the CC dose. There are multiple different protocols and management techniques employed for the use of CC to enable flexibility and tailoring of treatment to each individual patient and provider.
The initial dosing of CC is typically 50 mg orally for 5 days beginning on day 2–5 of the menstrual cycle with ovulation occurring 5–10 days afterwards. Literature shows that pregnancy outcomes are similar whether OI with CC is begun on day 2, 3, 4, or 5 in anovulatory patients, allowing some flexibility in administration [15]. This approach enables clinicians to ‘batch’ patients together by coordinating the start of treatment with an expected window for ovulation and avoids testing or follow-up appointments on weekends. Table 2 suggests treatment schedules when CC is initiated on day 5. If an unexpected scheduling challenge occurs, the patient may be started on an oral contraceptive pill to postpone ovarian recruitment. This technique avoids skipping a month of treatment, should the intended timing of OI become inconvenient.
Table 2.
Ovulation induction medications
| Medication | Dose Day (D) |
Initiate treatment Day (D) |
LBR | Side effects | Risk of M, OHSS, MC | Costs ($/cycle) |
|---|---|---|---|---|---|---|
| CC | 50 mg × 5 d [57] Max dose 250 mg |
D2–5 | 23.3% [13, 14] | Mood swings Hot flashes Diplopia Scotoma Photophobia [5, 58] |
M: <10% [45] OHSS: 0.5–2.5% [59] MC: 20% [56] |
$4 |
| Tamoxifen | 20 mg × 5 d Max dose 80 mg [24, 25] |
D5 [25] | 34% [20] | Hot flashes Atrophic vaginitis Irregular menses Heightened risk of cataracts and deep vein thrombosis [60] |
M: 0% [24] OHSS: 0% [24] MC: 6% [20] |
$5 |
| CC + Tamoxifen | 150 mg CC × 5 d 40 mg tamoxifen × 5 d [22] |
D3 [22] | 49.3% (triggered cycles) [22] | See CC and Tamoxifen | M: 0% [61] | $9 |
| Letrozole | 2.5 mg × 5 d [57] Max dose 7.5 mg [62] |
D3 [63] | 27.5% [21] | Decreased bone density Arthralgias/myalgias Vaginal dryness Loss of libido Dyspareunia [64] |
M:13% [38] OHSS: 9% [65] MC: 8–9% [20, 57] |
$6 |
| Metformin | Initial: 500 mg/d Titrate up 500 mg/d every week Max dose 2500 mg/d [32] |
Daily [32] | 7–52% [6, 67] | Abdominal pain Nausea Vomiting Diarrhea Rare lactic acidosis [67] |
M: 0–3% [66] MC: 6–12.3% [66] |
Free at select pharmacies $5 per 60 tablets |
| Metformin + Letrozole | 1500 mg metformin/d 2.5 mg × 5 d [33] |
Daily for 6–8 weeks Initiate Letrozole per normal protocol [33] |
34.5% [33] | See Metformin and Letrozole | MC: 0% [33] |
$11 |
| Metformin + CC | 50–250 mg/d CC 850 mg/d metformin [68] |
Daily for 6–8 weeks Initiate CC per normal protocol [32] |
Similar to CC alone [32] BMI >35: 22.9% [68] |
See Metformin and CC | MC: Refer to CC [32] | $4–10 |
| Gonadotropins | 75 IU starting dose Step-up protocol: Above plus 75 IU increase every 7 d in absence of recruited follicle Low-dose step-up protocol: 37.5–75 IU starting dose plus 37.5 IU increase every 7–14 d in absence of recruited follicle [57] Step-down protocol: 150 IU until dominant follicle >10 mm; then 112.5 IU for 5 d; 75 IU for 5 d until ovulation |
D5 [59] | 32.2% [38] | Potential formation of “anti-hormones” that counteract the administered hormone [69] Abd pain, bloating, decreased urine output, N/V/D, breast tenderness [70] |
M: 8–31.8% [38, 57] OHSS: 11% [47] MC: 4% [57] |
$285 per 300 IU injection cartridge |
M multiple gestations, OHSS Ovarian Hyperstimulation Syndrome, MC miscarriage
Historically, if ovulation did not occur after CC administration, the endometrium was allowed to shed with medroxyprogesterone (Provera) 10 mg for 7–10 days to induce a period and a higher dose of CC was administered the following cycle [16].However, if a dominant follicle is not identified at this time or serum progesterone levels indicate that ovulation has not occurred, the patient can be restarted immediately on a 50 mg higher dose of CC for another 5 days until maximum daily dose of 250 mg is reached or ovulation has occurred [16]. The mechanism behind this ‘stair-step’ protocol is uncertain, but proposed theories include hormone-induced ovarian resistance to the LH surge and thinning effects of progestin on the endometrial lining [14, 17]. Current literature shows that the individuals who undergo the stair-step protocol have shorter time to ovulation (20.5 ± 2.0 days) compared to the traditional approach (48.6 ± 2.4 days), limiting the duration of hormonal manipulation and potential exposure to side effects [13].
The majority (75%) of women undergoing CC treatment ovulate at doses at or below 150 mg [15]. Patients who fail to respond to CC at doses of 250 mg/day or do not ovulate after six treatment cycles are considered CC-resistant and will require alternative or combination therapies [15, 18]. Further evaluation of other infertility factors should be performed, and providers may consider other medications for OI, such as Letrozole or Gonadotropins, or initiate a referral to an REI specialist [2, 15].
Other SERMs
Mechanism and Clinical Outcomes
Other SERMS for OI include tamoxifen and raloxifene [19]. Tamoxifen has similar efficacy to CC in OI and has an LBR of 34% [20]. It is considered safe when used in monotherapy or combined with CC (Table 2) [21]. This OI protocol is associated with an LBR of 34% when administered as monotherapy and an LBR of 49.3% when combined with CC in triggered cycles [20, 22]. Raloxifene has comparable rates of OI and effect profile in PCOS patients versus CC; however, it is a uterine antagonist and not recommended for OI [23].
Administration and Dosing
As seen with CC administration, Tamoxifen is started on day 5 of the menstrual cycle at 20 mg for 5 days [24, 25]. Ovulation is thus expected to occur 5–10 days afterwards, at which time IUI or timed intercourse should be encouraged. A maximum dose of 80 mg has been suggested [24, 25].
Aromatase Inhibitors
Mechanism and Outcomes
Aromatase inhibitors (AIs), mainly the third-generation non-steroidal preparation known as letrozole, are another class of medication used for OI. It was initially used for estrogen-receptor-positive metastatic breast cancer, but in 2000 was determined to be effective for OI with PCOS and CC-resistant patients [26, 27]. Letrozole is widely used for OI; however, it is still considered ‘off label’ per FDA guidelines [26].
AIs inhibit aromatase, the enzyme responsible to catalyze the rate-limiting step in the process of converting testosterone to estrogen [10]. Aromatase is present in many tissues, including the brain, muscle, liver, and breast, though its activity is most prominent in the ovaries of premenopausal women and adipose tissue of postmenopausal women [10]. By this mechanism, AIs create a hypoestrogenic environment that stimulates endogenous estrogen production by the hypothalamus [6]. Peripheral inhibition of aromatase reduces estrogen levels without antagonizing estrogen receptors in the hypothalamus, so follicle growth in the ovary produces rising levels of estradiol and inhibin [10]. This hormonal milieu results in the usual HPO feedback loop, reducing FSH response [10]. Because of the relatively low FSH levels secondary to aromatase inhibition, only one or two mature follicles are triggered to develop [10]. This reduces the risk of ovarian hyperstimulation syndrome (OHSS) and multiple gestations. [10].
A recent RCT in women with PCOS (n = 750) demonstrated that those receiving Letrozole had significantly higher LBR (27.5%) compared those with CC (19.1%) (Table 2) [21]. A systematic review and meta-analysis in 2015 of 5000 OI cycles in PCOS women corroborated these findings (RR 1.55; 95% CI 1.26–1.90) [28].
Administration and Dosing
The timing and adjustments of AIs are very similar to CC, starting at a low dose on days 2–5 of the cycle, depending when physician and patient desire follow-up. The initial dose is usually 2.5 mg daily for 5 days, with ovulation normally occurring 5–10 days later. Comparable to CC, if a patient does not ovulate or recruit a dominant follicle, the patient can immediately be redosed with an additional 2.5 mg of Letrozole. A maximum dose of 7.5 mg daily is usually employed for individuals undergoing ovulation induction with Letrozole. If a patient does not ovulate after 6 cycles, then further review of infertility should be evaluated, along with consideration for other OI medications, or a referral to an REI specialist pending physician comfort.
Metformin
Mechanism and Outcomes
Metformin is a biguanide antihyperglycemic medication, often prescribed for type 2 diabetes. Metformin decreases insulin levels by reducing hepatic gluconeogenesis and enhancing the body’s ability to excrete insulin through the gastrointestinal tract and peripheral uptake [29]. Insulin resistance is a commonly seen in PCOS patients, and elevated insulin levels has a negative impact on ovulation by stimulating androgen production, decreasing sex hormone binding levels, and increasing insulin-like growth factor 1 (IGF-1) [30]. The alteration in these factors enhances androgen production and creates numerous small follicles [30].
The literature on pregnancy outcomes in CC-resistant patients is mixed. A recent systematic review in women with PCOS showed that although metformin used alone improves ovulation, no increase in pregnancy or LBR was seen when compared to placebo, and it was inferior to CC or letrozole [29]. When it was used in combination with CC, one study demonstrated an increase in ovulation rate and pregnancy rate (PR), without an improvement in LBR [29]. This study is contradicted by a multicenter randomized study, which found that metformin alone improved both PR and LBR compared to a control group (PR 53.6 vs. 40.4%, LBR 41.9 vs. 28.8%) [31].
Administration
Metformin may be administered as monotherapy or combination with CC or an AI. Monotherapy entails an initial dose of 500 mg/day with a 500 mg titration every week without response, up to a maximum dose of 2500 mg/day [32]. Numerous protocols for metformin-CC combination therapy have been used, but most commonly metformin is given as a 500 mg/day dose along with a 50–250 mg/day dose of CC, according to the CC stair-step protocol described above [32]. When used in conjunction with an AI, one source recommended that 1500 mg/day of metformin to be administered with 2.5 mg/day of AI for 5 days (Table 2) [33].
Gonadotropins
Indications, Mechanisms, and Clinical Outcomes
Individuals that are type I in the WHO classification system are ideal candidates for exogenous gonadotropin (Gn) treatment as these patients do not have an intact HPO axis. These patients would not respond to OI medications that act within this axis such as CC or Letrozole and, therefore, require a medication that supersedes the HPO axis’s normal ability to produce FSH in order to directly stimulate follicular growth and ovulation [34]. However, anovulatory or PCOS individuals who have failed traditional OI may also be good candidates for Gn treatment, with the idea that the FSH had not reached threshold level required to generate a dominant follicle [34, 35].
Gn treatments are FSH-based medications which include follitropin, a synthetic hormone, and urofollitropin, a purified form of FSH extracted from the urine of postmenopausal women, and all have similar efficacy [36]. ASRM recommends that the use of gonadotropins only be undertaken by those with appropriate training and experience [37]. Gn therapy produces favorable pregnancy outcomes with reported ovulatory rates of 70–72% in PCOS women and 15% to 30%. LBR per cycle (Table 2) [34, 37–39].
Administration
During Gn administration, estradiol levels can initially determine if the dose of Gn needs to be increased or decreased by the rate of its rise. Follicles need to be approximately 10 mm in diameter prior to detecting an appreciable doubling of estradiol every 2–3 days. If this rate is not seen, or if the rate is increasing much faster, the dose can be adjusted as appropriate. There is a delicate balance between attaining a sufficiently high level of estrogen in order to achieve ovulation, normally anywhere between 150 and 400 pg/mL (average 200 pg/mL), and avoiding the risk of multiple gestation and OHSS that is seen when estradiol is elevated [7, 37, 40].
There are three standard protocols for Gn therapy: step-up, low dose step-up, and step-down. The step-up protocol is most traditional, with Gn given at an initial dose of 75–150 IU for 2–4 days beginning on cycle day 2 or 3 [7, 40]. Following this, estradiol levels and transvaginal ultrasound (TVUS) are utilized to determine follicle recruitment. If no increase in estradiol or recruitment of follicles is noted, then the dose is slowly increased until an appropriate response is seen [40]. When estradiol levels start to rise, TVUS is required more frequently to ensure that a dominant follicle is observed when it has reached 16–18 mm, typically on day 7–12 with a goal of 1–2 dominant follicles [37]. Once the follicle is between 16 and 20 mm, hCG is given to trigger ovulation, which occurs 36–48 h later and intrauterine insemination (IUI) or intercourse is encouraged.
The step-up protocol is best utilized in WHO Group I as these individuals have not been previously exposed to FSH, have low estrogen, and thus will likely be sensitive to moderate levels of FSH [7, 40]. In contrast, PCOS individuals already have multiple small follicles and low levels of FSH; therefore, they need a lower dose of FSH to reach the “FSH window” and induce a single follicle. Higher doses place these women at risk of OHSS and multiple gestations.
The modified step-up protocol was designed with the goal of taking the numerous follicles and gently inducing the growth of a single dominant follicle [7, 40, 41]. Therefore, an extremely low dose of 37.5–75 IU is administered daily over 7–14 days. If no follicle is > 10 mm from the initial dose, it is increased by 37.5 IU [37, 42]. If estradiol levels are > 200 pg/ml or follicles are > 10 mm, then the same dose is continued until ovulation is triggered [40]. The benefit of this approach is fewer cancelled cycles and safer results. A concern for this low dose regimen is reduced pregnancy outcomes. However, in a review published by Birch Peterson et el., a 53.1% PR is seen, with mono-ovulation in 61.3% of patients, after six cycles at an initial dose of 50 IU.
The step-down regimen generally is used in older women who require ‘superovulation’ in order to overcome an age related decrease in oocytes, where a high dose of Gn is given to recruit a dominant follicle, then reduced to maintain the follicle until ovulation [7, 37]. Although exact protocols differ, a high dose (ie 150 IU daily) is administered until TVUS shows a dominant follicle > 10 mm, and then reduced serially reduced 5 days apart (112.5; 75 IU) until ovulation is triggered. (Table 2) [7, 40, 41].
Hyperstimulation rates have been reported to be as high 68% in the step-down protocol compared to 32% in the step-up protocol [43]. Although the step-down protocol is highly effective, it requires more expertise and is associated with more complications; therefore, the step-up protocol is considered a safer alternative [37].
Despite the positive results associated with Gn administration, they are reserved for second- or third-line use in anovulatory, unexplained, or CC failure patients due to their high cost and requirement for stringent monitoring during treatment [34]. For these reasons, OB/GYNs should consider consulting an REI specialist if Gn treatment is needed [15].
Side Effects
Many OI medications are similar in their side-effect profiles because of their antiestrogenic mechanisms, with symptoms such as mood changes, vaginal dryness/atrophy, hot flashes, and irregular menses. These side effects are noted particularly with CC, raloxifene, and tamoxifen. Metformin is mechanistically unique among OI medications, with gastrointestinal side effects and rare lactic acidosis. Because AIs do not function through downregulation of estrogen receptors, letrozole does not have the antiestrogenic side effects, including vasomotor symptoms and mood changes that are common with CC [21]. Side effects that have been reported include gastrointestinal symptoms, headache, and back pain [44]. Another potential advantage of AIs compared to CC is the relatively rapid elimination of AIs (half-life of 45 h), which may contribute to the more favorable side-effect profile [10].
Recent RCTs have shown no difference in overall congenital malformation or chromosomal abnormalities when comparing letrozole to CC (3.9 vs. 4.5%) [21]. Although there was no statistically significant difference in the rate of congenital defects, different congenital defects were seen between Letrozole and CC. Neurological and cardiac congenital anomalies may be seen with letrozole use, including cerebral palsy, spina bifida with tethered cord, and ventricular septal defect [21]. In contrast with CC, one case of atrial and ventricular septal defect with concomitant pulmonary stenosis was found in a 2014 study [21]. It is important when counseling patients that no difference has been noted in congenital anomalies when comparing individuals who utilize OI medications versus individuals who conceive naturally [10].
OI medications cause ovarian hyperstimulation and elevated FSH levels, which carry a risk of inducing multiple gestations, most often twins with rare triplets or higher order multiples. The specific rate of multiple gestation varies by medication; CC has <10% and Letrozole a 13% risk [38, 45]. This is in stark contrast to the 5–10-fold increase risk of multiple gestations seen in individuals utilizing Gn, even with low dose protocols [37]. Interestingly, metformin monotherapy and combination therapy with CC are effective in reducing the risk of multiple gestations compared to CC monotherapy (Table 2) [10].
Another concern with OI medication is OHSS (Table 2), in which the ovary responds in an exaggerated manner to hormonal stimulation [38, 46]. This triggers an outflowing of estrogens, progesterone and cytokines including vascular endothelial growth factor (VEGF) [46]. VEGF is implicated in fatal cases of OHSS due to its effects on local capillaries by increasing vascular permeability, leading to ascites, hydrothorax, and venous thrombosis [46]. Severe OHSS is relatively rare with oral OI and more commonly seen with Gn therapy (CC 0.5–2.5%, Letrozole 9%, step-down Gn 68%) [43, 47]. The reported rates of multiple gestation, OHSS, and miscarriage for each OI medications are listed in Table 2.
In a systematic review published by ASRM in 2016, there are limited high-quality studies that have investigated a link between OI medication and the risk for future malignancy. The concern behind the use of fertility drugs is that inducing multiple ovulations would increase the risk of ovarian cancer through the two-hit hypothesis and the unknown effect of altering the endogenous hormone level within a woman’s body [48]. However, a Cochrane review indicated no change in the risk assessment in those undergoing infertility treatments for invasive ovarian cancer [49]. There is a small elevation in the risk for borderline ovarian tumors (or tumors of uncertain malignant potential); however, ASRM did not recommend abstaining from any infertility treatment to prevent such tumors. Through their review, they also did not find any link between infertility treatment and breast, endometrial, colon, or cervical cancer, nor malignant melanoma and non-Hodgkin lymphomas [48].
Laparoscopic Ovarian Drilling
Mechanism and Indications
Although the majority of anovulatory PCOS women will respond to OI medications, approximately 20% will be CC-resistant [50, 51]. Laparoscopic ovarian surgery for anovulation-related infertility was first described in 1939 via wedge resection versus the more common techniques today of placing multiple perforations within each ovary utilizing cautery (either monopolar or bipolar) or laser vaporization [51]. Although the exact mechanism by which this method helps induce ovulation is unknown, the theory is that is destroys the androgen-producing cells (theca cells) within the ovarian stroma, thereby lowering the overall testosterone levels. Other endocrine changes include a decrease in estrogen and LH levels, and a slight increase in FSH and sex hormone binding globulin (SHBG) levels. Together, these changes normalize the ovarian milieu, enabling women to ovulate on their own or become more sensitive to OI medication. [50, 51].
Overall, laparoscopic ovarian drilling (LOD) is not considered first-line treatment for infertility in PCOS patients, as OI medications are deemed safer. Besides inherent surgical risks, other concerns include adhesions and possible decrease in ovarian function over time [50, 51]. Although the original ovarian surgery via wedge resection almost always caused adhesive disease, there has been no established link between adhesions and subsequent infertility [50]. Another concern is a reduction in ovarian reserve. Despite numerous studies showing a change in various ovarian reserve markers, no conclusive evidence has been determined that correlates LOD with an increase in premature ovarian failure. Therefore, the changes observed were likely related to normalization of ovarian function in PCOS individuals. [51].
Outcomes
The LBR after LOD was between 24 and 44% [51]. As LOD is usually undertaken only after CC failure, it is prudent to compare LOD versus Gns, which are also considered second-line treatment in CC-resistant patients. In the review by Fernandez et al, there was no statistically significant difference in LBR at 6 or 12 months or miscarriage rate, and no instances of OHSS were seen in LOD [50, 51]. A 2012 Cochrane Review summarized that when comparing LOD with or without the use of OI medications versus those with OI medications alone, no statistically significant difference in LBR was noted. Nevertheless, LOD is less expensive with a decreased risk of OHSS and, therefore, is a reasonable option in individuals with CC-resistant anovulatory infertility [50, 51].
Monitoring Methods for OI
Ultrasonography
Several methods may be employed for monitoring follicular recruitment through a cycle of OI. TVUS is routinely used after OI medication to visualize the number and size of recruited follicles [52]. Viable follicles destined for ovulation usually exceed 14 mm in diameter and will grow at a rate of 1.5–2 mm per day, reaching 20 mm just before ovulation or approximately 4 days later [53, 54]. By tracking follicular growth, TVUS monitoring allows for accurate timing of IUI or timed intercourse.
Basal Body Temperature
During the follicular phase of the menstrual cycle, basal body temperatures hovers around 97–98 F. A day prior to ovulation temperature reaches its nadir and then slowly increases in response to the rising progesterone levels to its maximum level 0.5–1 F above their baseline follicular temperature [55]. Basal body temperature may be tracked to predict ovulation, observing for a temperature increase 0.15F above the highest recorded level from the first 10 days of the cycle [55].
Urinary LH Kits
Reproductive hormone levels may be monitored by a number of methods. For home monitoring, urinary LH kits detect a peak in hormone levels 5–12 days after completion of OI medication and a surge confers impending ovulation and an interval of peak fertility [52]. Individuals usually ovulate 12–36 h after a positive indication of LH levels, so the timing of intercourse or IUI may be planned during the 6-day fertility window that ends on the predicted day of ovulation [15].
Progesterone Levels
Serum progesterone levels may be measured in the office or lab on day 21 of a 28-day cycle during the mid-luteal phase or 1 week prior to the onset of menses. Although progesterone levels fluctuate through the cycle, a level above 3 ng/mL indicates that ovulation has occurred. Salivary progesterone levels may be measured from home-collected samples, providing an estimate of active serum hormone levels. Patients collect saliva upon arising in the morning at the peak of the mid-luteal salivary progesterone levels. A level of 190 pmol/L indicates that ovulation has occurred, although this may vary by lab [56]. The one caveat to obtaining progesterone levels, is that it indicates that ovulation has already occurred, therefore cannot be used to time intercourse or IUI.
Conclusion
Infertility secondary to ovulatory dysfunction is a common condition that is often referred to REI specialists. However, initiating OI medications is a relatively simple treatment for female infertility that may be managed by the general OB-GYN. An understanding of reproductive physiology and common etiologies of anovulation informs administration of CC or Letrozole, which may be scheduled to optimize convenience for both clinician and patient, while promoting positive treatment outcomes. During treatment, a number of methods are available to monitor ongoing follicular development and ovulation, depending on the equipment capabilities of the individual practice. In those who fail initial treatment, further infertility evaluation, additional OI medication, or referral to REI specialist can be undertaken pending the generalist level of comfort and expertise. Ovulation induction offers a solution for the large percentage of women with infertility and should be adopted by the general OB/GYN.
Dr. Steven R. Lindheim
is a Professor of Obstetrics & Gynecology at Wright State University, Boonshoft School of Medicine and Section Chief of Reproductive Endocrine & Infertility. His areas of interest include assisted reproduction, reproductive surgery, and oncofertility.
Compliance with Ethical Standards
Conflict of interest
All the authors declare that they have no conflict of interest.
Footnotes
Steven R. Lindheim, MD, MMM is a professor in Division of Reproductive Endocrine Infertility, Department of Obstetrics and Gynecology, Boonshoft School of Medicine at the Wright State University, Dayton, OH, USA; Tanya L. Glenn, MD, Division of Reproductive Endocrine Infertility, Department of Obstetrics and Gynecology, Boonshoft School of Medicine at the Wright State University, Dayton, OH, USA and Department of Obstetrics and Gynecology, Wright-Patterson Air Force Base at the Wright-Patterson Medical Center, Dayton, OH, USA; Megan C. Smith, M.P.H, Division of Reproductive Endocrine Infertility, Department of Obstetrics and Gynecology, Boonshoft School of Medicine at the Wright State University, Dayton, OH, USA; Pascal Gagneux, Ph.D., Department of Cellular and Molecular Medicine at the University of California San Diego, San Diego, CA, USA.
References
- 1.Thoma ME, McLain AC, Louis JF, et al. The prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99(5):1324-1331.e1. doi: 10.1016/j.fertnstert.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindsay TJ, Vitrikas KR. Evaluation and treatment of infertility. Am Fam Physician. 2015;91(5):308–314. [PubMed] [Google Scholar]
- 3.Amer SA, Li TC, Metwally M, et al. Randomized controlled trial comparing laparoscopic ovarian diathermy with clomiphene citrate as a first-line method of ovulation induction in women with polycystic ovary syndrome. Hum Reprod. 2009;24(1):219–225. doi: 10.1093/humrep/den325. [DOI] [PubMed] [Google Scholar]
- 4.Eldein HN. Family physicians’ attitude and practice of infertility management at primary care—Suez Canal University. Egypt. Pan Afr Med J. 2013;15:106. doi: 10.11604/pamj.2013.15.106.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Collaborating Centre for Women’s and Children’s Health (UK). Ovulation disorders. In: Fertility: Assessment and treatment for people with fertility problems. London: Royal College of Obstetricians & Gynaecologists; 2013. [PubMed]
- 6.Mesiano S, Jones EE. Chapter 55: The female reproductive system. In: Boron WF, Boulpaep EL, editors. Medical physiology. 3. Philadelphia: Elsevier; 2017. [Google Scholar]
- 7.Speroff L, Fritz M, editors. Clinical gynecologic endocrinology and infertility. 8. Philadelphia: Lippincott, WIlliams & Wilkins; 2011. [Google Scholar]
- 8.Bao B, Garverick HA. Expression of steroidogenic enzyme and gonadotropin receptor genes in bovine follicles during ovarian follicular waves: a review. J Anim Sci. 1998;76(7):1903–1921. doi: 10.2527/1998.7671903x. [DOI] [PubMed] [Google Scholar]
- 9.Roy SK, Treacy BJ. Isolation and long-term culture of human preantral follicles**Supported by grant HD 18165 (to S.K.R.) from the national institutes of health, Bethesda, Maryland. Fertil Steril. 1993;59(4):783–790. doi: 10.1016/S0015-0282(16)55860-9. [DOI] [PubMed] [Google Scholar]
- 10.Martinez AM, Lindheim SR. Induction of ovulation. In: Falcone T, Hurd WW, editors. Clinical reproductive medicine and surgery: a practical guide. 2. London: Springer; 2013. [Google Scholar]
- 11.Brown JB. Pituitary control of ovarian function–concepts derived from gonadotrophin therapy. Aust N Z J Obstet Gynaecol. 1978;18(1):46–54. doi: 10.1111/j.1479-828X.1978.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 12.Niswender GD, Juengel JL, Silva PJ, et al. Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev. 2000;80(1):1–29. doi: 10.1152/physrev.2000.80.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Deveci CD, Demir B, Sengul O, et al. Clomiphene citrate ‘stair-step’ protocol vs. traditional protocol in patients with polycystic ovary syndrome: a randomized controlled trial. Arch Gynecol Obstet. 2015;291(1):179–184. doi: 10.1007/s00404-014-3398-y. [DOI] [PubMed] [Google Scholar]
- 14.Dong X, Zheng Y, Liao X, et al. Does progesterone-induced endometrial withdrawal bleed before ovulation induction have negative effects on IUI outcomes in patients with polycystic ovary syndrome? Int J Clin Exp Pathol. 2013;6(6):1157–1163. [PMC free article] [PubMed] [Google Scholar]
- 15.Moy I, Ekpo G. Clomiphene citrate use for ovulation induction: When, why, and how? Contemporary OB/GYN 2011.
- 16.Jones T, Gualtieri M, Bruno-Gaston J, et al. Evaluation of clomiphene citrate stair-step protocol for ovulation induction. Fertil Steril. 2014;102(3):e138. doi: 10.1016/j.fertnstert.2014.07.473. [DOI] [Google Scholar]
- 17.Diamond MP, Kruger M, Santoro N, et al. Endometrial shedding effect on conception and live birth in women with polycystic ovary syndrome. Obstet Gynecol. 2012;119(5):902–908. doi: 10.1097/AOG.0b013e31824da35c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Athaullah N, Proctor M, Johnson NP. Oral versus injectable ovulation induction agents for unexplained subfertility. Cochrane Database Syst Rev. 2002;3:CD003052. doi: 10.1002/14651858.CD003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallam HN, Abdel-Bak M, Sallam NH. Does ovulation induction increase the risk of gynecological cancer? Facts Views Vis Obgyn. 2013;5(4):265–273. [PMC free article] [PubMed] [Google Scholar]
- 20.Seyedoshohadaei F, Zandvakily F, Shahgeibi S. Comparison of the effectiveness of clomiphene citrate, tamoxifen and letrozole in ovulation induction in infertility due to isolated unovulation. Iran J Reprod Med. 2012;10(6):531–536. [PMC free article] [PubMed] [Google Scholar]
- 21.Legro RS, Brzyski RG, Diamond MP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371(2):119–129. doi: 10.1056/NEJMoa1313517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zakherah MS, Nasr A, El Saman AM, et al. Clomiphene citrate plus tamoxifen versus laparoscopic ovarian drilling in women with clomiphene-resistant polycystic ovary syndrome. Int J Gynaecol Obstet. 2010;108(3):240–243. doi: 10.1016/j.ijgo.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 23.de Paula Guedes Neto E, Savaris RF, von Eye Corleta H, et al. Prospective, randomized comparison between raloxifene and clomiphene citrate for ovulation induction in polycystic ovary syndrome. Fertil Steril. 2011;96(3):769–773. doi: 10.1016/j.fertnstert.2011.06.067. [DOI] [PubMed] [Google Scholar]
- 24.Dhaliwal LK, Suri V, Gupta KR, et al. Tamoxifen: an alternative to clomiphene in women with polycystic ovary syndrome. J Hum Reprod Sci. 2011;4(2):76–79. doi: 10.4103/0974-1208.86085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Gharib MN, Mahfouz AE, Farahat MA. Comparison of letrozole versus tamoxifen effects in clomiphen citrate resistant women with polycystic ovarian syndrome. J Reprod Infertil. 2015;16(1):30–35. [PMC free article] [PubMed] [Google Scholar]
- 26.Kar S. Current evidence supporting “letrozole” for ovulation induction. J Hum Reprod Sci. 2013;6(2):93–98. doi: 10.4103/0974-1208.117166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 28.Roque M, Tostes AC, Valle M, et al. Letrozole versus clomiphene citrate in polycystic ovary syndrome: systematic review and meta-analysis. Gynecol Endocrinol. 2015;31(12):917–921. doi: 10.3109/09513590.2015.1096337. [DOI] [PubMed] [Google Scholar]
- 29.Penzias A, Bendikson K, Butts S, et al. Role of metformin for ovulation induction in infertile patients with polycystic ovary syndrome (PCOS): A guideline. Fertil Steril. 2017;108(3):426–441. doi: 10.1016/j.fertnstert.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 30.Tock L, Carneiro G, Pereira AZ, et al. Adrenocortical production is associated with higher levels of luteinizing hormone in nonobese women with polycystic ovary syndrome. Int J Endocrinol. 2014;2014:620605. doi: 10.1155/2014/620605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morin-Papunen L, Rantala AS, Unkila-Kallio L, et al. Metformin improves pregnancy and live-birth rates in women with polycystic ovary syndrome (PCOS): a multicenter, double-blind, placebo-controlled randomized trial. J Clin Endocrinol Metab. 2012;97(5):1492–1500. doi: 10.1210/jc.2011-3061. [DOI] [PubMed] [Google Scholar]
- 32.Lashen H. Role of metformin in the management of polycystic ovary syndrome. Ther Adv Endocrinol Metab. 2010;1(3):117–128. doi: 10.1177/2042018810380215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sohrabvand F, Ansari S, Bagheri M. Efficacy of combined metformin-letrozole in comparison with metformin-clomiphene citrate in clomiphene-resistant infertile women with polycystic ovarian disease. Hum Reprod. 2006;21(6):1432–1435. doi: 10.1093/humrep/del020. [DOI] [PubMed] [Google Scholar]
- 34.Melo AS, Ferriani RA, Navarro PA. Treatment of infertility in women with polycystic ovary syndrome: approach to clinical practice. Clinics (Sao Paulo) 2015;70(11):765–769. doi: 10.6061/clinics/2015(11)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod. 2008;23(3):462–477. doi: 10.1093/humrep/dem426. [DOI] [PubMed] [Google Scholar]
- 36.Infertility in women. [Internet]; 2012. http://umm.edu/health/medical/reports/articles/infertility-in-women. Accessed 12 Mar 2018.
- 37.Practice Committee of American Society for Reproductive Medicine Use of exogenous gonadotropins in anovulatory women: a technical bulletin. Fertil Steril. 2008;90(5 Suppl):S7–S12. doi: 10.1016/j.fertnstert.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Diamond MP, Legro RS, Coutifaris C, et al. Letrozole, gonadotropin, or clomiphene for unexplained infertility. N Engl J Med. 2015;373(13):1230–1240. doi: 10.1056/NEJMoa1414827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawwass JF, Loucks TL, Berga SL. An algorithm for treatment of infertile women with polycystic ovary syndrome. Middle East Fertil Soc J. 2010;15(4):231–239. doi: 10.1016/j.mefs.2010.07.010. [DOI] [Google Scholar]
- 40.Palshetkar N, Roongta N. Protocols for ovulation induction. In: Mumbai Obstetric and Gynecological Society; 2011.
- 41.Fauser BC, Van Heusden AM. Manipulation of human ovarian function: physiological concepts and clinical consequences. Endocr Rev. 1997;18(1):71–106. doi: 10.1210/edrv.18.1.0290. [DOI] [PubMed] [Google Scholar]
- 42.Tarlatzis BC, Fauser BC, Kolibianakis EM, et al. GnRH antagonists in ovarian stimulation for IVF. Hum Reprod Update. 2006;12(4):333–340. doi: 10.1093/humupd/dml001. [DOI] [PubMed] [Google Scholar]
- 43.Birch Petersen K, Pedersen NG, Pedersen AT, et al. Mono-ovulation in women with polycystic ovary syndrome: a clinical review on ovulation induction. Reprod Biomed Online. 2016;32(6):563–583. doi: 10.1016/j.rbmo.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Casper RF, Mitwally MF. Use of the aromatase inhibitor letrozole for ovulation induction in women with polycystic ovarian syndrome. Clin Obstet Gynecol. 2011;54(4):685–695. doi: 10.1097/GRF.0b013e3182353d0f. [DOI] [PubMed] [Google Scholar]
- 45.Lee Y, Kim Y, Chang J, et al. Changes in oral mucosal MUCI expression and salivary hormones throughout the menstrual cycle. Oral Disease. 2015;21(962):968. doi: 10.1111/odi.12367. [DOI] [PubMed] [Google Scholar]
- 46.Fiedler K, Ezcurra D. Predicting and preventing ovarian hyperstimulation syndrome (OHSS): the need for individualized not standardized treatment. Reprod Biol Endocrinol. 2012;10(1):32. doi: 10.1186/1477-7827-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Inany H, Youssef MA, Ayeleke RO, et al. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev. 2016;4:CD001750. doi: 10.1002/14651858.CD001750.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Practice Committee of the American Society for Reproductive Medicine Fertility drugs and cancer: a guideline. Fertil Steril. 2016;106(7):1617–1626. doi: 10.1016/j.fertnstert.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 49.Rizzuto I, Behrens RF, Smith LA. Risk of ovarian cancer in women treated with ovarian stimulating drugs for infertility. Cochrane Database Syst Rev. 2013;8:CD008215. doi: 10.1002/14651858.CD008215.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernandez H, Morin-Surruca M, Torre A, et al. Ovarian drilling for surgical treatment of polycystic ovarian syndrome: a comprehensive review. Reprod Biomed Online. 2011;22(6):556–568. doi: 10.1016/j.rbmo.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 51.Farquhar C, Brown J, Marjoribanks J, Vandekerckhove P. Laparoscopic drilling by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database Syst Rev. 2012;6:CD001122. doi: 10.1002/14651858.CD001122.pub4. [DOI] [PubMed] [Google Scholar]
- 52.Practice Committee of the American Society for Reproductive Medicine Use of clomiphene citrate in infertile women: a committee opinion. Fertil Steril. 2013;100(2):341–348. doi: 10.1016/j.fertnstert.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 53.Fortune JE. Ovarian follicular growth and development in mammals. Biol Reprod. 1994;50(2):225–232. doi: 10.1095/biolreprod50.2.225. [DOI] [PubMed] [Google Scholar]
- 54.Chizen DR, Pierson R. Transvaginal ultrasonography and female infertility. In: The Global Library of Women’s Medicine; 2010.
- 55.Su H, Yi Y, Wei T, et al. Detection of ovulation, a review of currently available methods. Bioeng Transl Med. 2017;2(3):238–246. doi: 10.1002/btm2.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tourville TW, Shultz SJ, Vacek PM, et al. Evaluation of an algorithm to predict menstrual-cycle phase at the time of injury. J Athl Train. 2016;51(1):47–56. doi: 10.4085/1062-6050-51.3.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hassan A, Shehata N, Wahba A. Cost effectiveness of letrozole and purified urinary FSH in treating women with clomiphene citrate-resistant polycystic ovarian syndrome: a randomized controlled trial. Hum Fertil (Camb) 2017;20(1):37–42. doi: 10.1080/14647273.2016.1242783. [DOI] [PubMed] [Google Scholar]
- 58.Kumar P, Sait SF. Luteinizing hormone and its dilemma in ovulation induction. J Hum Reprod Sci. 2011;4(1):2–7. doi: 10.4103/0974-1208.82351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kamath MS, Maheshwari A, Bhattacharya S, Lor KY, Gibreel A. Oral medications including clomiphene citrate or aromatase inhibitors with gonadotropins for controlled ovarian stimulation in women undergoing in vitro fertilisation. Cochrane Database Syst Rev. 2017;1:CD008528. doi: 10.1002/14651858.CD008528.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.An KC. Selective estrogen receptor modulators. Asian Spine J. 2016;10(4):787–791. doi: 10.4184/asj.2016.10.4.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suginami H, Kitagawa H, Nakahashi N, et al. A clomiphene citrate and tamoxifen citrate combination therapy: a novel therapy for ovulation induction. Fertil Steril. 1993;59(5):976–979. doi: 10.1016/S0015-0282(16)55913-5. [DOI] [PubMed] [Google Scholar]
- 62.Franik S, Kremer J, Nelen W, Farquhar C. Aromatase inhibitors for subfertile women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2014;24(2):CD010287. doi: 10.1002/14651858.CD010287.pub2. [DOI] [PubMed] [Google Scholar]
- 63.Pritts EA, Yuen AK, Sharma S, et al. The use of high dose letrozole in ovulation induction and controlled ovarian hyperstimulation. ISRN Obstet Gynecol. 2011 doi: 10.5402/2011/242864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fabian CJ. The what, why and how of aromatase inhibitors: hormonal agents for treatment and prevention of breast cancer. Int J Clin Pract. 2007;61(12):2051–2063. doi: 10.1111/j.1742-1241.2007.01587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He Q, Liang L, Zhang C, et al. Effects of different doses of letrozole on the incidence of early-onset ovarian hyperstimulation syndrome after oocyte retrieval. Syst Biol Reprod Med. 2014;60(6):355–360. doi: 10.3109/19396368.2014.957879. [DOI] [PubMed] [Google Scholar]
- 66.Misso ML, Costello MF, Garrubba M, et al. Metformin versus clomiphene citrate for infertility in non-obese women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2013;19(1):2–11. doi: 10.1093/humupd/dms036. [DOI] [PubMed] [Google Scholar]
- 67.Pawlyk AC, Giacomini KM, McKeon C, et al. Metformin pharmacogenomics: current status and future directions. Diabetes. 2014;63(8):2590–2599. doi: 10.2337/db13-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barbieri RL. Clomiphene versus metformin for ovulation induction in polycystic ovary syndrome: the winner is. J Clin Endocrinol Metab. 2007;92(9):3399–3401. doi: 10.1210/jc.2007-1393. [DOI] [PubMed] [Google Scholar]
- 69.Cedrin-Durnerin I, Massin N, Galey-Fontaine J, et al. Timing of FSH administration for ovarian stimulation in normo-ovulatory women: comparison of an early or a mid follicular phase initiation of a short-term treatment. Hum Reprod. 2006;21(11):2941–2947. doi: 10.1093/humrep/del259. [DOI] [PubMed] [Google Scholar]
- 70.Follicle stimulating hormone and luteinizing hormone (intramuscular route, subcutaneous route) [Internet]: Truven Health Analytics; c20172018]. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMHT0010378/. Accessed 25 Jan 2018.