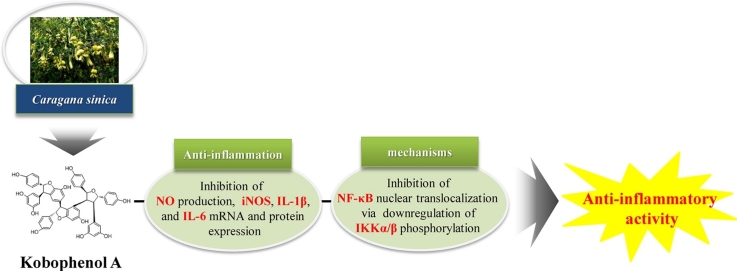
Graphical abstract
Abbreviations: C. sinica, Caragana sinica; IL-6, interleukin-6; IL-1β, interleukin-1β; iNOS, inducible nitric oxide synthase; IκB, inhibitory κB; IKKα/β, IκB kinase α/β; KPA, Kobophenol A; LPS, lipopolysaccharide; MAPKs, Mitogen-activated protein kinases; NF-κB, nuclear factor-κB; NO, nitric oxide; NSAIDs, nonsteroidal anti-inflammatory drugs; PGE2, Prostaglandin E2; TNF-α, tumor necrosis factor-α
Keywords: inducible nitric oxide synthase, kobophenol A, nitric oxide, nuclear factor-κB, pro-inflammatory cytokines
Abstract
Kobophenol A (KPA) is a biologically active natural compound isolated from the roots of Caragana sinica (Buc’hoz) Rehder (C. sinica). However, the anti-inflammatory effects of KPA have not been reported. This study aims to find out whether KPA isolated from roots of C. sinica can act as a potential substance on inflammation and analyze the molecular mechanism using the lipopolysaccharide (LPS)-stimulated J774 A.1 macrophage cell line. We showed that KPA treatment significantly suppressed the production of nitric oxide (NO) by inhibiting inducible nitric oxide synthase (iNOS) expression in a dose-dependent manner without cytotoxicity. In the KPA also inhibited pro-inflammatory cytokine gene expression and production, such as interleukin-1β (IL-1β) and interleukin-6 (IL-6) in LPS-stimulated J774 A.1 cells. As continuing study on the mechanisms involved, we confirmed that these effects of KPA were related to the inhibition of nuclear factor-κB (NF-κB) pathway including the suppression of IκB kinase α/β (IKKα/β) phosphorylation and translocation of NF-κB into the nucleus. Taken together, the present study is the first to demonstrate that KPA isolated from C. sinica suppresses the expression of inflammatory mediators and cytokines by inhibiting NF-κB nuclear translocation in LPS-stimulated J774 A.1 macrophages. KPA may be a potential candidate for the treatment of inflammatory diseases in the future.
1. Introduction
Carana sinica (Buc’hoz) Rehder (C. sinica) belongs to the genus Caragana in the family Fabaceae. Apoptotic, phytoestrogenic, neuroprotective, anti-bacterial, and anti-oxidant effects of C. sinica have been reported in [1,2]. C. sinica contains many oligostilbenes, such as (+)-α viniferin, caraganaphenol A, miyabenol C and kobophenol A (KPA) [3]. KPA, a tetrameric stilbene, is one of the principal active compounds in C. sinica [4,5]. Various studies have reported the apoptotic, anti-microbial and protein kinase C inhibitory activities of KPA [1,6].
Inflammation is a natural biological reaction to infection or injury in the human body involving activated immune cells such as monocytes and macrophages [7]. In particular, macrophages are important in the process of secretion of pro-inflammatory mediators and the host defense system [7,8]. During the inflammatory process, inducible nitric oxide synthase (iNOS) is an important regulator and induces the release of nitric oxide (NO). Additionally, lipopolysaccharide (LPS) induces the secretion of the pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) [[8], [9], [10], [11]]. Mitogen-activated protein kinases (MAPKs) play and important roles in activating the nuclear factor-κB (NF-κB) complex. IκB kinase (IKK) composed of IκB kinase α (IKKα) and IκB kinase β (IKKβ) is activated in response to LPS and pro-inflammatory cytokines. In inflammatory responses, the NF-κB complex activated by IKK leads to the degradation of inhibitory κB (IκB). Then active NF-κB complexes are phosphorylated, translocated into the nucleus and bind to the promoter of pro-inflammatory gene expression such as iNOS, TNF-α, IL-1β and IL-6 [[12], [13], [14], [15], [16]]. Therefore, blocking the action of the NF-κB signaling pathway and pro-inflammatory cytokines is known to target treatment for inflammatory diseases.
Recently, there has been a growing interest in physiologically active substances derived from natural products that have no or fewer side effects and toxicity. Previous studies have reported various physiologically active substances that suppress the production of NO and Prostaglandin E2 (PGE2) through anti-inflammatory mechanisms [8,9,[17], [18], [19]]. However, the anti-inflammatory activity of KPA isolated from the roots of C. sinica has not yet been elucidated. The present study investigated the anti-inflammatory effects and its molecular mechanism of KPA isolated from roots of C. sinica in LPS-stimulated J774 A.1 macrophage cell line.
2. Materials and methods
2.1. Plant materials
The roots of C.sinica [Caraganasinica(Buc’hoz)Rehder] were identified by Professor Joa Sub Oh, College of Pharmacy, Dankook University, Cheonan, South Korea and obtained from Gyeongdong Oriental Medicine Market, Jegi-dong, Dongdaemun-gu, Seoul, Republic of Korea in March 2015. A voucher specimen (G59) has been deposited at the Bio-center, Gyeonggi Institute of Science & Technology Promotion, Suwon, Republic of Korea.
Preparation of KPA
The dried roots of C. sinica (4.8 kg) were extracted two times with 70% EtOH (90 L × 2) at room temperature. The 70% EtOH extracts were evaporated in vacuum at 40 °C to yield 746 g of extract. The extract were suspended in H2O (4 L × 2) and solvent partitioned to n-hexane (8.2 g), CH2Cl2 (14 g), EtOAc (12.8 g) and n-BuOH (34.2 g) layers, respectively. The fraction of CH2Cl2-soluble layer (14 g) was subjected to liquid column chromatography [glass column (5 × 25 cm) packed with silica gel (70-230 mesh)] using stepwise gradient mixtures as eluents (CHCl3/MeOH = 1:0 to 0:1). The fractions G59 5-1∼5-13 were obtained through this process. Compound 1 (312.7 mg) was isolated from G59-5-9 by using liquid column chromatography [(glass column (3.0 × 76 cm) packed with Sephadex LH-20 gel)] using isocratic elution (CHCl3 : MeOH; 1:1).
2.2. Spectrometric analysis of KPA
KPA is yellow amorphous powder; [α]D22 216.79 (c 0.9, MeOH)] 1H-NMR (700 MHz, acetone-d6) δ 7.35 (2H, d, J = 8.4 Hz, H-2a, 6a), 7.08 (2H, d, J = 9.1 Hz, H- 2d, 6d), 6.89 (2H, d, J = 9.1 Hz, H-3a, 5a), 6.78 (2H, d, J = 8.4 Hz, H-3d, 5d), 6.60 (2H, d, J = 8.4 Hz, H-3c, 5c), 6.52 (2H, d, J = 2.1 Hz, H-12b), 6.50 (2H, d, J = 8.4 Hz, H -3b, 5b), 6.42 (1H, d, J = 2.1 Hz, H-14c), 6.41 (2H, d, J = 9.1 Hz, H-2c, 6c), 6.21 (2H, d, J = 8.4 Hz, H-2b, 6b), 6.09 (1H, t, J = 2.1 Hz, H-12d), 6.04 (1H, t, J = 2.1 Hz H-12a), 6.03 (2H, d, J = 2.1 Hz, H-10a, 14a), 6.01 (1H, d, J = 2.1 Hz, H-12c), 5.97 (1H, d, J = 2.1 Hz, H-14b), 5.82 (2H, d, J = 2.1 Hz, H-14d), 5.53 (1H, d, J = 2.1 Hz, H-14c), 5.16 (1H, d, J = 10.5 Hz, H-7d), 5.05 (1H, d, J = 4.9 Hz, H-7c), 5.01 (1H, d, J = 4.2 Hz, H-7b), 4.33 (1H, d, J = 2.1 Hz, H-8a), 3.50 (1H, d, J = 3.5 Hz, H-8b), 3.30 (1H, dd, J = 5.6, 4.9 Hz, H-8c), 3.11 (1H, dd, J = 10.5, 5.6 Hz, H-8d); 13C-NMR (175 MHz, acetone-d6) δ 160.9 (C-11b), 160.0 (C-11c), 159.8 (C-13b), 158.6 (C-11a, 13a), 157.7 (C-13c), 157.3 (C-11d, 13d), 157.0 (C-4a), 156.7 (C-4d), 156.4 (C-4b), 155.1 (C-4c), 146.5 (C-9a), 134.7(C-9b), 138.4(C-9d), 135.3 (C-9c), 134.4 (C-1a), 133.5 (C-1d), 132.6 (C-1b), 130.9 (C-1c), 127.7 (C-2d, 6d), 126.6 (C-2c, 6c), 126.2 (C-2b, 6b), 125.7 (C-2a, 6a), 123.4 (C-10c), 119.0 (C-10b), 115.5 (C-3a, 5a), 114.9 (C-3d, 5d), 114.9 (3b, 5b), 114.3 (C-3c, 5c), 109.4 (C-14c), 108.3 (C-10d, 14d), 107.4 (C-14b), 105.7 (C-10a, 14a), 102.5 (C-12d), 101.1 (C-12a), 95.2 (C-12b), 94.8 (C-12c), 92.4 (C-7b), 91.5 (C-7a), 84.2 (C-7d), 83.9 (C-7c), 61.2 (C-8d), 57.2 (C-8a), 51.4 (C-8c), 51.2 (C-8b); ESI-MS (negative) m/z 923 [M – H].
2.3. Cell culture conditions
J774 A.1 cells, derived from mouse monocyte-macrophages (TIB-67) and human embryonic kidney cells (HEK 293 T) were obtained American Type Culture Collection (ATCC, Manassas, VA, USA). All cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM; ATCC) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS; ATCC, Manassas, VA, USA) and 100 U/ml penicillin and 0.1 mg/ml streptomycin (Invitrogen, Carlsbad, CA, USA) in a humidified atmosphere of 5% CO2 at 37℃.
2.4. Cell viability assay
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT; Duchefa Biochemie, Haarlem, The Netherlands) assay was performed to determine cell viability. J774 A.1 macrophage cells were seeded in 96-well plates at a density of 1.25 × 105 cells/well and incubated for 16 h. Cells were pre-treated with KPA (3.125-100 μM) isolated from root of C. sinica for 1 h and then stimulated 1 μg/ml LPS (Sigma Aldrich, St. Louis, MO, USA) for 24 h. After incubation, the medium was removed and 100 μl MTT solution (Duchefa Biochemie, Haarlem, The Netherlands) was added to each well and the cells were more incubated for 2 h. After then the supernatant was removed and dissolved in 100 μl dimethyl sulfoxide (DMSO; Duchefa Biochemie, Haarlem, The Netherlands) in each well. Absorbance at a wavelength of 540 nm was measured using a SpectraMax 190PC microplate reader (Molecular Devices, Sunnyvale, CA, USA). The experiment data were expressed as the means ± standard deviation of triplicate assay.
2.5. Nitric oxide production assay
J774 A.1 macrophage cells were seeded in 96-well plates at a density of 1.25 × 105 cells/well and were pre-treated with KPA (3.125-100 μM) for 1 h and then stimulated 1 μg/ml LPS for 24 h. The culture medium was analyzed for the amount of nitric oxide by the Griess reaction. To analysis nitrite, 50 μl Griess reagent (Sigma Aldrich, St. Louis, MO, USA) was mixed with the culture medium and incubated 10 min at room temperature. To measure NO production, absorbance at wavelength of 540 nm was measured using a SpectraMax 190PC microplate reader (Molecular Devices, Sunnyvale, CA, USA). A Standard was used sodium nitrite.
2.6. Enzyme-linked immunosorbent assay (ELISA)
J774 A.1 macrophage cells were seeded in 6-well plates at a density of 4 × 106 cells/well and pre-treated with KPA (25–100 μM) for 1 h and then stimulated 1 μg/ml LPS for 16 h. The levels of the pro-inflammatory cytokine IL-1β (cat. no. BMS6002) and IL-6 (cat. no. BMS614/2) were measured by ELISA kits (Invitrogen, Thermo Fisher Scientific, Inc.) in cell culture supernatants according to the manufacturer's recommended instructions.
2.7. Reverse transcription-polymerase chain reaction (RT-PCR)
J774 A.1 macrophage cells were seeded in 6-well plates at a density of 4 × 106 cells/well and pre-treated with KPA (25–100 μM) for 1 h and then stimulated 1 μg/ml LPS for 24 h. Total RNA was extracted by Trizol reagent (Invitrogen, Thermo Fisher Scientific, Inc.) according to the manufacturer's recommended instructions. 4 μg of total RNA was reverse transcribed to single stranded cDNA with random primers using the Taq DNA polymerase and SuperScript®III First-Strand Synthesis System (Invitrogen, Thermo Fisher Scientific, Inc.). The cDNA was amplified using specific primers and AccuPower® Pfu PCR premix (Bioneer Corporation, Daejeon, Republic of Korea) in MyGene™Series Peltier Thermal Cycler Model MG96 G (LongGene Scientific Instruments, Hangzhou, China). PCR cycling conditions were 5 min at 95 °C followed by 25 cycles of 30 sec at 95 °C, 40 sec at between 50 and 60 °C, 1 min at 72 °C and final extension for 10 min at 72 °C. After amplification, PCR products were electrophoresed on 1% agarose gel containing ethidium bromide. The following primers (Bioneer Corporation, Daejeon, Republic of Korea) were used for PCR amplification: IL-1β, 5’-CTTTGAAGAAGAGCCCATCC-3’ (sense) and 5’-TTTGTCGTTGCTTGGTTCTC-3’ (antisense); IL-6, 5’-CACTTCACAAGTCGGAGGCTT-3’ (sense) and 5’-GCAAGTGCATCATCGTTGTTC-3’ (antisense); iNOS, 5’-GAGTTCGAGACTTCTGTGA-3’ (sense) and 5’-GGCGATCTGGTAGTAGTAG-3’ (antisense); GAPDH, 5'-CAGGTAAACTCAGGAGAGTG-3' (sense) and 5'-GTAGACTCCACGACATACT C-3' (antisense).
2.8. Western blot analysis
J774 A.1 macrophage cells were seeded in 6-well plates at a density of 4 × 106 cells/well and pre-treated with KPA (25–100 μM) for 1 h and then stimulated 1 μg/ml LPS for 30 min and 24 h. After incubation, cells were washed 2 times with cold PBS and harvested using RIPA buffer (Sigma Aldrich) including protease inhibitor and phosphatase inhibitors (Sigma Aldrich). Total protein was extracted from the cells. Total protein was separated on 8% to 12% Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under denaturing condition and transferred to nitrocellulose (NC) membranes (Whatman, Dassel, Germany). The membranes were blocked with 5% BSA dissolved in Tris buffered saline (TBS) with 0.1% Tween-20 (TBST) for 1 h at room temperature and incubated with primary antibodies at 1:1000 (v/v) dilution in TBST against iNOS (cat. no. ab3523, 1:500 dilution; Abcam, Cambridge, UK), p-IKK α /β (cat. no. 2697), IKKα (cat. no. 2682), NF-κB p65 (cat. no. 8242), NF-κB p50 (cat. no. 12540), β-actin (cat. no. 5125, 1:1,000 dilution; Cell Signaling Technology, Danvers, MA, USA) and Lamin B (cat. no. sc-6216, 1:1,000 dilution; Santa Cruz Biotechnology, Dallas, TX, USA) using gentle shaking overnight at 4 °C. And then, membranes were washed 4 times for 20 min with TBST and incubated with Horesradish peroxidase (HRP)-conjugated secondary antibodies (cat. no. sc-2354, 1:5,000 dilution; Santa Cruz Biotechnology, Dallas, TX, USA) using shaker for 1 h at room temperature. Membranes were washed 3 times with TBST and detected by SuperSignal® West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL, USA).
2.9. NF-κB luciferase reporter assay
HEK 293 T cells were seeded in 24-well plates at a density of 1 × 105 cells/well. After overnight, the recombinant vector obtained by inserting the NF-κB gene into the pGL3-basic luciferase expression vector contained a coding region for Firefly luciferase and the pRL-SV-40 plasmid contained the cDNA encoding Renilla luciferase as a control reporter was co-transfected in DMEM, serum free media using lipofectamine (cat. no. 1857477, Invitrogen, Thermo Fisher Scientific, Inc.) for 24 h. After KPA (25–100 μM) treatment for 24 h, the cells were washed with PBS and lysed with 100 μl 1X Passive Lysis Buffer and NF-κB luciferase reporter activity was measured by a Dual Luciferase Kit (cat. no. E1910, Promega, Madison, WI, USA).
2.10. Cytosolic and nuclear proteins fraction
J774 A.1 macrophage cells were seeded in 6-well plates at a density of 1 × 106 cells/well and pre-treated with KPA (25–100 μM) for 1 h and then stimulated 1 μg/ml LPS for 30 min. The cells were washed 2 times with PBS and harvested. For isolation of cytosolic fractions, harvested cells were centrifuged at 12,000 × g at 4 °C for 5 min and incubated on ice for 10 min in Buffer A (10 mM HEPES pH7.9, 10 mM KCl, 0.1 mM EDTA, 0.5 mM PMSF, 1 mM DTT) with 10% NP-40. The supernatant, cytosolic extract was collected by centrifugation at 12,000 × g for 2 min at 4 °C. For isolation of nuclear fractions, the remaining cell pellets were resuspended and incubated on ice for 10 min in Buffer B (20 mM HEPES, 0.4 M NaCl, 1 mM EDTA, amM PMSF, amM DTT, 1% Nonidet P-40). The pellets were hardly shaked for 10 min and centrifuged at 17,000 × g at 4 °C for 15 min to collect the supernatant, nuclear protein extract.
2.11. Statistical analysis
All experiments were performed in triplicate wells and triplicate experiments. Statistical analysis was determined by one-way analysis of variance (ANOVA) followed by Dunnett's test and Student’s t-test between control and treated groups. All data were expressed as the means ± standard deviation (SD). Values of P < 0.05 were indicated to be statistically significant (*P < 0.05).
3. Results
3.1. Effects of KPA on cell viability and NO production in LPS-stimulated J774 A.1 cells
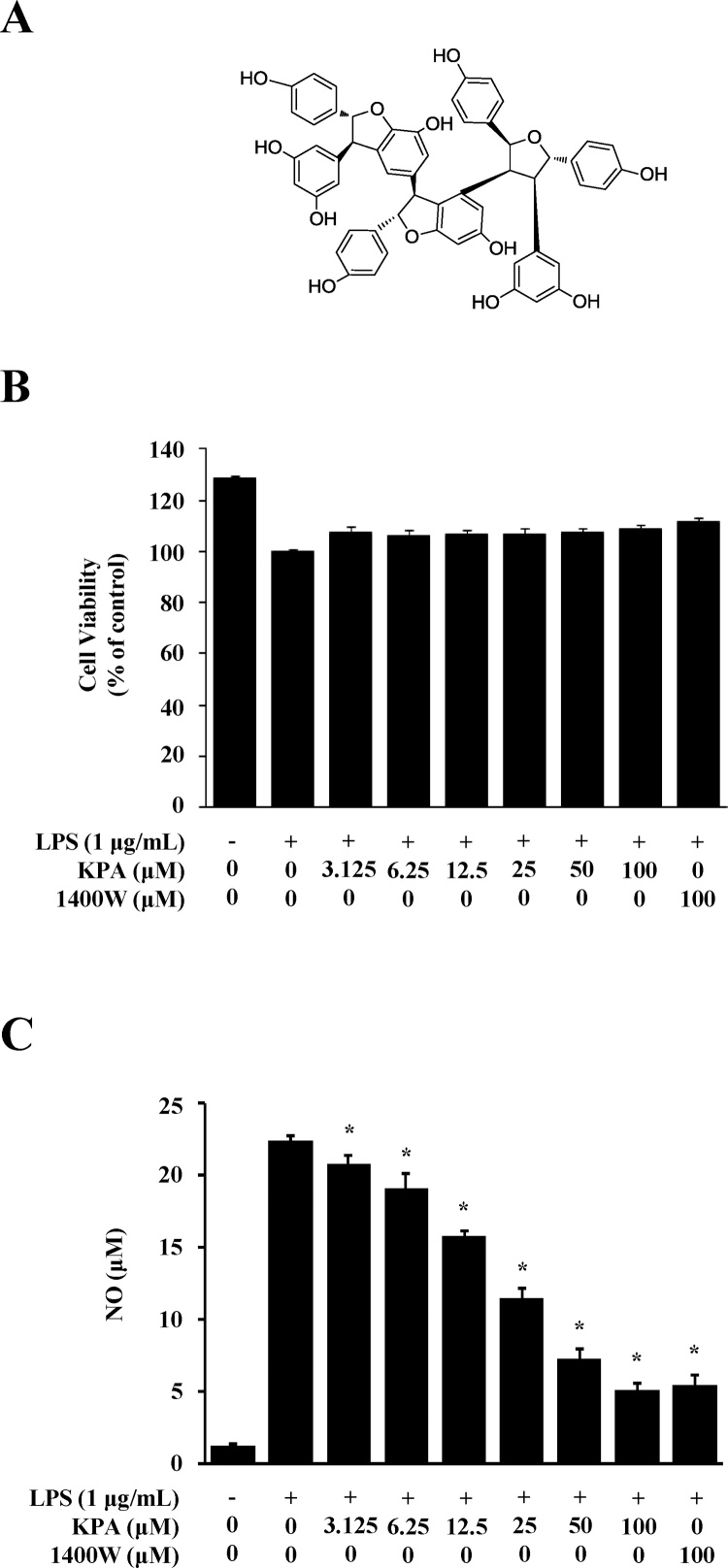
The chemical structure of KPA isolated from the roots of C. sinica is shown in Fig. 1A. To determine the effect of KPA on cell viability in LPS-stimulated J774 A.1 cells by MTT assay, cells were treated with different concentrations of KPA (3.125–100 μM) for 1 h prior to 1 μg/ml LPS stimulation for 24 h. KPA did not change cell viability at all concentrations up to 100 μM KPA. Therefore, these results suggest that KPA has no effect on the viability of J774 A.1 cells (Fig. 1B). We examined the inhibitory effect of KPA on NO production in LPS-stimulated J774 A.1 cells by using Griess reagent. Cells were treated with different concentrations of KPA (3.125–100 μM) for 1 h prior to 1 μg/ml LPS stimulation for 24 h. As shown in Fig. 1C, KPA dramatically inhibited NO production in a dose-dependent manner in LPS-stimulated inflammatory response in J774 A.1 cells. In particular, 50 and 100 μM KPA treatment reduced LPS-stimulated NO production by 65% and 78%, respectively. The results observed following treatment with 100 μM KPA were similar to those after treatment with 100 μM 1400 W dihydrochloride, an inhibitor of NO synthase [20]. Therefore, these results indicate that KPA has an anti-inflammatory effect through the suppression of NO production without cytotoxicity in LPS-stimulated J774 A.1 cells.
Fig. 1.
Effects of KPA on cytotoxicity and NO production in LPS-stimulated J774 A.1 cells. Cells were treated with KPA (3.125-100 μM) for 1 h prior to LPS (1 μg/ml) stimulation for 24 h. (A) The structure of kobophenol A. (B) Cytotoxicity of KPA was determined by MTT assay. (C) The NO production was analyzed by measuring the amount of nitric oxide using Griess reagent. 1400 W dihydrochloride was used as a positive control. Values represent the mean ± SD of three independent experiments and statistical significant values are indicated (*p < 0.05, compared with LPS-stimulated group).
3.2. Effect of KPA on the expression of iNOS in LPS-stimulated J774 A.1 cells
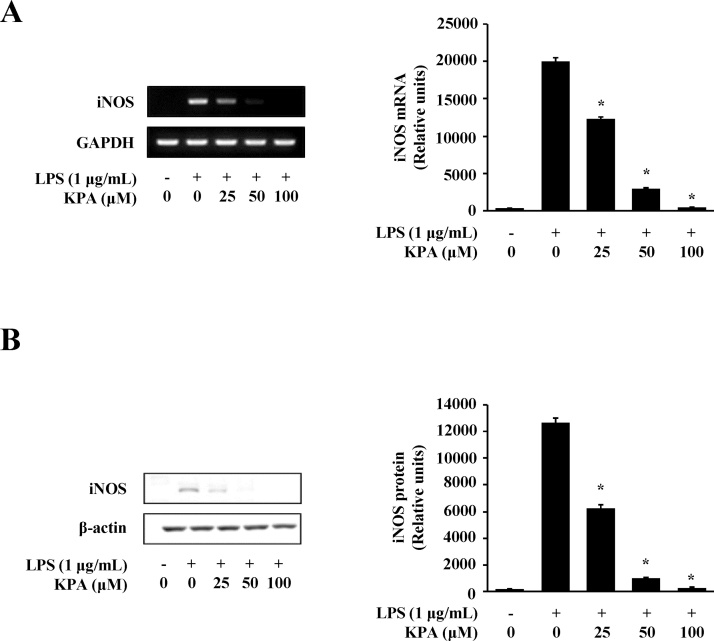
NO production in LPS-stimulated J774 A.1 cells is mediated by iNOS [21]. We examined the effect of KPA on iNOS activity by RT-PCR and western blotting at the gene and protein level. As shown in Fig. 2A, KPA treatment (25-100 μM) significantly inhibited iNOS expression in a dose-dependent manner at mRNA level in LPS-stimulated J774 A.1 cells. We also showed that iNOS protein production was significantly inhibited by treatment with 25, 50, and 100 μM KPA (Fig. 2B). This effect of KPA on the protein expression of iNOS was similar to that of its inhibition of the mRNA expression of iNOS (Fig. 2A and B). This finding suggests that KPA inhibits LPS-stimulated NO production by down-regulating iNOS expression in J774 A.1 cells.
Fig. 2.
Effects of KPA on expression of iNOS in LPS-stimulated J774 A.1 cells. Cells were treated with KPA (25-100 μM) for 1 h prior to LPS (1 μg/ml) stimulation for 24 h. (A) The expression of iNOS at the mRNA was determined by RT-PCR and the representation of iNOS mRNA was analyzed by densitometry protocol. (B) The expression of iNOS at the protein levels was determined by western blotting and the representation of iNOS protein was analyzed by densitometry protocol. Values represent the mean ± SD of three independent experiments and statistical significant values are indicated (*p < 0.05, compared with LPS-stimulated group).
3.3. Effect of KPA on expression of pro-inflammatory cytokines in LPS-stimulated J774 A.1 cells
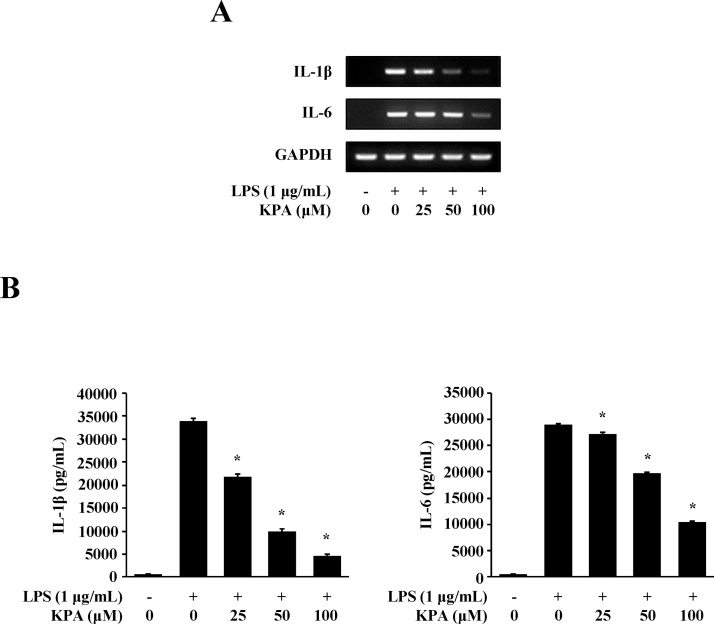
Pro-inflammatory cytokines such as IL-1β and IL-6 are released through LPS stimulation in macrophage cells [22,23]. To examine the effect of KPA on pro-inflammatory cytokine expression, we investigated the effect of KPA on IL-1β and IL-6 at the mRNA and protein levels in LPS-stimulated J774 A.1 cells. As shown in Fig. 3A, treatment with 100 μM KPA inhibited the expression of IL-1β and IL-6 at the mRNA level. Furthermore, we confirmed the effect of KPA on their protein releases by using ELISA. The production of IL-1β and IL-6 significantly reduced after treatment with KPA (25–100 μM) (Fig. 3B). These findings also indicate that KPA has an anti-inflammatory effect by inhibiting pro-inflammatory cytokines expression.
Fig. 3.
Effect of KPA on expression of pro-inflammatory cytokine in LPS-stimulated J774 A.1 cells. Cells were treated with KPA (25-100 μM) for 1 h prior to LPS (1 μg/ml) stimulation for 16 h. (A) The expressions of IL-1β, and IL-6 at mRNA level were determined by RT-PCR. (B) IL-1β and IL-6 in culture supernatants with 1 μg/ml LPS stimulation were measured by ELISA. Values represent the mean ± SD of three independent experiments and statistical significant values are indicated (*p < 0.05, compared with LPS-stimulated group).
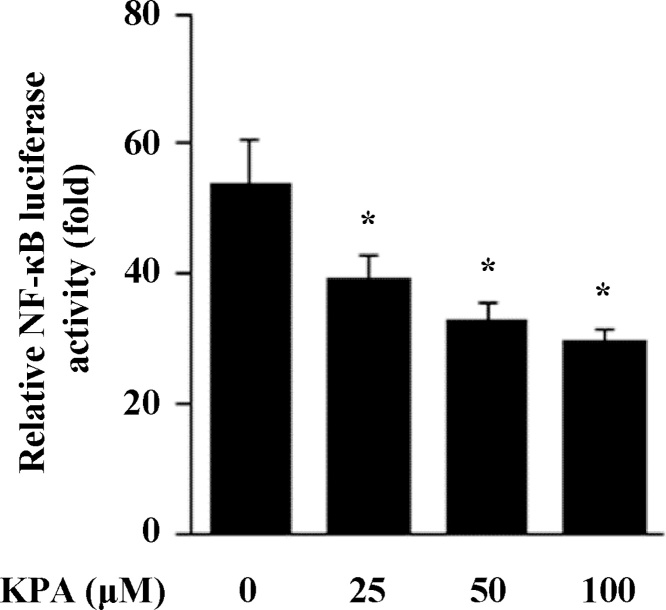
3.4. Effect of KPA on NF-κB transcriptional activity in HEK 293 T cells
NF-κB is known to play a crucial role in regulating cell growth, differentiation and the expression of inflammatory factors such as IL-1β, IL-6 and iNOS [24,25]. To determine the pharmacological roles and molecular mechanism of KPA on LPS-stimulated inflammatory response, we performed a reporter assay of NF-κB transcriptional activity after KPA treatment in HEK 293 T cells. We examined the reduction of NF-κB activity using 293 T cells which are sensitive to lentivirus infection [26]. Cells exposed to KPA treatment significantly decreased NF-κB transcriptional activity in a dose-dependent manner in HEK 293 T cells (Fig. 4).
Fig. 4.
Effect of KPA on NF-κB transcriptional activity. Effect of KPA on NF-κB luciferase reporter assay in HEK 293 T cells. Cells were co-transfected with pRL-SV-40 plasmid and NF-κB luciferase reporter plasmid for 24 h. And then, cells were treated various concentration of KPA (25-100 μM). Luciferase activities was measured by firefly and Renilla luciferase reporter. Values represent the mean ± SD of three independent experiments and statistical significant values are indicated (*p < 0.05, compared with LPS-stimulated group).
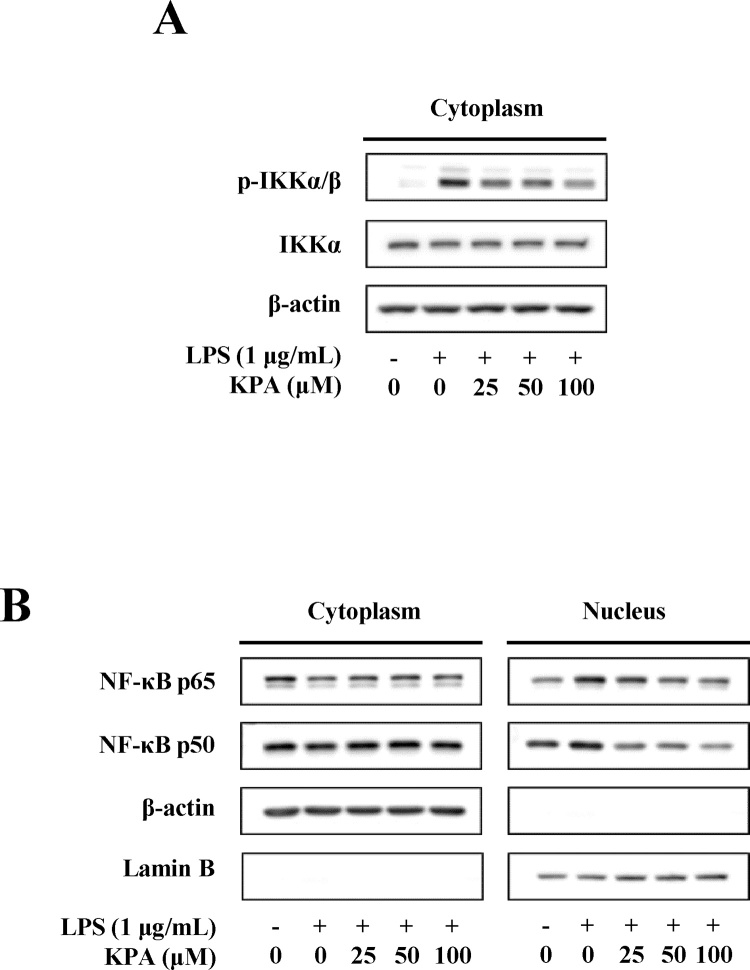
3.5. Effect of KPA on nuclear localization of NF-κB in LPS-stimulated J774 A.1 cells
We examined the effect of KPA on NF-κB phosphorylation and nuclear translocation in LPS-stimulated J774 A.1 cells. The IKK complex (consisting of IKKα and IKKβ) is known to be related to IκB phosphorylation and signal transduction to NF-κB [27]. As shown in Fig. 5A, LPS stimulation for 30 min dramatically induced the phosphorylation of IKKα/β in the cytosolic compartments. However, KPA treatment dose-dependently inhibited LPS-stimulated phosphorylation of IKKα/β in J774 A.1 cells. We investigated whether KPA regulates the translocation of NF-κB into the nucleus in LPS-stimulated J774 A.1 cells. LPS stimulation for 30 min induced the nuclear localization of NF-κB in the nuclear compartments, and KPA treatment inhibited LPS-induced nuclear localization of NF-κB p65 and p50 in a dose-dependent manner (Fig. 5B). These results demonstrate that KPA can modulate the LPS-stimulated inflammatory response by suppressing the NF-κB signaling pathway in J774 A.1 cells.
Fig. 5.
Effect of KPA on NF-κB translocation. (A) Effect of KPA on IKK activation in LPS-stimulated J774 A.1 cells. Cells were treated with KPA (25-100 μM) for 1 h prior to LPS (1 μg/ml) stimulation for 30 min. (B) Effect of KPA on nuclear translocation of NF-κB p65 and p50 in LPS-induced J774 A.1 cells. Cells were treated with KPA (25-100 μM) for 1 h prior to LPS (1 μg/ml) stimulation for 30 min. The data are representative of western blotting and the results were performed 3 independent experiments.
4. Discussion
In inflammation processes, macrophages and monocytes play a crucial role in maintaining homeostasis and the host defense system. The expression of pro-inflammatory mediators and cytokines is directly related to the inordinate inflammation response and pathogenesis of chronic diseases [28]. Nonsteroidal anti-inflammatory drugs (NSAIDs) are the generally used anti-inflammatory drugs; however, they have been known to induce side effects and lead to various other diseases [29]. Recently, there has been an increased interest in the development of new medicines from natural products with few side effects.
Inflammatory responses are regulated by pro-inflammatory mediator, NO, which is a useful target for inhibition of inflammatory symptoms. NO production in macrophages is primary regulated by iNOS in the inflammatory response [30,31]. In our previous study, we observed that the ethanol extract of C. sinica inhibits NO production in LPS-stimulated J774 A.1 cells (data not shown). Therefore, in the present study, we isolated KPA from the ethanol extract of C. sinica and examined its effect on inflammatory response by using J774 A.1 macrophages. We showed that KPA treatment inhibits NO production and iNOS expression without cytotoxicity (Fig. 1, Fig. 2). To evaluate NO response, we used 1400 W dihydrochloride, which is a positive control for the inhibition of NO synthase, and its inhibitory effect was very similar to the effect of KPA treatment (100 μM). These results provide evidence that KPA suppresses LPS-stimulated NO production via down-regulating iNOS expression in macrophages.
We also showed that KPA inhibits the release of pro-inflammatory cytokines such as IL-1β and IL-6 in LPS-stimulated J774 A.1 cells (Fig. 3). Previous studies reported that LPS-stimulated macrophages and monocytes were able to activate and generate IL-1β and IL-6 [32]. These pro-inflammatory cytokines have critical roles in regulating inflammation response via activation of NF-κB and MAPKs [33,34].
NF-κB is a major transcription factor in intercellular signaling pathways, regulates inflammatory gene expression, and is induced by LPS stimulation. NF-κB is composed of the subunits p65 and p50, which are bound to IκB in the cytoplasm in the un-stimulated condition. In condition of LPS stimulation, IκB is phosphorylated by IKK [27,35]. In this study, we observed that KPA suppresses NF-κB transcriptional activity in HEK 293 T cells (Fig. 4). Moreover, KPA inhibited the phosphorylation of IκB kinase α/β (IKKα/β) and NF-κB translocation from the cytoplasm into the nucleus (Fig. 5). These findings indicate that KPA suppressed pro-inflammatory mediators by blocking the nuclear translocation of NF-κB in LPS-stimulated J774 A.1 cells.
NF-κB can be activated by the LPS-stimulated activation of the MAPKs signaling pathway [36,37] and MAPKs are important regulators in the innate and adaptive immune response [38]. In addition, inflammation related studies have been reported through animal experiments [[39], [40], [41]]. Therefore, further research should investigate the inhibitory effect of KPA on MAPKs related to other molecular mechanisms in animal experiments and LPS-stimulated response.
5. Conclusion
Taken together, these findings demonstrated that KPA isolated from the roots of C. sinica shows anti-inflammatory activity by inhibiting the expression of inflammatory mediators through the down-regulation the of NF-κB signaling pathway in LPS-stimulated J774 A.1 cells. This is the first report to show that KPA might have potential as a therapeutic agent for the regulation of inflammatory responses.
Funding
This study was supported by the research fund of Dankook University in 2015.
Conflictof interest
The authors have no conflicts of interest to declare.
Transparency document
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2018.05.011.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Lee S.R., Kwak J.H., Kim H.J., Pyo S. Neuroprotective effects of kobophenol A against the withdrawal of tropic support, nitrosative stress, and mitochondrial damage in SH-SY5Y neuroblastoma cells. Bioorg Med Chem Lett. 2007;17:1879–1882. doi: 10.1016/j.bmcl.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 2.Meng Q., Niu Y., Niu X., Roubin R.H., Hanrahan J.R. Ethnobotany, phytochemistry, and pharmacology of the genus Caragana used in traditional Chinese medicine. J Ethnopharmacol. 2009;124:350–368. doi: 10.1016/j.jep.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 3.Lee S.R., Kwak J.H., Park D.S., Pyo S. Protective effect of kobophenol A on nitric oxide-induced cell apoptosis in human osteoblast-like MG-63 cells: involvement of JNK, NF-κB and AP-1 pathways. Int Immunopharmacol. 2011;11:1251–1259. doi: 10.1016/j.intimp.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Kulanthaivel P., Janzen W.P., Ballas L.M., Jiang J.B., Hu C.Q., Darges J.W., Seldin J.C., Cofield D.J., Adams L.M. Naturally occurring protein kinase C inhibitors; II. Isolation of Oligomeric Stilbenes from Caragana sinica. Planta Med. 1995;61:41–44. doi: 10.1055/s-2006-957996. [DOI] [PubMed] [Google Scholar]
- 5.Kurihara H., Kawabata J., Ichikawa S., Mishima M., Mizutani J. Oligostilbenes from Carex kobomugi. Phytochemistry. 1991;30:649–653. [Google Scholar]
- 6.Kim D.H., Kim S.H., Kim H.J., Jin C., Chung K.C., Rhim H. Stilbene derivatives as human 5-HT6 receptor antagonists from the root of Caragana sinica. Biol Pharm Bull. 2010;33:2024–2028. doi: 10.1248/bpb.33.2024. [DOI] [PubMed] [Google Scholar]
- 7.Jnawali H.N., Lee E., Jeong K.W., Shin A., Heo Y.S., Kim Y. Anti-inflammatory activity of rhamnetin and a model of its binding to c-Jun NH2-terminal kinase 1 and p38 MAPK. J Nat Prod. 2014;77:258–263. doi: 10.1021/np400803n. [DOI] [PubMed] [Google Scholar]
- 8.Kim K.S., Lee D.S., Bae G.S., Park S.J., Kang D.G., Lee H.S., Oh H., Kim Y.C. The inhibition of JNK MAPK and NF-κB signaling by tenuifoliside A isolated from Polygala tenuifolia in lipopolysaccharide-induced macrophages is associated with its anti-inflammatory effect. Eur J Pharmacol. 2013;721:267–276. doi: 10.1016/j.ejphar.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence T., Willoughby D.A., Gilroy D.W. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002;10:787–795. doi: 10.1038/nri915. [DOI] [PubMed] [Google Scholar]
- 10.Pautz A., Art J., Hahn S., Nowag S., Voss C., Kleinert H. Regulation of the expression of inducible nitric oxide synthase. Nitric oxide. 2010;23:75–93. doi: 10.1016/j.niox.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Raso G.M., Pacilio M., Esposito E., Coppola A., Di Carlo R., Meli R. Leptin potentiates IFN-γ-induced expression of nitric oxide synthase and cyclo-oxygenase-2 in murine macrophage J774A 1. Br J Pharmacol. 2002;137:799–804. doi: 10.1038/sj.bjp.0704903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayden M.S., Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Jang B.C., Paik J.H., Kim S.P., Bae J.H., Mun K.C., Song D.K., Cho C.H., Shin D.H., Kwon T.K., Park J.W., Park J.G., Baek W.K., Suh M.H., Lee S.H., Baek S.H., Lee I.S., Suh S.I. Catalase induces the expression of inducible nitric oxide synthase through activation of NF-kappaB and PI3K signaling pathway in Raw 264.7 cells. Biochem Pharmacol. 2005;68:2167–2176. doi: 10.1016/j.bcp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Oh W.J., Jung U., Eom H.S., Shin H.J., Park H.R. Inhibition of lipopolysaccharide-induced proinflammatory responses by Buddleja officinalis extract in BV-2 microglial cells via negative regulation of NF-kB and ERK1/2 signaling. Molecules. 2013;18:9195–9206. doi: 10.3390/molecules18089195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkins N.D. Integrating cell-signalling pathway with NF-κB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 16.Tak P.P., Firestein G.S. NF-κB a key role in inflammatory disease. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang S.H., Le B., Androutsopoulos V.P., Tsukamoto C., Shin T.S., Tsatsakis A.M., Chung G. Anti-inflammatory effects of soyasapogenol I-αa via downregulation of the MAPK signaling pathway in LPS-induced RAW 264.7 macrophages. Food Chem Toxicol. 2018;113:211–217. doi: 10.1016/j.fct.2018.01.050. [DOI] [PubMed] [Google Scholar]
- 18.Guo Y., Sakulnarmrat K., Konczak I. Anti-inflammatory potential of native Australian herbs polyphenols. Toxicol Rep. 2014;1:385–390. doi: 10.1016/j.toxrep.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon T., Cheon M.S., Lee A.Y., Lee D.Y., Moon B.C., Chun J.M., Choo B.K., Kim H.K. Anti-inflammatory activity of methylene chloride fraction from Glehnia littoralis extract via suppression of NF-kappa B and mitogen-activated protein kinase activity. J Pharmacol Sci. 2010;112:46–55. doi: 10.1254/jphs.09168fp. [DOI] [PubMed] [Google Scholar]
- 20.Jafarian-Tehrani M., Louin G., Royo N.C., Besson V.C., Bohme G.A., Plotkine M., Marchand-Verrecchia C. 1400W, a potent selective inducible NOS inhibitor, improves histopathological outcome following traumatic brain injury in rats. Nitric Oxide. 2005;12:61–69. doi: 10.1016/j.niox.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Hattori Y., Akimoto K., Matsumura M., Tseng C.C., Kasai K., Shimoda S. Effect of cycloheximide on the expression of LPS-inducible iNOS, IFN-beta, and IRF-1 genes in J774 macrophages. Biochem Mol Biol Int. 1996;40:889–896. doi: 10.1080/15216549600201503. [DOI] [PubMed] [Google Scholar]
- 22.Meng F., Lowell C.A. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J Exp Med. 1997;9:1661–1670. doi: 10.1084/jem.185.9.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soromou L.W., Zhang Z., Li R., Chen N., Guo W., Huo M., Guan S., Lu J., Deng X. Regulation of inflammatory cytokines in lipopolysaccharide-stimulated RAW 264.7 murine macrophage by 7-O-Methyl-naringenin. Molecules. 2012;3:3574–3585. doi: 10.3390/molecules17033574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Won J.H., Im H.T., Kim Y.H., Yun K.J., Park H.J., Choi J.W., Lee K.T. Anti-inflammatory effect of buddlejasaponin IV through the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via the NF-kappaB inactivation. Br J Pharmacol. 2006;148:216–225. doi: 10.1038/sj.bjp.0706718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu L., Fan Y., Fan C., Yu Y., Sun L., Jin Y., Zhang Y., Ye R.D. Licocoumarone isolated from Glycyrrhiza uralensis selectively alters LPS-induced inflammatory responses in RAW 264.7 macrophages. Eur J Pharmacol. 2017;15:46–53. doi: 10.1016/j.ejphar.2017.02.049. [DOI] [PubMed] [Google Scholar]
- 26.Li X., Yang X., Liu Y., Gong N., Yao W., Chen P., Qin J., Jin H., Li J., Chu R., Shan L., Zhang R., Zhang W., Wang H. Japonicone A suppresses growth of Burkitt lymphoma cells through its effect on NF-κB. Clin Cancer Res. 2013;19:2917–2928. doi: 10.1158/1078-0432.CCR-12-3258. [DOI] [PubMed] [Google Scholar]
- 27.Oeckinghaus A., Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guslandi M. Nitric oxide and inflammatory bowel diseases. Eur J Clin Invest. 1998;28:904–907. doi: 10.1046/j.1365-2362.1998.00377.x. [DOI] [PubMed] [Google Scholar]
- 29.Samad T.A., Moore K.A., Sapirstein A., Billet S., Allchorne A., Poole S., Bonventre J.V., Woolf C.J. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 30.Ahmad S.F., Ansari M.A., Zoheir K.M., Bakheet S.A., Korashy H.M., Nadeem A., Ashour A.E., Attia S.M. Regulation of TNF-α and NF-κB activation through the JAK/STAT signaling pathway downstream of histamine 4 receptor in a rat model of LPS-induced joint inflammation. Immunobiology. 2015;220:889–898. doi: 10.1016/j.imbio.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Moncada S., Palmer R.M., Higgs E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 32.Guha M., Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 33.Blaser H., Dostert C., Mak T.W., Brenner D. TNF and ROS crosstalk in inflammation. Trends Cell Biol. 2016;26:249–261. doi: 10.1016/j.tcb.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen D.P., Li J., Tewari A.K. Inflammation and prostate cancer: the role of interleukin 6 (IL-6) BJU Int. 2014;113:986–992. doi: 10.1111/bju.12452. [DOI] [PubMed] [Google Scholar]
- 35.Li Q., Verma I.M. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 36.Lin C.Y., Lee C.H., Chang Y.W., Wang H.M., Chen C.Y., Chen Y.H. Pheophytin A inhibits inflammation via suppression of LPS-induced nitric oxide synthase-2, prostaglandin E2, and interleukin-1β of macrophages. Int J Mol Sci. 2014;15:22819–22834. doi: 10.3390/ijms151222819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu C.Q., Liu B.J., Wu J.F., Xu Y.C., Duan X.H., Cao Y.X., Dong J.C. Icariin attenuates LPS-induced acute inflammatory responses: involvement of PI3K/Akt and NF-kappaB signaling pathway. Eur J Pharmacol. 2010;642:146–153. doi: 10.1016/j.ejphar.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 38.Dawn B., Xuan Y.T., Guo Y., Rezazadeh A., Stein A.B., Hunt G., Wu W.J., Tan W., Bolli R. IL-6 plays an obligatory role in late preconditioning via JAK-STAT signaling and upregulation of iNOS and COX-2. Cardiovasc Res. 2004;64:61–71. doi: 10.1016/j.cardiores.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarkhel S. Evaluation of the anti-inflammatory activities of Quillaja saponaria Mol. saponin extract in mice. Toxicol Rep. 2016;3:1–3. doi: 10.1016/j.toxrep.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niu X., Wang Y., Li W., Zhang H., Wang X., Mu Q., He Z., Yao H. Esculin exhibited anti-inflammatory activities in vivo and regulated TNF-α and IL-6 production in LPS-stimulated mouse peritoneal macrophages in vitro through MAPK pathway. Int Immunopharmacol. 2015;29:779–786. doi: 10.1016/j.intimp.2015.08.041. [DOI] [PubMed] [Google Scholar]
- 41.Dong L., Zhang Y., Wang X., Dong Y.X., Zheng L., Li Y.J., Ni J.M. In vivo and in vitro anti-inflammatory effects of ethanol fraction from Periploca forrestii Schltr. Chin J Integr Med. 2017;23:528–534. doi: 10.1007/s11655-017-2803-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.