Abstract
Objective
To compare the efficacy and safety of dienogest (DNG) with depot leuprolide acetate (LA) in patients with recurrent pelvic pain following laparoscopic surgery for endometriosis.
Design
Prospective randomized trial.
Setting
Zagazig University hospitals, Egypt.
Patients
Two hundred and forty-two patients with recurrent pelvic pain following laparoscopic surgery for endometriosis.
Intervention
Dienogest (2 mg/day, orally) or depot LA (3.75 mg/4 weeks, intramuscularly) for 12 weeks.
Main Outcome Measures
A visual analogue scale was used to test the intensity of pain before and after the end of treatment.
Results
There was highly significant reduction in pelvic pain, back pain and dyspareunia in both groups with mean of difference in dienogest group (28.7 ± 5.3, 19.0 ± 4.3 and 20.0 ± 3.08 mm, respectively) and in LA group (26.2 ± 3.01, 19.5 ± 3.01 and 17.9 ± 2.9 mm, respectively). The most frequent drug-related adverse effects in dienogest group were vaginal bleeding and weight gain (64.5 and 10.8%, respectively) which were significantly higher than LA group (21.5 and 3.3%, respectively). While the most frequent drug-related adverse effects in LA group were hot flushes and vaginal dryness (46.3 and 15.7%, respectively) which were significantly higher than dienogest group (15.7 and 3.3%, respectively).
Conclusion
Daily dienogest is as effective as depot LA for relieving endometriosis-associated pelvic pain, low back pain and dyspareunia. In addition, dienogest has acceptable safety, tolerability and lower incidence of hot flushes. Thus, it may offer an effective and well-tolerated treatment in endometriosis.
Keywords: Endometriosis, Pelvic pain, Leuprolide acetate, Dienogest
Introduction
Endometriosis is defined as the presence of ectopic endometrial tissue outside the uterine cavity inducing a chronic inflammatory reaction [1].
It is a common condition affecting adult women, and the exact prevalence of which is unknown but estimated to range from 2 to 10% of the general female population and up to 50% of infertile women [2]. The prevalence of endometriosis in adolescents undergoing laparoscopy for pelvic pain was reported to be 47% [3]. Although some patients with endometriosis suffer from painful symptoms and/or infertility, others are asymptomatic. Diagnosis is made by physical examination, imaging techniques, and finally proved by histopathology [1].
There is no cure for endometriosis up till now. Surgery is a commonly used treatment option, but the recurrence is high: approximately 40–50% after 5 years [4–6]. Laparoscopic surgery may reduce painful symptoms and improve quality of life in 67–80% of patients [7], but lesions and symptoms frequently recur within 2–5 years [8, 9]. Repeated surgery is associated with increased morbidity, health care costs and reduction in the ovarian reserve in patients with ovarian endometriosis [4].
Medical treatment can alleviate the pelvic pain, but unfortunately the relief is for a limited duration, and symptoms often recur after cessation of treatment. The most widely used therapies are progestins, combined oral contraceptives (COCs), GnRH agonists [10]. GnRH agonists induce a hypoestrogenic state causing menopausal symptoms such as hot flushes, and reduced bone mineral density. Their use is therefore limited to short-term use [11].
Dienogest is a derivative of 19-nortestosterone with a high selectivity for the progesterone receptors. The oral use has been approved for medical treatment of endometriosis in Europe, Japan, and Australia. It has the ability of creating a hypoestrogenic and hyperprogestogenic environment, which causes decidualization of the ectopic endometrium [12].
The Aim of the Study
To compare the efficacy of DNG with depot leuprolide acetate (LA) in patients with recurrent pelvic pain following laparoscopic surgery for endometriosis.
Patients and Methods
The study was conducted in the Department of Obstetrics and Gynecology, Faculty of medicine, Zagazig University hospitals, Egypt, between May 2014 and December 2016.
Study Design
The study was a 12-week prospective randomized trial of dienogest versus depot LA. After approval of the local ethics committee, a written informed consent was obtained from all patients before starting. The flowchart of the participants is shown in Fig. 1.
Fig. 1.
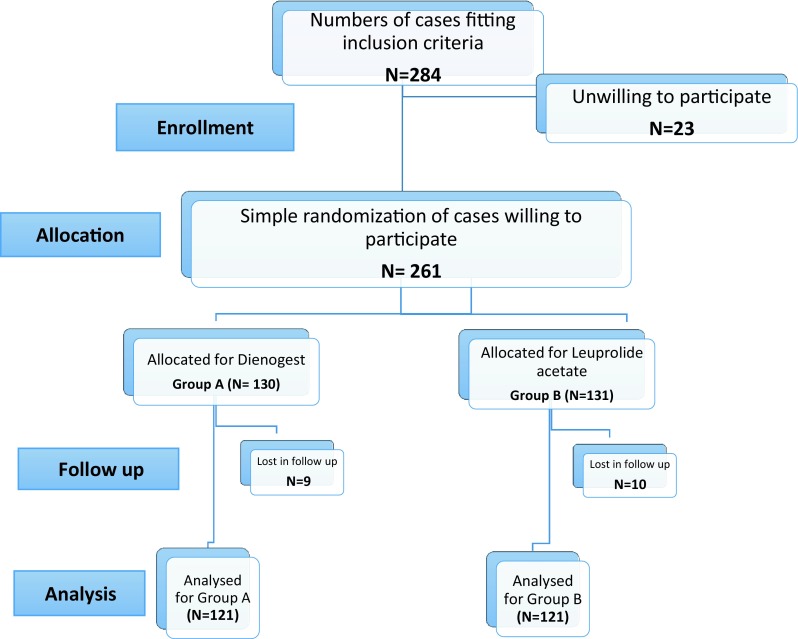
Flowchart of the study population
The study included 242 women aged 20–45 years with recurrent pelvic pain within 1 year following laparoscopic surgery for histopathologically proven endometriosis in revised-American Fertility Society (r-AFS, 1985) stages I–IV based on the total surface size of the lesions, presence of adhesions, and ovarian lesions [13]. Endometriosis had to be confirmed by either diagnostic laparoscopy within 3 months or by therapeutic laparoscopy within 12 months of enrollment into the study with subsequent recurrence of pain.
Exclusion criteria were pregnancy, breast feeding, amenorrhea within 3 months of enrollment, previous use of hormonal agents (e.g., GnRH agonists, progestins, danazol or oral contraceptives) following laparoscopy, undiagnosed genital bleeding, history of severe adverse drug reactions or hypersensitivity to steroid hormones or GnRH agonists, history of thrombosis/embolism or depression and patients at risk of decreased bone mineral density (BMD) (e.g., a family history of osteoporosis or use of corticosteroids).
The indication for laparoscopy, in all included patients, was chronic pelvic pain, and there were no cases concerned with fertility.
The study protocol, the intervention involved, possible early and long-term side effects of interventions were explained to the patients. Those who were willing to participate in the study and consented for participation were subjected to the following:
Full history taking with special attention to the pelvic pain.
Endometriosis-associated pelvic pain, back pain and dyspareunia were assessed by a visual analogue scale (VAS; 0 mm = absence of pain, 100 mm = unbearable pain). The VAS score was primarily selected in this study because it is considered a well-established tool for the measurement of pelvic pain associated with endometriosis [14].
Conventional transvaginal ultrasonography for detection of the uterine size, endometrial thickness, together with evaluation of the ovaries for the presence of endometriomas, was done with B-mode imaging using wide-band micro-convex endocavity probe with 4.5–8.5/D6 MHz frequency specially used for obstetrics and gynecological purpose.
Patients were divided randomly by using random number table (computer), software Open Epi version 3.21 into two groups (A and B). Patients were assigned to either group by the randomization known, while allocation concealment concentrated on preventing selection and confusing biases. In group A, patients received dienogest at a dose of 2 mg given orally once daily at the same time for 12 weeks with the first tablet taken on the first day after onset of menstrual bleeding. In group B, patients received leuprolide acetate at a standard dose of 3.75 mg as a depot intramuscular injection every 4 weeks for 12 weeks with the first injection given during the first 3 days of menstrual bleeding.
Patients were followed up during the treatment period; they were asked about the compliance of drug intake, assessment of pain improvement using visual analogue score at 12 weeks, also they were asked about development of side effects. Patients initially diagnosed to have endometriomas at enrollment had ultrasonography for assessment of the size of endometriomas after 12 weeks of treatment.
The primary outcome measured was the change of severity of pelvic pain, back pain and dyspareunia from the baseline to the end of treatment (12 weeks after administration of dienogest or LA), as assessed by a visual analogue scale (VAS; 0 mm = absence of pain, 100 mm = unbearable pain).
Statistical Analysis
Collected data were coded, entered and analyzed using Microsoft Excel software. Data were then imported into Statistical Package for the Social Sciences (SPSS) version 20.0 software for analysis. Data were expressed as number and percentage for qualitative variable while they were expressed as mean ± SD for quantitative variables. The following tests were used to test differences for significance; differences between frequencies (qualitative variables) and percentages in groups were compared by Chi-square test and differences between parametric quantitative independent groups by t test in paired by paired t. p value was set at < 0.05 for significant results & < 0.001 for high significant result.
Results
Total of 317 new cases attended the outpatient clinic in the Department of Obstetrics and Gynecology, Faculty of medicine, Zagazig University hospitals, during the study period and were assessed for eligibility, and 284 patients met requirements of inclusion criteria. The study protocol, the intervention involved, possible early and long-term side effects of interventions were explained to the patients. Out of which, 261 patients were willing to participate in the study and consented for participation. Simple randomization of those 261 patients was done and 130 cases were allocated in group A (dienogest 2 mg oral tablet once daily for 12 weeks) and 131 cases in group B (leuprolide acetate depot 3.75 mg intramuscular injection every 4 weeks for 12 weeks). Patients who dropped from follow-up were excluded from the study statistics and results (9 patients from group A and 10 patients from group B). So, finally 242 patients were analyzed (121 patients from group A and 121 patients from group B). Study flowchart is shown in Fig. 1.
There were no statistically significant differences (p > 0.05) between the two studied groups regarding the demographic parameters as shown in Table 1. Patients were of comparable age (29.52 ± 3.32 years in dienogest group and 29.77 ± 3.09 years in leuprolide acetate group), weight (67.55 ± 6.65 kg in dienogest group and 67.19 ± 6.97 kg in leuprolide acetate group) and body mass index (25.03 ± 1.45 kg/m2 in dienogest group and 24.84 ± 1.47 kg/m2 in leuprolide acetate group) in both groups. There was no significant difference between the visual analogue score in both groups before the study as regards pelvic pain (59.27 ± 11.02 in dienogest group and 58.73 ± 11.01 in leuprolide acetate group), back pain (45.91 ± 3.33 in dienogest group and 46.68 ± 3.29 in leuprolide acetate group) and dyspareunia (36.53 ± 3.87 in dienogest group and 34.98 ± 4.96 in leuprolide acetate group) (Table 1).
Table 1.
Demographic data of the study population
| Dienogest | Leuprolide acetate | Test of significance | p value | |
|---|---|---|---|---|
| Age | 29.52 ± 3.32 | 29.77 ± 3.09 | t = − 0.601 | 0.548 |
| Weight | 67.55 ± 6.65 | 67.19 ± 6.97 | t = 0.406 | 0.685 |
| BMI | 25.03 ± 1.45 | 24.84 ± 1.47 | t = 0.991 | 0.323 |
| VAS of pelvic pain | 59.27 ± 11.02 | 58.73 ± 11.01 | t = 0.343 | 0.732 |
| VAS of back pain | 45.91 ± 3.33 | 46.68 ± 3.29 | t = − 1.358 | 0.177 |
| VAS of dyspareunia | 36.53 ± 3.87 | 34.98 ± 4.96 | t = 1.859 | 0.066 |
| Stage of endometriosis | ||||
| I | 30 (24.8%) | 27 (22.3%) | X2 = 0.42 | 0.93 |
| II | 36 (29.8%) | 39 (32.2%) | ||
| III | 32 (26.4%) | 34 (28.1%) | ||
| IV | 23 (19.0%) | 21 (17.4%) | ||
Both dienogest and leuprolide acetate were associated with highly significant reduction in VAS for pelvic pain by the end of the study. At baseline, the mean VAS was 59.27 ± 11.02 mm in the dienogest group and 58.73 ± 11.01 mm in the leuprolide acetate group. By 12 weeks, mean VAS for pelvic pain had decreased to 30.61 ± 10.65 mm in the dienogest group and to 32.53 ± 8.74 mm in the leuprolide acetate group. There was no significant difference between both groups by 12 weeks (p = 0.17) (Table 2).
Table 2.
VAS for pelvic pain
| Dienogest (N = 101) Mean ± SD |
Leuprolide acetate (N = 96) Mean ± SD |
T | p value | |
|---|---|---|---|---|
| Baseline VAS | 59.27 ± 11.02 | 58.73 ± 11.01 | 0.343 | 0.732 |
| VAS by 12 weeks | 30.61 ± 10.65 | 32.53 ± 8.74 | − 1.377 | 0.170 |
| Paired t | 32.348 | 83.246 | – | – |
| P | 0.000** | 0.000** | – | – |
**Highly significant difference
Regarding back pain, both dienogest and leuprolide acetate were associated with highly significant reductions in VAS by the end of the study (12 weeks). In dienogest group, the mean VAS was 45.91 ± 3.33 mm at the baseline which decreased to 26.92 ± 4.40 mm by 12 weeks. While in leuprolide acetate group, the mean VAS was 46.68 ± 3.29 mm at the baseline which decreased to 27.22 ± 1.79 mm by 12 weeks. There was no significant difference between both groups by 12 weeks (p = 0.597) (Table 3).
Table 3.
VAS for back pain
| Dienogest (N = 72) Mean ± SD |
Leuprolide acetate (N = 68) Mean ± SD |
T | p value | |
|---|---|---|---|---|
| Baseline VAS | 45.91 ± 3.33 | 46.68 ± 3.29 | − 1.358 | 0.177 |
| VAS by 12 weeks | 26.92 ± 4.40 | 27.22 ± 1.79 | − 0.529 | 0.597 |
| Paired t | 37.476 | 51.714 | – | – |
| P | 0.000** | 0.000** | – | – |
**Highly significant difference
Also, VAS for dyspareunia was highly significantly reduced in both dienogest and leuprolide acetate groups by the end of the study. At baseline, the mean VAS in the dienogest and the leuprolide acetate groups was 36.53 ± 3.87 and 34.98 ± 4.96 mm, respectively, which was reduced to 16.53 ± 3.10 and 17.11 ± 2.53 mm, respectively, by 12 weeks. There was no significant difference between both groups by 12 weeks (p = 0.263) (Table 4).
Table 4.
VAS for dyspareunia
| Dienogest (N = 55) Mean ± SD |
Leuprolide acetate (N = 62) Mean ± SD |
T | p value | |
|---|---|---|---|---|
| Baseline VAS | 36.53 ± 3.87 | 34.98 ± 4.96 | 1.859 | 0.066 |
| VAS by 12 weeks | 16.53 ± 3.10 | 17.11 ± 2.53 | − 1.125 | 0.263 |
| Paired t | 48.076 | 25.656 | – | – |
| P | 0.000** | 0.000** | – | – |
**Highly significant difference
At the beginning of the study, 23 patients of the dienogest group and 19 patients of leuprolide acetate group were diagnosed to have endometrioma. The mean size of endometriomas was 32.48 ± 4.93 and 33.00 ± 5.29 mm, respectively. By 12 weeks, endometrioma size was reduced to 28.74 ± 6.39 and 30.11 ± 5.48 mm, respectively There was no significant difference between both groups by 12 weeks (p = 0.467) (Table 5).
Table 5.
Endometrioma size
| Dienogest (N = 23) Mean ± SD |
Leuprolide acetate (N = 19) Mean ± SD |
t/Mann–Whitney | p value | |
|---|---|---|---|---|
| At baseline | 32.48 ± 4.93 | 33.00 ± 5.29 | − 0.330 | 0.743 |
| By 12 weeks | 28.74 ± 6.39 | 30.11 ± 5.48 | − 0.735 | 0.467 |
| Paired t | 4.789 | 3.886 | – | – |
| P | 0.000** | 0.001** | – | – |
**Highly significant difference
The most frequent drug-related adverse effects in dienogest group were vaginal bleeding and weight gain (64.5 and 10.8%, respectively) which were significantly higher than leuprolide acetate group (21.5 and 3.3%, respectively), p value was (0.000 and 0.020, respectively). While the most frequent drug-related adverse effects in leuprolide acetate group were hot flushes and vaginal dryness (46.3 and 15.7%, respectively) which were significantly higher than dienogest group (15.7 and 3.3%, respectively), p value was (0.000 and 0.001, respectively) (Table 6).
Table 6.
Drug-related adverse effects
| Dienogest (N = 121) |
Leuprolide acetate (N = 121) |
X2 | p value | |
|---|---|---|---|---|
| Headache | 17 (14%) | 26 (21.5%) | 2.29 | 0.13 |
| Weight gain | 13 (10.8%) | 4 (3.3%) | 5.1 | 0.020* |
| Vaginal bleeding | 78 (64.5%) | 26 (21.5%) | 45.5 | 0.000** |
| Vaginal dryness | 4 (3.3%) | 19 (15.7%) | 10.81 | 0.001** |
| Hot flushes | 19 (15.7%) | 56 (46.3%) | 26.45 | 0.00** |
**Highly significant difference
*Significant difference
There was highly significant reduction in pelvic pain, back pain and dyspareunia in both groups with mean of difference in dienogest group (28.7 ± 5.3, 19.0 ± 4.3 and 20.0 ± 3.08 mm, respectively) and in leuprolide acetate group (26.2 ± 3.01, 19.5 ± 3.01 and 17.9 ± 2.9 mm, respectively).
Discussion
This study was an attempt to find the best treatment for recurrent pelvic pain following laparoscopic surgery for histopathologically proven endometriosis, comparing the effect of dienogest and leuprolide acetate.
Recurrence is a major concern after surgical excision of ovarian endometriomas especially in patients wishing to preserve the ovarian function. The recurrence rate is reported to be as high as 50% at 5 years [6].
There is worldwide concern regarding the risk of repeated operations for endometriosis [15].
Gonadotrophin-releasing hormone analogues or progestins are the options most usually adopted.
In recent years, the attention of clinicians has been focused on dienogest, which is currently the only steroidal drug marketed in most of Europe. Dienogest reduces endometriotic lesions by creating a local progestogenic environment, suppressing the systemic estrogen level moderately [16].
Pain was evaluated with a VAS score, one of the most utilized tests in clinical research even if it is a subjective measure [17]; it has, however, the advantage of simplicity, is language independent and is easily understood by most patients. The VAS score was assessed at the beginning of the study and again at 12 weeks.
In the current study, both dienogest and leuprolide acetate were associated with highly significant reductions in VAS for pelvic pain, back pain and dyspareunia by 12 weeks. There was no significant difference between both groups.
This result was in agreement with Strowitzki et al., who studied 252 Patients with confirmed endometriosis that were randomized to treatment with dienogest (2 mg/day, orally) or LA (3.75 mg, depot i.m. injection, every 4 weeks) for 24 weeks and found that both dienogest and LA were associated with substantial reductions in VAS score between baseline and week 24. At baseline, the mean (± SD) VAS score was 60.2 (± 24.2) mm in the dienogest group and 57.9 (± 21.0) mm in the LA group. By week 24, mean VAS scores had decreased to 12.7 (± 20.3) mm in the dienogest group and to 11.9 (± 16.9) mm in the LA group [17].
Harada et al. in 2009 compared dienogest (2 mg/day, orally) to buserelin acetate (BA) (900 μg/day, intranasally) for 24 weeks in 271 patients with endometriosis complaining of at least one of five subjective symptoms (lower abdominal pain, lumbago, defecation pain, dyspareunia, and pain on internal examination) and concluded that dienogest reduced the scores of all symptom at the end of treatment that were comparable to those obtained with BA [18].
Caruso et al. in 2015 studied the effect of dienogest 2 mg on quality of life and sexual function in women with endometriosis-associated pelvic pain. Pain improvement was observed in the study group at 3 (p < 0.05) and 6 months (p < 0.001) of treatment, and no change was observed in the control group (p = NS) [19].
The most frequent drug-related adverse effect in dienogest group was vaginal bleeding (64.5%) which was significantly higher than leuprolide acetate group (21.5%). None of the patients in either group had discontinued the medication due to abnormal uterine bleeding which suggests that the break through bleeding had a minimal impact on the drug compliance especially with the concomitant improvement in pelvic pain associated with treatment. Informing the patients about this possible adverse effect of dienogest therapy may enhance further compliance.
Many studies reported that dienogest is associated with irregular uterine bleeding in the short-term therapy [20–22]. Dienogest-induced genital bleeding originated mainly from breakthrough bleeding from pseudodecidua that occurs commonly with progestational agents. The incidence of genital bleeding decreased with continued treatment and resolved after the end of treatment [23].
There were minimal changes in the body weight which occurred in 10.8% of dienogest-treated cases. This was consistent with previous studies [24, 25].
The most frequent drug-related adverse effects in leuprolide acetate group were hot flushes and vaginal dryness (46.3 and 15.7%, respectively) which were significantly higher than dienogest group (15.7 and 3.3%, respectively). Consistent with this observation, GnRH agonists are associated with estrogen deprivation symptoms (hot flushes, vaginal dryness, headache and decreased libido) and bone demineralization which limits the duration of treatment to 6 months in the absence of add-back therapy [17]. The European Society for Human Reproduction and Embryology (ESHRE) guideline recommends careful use of GnRH agonists in younger women who have not yet achieved the maximum bone density [1].
The safety profile of dienogest in treatment of endometriosis that was reported in this study is supported by previous studies [26–28].
In this study, there was highly significant reduction in the endometrioma size in both dienogest and leuprolide acetate groups which may offer a new treatment option in cases with pelvic pain and endometrioma. Unfortunately, there was a small number of cases having endometrioma in this study.
Limitation of this Study
A potential limitation of the study was the relatively short duration of treatment which was restricted to 12 weeks. A longer study duration on a larger number of patients is therefore recommended to properly assess the long-term efficacy and safety of dienogest. Another limitation was the VAS scoring for pain which is unfortunately a subjective measure, so the process of obtaining patients’ scores by the investigators could have been biased.
Conclusion
Daily dienogest is as effective as depot LA for relieving endometriosis-associated pelvic pain, low back pain and dyspareunia. In addition, dienogest has acceptable safety, tolerability and lower incidence of hot flushes. Thus, it may offer an effective and well-tolerated treatment in endometriosis.
Acknowledgements
The authors would like to thank all the staff members of the Department of Obstetrics and Gynecology, Faculty of medicine, Zagazig University hospitals as well as all included women for their valuable contribution in this work.
Ahmed Mahmoud Abdou
is a Lecturer and Consultant of Obstetrics and Gynecology, Faculty of Medicine, Zagazig University, Egypt. He has Bachelor degree (M.B. Bch) in Medicine and Surgery, Master (M.Sc.) and Doctorate degrees (M.D. degree) in Obstetrics and Gynecology from the previously mentioned institute. His field of interest is reproductive endocrinology and gynecological endoscopy.
Authors’ Contribution
AMA: project development, IMMA: data analysis and manuscript revision, AAAA: data collection, AAA: manuscript writing
Compliance with Ethical Standards
Conflict of interest
All authors declare that there is no conflict of interest with other people or organizations that could inappropriately influence or bias the content of the paper.
Ethical approval
All procedures performed in this study involving human participants (administration of oral dienogest 2 mg once daily or intramuscular leuprolide acetate depot 3.75 mg injection every 4 weeks for 12 weeks for patients with recurrent pelvic pain following laparoscopy for endometriosis) were in accordance with the ethical standards of the Faculty of Human Medicine—Zagazig University and with the 1964 Declaration of Helsinki and its later amendments and were approved by the IRB (Institutional Review Board). The study protocol, the intervention involved, possible early and long-term side effects of interventions were explained to the patients. After approval of the local ethics committee, a written informed consent was obtained from all patients before participating in the study.
Informed Consent
Written informed consent was obtained from all patients before participating in the study.
Footnotes
Ahmed Mahmoud Abdou, M.D., is a Lecturer and Consultant of Obstetrics and Gynecology, Faculty of Medicine, Zagazig University, Egypt.
References
- 1.Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412. doi: 10.1093/humrep/det457. [DOI] [PubMed] [Google Scholar]
- 2.Meuleman C, Vandenabeele B, Fieuws S, et al. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil Steril. 2009;92(1):68–74. doi: 10.1016/j.fertnstert.2008.04.056. [DOI] [PubMed] [Google Scholar]
- 3.Smorgick N, As-Sanie S, Marsh CA, et al. Advanced stage endometriosis in adolescents and young women. J Pediatr Adolesc Gynecol. 2014;27(6):320–323. doi: 10.1016/j.jpag.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Guo SW. Recurrence of endometriosis and its control. Hum Reprod Update. 2009;15(4):441–461. doi: 10.1093/humupd/dmp007. [DOI] [PubMed] [Google Scholar]
- 5.Vercellini P, Crosignani PG, Abbiati A, et al. The effect of surgery for symptomatic endometriosis: the other side of the story. Hum Reprod Update. 2009;15(2):177–188. doi: 10.1093/humupd/dmn062. [DOI] [PubMed] [Google Scholar]
- 6.Singh SS, Suen MW. Surgery for endometriosis: beyond medical therapies. Fertil Steril. 2017;107:549–554. doi: 10.1016/j.fertnstert.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Muzii L, Marana R, Angioli R, et al. Histologic analysis of specimens from laparoscopic endometrioma excision performed by different surgeons: does the surgeon matter? Fertil Steril. 2011;95:2116–2119. doi: 10.1016/j.fertnstert.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 8.Vercellini P, Somigliana E, Viganò P, et al. Post-operative endometriosis recurrence: a plea for prevention based on pathogenetic, epidemiological and clinical evidence. Reprod Biomed Online. 2010;21:259–265. doi: 10.1016/j.rbmo.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Vercellini P, Somigliana E, Daguati R, et al. Post-operative oral contraceptive exposure and risk of endometrioma recurrence. Am J Obstet Gynecol. 2008;198:504–505. doi: 10.1016/j.ajog.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Becker CM, Gattrell WT, Gude K, et al. Reevaluating response and failure of medical treatment of endometriosis: a systematic review. Fertil Steril. 2017;108(1):125–136. doi: 10.1016/j.fertnstert.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DY, Lee JY, Seo JW, et al. Gonadotropin-releasing hormone agonist with add–back treatment is as effective and tolerable as dienogest in preventing pain recurrence after laparoscopic surgery for endometriosis. Arch Gynecol Obstet. 2016;294(6):1257–1263. doi: 10.1007/s00404-016-4184-9. [DOI] [PubMed] [Google Scholar]
- 12.Zito G, Luppi S, Giolo E, et al. Medical treatments for endometriosis-associated pelvic pain. BioMed Res Int. 2014;2014:191967. doi: 10.1155/2014/191967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahmioglu N, Missmer SA, Montgomery GW, et al. Insights into assessing the genetics of endometriosis. Curr Obstet Gynecol Rep. 2012;1(3):124–137. doi: 10.1007/s13669-012-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fauconnier A, Fritel X, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Gynecol Obstet Fertil. 2009;37(1):57–69. doi: 10.1016/j.gyobfe.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Takae S, Kawamura K, Sato Y, et al. Analysis of late-onset ovarian insufficiency after ovarian surgery: retrospective study with 75 patients of post-surgical ovarian insufficiency. PLoS ONE. 2014;9(5):e98174. doi: 10.1371/journal.pone.0098174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vercellini P, Frattaruolo MP, Somigliana E, et al. Surgical versus low-dose progestin treatment for endometriosis-associated severe deep dyspareunia II: effect on sexual functioning, psychological status and health-related quality of life. Hum Reprod. 2013;28:1221–1230. doi: 10.1093/humrep/det041. [DOI] [PubMed] [Google Scholar]
- 17.Strowitzki T, Marr J, Gerlinger C, et al. Dienogest is as effective as leuprolide acetate in treating the painful symptoms of endometriosis: a 24-week, randomized, multicentre, open-label trial. Hum Reprod. 2010;25(3):633–641. doi: 10.1093/humrep/dep469. [DOI] [PubMed] [Google Scholar]
- 18.Harada T, Momoeda M, Taketani Y, et al. Dienogest is as effective as intranasal buserelin acetate for the relief of pain symptoms associated with endometriosis—a randomized, double-blind, multicenter, controlled trial. Fertil Steril. 2009;91(3):675–681. doi: 10.1016/j.fertnstert.2007.12.080. [DOI] [PubMed] [Google Scholar]
- 19.Caruso S, Iraci M, Cianci S, et al. Quality of life and sexual function of women affected by endometriosis-associated pelvic pain when treated with dienogest. J Endocrinol Invest. 2015;38:1211. doi: 10.1007/s40618-015-0383-7. [DOI] [PubMed] [Google Scholar]
- 20.Harada T, Momoeda M, Taketani Y. Dienogest is as effective as intranasal buserelin acetate for the relief of pain symptoms associated with endometriosis—a randomized, double-blind, multicenter, controlled trial. Fertil Steril. 2009;91:675–681. doi: 10.1016/j.fertnstert.2007.12.080. [DOI] [PubMed] [Google Scholar]
- 21.Strowitzki T, Faustmann A, Christoph G, et al. Dienogest in the treatment of endometriosis-associated pelvic pain: a 12-week, randomized, double-blind, placebo-controlled study. Eur J Obstet Gynecol Reprod Biol. 2010;151:193–198. doi: 10.1016/j.ejogrb.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Köhler G, Faustmann TA, Gerlinger C, et al. A dose-ranging study to determine the efficacy and safety of dienogest 1, 2, and 4 mg daily in the treatment of endometriosis. Int J Gynaecol Obstet. 2010;108:21–25. doi: 10.1016/j.ijgo.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Irahara M, Harada T, Momoeda M, et al. Hormonal and histological study on irregular genital bleeding in patients with endometriosis during treatment with dienogest, a novel progestational therapeutic agent. Reprod Med Biol. 2007;6:223–228. doi: 10.1111/j.1447-0578.2007.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seitz C, Gerlinger C, Marr J, et al. A double-blind placebo-controlled trial investigating the efficacy of 12 weeks of dienogest 2 mg/day for the treatment of endometriosis-associated pain. Fertil Steril. 2008;90:S140. doi: 10.1016/j.fertnstert.2008.07.230. [DOI] [Google Scholar]
- 25.Strowitzki T, Marr J, Gerlinger C, et al. Dienogest is as effective as leuprolide acetate in treating the painful symptoms of endometriosis: a 24-week, randomized, multicentre, open-label trial. Hum Reprod. 2010;25:633–641. doi: 10.1093/humrep/dep469. [DOI] [PubMed] [Google Scholar]
- 26.Momoeda M, Taketani Y, Terakawa N, et al. A randomized, double-blind, multicenter, parallel, dose–response study of dienogest in patients with endometriosis. Jpn Pharmacol Ther. 2007;35:769–783. [Google Scholar]
- 27.Momoeda M, Harada T, Terakawa N. Long-term use of dienogest for the treatment of endometriosis. J Obstet Gynaecol Res. 2009;35(6):1069–1076. doi: 10.1111/j.1447-0756.2009.01076.x. [DOI] [PubMed] [Google Scholar]
- 28.Kitawaki J, Kusuki I, Yamanaka K, et al. Maintenance therapy with dienogest following gonadotropin-releasing hormone agonist treatment for endometriosis-associated pelvic pain. Eur J Obstet Gynecol Reprod Biol. 2011;157(2):212–216. doi: 10.1016/j.ejogrb.2011.03.012. [DOI] [PubMed] [Google Scholar]


