Abstract
A lead generation campaign identified indole-based sPLA2-X inhibitors with a promising selectivity profile against other sPLA2 isoforms. Further optimization of sPLA2 selectivity and metabolic stability resulted in the design of (−)-17, a novel, potent, and selective sPLA2-X inhibitor with an exquisite pharmacokinetic profile characterized by high absorption and low clearance, and low toxicological risk. Compound (−)-17 was tested in an ApoE–/– murine model of atherosclerosis to evaluate the effect of reversible, pharmacological sPLA2-X inhibition on atherosclerosis development. Despite being well tolerated and achieving adequate systemic exposure of mechanistic relevance, (−)-17 did not significantly affect circulating lipid and lipoprotein biomarkers and had no effect on coronary function or histological markers of atherosclerosis.
Keywords: Secreted phospholipase A2 type X, sPLA2-X, inhibitor, atherosclerosis, coronary artery disease, carotid ligation
Based on our previously reported hit identification efforts,1 the discovery and in vivo characterization of (−)-2-{2-[carbamoyl-6-(trifluoromethoxy)-1H-indol-1-yl]pyridine-2-yl}propanoic acid, a novel sPLA2-X inhibitor with significant selectivity over the other main sPLA2 isoforms, is presented, alongside its lack of efficacy in the murine carotid artery ligation model of atherosclerosis.2
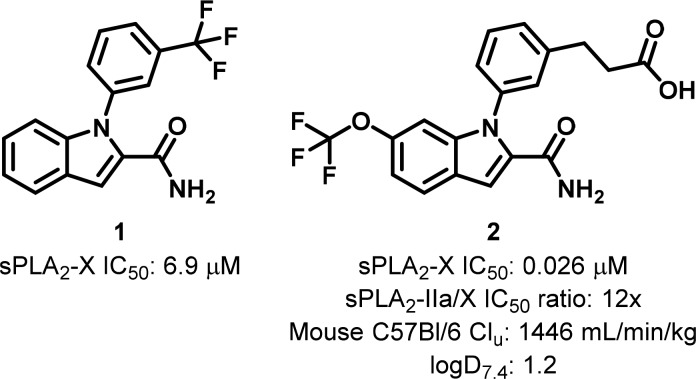
Preliminary structure-based evolution of the initial fragment hit 1 resulted in a lead series offering a bidentate coordination of the calcium ion present in the sPLA2-X catalytic site.1 One of the most promising compounds in the series yielded a greater than 250-fold sPLA2-X potency improvement over the original fragment hit (2, Figure 1). However, in order to fulfill the project goal of evaluating the therapeutic potential of selective sPLA2-X inhibition in a rodent model of atherosclerotic disease, further optimization was required. Specifically, maximizing selectivity toward sPLA2-X was of interest, in order to minimize confounding effects originating from inhibition of alternative sPLA2 isoforms. Furthermore, a significant improvement of the metabolic properties of 2 was needed to afford a pharmacokinetic profile compatible with prolonged in vivo dosing. Based on the existing data, we decided to target a 3-fold improvement in both sPLA2-X selectivity and mouse unbound clearance over 2 (Figure 1).
Figure 1.
Initial fragment hit 1 and derived lead 2.
Anticipating that the second objective would have been more stringent from a design point of view, due to its partial dependency on lipophilicity, it was decided to (a) identify areas on the molecule where polarity could be introduced and (b) incorporate molecular modifications in accordance with the available sPLA2 structural framework and associated selectivity hypotheses. The aim was to balance the contrasting effects of reduced lipophilicity on sPLA2-X inhibition and pharmacokinetics while monitoring sPLA2 selectivity. Considering the ionized class and physicochemical nature of the compounds and associated implications for DMPK properties,3 we opted to target a ca. 0.5 logD7.4 reduction from 2.
Compounds 3–9 (Table 1) were designed to introduce polar elements in the region that, being furthest away from the bottom of the lipophilic binding pocket, appeared most amenable to modification, based on the X-ray of 2 bound to sPLA2-X.1 Synthesis started by preparation of the adequate heterocyclic halides, bearing a propanoate side chain in analogy to 2, as shown in Scheme 1. The halides so-obtained were coupled under N-arylation/Ullmann conditions4 with 6-trifluoromethoxy-indole-2-carboxylic acid to generate the corresponding N-heteroaryl-indoles in modest yields. Standard TBTU coupling produced the indole-2-carboxamides in good to quantitative yields. Finally, hydrolysis of the corresponding esters yielded the carboxylic acid products 3–9 (see Supporting Information).
Table 1. sPLA2 Isoform Potencies and Human Hepatocyte Intrinsic Clearance for Compounds 2–9.
Results are mean of at least two experiments. Experimental errors within 20% of value.
Not active at highest tested concentration (10 μM).
Not determined.
Scheme 1. General Synthesis of 1-Heteroaryl-1H-indole-2-carboxamide Derivatives.
Reagents and conditions: (a) Cu(OAc)2, DBU, DMSO, μw 110–180 °C, 7 min to 24 h (15–25%); (b) NH4Cl, TBTU, NMM, DMF, rt. 1.5–16 h (74–100%); (c) chiral chromatography; (d) LiOH, THF, MeOH, water, rt. 3–19h (45–83%).
Of the three pyridine regioisomers evaluated (3–5), the 2,6-disubstituted one (5) was the only one that maintained comparable potency to its phenyl counterpart. It is noteworthy that such modification had no effect on sPLA2-X selectivity. Inhibition of HDL hydrolysis as mediated by sPLA2-X, an important translational biomarker of lipoprotein modification, was also maintained. Introduction of marked polarity on the aryl moiety via an N-substituted pyridine-2-one (6) reduced potency by more than 100-fold. Decreased lipophilicity by installment of five-membered heterocyclic rings also diminished sPLA2-X inhibition with pyrazoles (7, 8) having a more pronounced effect than furane (9). Importantly, all the pyridine heterocycles had the sought effect of reducing metabolism, as shown in Table 1.
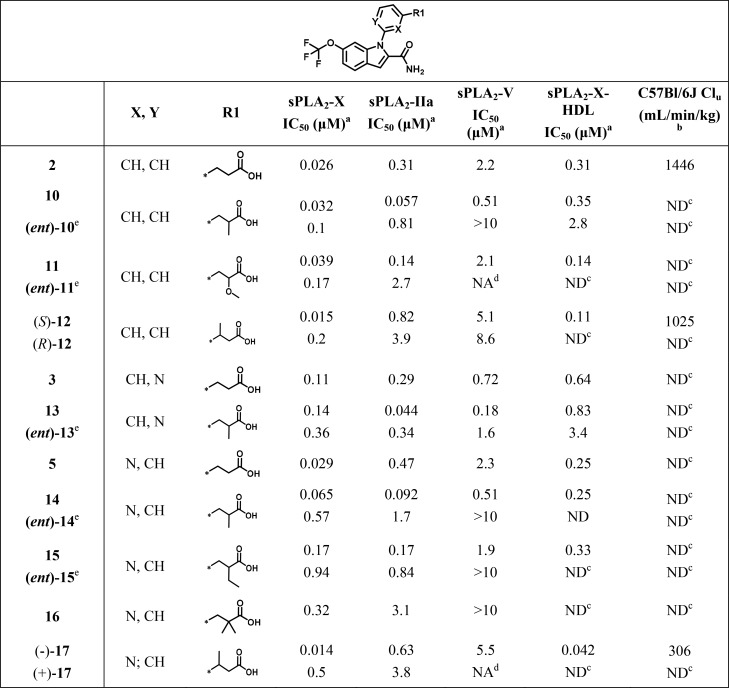
Having successfully identified a polar element with neutral effect on potency and favorable impact on metabolism, optimization of sPLA2-X selectivity was in focus. Multiple sequence and crystallographic structure alignments of the three main sPLA2 isoforms considered in this study indicated amino acid variation across the proteins in the area immediately surrounding the pyridine and carboxy linker.1 Although the side chain differences were limited (I2-L29, L2-V29, and L2-I29, in sPLA2-X, -IIa, and -V, respectively), we hypothesized that these could react differently to ligand contacts and therefore drive selectivity between sPLA2 isoforms, as previously reported.5,6 Design of compounds 10–21 served to verify those assumptions, as shown in Table 2.
Table 2. sPLA2 Potency and in Vivo Mouse Unbound Clearance for Compounds 10–29.
Results are mean of at least two experiments. Experimental errors within 20% of value.
Unbound clearance (Clu) is defined as Cl/Fu where Fu is the compound’s fraction that is unbound in plasma. Clearance is calculated from noncompartmental analysis concentrations in fasted C57Bl/6J male mice (i.v./p.o., N = 2).
Not determined.
Not active at highest tested concentration (10 μM).
Enantiomers that were not characterized further are labeled using the (ent)-prefix.
Compounds 10–21 were synthesized according to a similar synthetic scheme using the previously described Ullman coupling as a key step. Separation of the enantiomeric and diastereoisomeric pairs obtained was efficiently performed using preparative chiral HPLC (see Supporting Information).
Introduction of substituents at the α position respective to the carboxylic acid typically resulted in significant, stereoselective potency gains across sPLA2-IIa and -V isoforms and, as a result, reduced sPLA2-X selectivity (cf. 10, (ent)-10, and 2; 13, (ent)-13, and 3; 14, (ent)-14, and 5; Table 2), possibly due to the amino acid differences previously described. Here, methyl groups had the most profound effect, followed by methoxy and ethyl substituents (cf. 10 and 11; 5 and 15; Table 2). On the contrary, gem-dimethyl substitution at the same position was not tolerated (cf. 2, 10, and 16; Table 2). Intriguingly, when the substituents were installed on the beta position to the acid, improved selectivity between sPLA2-X and, in an increasing order, sPLA2-IIa and sPLA2-V was apparent (cf. (S)-12 and 2; (−)-17 and 5; Table 2).
Compounds (S)-12 and (−)-17 emerged from this exploration as highly potent sPLA2-X inhibitors (IC50, 15 and 14 nM; logD7.4, 1.3 and 0.9, respectively) of comparable selectivity over sPLA2-IIa (60- and 45-fold, respectively) and sPLA2-V (360- and 390-fold, respectively). The two derivatives also inhibited the sPLA2-X-mediated lipolytic effect on HDL with (−)-17 being the most potent (IC50, 42 and 110 nM, respectively). When their pharmacokinetic profile was evaluated in mice, (−)-17 demonstrated superior properties, as indicated by a lower unbound clearance (Table 2). This is probably the result of its reduced lipophilicity (0.4 logD7.4 units difference compared to (S)-12) and its combined impact on both metabolic stability and plasma protein binding. Importantly, the more polar character of (−)-17 did not deteriorate its absorption profile, as indicated by adequate passive diffusion (Caco-2 Papp: 5.8 × 10–6 cm/s) and oral bioavailability in mice (F: 95%). The absolute structure was assumed to be (S), as from molecular modeling based on the X-ray of (S)-12 (Figure 2) and the comparable separation of sPLA2-X activity in the corresponding enantiomeric pairs (S)-12–(R)-12 and (−)-17–(+)-17 (Table 3). The binding mode of (S)-12 closely resembled that of 2, except for a slight upward shift of its phenyl ring (Figure 2). The additional methyl group at the benzylic position establishes van der Waals contacts with Y50 and K61. Further, the introduction of the methyl group slightly alters the conformation of the I2 side chain and subsequently alters its packing against K61. This suggests that the observed isoform selectivity results might stem from the slightly smaller space in the area around the pyridine, primarily caused by the I2 to L2 substitution. It is also likely that sequence differences in other parts of the active site, i.e., L5, V9, and L98 (F/L, I/I, and F/L in IIa/V, respectively), may alter the binding mode of the entire ligand,1 thus contributing to the effect. Furthermore, (−)-17 maintained adequate potency for the mouse sPLA2-X homologue (IC50: 75 nM). Based on these promising preliminary characteristics, (−)-17 was evaluated further to identify potential shortcomings. The compound showed no inhibition of ion channels implicated in cardiovascular function (hERG, Nav1.5, IKs, Cav1.2, Kv4.3) at the highest tested concentration (33 μM). Compound (−)-17 did not display any significant binding in a selectivity panel consisting of >100 different proteins when tested at 10 μM. No CYP450 isoforms were inhibited by (−)-17 when dosed at up to 20 μM. In line with previously reported derivatives from the same series and lipophilicity range, (−)-17 possessed adequate solubility and did not form any reactive metabolites (Table 3).
Figure 2.

Overlay between the crystal structures of (S)-12 (orange stick) and 2 (green stick) bound to sPLA2-X (white and green sticks, respectively) (please see the Supporting Information for experimental details).
Table 3. In Vitro–in Vivo Profilea of (−)-17.
| solubility (μM) | 100 |
| Caco-2 Papp (10–6 cm/s), efflux ratio | 5.8, 0.4 |
| human hepatocytes T1/2 (min) | >173 |
| hERG, Nav1.5, IKs, Kv4.3 Cav3.2, Cav1.2 IC50 (μM) | >33 |
| reactive metabolite formation | no (0.0) |
| cytochrome P450 IC50 (μM) | >20 |
| pharmacokinetics | C57Bl/6J mouse |
| plasma protein binding Fu (%) | 5 |
| dose iv/po (μmol/kg) | 10/50 |
| CLu (mL/min/kg) | 306 |
| F (%) | 95 |
| Vss (L/kg) | 0.52 |
Please see the Supporting Information for experimental details.
Owing to its favorable in vitro–in vivo profile as well as its selective sPLA2-X action, (−)-17 was deemed suitable for long-term efficacy studies using the mouse carotid artery flow cessation model, modified as previously described.2 Here, pharmacokinetic–pharmacodynamic (PK–PD) modeling based on the in vitro inhibitory potency (sPLA2-X-mediated HDL hydrolysis IC50 and IC90, 42 and 362 nM; mouse sPLA2-X IC50 and IC90, 75 and 820 nM), mouse plasma protein binding (Fu, 5%), and the measured PK profile (Table 3) predicted that once daily oral doses for (−)-17 of 75 and 150 μmol/kg would allow for 24 h coverage of IC50 and IC90, respectively. This PK–PD hypothesis enabled testing a dynamic range of sPLA2-X inhibition and its relevance to several pharmacological effects: (a) modification of circulating lipoproteins and lipids, (b) improvement of vascular function, and (c) reduction of atherosclerosis. The results from a 3 week administration of (−)-17 to ApoE–/– mice that underwent carotid artery ligation are summarized in Figure 3 (see Supporting Information for experimental details and additional results).
Figure 3.
Effect of (−)-17 in an ApoE–/– murine carotid artery ligation model of atherosclerosis (N = 16/group). (a) Plasma unbound concentrations of (−)-17 at study termination. ApoE–/– mice body weight (b), heart rate (c), and total cholesterol (d). Coronary flow reserve (CFR) analysis (e) and left carotid artery neointima area (f) (please see the Supporting Information for experimental details).
Treatment with (−)-17 was overall well tolerated with no significant changes in the body weight of the animals and their liver enzyme levels. Histopathological analysis of vital organs did not reveal any significant findings. Importantly, plasma concentrations of (−)-17 confirmed the PK–PD predictions with the 75 and 150 μmol/kg doses resulting in free compound exposures well in excess (>7-fold) of the in vitro sPLA2-X HDL IC50 and IC90, respectively, both after 1 week treatment and at the end of the study. When compared to the control group receiving only Western diet, dosing of (−)-17 did not significantly alter the plasma levels of triglycerides, free fatty acids, and cholesterol (including esterified cholesterol, free cholesterol, or any of its lipoprotein-associated fractions), despite their relative reductions with the 150 μmol/kg dose. Unfortunately, similar nonstatistically significant trends were observed when the same analysis focused on liver, aorta, and heart biosamples (data not shown). Coronary flow reserve (CFR) analysis of the left coronary artery using Doppler ultrasound-based echocardiography7 did not reveal any differences between control and treatment groups. Furthermore, no significant changes in heart rate were observed upon treatment with (−)-17. Histological analysis of the left common carotid artery after ligation surgery8 served to assess the effect of (−)-17 on the development of atherosclerosis. Here, no significant reduction of artery wall thickness or increase in the overall lumen thickness was apparent compared to the untreated animal group. Additionally, administration of (−)-17 did not result in a significant decrease of neointima and media formation, as highlighted in Figure 3.
When compared to the vehicle group or positive treatment precedents using alternative modalities in the same in-house disease model (e.g., 25 μmol/kg fluvastatin qd, as previously reported9), it was clear that, while being well tolerated, (−)-17 failed to significantly affect any of the study end points. Lipoproteins and lipid biomarkers were postulated to be the most direct proof of concept end points due to the published lipid modifying and pro-inflammatory properties of sPLA2-X.10−12 The mechanistic hypothesis we followed implied that, because of marked improvements in lipid/lipoprotein metabolism and homeostasis, as mediated by sPLA2-X inhibition, a significant reduction in the progression of atherosclerosis and therefore an overall improved coronary function would result. The data obtained with (−)-17 would not seem to support such hypothesis. Potential causes for the observed lack of efficacy could include an underappreciated difference between in vitro and in vivo sPLA2-X inhibition efficiency, requiring much higher compound exposure than the one sampled, although the latter was purposely designed with a higher margin to mitigate this risk. It is possible that the beneficial effects of sPLA2-X inhibition on atherosclerosis would require much longer treatment times given the pharmacodynamic coupling between lipid/lipoprotein homeostasis and atherosclerosis development in the chosen ApoE–/– carotid ligation model.13 Alternatively, pharmacological inhibition of sPLA2-X might not have direct enough relevance to a complex, multifactorial disease such as atherosclerosis in the ApoE–/– model employed here. Interestingly, a recent study has shown no association between genetic variants of PLA2G10, encoding sPLA2-X, and coronary heart disease risk traits and outcome,14 as opposed to PLA2G2A, encoding sPLA2-IIa.15
In summary, started from a fragment-derived chemical series of sPLA2-X inhibitors, optimization of the sPLA2 isoform selectivity and pharmacokinetic profile led to the design of (−)-2-{2-[carbamoyl-6-(trifluoromethoxy)-1H-indol-1-yl]pyridine-2-yl}propanoic acid, (−)-17, a novel, potent, and selective sPLA2-X inhibitor. Three weeks of oral administration of (−)-17 to carotid artery-ligated ApoE–/– mice fed on a Western diet failed to reduce development of atherosclerosis, questioning the therapeutic validity of selective pharmacological sPLA2-X inhibition.16−18 Considering recent clinical failures in cardiovascular diseases treatment with the broad spectrum sPLA2 inhibitor varespladib and lipoprotein-associated PLA2 (Lp-PLA2) inhibitor darapladib,19 new insights into the causative association between these enzymes and atherosclerosis are required to progress novel, effective therapies.
Acknowledgments
The authors would like to acknowledge the support of the Structure Analysis and Separation Science groups of AstraZeneca Gothenburg.
Glossary
Abbreviations Used
- sPLA2
secreted phospholipase A2
- ApoE
apolipoprotein E
- HEP
hepatocytes
- PK–PD
pharmacokinetics–pharmacodynamics
- CFR
coronary flow reserve
- Lp-PLA2
lipoprotein-associated phospholipase A2.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.7b00507.
Experimental details, synthesis, assay protocols, and X-ray crystallographic statistics (PDF)
Author Present Address
○ (F.G.) D.E. Shaw Research, 120 W 45th Street, New York, New York 10036, United States.
Author Present Address
◆ (H.-G.B.) Medivir AB, SE-141 22 Huddinge, Sweden.
Author Present Address
□ (H.d.l.M.) RISE Research Institutes of Sweden Division Bioeconomy, SE-417 56 Gothenburg, Sweden.
Author Present Address
▼ (Å.M.) Alfa Laval Lund AB, SE-226 55 Lund, Sweden.
Author Present Address
⬡ (G.S.) SCA Hygiene products AB, SE-851 88 Stockholm, Sweden.
Author Present Address
¶ (F.K.) SciLifeLab, Drug Discovery & Development Platform, SE-171 21 Solna, Sweden.
The authors declare no competing financial interest.
Supplementary Material
References
- Knerr L.; Giordanetto F.; Nordberg P.; Pettersen D.; Selmi N.; Beisel H.-G.; de la Motte H.; Olsson T.; Perkins T. D. J.; Herslöf M.; Månsson Å.; Dahlström M.; Broddefalk J.; Saarinen G.; Klingegård F.; Hurt-Camejo E.; Rosengren B.; Brengdahl J.; Janssen J.; Rohman M.; Sandmark J.; Hallberg K.; Åkerud T.; Roth R. G.; Ahlqvist M. Discovery of a series of indole-2 carboxamides as selective secreted phospholipase A2 type X (sPLA2-X) inhibitors. ACS Med. Chem. Lett. 2018, 10.1021/acsmedchemlett.7b00505. [DOI] [PMC free article] [PubMed] [Google Scholar]; Companion paper.
- Kumar A.; Lindner V. Remodeling With Neointima Formation in the Mouse Carotid Artery After Cessation of Blood Flow. Arterioscler., Thromb., Vasc. Biol. 1997, 17, 2238–2244. 10.1161/01.ATV.17.10.2238. [DOI] [PubMed] [Google Scholar]
- Charifson P. S.; Walters W. P. Acidic and Basic Drugs in Medicinal Chemistry: A Perspective. J. Med. Chem. 2014, 57, 9701–9717. 10.1021/jm501000a. [DOI] [PubMed] [Google Scholar]
- Huang H.; Yan X.; Zhu W.; Liu H.; Jiang H.; Chen K. Efficient copper-promoted N-arylations of aryl halides with amines. J. Comb. Chem. 2008, 10, 617–619. 10.1021/cc800048p. [DOI] [PubMed] [Google Scholar]
- Giordanetto F.; Pettersen D.; Starke I.; Nordberg P.; Dahlström M.; Knerr L.; Selmi N.; Rosengren B.; Larsson L.-O.; Sandmark J.; et al. Discovery of AZD2716: A Novel Secreted Phospholipase A2 (sPLA2) Inhibitor for the Treatment of Coronary Artery Disease. ACS Med. Chem. Lett. 2016, 7, 884–889. 10.1021/acsmedchemlett.6b00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchlis V. D.; Magrioti V.; Barbayianni E.; Cermak N.; Oslund R. C.; Mavromoustakos T. M.; Gelb M. H.; Kokotos G. Inhibition of secreted phospholipases A2 by 2-oxoamides based on α-amino acids: Synthesis, in vitro evaluation and molecular docking calculations. Bioorg. Med. Chem. 2011, 19, 735–743. 10.1016/j.bmc.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergren H. U.; Grönros J.; Heinonen S. E.; Miliotis T.; Jennbacken K.; Sabirsh A.; Ericsson A.; Jönsson-Rylander A. C.; Svedlund S.; Gan L. M. Impaired Coronary and Renal Vascular Function in Spontaneously Type 2 Diabetic Leptin-Deficient Mice. PLoS One 2015, 10, e0130648. 10.1371/journal.pone.0130648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wilde D.; Trachet B.; De Meyer G. R.; Segers P. Shear Stress Metrics and Their Relation to Atherosclerosis: An In Vivo Follow-up Study in Atherosclerotic Mice. Ann Biomed. Ann. Biomed. Eng. 2016, 44, 2327–2338. 10.1007/s10439-015-1540-z. [DOI] [PubMed] [Google Scholar]
- Nakamura K.; Sasaki T.; Cheng X. W.; Iguchi A.; Sato K.; Kuzuya M. Statin prevents plaque disruption in apoE-knockout mouse model through pleiotropic effect on acute inflammation. Atherosclerosis 2009, 206, 355–361. 10.1016/j.atherosclerosis.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Singer A. G.; Ghomashchi F.; Le Calvez C.; Bollinger J.; Bezzine S.; Rouault M.; Sadilek M.; Nguyen E.; Lazdunski M.; Lambeau G.; Gelb M. H. Interfacial kinetic and binding properties of the complete set of human and mouse groups I, II, V, X, and XII secreted phospholipases A2. J. Biol. Chem. 2002, 277, 48535–48549. 10.1074/jbc.M205855200. [DOI] [PubMed] [Google Scholar]
- Hanasaki K.; Yamada K.; Yamamoto S.; Ishimoto Y.; Saiga A.; Ono T.; Ikeda M.; Notoya M.; Kamitani S.; Arita H. Potent modification of low density lipoprotein by group X secretory phospholipase A2 is linked to macrophage foam cell formation. J. Biol. Chem. 2002, 277, 29116–29124. 10.1074/jbc.M202867200. [DOI] [PubMed] [Google Scholar]
- Curfs D. M.; Ghesquiere S. A.; Vergouwe M. N.; van der Made I.; Gijbels M. J.; Greaves D. R.; Verbeek J. S.; Hofker M. H.; de Winther M. P. Macrophage secretory phospholipase A2 group X enhances anti-inflammatory responses, promotes lipid accumulation, and contributes to aberrant lung pathology. J. Biol. Chem. 2008, 283, 21640–21648. 10.1074/jbc.M710584200. [DOI] [PubMed] [Google Scholar]
- Ait-Oufella H.; Herbin O.; Lahoute C.; Coatrieux C.; Loyer X.; Joffre J.; Laurans L.; Ramkhelawon B.; Blanc-Brude O.; Karabina S.; Girard C. A.; Payré C.; Yamamoto K.; Binder C. J.; Murakami M.; Tedgui A.; Lambeau G.; Mallat Z. Group X secreted phospholipase A2 limits the development of atherosclerosis in LDL receptor–null mice. Arterioscler., Thromb., Vasc. Biol. 2013, 33, 466–473. 10.1161/ATVBAHA.112.300309. [DOI] [PubMed] [Google Scholar]
- Guardiola M.; Exeter H. J.; Perret C.; Folkersen L.; Van’t Hooft F.; Eriksson P.; Franco-Cereceda A.; Paulsson-Berne G.; Palmen J.; Li K.; et al. PLA2G10 Gene Variants, sPLA2 Activity, and Coronary Heart Disease Risk. Circ.: Cardiovasc. Genet. 2015, 8, 356–362. 10.1161/CIRCGENETICS.114.000633. [DOI] [PubMed] [Google Scholar]
- Kugiyama K.; Ota Y.; Takazo K.; Moriyama Y.; Kawano H.; Miyao Y.; Sakamoto T.; Soejima H.; Ogawa H.; Doi H.; Sugiyama S.; Yasue H. Circulating levels of secretory type II phospholipase A(2) predict coronary events in patients with coronary artery disease. Circulation 1999, 100, 1280–1284. 10.1161/01.CIR.100.12.1280. [DOI] [PubMed] [Google Scholar]
- Watanabe K.; Fujioka D.; Saito Y.; Nakamura T.; Obata J. E.; Kawabata K.; Watanabe Y.; Mishina H.; Tamaru S.; Hanasaki K.; Kugiyama K. Group X secretory PLA2 in neutrophils plays a pathogenic role in abdominal aortic aneurysms in mice. Am. J. Physiol. Heart. Circ. Physiol. 2012, 302, H95–104. 10.1152/ajpheart.00695.2011. [DOI] [PubMed] [Google Scholar]
- Schewe M.; Franken P. F.; Sacchetti A.; Schmitt M.; Joosten R.; Böttcher R.; van Royen M. E.; Jeammet L.; Payré C.; Scott P. M.; Webb N. R.; Gelb M.; Cormier R. T.; Lambeau G.; Fodde R. Secreted phospholipases A2 are intestinal stem cell niche factors with distinct roles in homeostasis, inflammation, and cancer. Cell Stem Cell. 2016, 19, 38–51. 10.1016/j.stem.2016.05.023. [DOI] [PubMed] [Google Scholar]
- Hallstrand T. S.; Lai Y.; Hooper K. A.; Oslund R. C.; Altemeier W. A.; Matute-Bello G.; Gelb M. H. Endogenous secreted phospholipase A2 group X regulates cysteinyl leukotrienes synthesis by human eosinophils. J. Allergy Clin. Immunol. 2016, 137, 268–277. 10.1016/j.jaci.2015.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokotou M. G.; Limnios D.; Nikolaou A.; Psarra A.; Kokotos G. Inhibitors of phospholipase A2 and their therapeutic potential: an update on patents (2012–2016). Expert Opin. Ther. Pat. 2017, 27, 217–225. 10.1080/13543776.2017.1246540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.