Abstract
Recent visible-light photoredox catalyzed C(sp3)–C(sp2) cross-coupling provides a novel transformation to potentially enable the synthesis of medicinal chemistry targets. Here, we report a profiling study of photocatalytic C(sp3)–C(sp2) cross-coupling, both decarboxylative coupling and cross-electrophile coupling, with 18 pharmaceutically relevant aryl halides by using either Kessil lamp or our newly developed integrated photoreactor. Integrated photoreactor accelerates reaction rate and improves reaction success rate. Cross-electrophile coupling gives higher success rate with broad substrate scope on alkyl halides than that of the decarboxylative coupling. In addition, a successful application example on a discovery program demonstrates the efficient synthesis of medicinal chemistry targets via photocatalytic C(sp3)–C(sp2) cross-coupling by using our integrated photoreactor.
Keywords: Photoredox catalysis, C(sp3)−C(sp2) cross-coupling, integrated photoreactor, informer library
In recent years, visible-light-mediated photoredox catalysis has emerged as a powerful synthetic strategy that enables elusive bond construction and challenging chemical transformations.1−8 The merger of photoredox catalysis with other modes of catalysis has led to unique bond-forming reactions of high value to medicinal and process chemistry.9 Several photoredox mediated transformations, such as C(sp3)–C(sp2) decarboxylative coupling, C(sp3)–C(sp2) cross-electrophile coupling, C(sp3)–C(sp3) coupling, and C–N coupling, are increasingly being applied by medicinal and process chemists in biopharmaceutical companies to access challenging molecular targets. However, the lack of standardized reaction setup often leading to poor or inconsistent photon efficiency, thus hamper the broad adoption of these novel chemistries in both academia and industry. Our recent collaboration with MacMillan group has led to an integrated photoreactor that enhances photon capture and catalyst excitation, and standardizes the reaction setup. This reactor was evaluated with eight commonly used photocatalytic transformations. In all cases, the reaction was significantly accelerated compared to the previously reported reaction set-ups.10
In medicinal chemistry, for the first-batch analogue synthesis on discovery programs, the speed to successfully make target molecules is often more important than the chemical yield of the reaction. Although we demonstrated that our integrated photoreactor significantly enhances the reaction rate for these eight transformations with relatively simple substrates, our ultimate goal is to translate the improved photon efficiency to higher success rate for diverse and pharmaceutically relevant substrates in order to enable the broad application of photoredox catalysis in medicinal chemistry. Wide substrate scope and high success rate is essential for broad adoption of novel synthetic methodologies in medicinal chemistry.11 With this goal in mind, we extensively evaluated our integrated photoreactor with a photocatalytic C(sp3)–C(sp2) cross-coupling reaction, which is one of the most commonly used photoredox reactions in pharma industry, for a diverse set of pharmaceutically relevant substrates using collections of drug-like molecules, which are called chemistry informer libraries.12 These compounds represent the penultimate intermediates from various drug discovery and development programs and thus ideally typify pharmaceutical substrates. This strategy has been successfully applied to evaluate enabling chemistries.13−17 In our profiling studies, we used the following 18 aryl halides (13 bromides, 3 chlorides, and 2 iodides) with high complexity and various functional groups (Figure 1).
Figure 1.
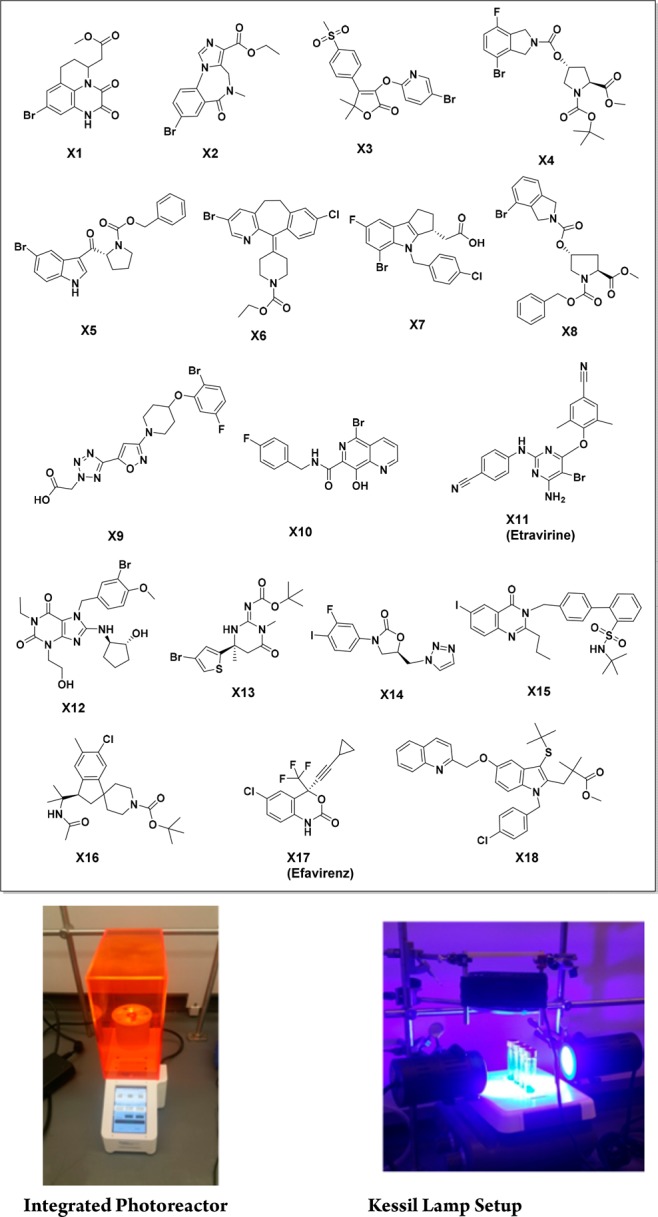
MSD informer library of 18 aryl halides.
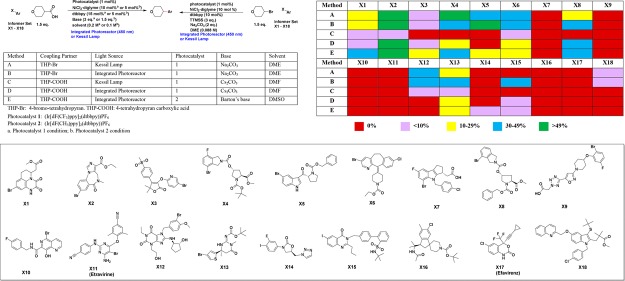
MacMillan group reported two photoredox and nickel dual-catalysis methods, which afford the C(sp3)–C(sp2) cross-coupling product from aryl halide, either with radicals derived from aliphatic carboxylic acid via decarboxylative coupling18,19 or with radicals derived from alkyl bromide via cross-electrophile coupling.20 These chemistries have been increasingly applied to enable the synthesis of drug-like medicinal chemistry targets with higher C(sp3) fractions. We first evaluated both chemistries for 18 informer aryl halides with either 4-bromo-tetrahydropyran or 4-tetrahydropyran carboxylic acid by using the integrated photoreactor or following the standard Kessil lamp procedures that were previously reported (Scheme 1).18−20 For decarboxylative cross-coupling, we examined both Ir[dF(CF3)ppy]2(dtbbpy))PF6 (photocatalyst 1) and Ir[dF(CH3)ppy]2(dtbbpy))PF6 (photocatalyst 2). In all reactions, progress of the reaction was monitored by LCMS of the reaction mixture. The table in Scheme 1 summarizes the range of percentage of the desired product in each reaction mixture based on the LCMS data (see Supporting Information for details with both the percentage of remaining starting material and the percentage of desired product, together with the reaction time). For the data analysis, we consider >10% of the desired product in the reaction mixture a successful reaction since typically this allows us to isolate the desired product via modern purification techniques to obtain enough material for primary assays on discovery programs.
Scheme 1. Photocatalytic C(sp3)–C(sp2) Cross-Coupling of Informer Aryl Halides.
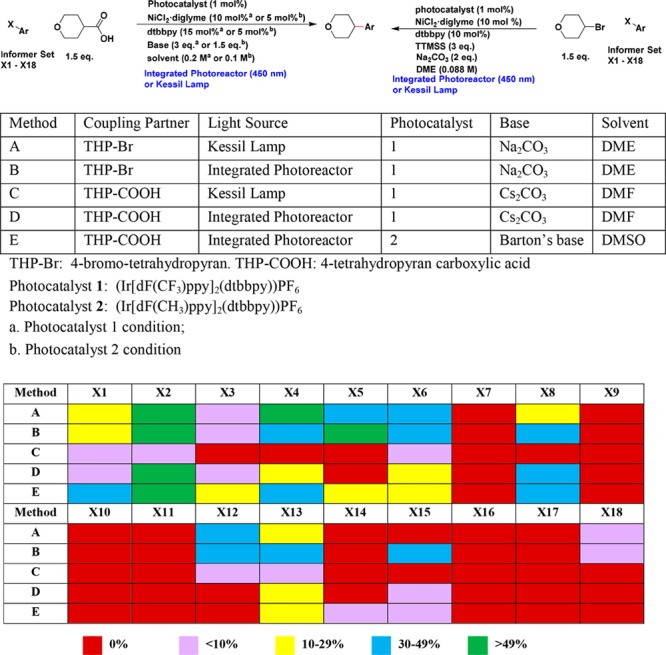
Percentage of the desired product in the crude reaction mixture based on the AUC in LCMS at UV 254 nm (see SI for details).
For the decarboxylative C(sp3)–C(sp2) coupling, we observed the dehalogenation of the heteroaryl halide as the common side reaction for many of the 18 substrates. None of the reactions achieved >10% conversion with (Ir[dF(CF3)-ppy]2(dtbbpy))PF6 (photocatalyst 1) using the Kessil lamp setup after 16 h (Method C, Scheme 1). However, five out of 18 aryl halides gave >10% desired product with photocatalyst 1 by using our integrated photoreactor (Method D). When (Ir[dF(CH3)ppy]2(dtbbpy))PF6 (photocatalyst 2) was employed, eight out of 18 reactions gave >10% of the desired product by using the integrated photoreactor (Method E). X1, X3, and X5 gave the desired decarboxylative coupling products only with photocatalyst 2 and using the integrated photoreactor. These results suggest that our integrated photoreactor improves the success rate of the photocatalytic C(sp3)–C(sp2) decarboxylative cross-coupling for complex substrates. Photocatalyst 2 appears to give higher success rate compared to photocatalyst 1. This is probably due to the better stability of the photocatalyst 2 under the reaction conditions.21 In this particular study, the success rate increased from 0% (photocatalyst 1, Kessil lamp) to 44% (photocatalyst 2, integrated photoreactor).
For cross-electrophile coupling, eight out of these 18 aryl halides gave >10% desired product by using the Kessil lamp setup, and nine out of 18 aryl halides gave >10% desired product with the integrated photoreactor. Similar to the decarboxylative coupling, the dehalogenation of the aryl halide is the major side reaction. It is worth to note that in general the integrated photoreactor accelerates the reaction rate and reduces the reaction time (see Supporting Information for details). X15, an aryl iodide, gave the desired coupling product (35%) only with the integrated photoreactor. In addition, we demonstrated that the cross-electrophile coupling increased the success rate and yield compared to the decarboxylative cross-coupling (method B vs method E). X12 and X15 gave desired coupling products only with the cross-electrophile coupling but not with the decarboxylative coupling. We reason that the higher success rate of the cross-electrophile coupling is due to the more efficient sp3 carbon radical generation via halogen atom abstraction with photocatalytically generated silyl radical.20 Protecting groups such as Boc, Cbz, and functional groups such as ester, amide, nitrile, and hydroxyl are tolerated in the reaction with the substrates we tested.
Combining the results from five conditions evaluated, 12 out of 18 informer aryl halides gave desired products, which were purified by a high-throughput reverse-phase mass-directed purification system and were fully characterized (see SI for details). The remaining six aryl halides (X7, X9, X10, X11, X16, and X17) failed to give desired coupling products. X7 and X9 contain a carboxylic acid group, which could be decarboxylated to generate radicals and potentially interfere with the reaction. Under both decarboxylative coupling and cross-electrophile coupling conditions, X10 gave des-bromide and hydrolysis product (−Br to – OH) as major products. X11 gave the des-bromide as the major product. X16 and X17 were largely unchanged (no or little des-Cl byproduct) under all conditions evaluated, suggesting that aryl chlorides are much less reactive than aryl bromides. In fact, X6 chemoselectively yielded the coupling product with aryl bromide in the presence of the chloride under decarboxylative coupling or cross-electrophile coupling conditions. Six aryl halides that failed to give desired products will be subjected to our high-throughput experimentation (HTE) platform for reaction screening to identify suitable coupling conditions.
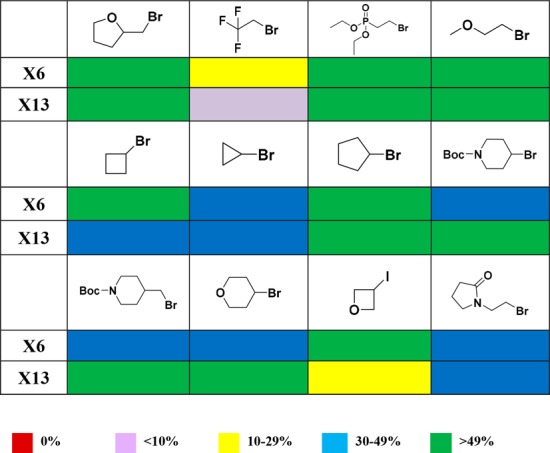
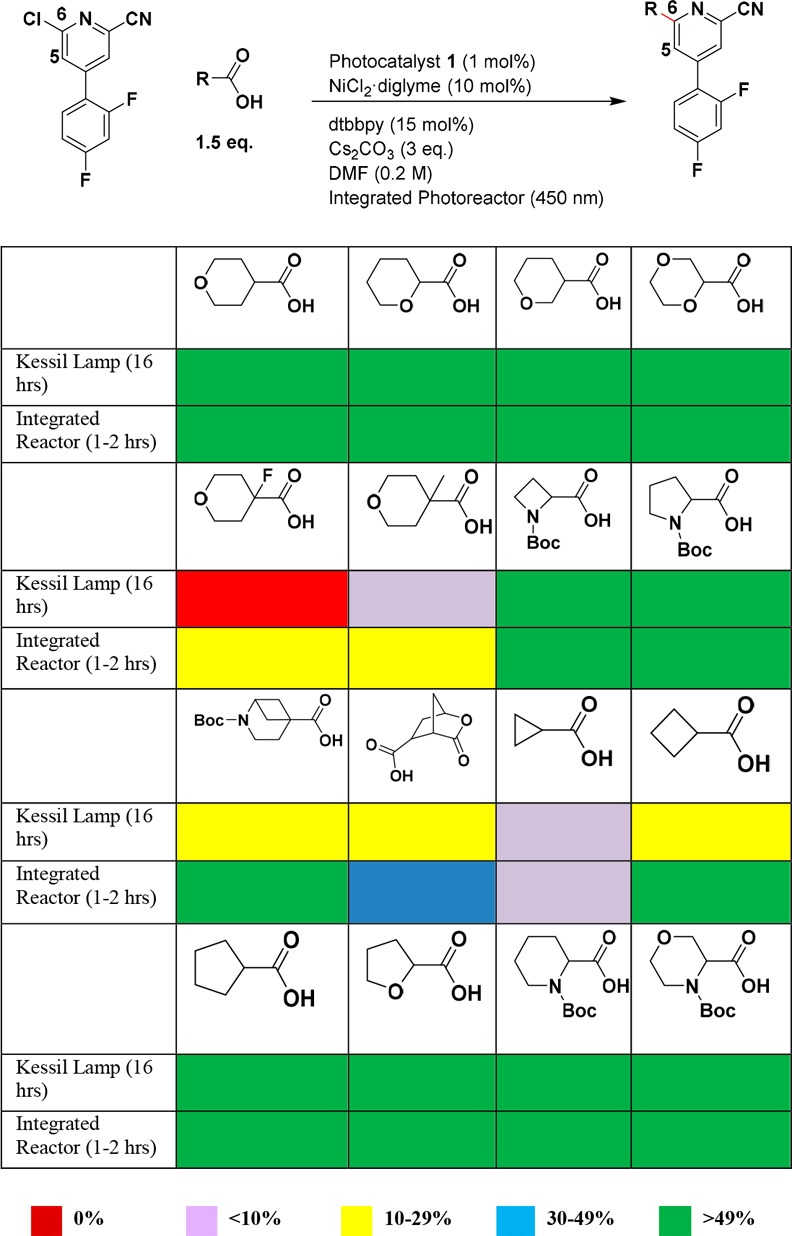
Since cross-electrophile coupling using the integrated photoreactor gave the best success rate among all five conditions we evaluated, we then studied the scope of the aliphatic halides for the cross-electrophile coupling reaction. Two heteroaryl bromides X6 and X13 were selected to couple with 12 diverse aliphatic bromides or iodides using the integrated photoreactor (Table 1). These diverse aliphatic halides were selected to represent targets that medicinal chemists often design, including cyclopropyl, oxetanyl, and trifluoroethyl groups, very attractive medicinal chemistry targets, but are often hard to make due to the challenging coupling chemistry. We used 2,6-lutidine (2 equiv) as the base instead of sodium carbonate in this experiment to keep reactions under homogeneous condition.10Table 1 summarizes the reaction results (see SI for percentage conversion of each reaction). It is gratifying that 23 out of 24 reactions gave >20% desired product, a 95% success rate for this mini-library. Cyclopropyl bromide, cyclobutyl bromide, and oxetane-iodide all gave desired coupling products. Even a challenging substrate, trifluoroethyl bromide, showed promising results. These 23 compounds were purified via a high throughput reverse-phase mass-directed HPLC purification system and fully characterized (see SI for details). These results suggest that cross-electrophile coupling can be widely applicable in discovery chemistry to enable C(sp3)–C(sp2) cross-coupling. Since then, we have applied this chemistry on several internal programs across MSD research sites to enable the synthesis of molecular targets and have demonstrated program impact.
Table 1. Percentage of the Desired Product in the Crude Reaction Mixture for Photocatalytic C(sp3)–C(sp2) Cross-Electrophile Coupling with 12 Aliphatic Halides Using Integrated Photoreactor.
One limitation of the cross-electrophile coupling is that the number of commercially available alkyl bromides or iodides is significantly lower than the number of commercially available carboxylic acids.22 Moreover, carboxylic acids are more suitable substrates than bromides for coupling α-O- or N-substituted alkyl radicals with aryl halides. So the decarboxylative C(sp3)–C(sp2) cross-coupling is still a very attractive method for medicinal chemists even though the overall success rate is lower than that of the cross-electrophile coupling based on our study. On a recent internal medicinal chemistry program, we designed a set of targets in an effort to reduce the aromaticity and improve metabolic stability of the lead compound. We envisioned these targets could be readily made via C(sp3)–C(sp2) photocatalytic cross-coupling. In surveying the availability of the carboxylic acid or alkyl bromide monomers, it became clear that we should apply the decarboxylative cross-coupling to access these targets (Scheme 2). We ran this 16-member library with the Kessil lamp setup or our integrated photoreactor at 20 mg scale as part of the evaluation study. Most of the reactions proceeded readily to give modest to good yield except for the substrates with quaternary carbon or cyclopropyl carboxylic acid (see SI for percentage conversion of each reaction). Consistent with the reported mechanism,18,19 substrates with a heteroatom (O or N) at α-position of the carboxylic acid gave higher yield presumably due to the stabilization of the radical with the α-heteroatom. From this study, our integrated photoreactor improved success rate from 81% to 94% compared to the Kessil lamp setup. In addition, the integrated photoreactor also significantly reduced the reaction time from 16 h with the Kessil lamp to typically 1–2 h and improved reaction yield for many substrates (see SI for details). It is worth to note that the quaternary carbon substrates 4-fluoro or 4-methyltetrahydropyran-4-carboxylic acid gave >10% desired product with the integrated photoreactor but not with the Kessil lamp setup. Photocatalytic C(sp3)–C(sp2) cross-coupling arguably is the most direct way to make these two targets. Cyclopropyl analogue was the only one that had <10% conversion from this mini-library. Based on our above study, we hypothesized that C(sp3)–C(sp2) cross-electrophile coupling could give better yield. Indeed, we achieved 30% crude yield through the coupling of 6-chloro-4-(2,4-difluorophenyl)-picolinonitrile and bromocyclopropane by using the integrated photoreactor (see example 73 in SI for details). We also observed the formation of 5-substituted regioisomers for some of the carboxylic acid substrates in this experiment. The mechanism of the formation of the 5-substituted isomer is a subject of our current study. From this array, all 16 desired targets were successfully isolated, purified, and submitted to the program in vitro assays. These results demonstrated the efficient synthesis of medicinal chemistry targets via photocatalytic cross-couplings by using the integrated photoreactor.
Scheme 2. Sixteen-Member Decarboxylative Cross-Coupling Library.
The integrated photoreactor can also accommodate different sizes of the reaction vials (1, 4, 8, 20, and 40 mL vials) for reactions from milligram to gram scale. Using the integrated photoreactor, one of the members of the above library, compound (4-(2,4-difluorophenyl)-6-(tetrahydro-2H-pyran-4-yl)picolinonitrile), was scaled up at 200 and 500 mg scales with 20 and 40 mL vials, respectively. The yields for these reactions are consistent with the 20 mg scale (see example 49 in SI for details).
In conclusion, the results of photocatalytic C(sp3)–C(sp2) cross-coupling with the informer aryl halides suggest that this chemical transformation has a great potential to enable the synthesis of targets in medicinal chemistry. Our studies have showed that cross-electrophile coupling gives higher success rate than the decarboxylative cross-coupling under the conditions we evaluated. This study also demonstrated that the integrated photoreactor improves success rate and reduces the reaction time for the C(sp3)–C(sp2) photocatalytic cross-coupling reactions compared to the Kessil lamp setup. The integrated photoreactor has been widely used by chemists across MSD research sites and demonstrated impact on our discovery programs. We anticipate the integrated photoreactor will enable broad application of photoredox catalysis in the pharmaceutical industry.
Acknowledgments
The auothers wish to thank Prof. David W. C. MacMillan for helpful discussions. We would like to thank the following MSD scientists and engineers for technical support and discussions: Ashok Arasappan, Olugbeminiyi Fadeyi, Spencer Dreher, Shane Krska, Donald Conway, Abdellatif El Marrouni, Kuanchang Chen, Cristina Grosanu, Cangming Yang, Tao Meng, Qi Gao, Melissa Lin, Peter G. Dormer, Li-Kang Zhang, and Rong-Sheng Yang.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00183.
Materials and methods, experimental and NMR spectra (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Twilton J.; Le C.; Zhang P.; Shaw M. H.; Evans R. W.; MacMillan D. W. C. The merger of transition metal and photocatalysis. Nature Reviews Chemistry 2017, 1, 0052. 10.1038/s41570-017-0052. [DOI] [Google Scholar]
- Xie J.; Jin H.; Hashmi A. S. K. The recent achievements of redox-neutral radical C–C cross-coupling enabled by visible-light. Chem. Soc. Rev. 2017, 46, 5193. 10.1039/C7CS00339K. [DOI] [PubMed] [Google Scholar]
- Shaw M. H.; Twilton J.; MacMillan D. W. C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898–6926. 10.1021/acs.joc.6b01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staveness D.; Bosque I.; Stephenson C. R. J. Free Radical Chemistry Enabled by Visible Light-Induced Electron Transfer. Acc. Chem. Res. 2016, 49, 2295–2306. 10.1021/acs.accounts.6b00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero N. A.; Nicewicz D. A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]
- Skubi K. L.; Blum T. R.; Yoon T. P. Dual Catalysis Strategies in Photochemical Synthesis. Chem. Rev. 2016, 116, 10035–10074. 10.1021/acs.chemrev.6b00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.-R.; Hu X.-Q.; Lu L.-Q.; Xiao W.-J. Exploration of Visible-Light Photocatalysis in Heterocycle Synthesis. Acc. Chem. Res. 2016, 49, 1911–1923. 10.1021/acs.accounts.6b00254. [DOI] [PubMed] [Google Scholar]
- Cavalcanti L. N.; Molander G. A. Photoredox Catalysis in Nickel-Catalyzed Cross-Coupling. Top Curr. Chem. 2016, 374, 39. 10.1007/s41061-016-0037-z. [DOI] [PubMed] [Google Scholar]
- Douglas J. J.; Sevrin M. J.; Stephenson C. R. J. Visible Light Photocatalysis: Applications and New Disconnections in the Synthesis of Pharmaceutical Agents. Org. Process Res. Dev. 2016, 20, 1134. 10.1021/acs.oprd.6b00125. [DOI] [Google Scholar]
- Le C.; Wismer M. K.; Shi Z.-C.; Zhang R.; Conway D. V.; Li G.; Vachal P.; Davies I. W.; MacMillan D. W. C. A General Small-Scale Reactor To Enable Standardization and Acceleration of Photocatalytic Reactions. ACS Cent. Sci. 2017, 3, 647–653. 10.1021/acscentsci.7b00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore D. C.; Castro L.; Churcher I.; Rees D. C.; Thomas A. W.; Wilson D. M.; Wood A. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 2018, 10, 383–394. 10.1038/s41557-018-0021-z. [DOI] [PubMed] [Google Scholar]
- Kutchukian P. S.; Dropinski J. F.; Dykstra K. D.; Li B.; DiRocco D. A.; Streckfuss E. C.; Campeau L.-C.; Cernak T.; Vachal P.; Davies I. W.; Krska S. W.; Dreher S. D. Chemistry informer libraries: a chemoinformatics enabled approach to evaluate and advance synthetic methods. Chem. Sci. 2016, 7, 2604–2613. 10.1039/C5SC04751J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran E. B.; Pirnot M. T.; Dreher S. D.; DiRocco D. A.; Davies I. W.; Buchwald S. L.; MacMillan D. W. C. Aryl amination using ligand-free Ni(II) salts and photoredox catalysis. Science 2016, 353, 279–283. 10.1126/science.aag0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greshock T. J.; Moore K. P.; McClain R. T.; Bellomo A.; Chung C. K.; Dreher S. D.; Kutchukian P. S.; Peng Z.; Davies I. W.; Vachal P.; Ellwart M.; Manolikakes S. M.; Knochel P.; Nantermet P. G. Synthesis of Complex Drug-like Molecules by the Use of Highly Functionalized Bench-Stable Organozinc Reagents. Angew. Chem., Int. Ed. 2016, 55, 13714–13718. 10.1002/anie.201604652. [DOI] [PubMed] [Google Scholar]
- Fier S. P.; Maloney M. K. Synthesis of Complex Phenols Enabled by a Rationally Designed Hydroxide Surrogate. Angew. Chem., Int. Ed. 2017, 56, 4478–4482. 10.1002/anie.201700244. [DOI] [PubMed] [Google Scholar]
- Krska S. W.; DiRocco D. A.; Dreher S. D.; Shevlin M. The Evolution of Chemical High-Throughput Experimentation To Address Challenging Problems in Pharmaceutical Synthesis. Acc. Chem. Res. 2017, 50, 2976–2985. 10.1021/acs.accounts.7b00428. [DOI] [PubMed] [Google Scholar]
- Vara B.; Li X.; Berritt S.; Walters C.; Petersson E.; Molander G. Scalable thioarylation of unprotected peptides and biomolecules under Ni/photoredox catalysis. Chem. Sci. 2018, 9, 336–344. 10.1039/C7SC04292B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z.; Ahneman D. T.; Chu L.; Terrett J. A.; Doyle A. G.; MacMillan D. W. C. Merging photoredox with nickel catalysis: Coupling of a-carboxyl sp3-carbons with aryl halides. Science 2014, 345, 437–440. 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z.; Cong H.; Li W.; Choi J.; Fu G. C.; MacMillan D. W. C. Enantioselective Decarboxylative Arylation of α-Amino Acids via the Merger of Photoredox and Nickel Catalysis. J. Am. Chem. Soc. 2016, 138, 1832–1835. 10.1021/jacs.5b13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P.; Le C. C.; MacMillan D. W. C. Silyl Radical Activation of Alkyl Halides in Metallaphotoredox Catalysis: A Unique Pathway for Cross-Electrophile Coupling. J. Am. Chem. Soc. 2016, 138, 8084–808. 10.1021/jacs.6b04818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metallaphotoredox Decarboxylative Arylation. http://chemlabs.princeton.edu/macmillan/reactions/metallaphotoredox-decarboxylative-arylation-2/.
- An internal analysis of in-house building block collection showed the number of aliphatic acids is 5 times more than the number of aliphatic bromides
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.