Abstract
Carbonic anhydrase IX (CA IX) expression is important for the regulation of pH in hypoxic tumors and is emerging as a therapeutic target for the treatment of various cancers. Recent studies have demonstrated the selectivity of sucrose, saccharin, and acesulfame potassium for CA IX over other CA isoforms. Reported here is the X-ray crystal structure of CA IX-mimic in complex with sucralose determined to ∼1.5 Å resolution. Furthermore, this structure is compared to the aforementioned sweetener/carbohydrate structural studies in order to determine active site properties of CA IX that promote selective binding. This structural analysis provides a further understanding of CA IX isoform specific inhibition to facilitate the design of new inhibitors and anticancer drugs.
Keywords: Carbonic anhydrase, breast cancer, artificial sweeteners, sucralose, sucrose, saccharin, acesulfame potassium
Solid tumors often exhibit an acidic tumor microenvironment that can be attributed in part to hypoxia.1 Hypoxia is predominantly characterized by a low level of oxygen diffusion due to the development of abnormal vasculature in response to tumor growth.2 Subsequently, rapidly proliferating cells undergo a metabolic switch from oxidative phosphorylation to anaerobic glycolysis, termed the Warburg effect. This phenomenon results in an increased production of lactic acid that is subsequently exported from the cell, decreasing the extracellular pH.3 While healthy cells are unable to thrive in these unfavorable conditions, neoplastic cells adapt in order to grow and proliferate.4 Hypoxic conditions induce the expression of genes regulated by hypoxia inducible factor 1 (HIF-1), such as carbonic anhydrase IX (CA IX).5,6 CA IX is an isoform from a family of zinc metalloenzymes that catalyze the interconversion of carbon dioxide and water to bicarbonate and a proton.7,8 In healthy tissue, CA IX expression is limited to the GI tract; however, overexpression of this isozyme has been observed in several aggressive cancers, including breast cancer.9−11 The catalytic activity of CA IX produces bicarbonate that can act as a buffer in the surrounding microenvironment or be transported into the cell to maintain intracellular pH.12−14 CA IX has therefore been recognized as a biomarker and therapeutic target for the development of potential breast cancer treatments due to its role in tumorigenesis.12,15−18 Previous mouse studies have shown the therapeutic benefits of CA IX inhibition in relation to decreased tumor volume and prolonged survival.15,19,20
CAs have been the target of drug development for several disorders including glaucoma, altitude sickness, epilepsy, and obesity.21−25 CAs are classically inhibited by sulfonamide-based compounds (SO2NH2) that bind directly to the active site zinc, displacing a zinc-bound solvent (ZBS) that is essential for catalysis.8 However, there are 15 CA isoforms expressed in humans that share structural homology within the active site. Therefore, many of the current clinically administered CA inhibitors (CAIs) bind multiple isoforms nonspecifically, thus decreasing the bioavailability of the compounds.26,27 Consequently, nonclassical CAIs are being sought to identify new classes of compounds that selectively inhibit CA IX.28
Recent studies of nonclassical CAIs have identified classes of compounds, such as carboxylic acids, diols, and coumarins, that inhibit CA activity by anchoring through the ZBS or occluding the entrance of the active site.28−30 These binding modes increase the probability of forming interactions with isoform specific residues, potentially increasing the selectivity of such compounds for CA IX. Several recent studies have also indicated artificial sweetener- and carbohydrate-based inhibitors as promising lead compounds for selective CA IX inhibition, including sucrose, saccharin, and acesulfame potassium (Ace K).31−33 Such compounds have been observed to exhibit multiple binding modes binding directly to zinc, anchoring to ZBS, and binding to the entrance of the active site. This class of CAIs is attractive for drug development since these sweeteners have already been approved for safe human consumption (Title 21 US Code of Federal Regulations (CFR) Sec. 172.800 (Ace K) and 180.37 (saccharin)). Therefore, other sugars and sweeteners are being studied to identify pharmacophores to use in the design of isoform specific inhibitors.
Here, the X-ray crystal structure of CA IX-mimic in complex with sucralose is presented at ∼1.5 Å resolution and compared to the binding of aforementioned sweeteners/carbohydrates to identify interactions within the CA IX active site that promote preferential binding. This structural analysis provides an understanding of CA IX isoform specific inhibition for the design of new anticancer drugs.
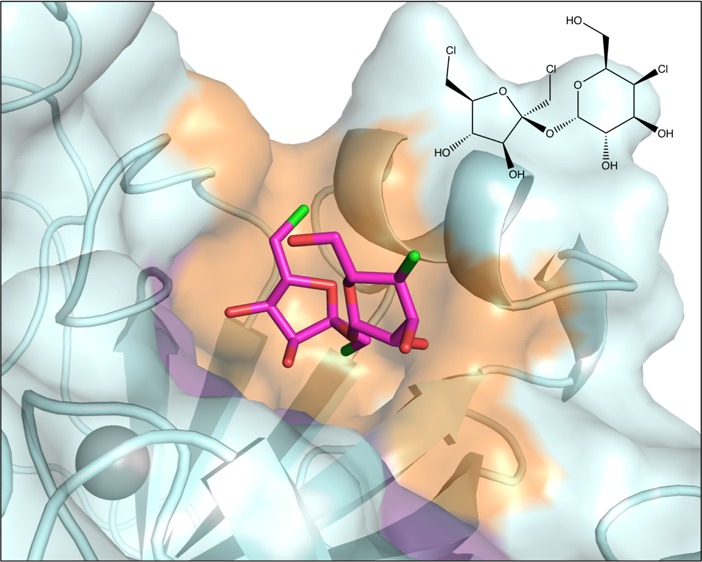
The sucralose binding site was identified using X-ray crystallography. CA IX-mimic crystals soaked in 1 M sucralose diffracted to 1.5 Å resolution (crystallography statistics in Supplementary Table 1). Unambiguous electron density in the initial Fo–Fc omit map was observed for sucralose at the entrance of the active site (Figure 1). Sucralose binding is primarily stabilized through hydrogen bonds with residues on the hydrophilic side of the active site. The only direct side chain hydrogen bond was observed between the C3 hydroxyl of the fructofuranose moiety and Q92 (3.2 Å). Additionally, several other hydrogen bonds are observed between hydroxyl groups of sucralose and solvent molecules in the active site, which further bridge to residues Q67 and T200. Sucralose is also stabilized by van der Waals interactions with residues on the hydrophobic side of the active site, including L91, V121, V131, and L141 (Figure 1). The CO2 substrate binding site was not impeded, and the proton wire was conserved in the presence of sucralose binding. Therefore, inhibition is most likely a result of obstruction of the hydrophobic side of the active site, inhibiting the access of CO2 to the catalytic zinc. In the presence of sucralose, there is still a small opening of ∼6 Å on the hydrophilic side of the active site entrance, but due to the hydrophobic nature of CO2, it is energetically unlikely that it would enter through this opening (Figure 2A).
Figure 1.
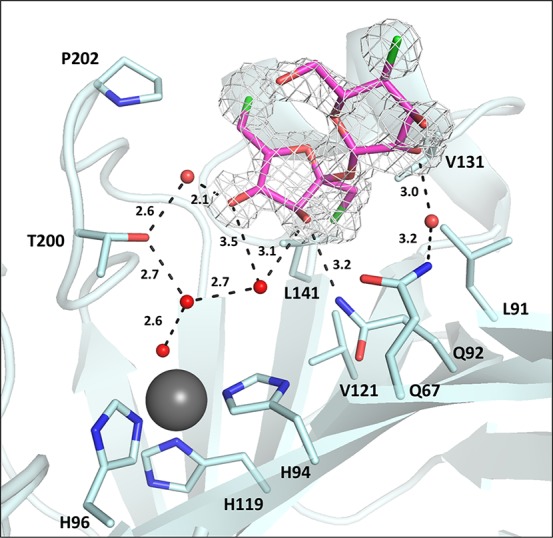
Sucralose binding site in CA IX-mimic. The 2Fo–Fc electron density map is contoured to σ = 0.8. Hydrogen bonds are shown as black dashes with lengths in Angstroms.
Figure 2.
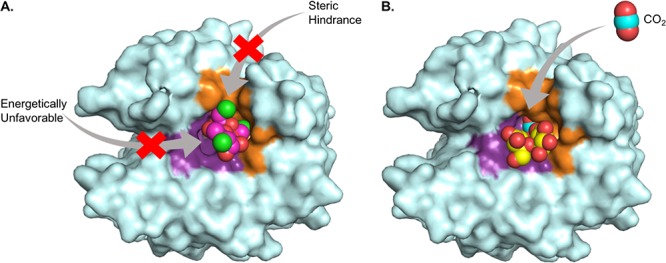
Surface representation of CA IX-mimic illustrating the CO2 substrate pathway to the active site. (A) Bound sucralose (pink) and (B) sucrose (yellow) are represented as spheres. The size of each sphere is representative of the van der Waals radii of that atom. Proposed CO2 pathways are indicated by gray arrows.
To further annotate the binding mode of sucralose, it was compared to the previously published structures of CA IX-mimic in complex with other sweeteners, including sucrose, saccharin, and ace K (Figure 3).31−33 These sweeteners cover an interface surface area of 200–300 Å2 within the active site, with the compounds that bind at the entrance exhibiting a larger buried surface area. Compounds that bind directly to the zinc displace more water molecules, including three waters of the proton wire (ZBS, DW, W1), and form fewer hydrogen bonds in comparison to the disaccharides (Table 1). Not surprisingly, sucralose binds in a similar position to sucrose due to their common chemical structures.31 Sucralose is produced by substituting chlorine atoms for hydroxyl groups at the C4 and C1, C6 positions of the glucopyranose and fructofuranose rings in sucrose, respectively.34 Both compounds interact primarily through hydrogen bonding and do not displace the solvent molecules of the proton wire. Additionally, both sucralose and sucrose maintain van der Waal interactions with residues in the hydrophobic cleft (Figure 3A,B). Interestingly, sucralose inhibits CA IX activity (Ki = 2 μM), whereas sucrose does not. This difference in inhibitory potential is most likely attributed to the aforementioned chlorine atom substitutions. In comparison to sucrose binding, the fructofuranose ring of sucralose is rotated nearly 180° and shifted ∼4–5 Å toward the hydrophobic pocket (Figure 4). This conformational shift is likely caused by the increase in bond length of C–Cl (1.8 Å) in comparison to C–OH (1.4–1.5 Å), which induces rotation of the compound to prevent steric clashes with active site residues. This reorientation also allows the formation of interactions between hydrophobic chloride ions of the fructofuranose ring and residues of the hydrophobic pocket.35,36 These interactions are not observed in sucrose binding, leaving an opening along the hydrophobic pocket through which CO2 could enter to initiate catalysis (Figure 2B).
Figure 3.
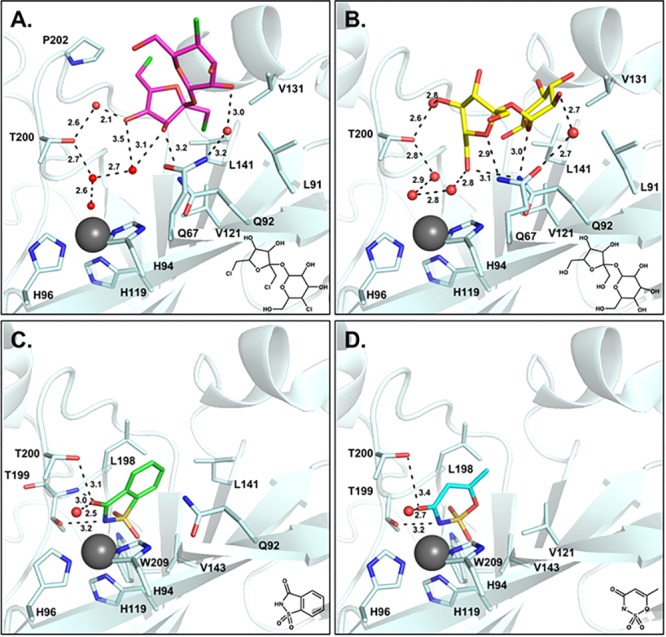
Sweetener binding in CA IX-mimic. (A) Sucralose (pink), (B) sucrose (yellow, PDB: 4YWP), (C) saccharin (green, PDB: 4RIV), and (D) ace K (blue, PDB: 5WGP). Hydrogen bonds are represented by black dashes (lengths given in Angstroms), and active site residues are labeled.
Table 1. Sweetener Binding Properties.
|
Ki (nM) |
|||||
|---|---|---|---|---|---|
| CA II | CA IX | # waters displaced | # H-bonds | interface surface area (Å2) | |
| sucralose | 300 | 2200 | 3 | 5 | 300 |
| sucrose | >20000 | >20000 | 5 | 6 | 260 |
| saccharin | 6000 | 100 | 6 | 4 | 200 |
| ace K | >20000 | 2400 | 6 | 3 | 180 |
Figure 4.
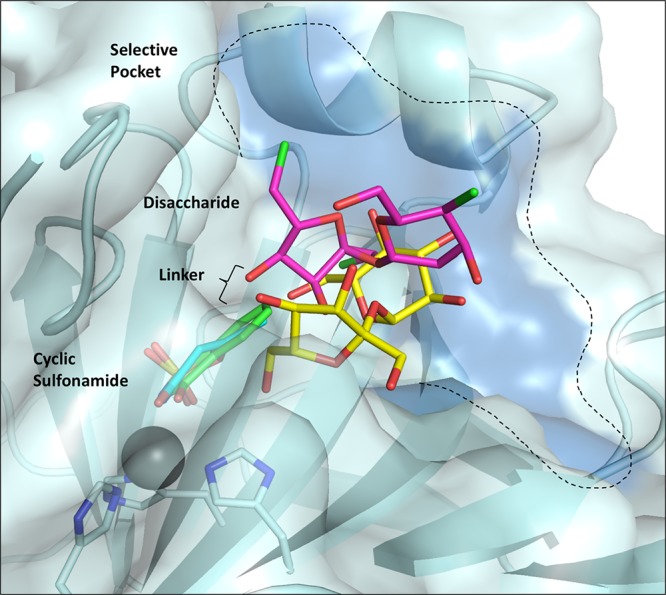
Overlay of sweeteners in complex with CA IX-mimic. Sucralose (pink), sucrose (yellow), saccharin (green), and ace K (blue) are shown as sticks. CA IX isoform unique residues are shaded dark blue on the surface.
In contrast to sucralose and sucrose binding, saccharin and ace K bind further into the CA IX active site and interact directly with the zinc, inhibiting activity by displacement of the ZBS (Figure 3C,D).32,33 Both saccharin and ace K are stabilized through hydrogen bonds with conserved residues T199 and T200. As fragmentation studies show that the most potent inhibitors result from the combination of small molecules, the combination of a cyclic sulfonamide (such as saccharin or ace K) linked to a disaccharide (such as sucralose or sucrose) has the potential to produce efficacious CA IX inhibitors. The chemical linker would provide the spacial requirements to optimize interactions with residues in the selective pocket of the active site, improving selectivity for CA IX and preventing off target inhibition of CA II. Such compounds would simultaneously inhibit activity through two mechanisms- displacing the ZBS essential for catalysis and occluding the active site for substrate binding (Figure 4).
In conclusion, the field of CA inhibition research is expanding to include nonclassical/nonsulfonamide compounds. New modes of binding have the potential to increase inhibitor specificity for cancer associated CA IX by promoting interactions with isoform unique residues located in the selective pocket of the active site. Recent studies have identified inhibitors with novel binding modes that anchor through the zinc-bound water, block substrate entry into the active site, or bind outside of the active site. Sweetener- and carbohydrate-based compounds are now being studied due to their inhibitory properties of CA activity. Further benefits of carbohydrate-based compounds include properties of delivery and digestion. Sweeteners are typically water-soluble but can also be modified to ensure impermeability of the cell membrane, selecting for the extracellular catalytic domain of CA IX and avoiding off-target inhibition of cytosolic CAs. In addition, artificial carbohydrates such as sucralose are not naturally digested and have therefore been optimized for stability in the range of conditions and temperatures experienced during digestion.
The insights gained from the structural comparisons of saccharin, ace K, sucrose, and sucralose binding can be utilized to rationalize structure-guided drug design of CA IX specific inhibitors. The fructofuranose ring of a disaccharide was confirmed to orient toward the active site and interact with residues in the selective pocket, determining the specificity of binding. A disaccharide can therefore be utilized as a lead compound and the stereochemistry or composition of the six-membered ring sugar altered to optimize hydrogen bonding with isoform specific residues. Combining these compounds with the sweeteners that bind directly to the catalytic zinc demonstrate the potential to inhibit CA activity through two simultaneous mechanisms. Therefore, carbohydrate-based compounds provide a promising approach for the design of CA IX specific inhibitors in the treatment of breast cancer.
Acknowledgments
Experiments were performed at the F1 beamline of CHESS. The authors would like to acknowledge the expertise and guidance provided by the CHESS experimental staff.
Glossary
ABBREVIATIONS
- CA IX
carbonic anhydrase IX
- ZBS
zinc-bound solvent
- CAIs
carbonic anhydrase inhibitors
- Ace K
acesulfame potassium
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00100.
Experimental methods (PDF)
Author Contributions
C.L.L. performed the experiments. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
C.L.L. is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under University of Florida Clinical and Translational Science Awards TL1TR001428 and UL1TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work is based upon research conducted at the Cornell High Energy Synchrotron Source (CHESS), which is supported by the National Science Foundation under Award DMR-1332208, using the Macromolecular Diffraction at CHESS (MacCHESS) facility, which is supported by Award GM-103485 from the National Institute of General Medical Sciences, National Institutes of Health.
The authors declare no competing financial interest.
Supplementary Material
References
- Kato Y.; Ozawa S.; Miyamoto C.; Maehata Y.; Suzuki A.; Maeda T.; Baba Y. Acidic Extracellular Microenvironment and Cancer. Cancer Cell Int. 2013, 13, 89. 10.1186/1475-2867-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muz B.; de la Puente P.; Azab F.; Azab A. K. The Role of Hypoxia in Cancer Progression, Angiogenesis, Metastasis, and Resistance to Therapy. Hypoxia 2015, 3, 83–92. 10.2147/HP.S93413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O.; Wind F.; Negelein E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927, 8 (6), 519–530. 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J. S.; Gillies R. D.; Gatenby R. A. Adaptation to Hypoxia and Acidosis in Carcinogenesis and Tumor Progression. Semin. Cancer Biol. 2008, 18 (5), 330–337. 10.1016/j.semcancer.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. L.; Semenza G. L. Purification and Characterization of Hypoxia-Inducible Factor 1. J. Biol. Chem. 1995, 270 (3), 1230–1237. 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- Semenza G. L. Targeting HIF-1 for Cancer Therapy. Nat. Rev. Cancer 2003, 3 (10), 721–732. 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Lindskog S. Structure and Mechanism of Carbonic Anhydrase. Pharmacol. Ther. 1997, 74 (1), 1–20. 10.1016/S0163-7258(96)00198-2. [DOI] [PubMed] [Google Scholar]
- Supuran C. T. Carbonic Anhydrases: Novel Therapeutic Applications for Inhibitors and Activators. Nat. Rev. Drug Discovery 2008, 7 (2), 168–181. 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- Mahon B. P.; Pinard M. A.; McKenna R. Targeting Carbonic Anhydrase IX Activity and Expression. Molecules 2015, 20 (2), 2323–2348. 10.3390/molecules20022323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Tu C.; Wang H.; Silverman D. N.; Frost S. C. Catalysis and PH Control by Membrane-Associated Carbonic Anhydrase IX in MDA-MB-231 Breast Cancer Cells. J. Biol. Chem. 2011, 286 (18), 15789–15796. 10.1074/jbc.M110.188524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone G.; Supuran C. T. Carbonic Anhydrase IX: Biochemical and Crystallographic Characterization of a Novel Antitumor Target. Biochim. Biophys. Acta, Proteins Proteomics 2010, 1804 (2), 404–409. 10.1016/j.bbapap.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Hilvo M.; Baranauskiene L.; Salzano A. M.; Scaloni A.; Matulis D.; Innocenti A.; Scozzafava A.; Monti S. M.; Di Fiore A.; De Simone G.; Lindfors M.; Janis J.; Valjakka J.; Pastorekova S.; Pastorek J.; Kulomaa M. S.; Nordlund H. R.; Supuran C. T.; Parkkila S. Biochemical Characterization of CA IX, One of the Most Active Carbonic Anhydrase Isozymes. J. Biol. Chem. 2008, 283 (41), 27799–27809. 10.1074/jbc.M800938200. [DOI] [PubMed] [Google Scholar]
- Frost S. C.; McKenna R.. Carbonic Anhydrase: Mechanism, Regulation, Links to Disease, and Industrial Applications; Springer Science & Business Media, 2013. [Google Scholar]
- Swietach P.; Vaughan-Jones R. D.; Harris A. L. Regulation of Tumor pH and the Role of Carbonic Anhydrase 9. Cancer Metastasis Rev. 2007, 26 (2), 299–310. 10.1007/s10555-007-9064-0. [DOI] [PubMed] [Google Scholar]
- Lou Y.; McDonald P. C.; Oloumi A.; Chia S.; Ostlund C.; Ahmadi A.; Kyle A.; Auf dem Keller U.; Leung S.; Huntsman D.; Clarke B.; Sutherland B. W.; Waterhouse D.; Bally M.; Roskelley C.; Overall C. M.; Minchinton A.; Pacchiano F.; Carta F.; Scozzafava A.; Touisni N.; Winum J. Y.; Supuran C. T.; Dedhar S. Targeting Tumor Hypoxia: Suppression of Breast Tumor Growth and Metastasis by Novel Carbonic Anhydrase IX Inhibitors. Cancer Res. 2011, 71 (9), 3364–3376. 10.1158/0008-5472.CAN-10-4261. [DOI] [PubMed] [Google Scholar]
- Hussain S. A.; Ganesan R.; Reynolds G.; Gross L.; Stevens A.; Pastorek J.; Murray P. G.; Perunovic B.; Anwar M. S.; Billingham L.; James N. D.; Spooner D.; Poole C. J.; Rea D. W.; Palmer D. H. Hypoxia-Regulated Carbonic Anhydrase IX Expression Is Associated with Poor Survival in Patients with Invasive Breast Cancer. Br. J. Cancer 2007, 96 (1), 104–109. 10.1038/sj.bjc.6603530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald P. C.; Winum J.-Y.; Supuran C. T.; Dedhar S. Recent Developments in Targeting Carbonic Anhydrase IX for Cancer Therapeutics. Oncotarget 2012, 3 (1), 84–97. 10.18632/oncotarget.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Glaberman U.; Marron M.; Chalasani P.; Livingston R.; Iannone M.; Specht J.; Stopeck A. T. Circulating Carbonic Anhydrase IX and Antiangiogenic Therapy in Breast Cancer. Dis. Markers 2016, 2016, 1. 10.1155/2016/9810383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacchiano F.; Carta F.; McDonald P. C.; Lou Y.; Vullo D.; Scozzafava A.; Dedhar S.; Supuran C. T. Ureido-Substituted Benzenesulfonamides Potently Inhibit Carbonic Anhydrase IX and Show Antimetastatic Activity in a Model of Breast Cancer Metastasis. J. Med. Chem. 2011, 54 (6), 1896–1902. 10.1021/jm101541x. [DOI] [PubMed] [Google Scholar]
- Zatovicova M.; Jelenska L.; Hulikova A.; Csaderova L.; Ditte Z.; Ditte P.; Goliasova T.; Pastorek J.; Pastorekova S. Carbonic Anhydrase IX as an Anticancer Therapy Target: Preclinical Evaluation of Internalizing Monoclonal Antibody Directed to Catalytic Domain. Curr. Pharm. Des. 2010, 16 (29), 3255–3263. 10.2174/138161210793429832. [DOI] [PubMed] [Google Scholar]
- Becker B. Diamox and the Therapy of Glaucoma. Am. J. Ophthalmol. 1954, 38, 109–111. 10.1016/0002-9394(54)90051-3. [DOI] [PubMed] [Google Scholar]
- Becker B. The Mechanism of the Fall in Intraocular Pressure Induced by the Carbonic Anhydrase Inhibitor, Diamox*. Am. J. Ophthalmol. 1955, 39, 177–184. 10.1016/0002-9394(55)90022-2. [DOI] [PubMed] [Google Scholar]
- Swenson E. R.Carbonic Anhydrase Inhibitors and High Altitude Illnesses. In Carbonic Anhydrase: Mechanism, Regulation, Links to Disease, and Industrial Applications; Subcellular Biochemistry; Springer: Dordrecht, 2014; pp 361–386. [DOI] [PubMed] [Google Scholar]
- Bruno E.; Buemi M. R.; DeLuca L.; Ferro S.; Monforte A.-M.; Supuran C. T.; Vullo D.; De Sarro G.; Russo E.; Gitto R. In Vivo Evaluation of Selective Carbonic Anhydrase Inhibitors as Potential Anticonvulsant Agents. ChemMedChem 2016, 11 (16), 1812–1818. 10.1002/cmdc.201500596. [DOI] [PubMed] [Google Scholar]
- Poulsen S.-A.; Wilkinson B. L.; Innocenti A.; Vullo D.; Supuran C. T. Inhibition of Human Mitochondrial Carbonic Anhydrases VA and VB with Para-(4-Phenyltriazole-1-Yl)-Benzenesulfonamide Derivatives. Bioorg. Med. Chem. Lett. 2008, 18 (16), 4624–4627. 10.1016/j.bmcl.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Supuran C. T. Carbonic Anhydrase Inhibitors. Bioorg. Med. Chem. Lett. 2010, 20 (12), 3467–3474. 10.1016/j.bmcl.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Supuran C. T. Structure and Function of Carbonic Anhydrases. Biochem. J. 2016, 473 (14), 2023–2032. 10.1042/BCJ20160115. [DOI] [PubMed] [Google Scholar]
- Lomelino C. L.; Supuran C. T.; McKenna R. Non-Classical Inhibition of Carbonic Anhydrase. Int. J. Mol. Sci. 2016, 17 (7), 1150. 10.3390/ijms17071150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supuran C. T. How Many Carbonic Anhydrase Inhibition Mechanisms Exist?. J. Enzyme Inhib. Med. Chem. 2016, 31, 345. 10.3109/14756366.2015.1122001. [DOI] [PubMed] [Google Scholar]
- Maresca A.; Temperini C.; Vu H.; Pham N. B.; Poulsen S.-A.; Scozzafava A.; Quinn R. J.; Supuran C. T. Non-Zinc Mediated Inhibition of Carbonic Anhydrases: Coumarins Are a New Class of Suicide Inhibitors. J. Am. Chem. Soc. 2009, 131 (8), 3057–3062. 10.1021/ja809683v. [DOI] [PubMed] [Google Scholar]
- Pinard M. A.; Aggarwal M.; Mahon B. P.; Tu C.; McKenna R. A Sucrose-Binding Site Provides a Lead towards an Isoform-Specific Inhibitor of the Cancer-Associated Enzyme Carbonic Anhydrase IX. Acta Crystallogr., Sect. F: Struct. Biol. Commun. 2015, 71, 1352–1358. 10.1107/S2053230X1501239X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. B.; Lomelino C. L.; Supuran C. T.; McKenna R. Seriously Sweet”: Acesulfame K Exhibits Selective Inhibition Using Alternative Binding Modes in Carbonic Anhydrase Isoforms. J. Med. Chem. 2017, 61 (3), 1176–1181. 10.1021/acs.jmedchem.7b01470. [DOI] [PubMed] [Google Scholar]
- Mahon B. P.; Hendon A. M.; Driscoll J. M.; Rankin G. M.; Poulsen S.-A.; Supuran C. T.; McKenna R. Saccharin: A Lead Compound for Structure-Based Drug Design of Carbonic Anhydrase IX Inhibitors. Bioorg. Med. Chem. 2015, 23 (4), 849–854. 10.1016/j.bmc.2014.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman S. S.; Rother K. I. Sucralose, a Synthetic Organochlorine Sweetener: Overview of Biological Issues. J. Toxicol. Environ. Health, Part B 2013, 16 (7), 399–451. 10.1080/10937404.2013.842523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla N.; Pomarico E.; Hecht C. J. S.; Taylor E. A.; Chergui M.; Othon C. M. Hydrophobic Interactions of Sucralose with Protein Structures. Arch. Biochem. Biophys. 2018, 639, 38–43. 10.1016/j.abb.2017.12.013. [DOI] [PubMed] [Google Scholar]
- Kortagere S.; Ekins S.; Welsh W. J. Halogenated Ligands and Their Interactions with Amino Acids: Implications for Structure–activity and Structure–toxicity Relationships. J. Mol. Graphics Modell. 2008, 27 (2), 170–177. 10.1016/j.jmgm.2008.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


