Abstract
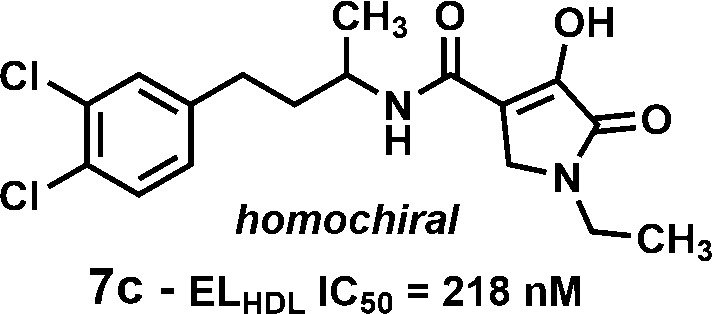
Screening of a small set of nonselective lipase inhibitors against endothelial lipase (EL) identified a potent and reversible inhibitor, N-(3-(3,4-dichlorophenyl)propyl)-3-hydroxy-1-methyl-2-oxo-1,2-dihydropyridine-4-carboxamide (5; EL IC50 = 61 nM, ELHDL IC50 = 454 nM). Deck mining identified a related hit, N-(3-(3,4-dichlorophenyl)propyl)-4-hydroxy-1-methyl-5-oxo-2,5-dihydro-1H-pyrrole-3-carboxamide (6a; EL IC50 = 41 nM, ELHDL IC50 = 1760 nM). Both compounds were selective against lipoprotein lipase (LPL) but nonselective versus hepatic lipase (HL). Optimization of compound 6a for EL inhibition using HDL as substrate led to N-(4-(3,4-dichlorophenyl)butan-2-yl)-1-ethyl-4-hydroxy-5-oxo-2,5-dihydro-1H-pyrrole-3-carboxamide (7c; EL IC50 = 148 nM, ELHDL IC50 = 218 nM) having improved PK over compound 6a, providing a tool molecule to test for the ability to increase HDL-cholesterol (HDL-C) levels in vivo using a reversible EL inhibitor. Compound 7c did not increase HDL-C in vivo despite achieving plasma exposures targeted on the basis of enzyme activity and protein binding demonstrating the need to develop more physiologically relevant in vitro assays to guide compound progression for in vivo evaluation.
Keywords: Endothelial lipase (EL), high density lipoprotein (HDL), reverse cholesterol transport (RCT), coronary artery disease (CAD)
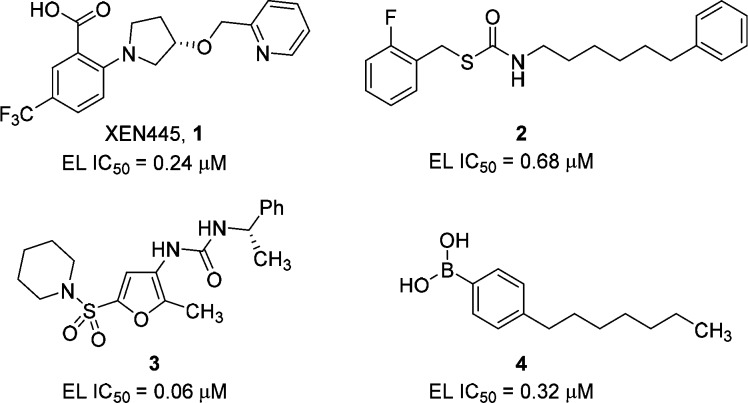
Endothelial lipase (EL; gene nomenclature LIPG)1,2 exerts pleiotropic effects on cardiovascular biology through its role in high density lipoprotein (HDL) catabolism,3−6 vessel wall inflammation,7−15 and subsequent effects on reverse cholesterol transport (RCT).4,16−19 Inhibition of EL using neutralizing polyclonal antibodies in mice20 or pharmacologically using irreversible small molecule inhibitors XEN445 (1)21 and compound 2(22) (Figure 1) has been reported to raise HDL-C levels in mice, whereas loss of function EL variants in humans (e.g., Asn396Ser) have been associated with increased HDL-C levels.23 A recently published Mendelian randomization study showed no correlation between increased HDL-C levels resulting from a loss of function EL variant and the risk of myocardial infarction despite an expected 13% reduction of risk estimated from the amount of HDL-C increase associated with the loss of function allele.24 This study throws doubt into the hypothesis that raising HDL-C levels by inhibiting EL enzymatic activity will decrease coronary artery disease (CAD). To fully investigate the effect of raising HDL-C levels on CAD through pharmacological inhibition of EL will require high quality drug molecules with potent in vivo efficacy.
Figure 1.
Examples of literature EL inhibitors.
EL is a member of the family of enzymes that includes lipoprotein lipase (LPL), hepatic lipase (HL), and pancreatic lipase (PL). In contrast to LPL and PL, which selectively hydrolyze triglycerides (TGs) and HL that hydrolyzes both TGs and phospholipids, EL shows a preference for the hydrolysis of the sn1 ester of phosphatidylcholines found in HDL producing lyso-phosphatidylcholines (LPCs) and free fatty acids (FFAs), resulting in lipid-depleted HDL particles that are cleared more rapidly from the circulation.25
Several small molecule inhibitors of EL have been reported in the literature, e.g., anthranilic acids (XEN445, 1),21 thiocarbamates (2),22 sulfanylfuran ureas (3),26,27 and phenyl boronic acids (4)28 (Figure 1). We report herein our initial efforts to identify potent, reversible inhibitors of EL for the purpose of elevating HDL-C blood levels in vivo.
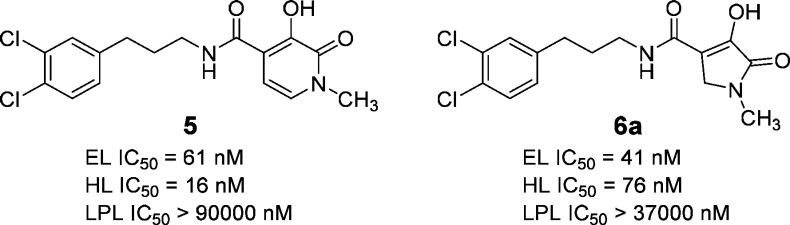
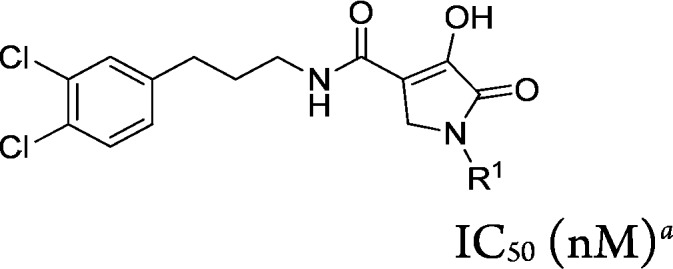
In conjunction with virtual and high throughput screens, nonselective in-house lipase inhibitors were screened for EL inhibition using a mixed vesicle substrate (EL assay; see Supporting Information). The screen led to the identification of N-(3-(3,4-dichlorophenyl)propyl)-3-hydroxy-1-methyl-2-oxo-1,2-dihydropyridine-4-carboxamide (compound 5, Figure 2) as a potent inhibitor of EL activity (EL IC50 = 61 nM) containing a weakly acidic to neutral heterocycle (measured pKa = 6.5). Compound 5 was also a potent inhibitor of HL (HL IC50 = 16 nM) but was found to be highly selective against LPL (LPL IC50 > 90,000 nM). When EL was inhibited with a high concentration of compound 5 (2 μM), enzymatic function was restored by dialyzing away unbound inhibitor from the assay buffer (data not shown), demonstrating the reversibility of inhibition. Further deck mining around compound 5 identified a structurally related EL inhibitor, N-(3-(3,4-dichlorophenyl)-propyl)-4-hydroxy-1-methyl-5-oxo-2,5-dihydro-1H-pyrole-3-cabox-amide (compound 6a, Figure 2), with EL IC50 = 41 nM that contained a slightly more acidic heterocycle (measured pKa = 4.8). Compound 6a was also not selective against HL (HL IC50 = 76 nM) but had excellent selectivity versus LPL (LPL IC50 > 37,000 nM). Pharmacokinetic (PK) profiling of compounds 5 and 6a in CD1 mice (Table 1) showed both compounds to have excellent oral bioavailability (82% and 76%, respectively). Owing to slightly better selectivity against HL and facile synthetic methods available for compound 6a, we focused our initial SAR efforts on this chemotype, seeking to improve potency, to maintain the favorable PK properties, and to establish a pharmacodynamic (PD) profile and PK/PD relationship so the EL mechanism of action could be evaluated pharmacologically in vivo.
Figure 2.
Initial EL hits from focused deck screening.
Table 1. Pharmacokinetic Parameters for Compounds 5 and 6a in CD1 Mice.
| compound 5 |
compound 6a |
|||
|---|---|---|---|---|
| parameter | i.v. | p.o | i.v. | p.o. |
| dose (mpk)a | 1.0 | 1.0 | 1.0 | 1.0 |
| Cmax (μM) | 25 | 2.0 | 32 | 8.2 |
| AUCtotal (μM·h) | 19 | 16 | 24 | 18 |
| CL (mL/min/kg) | 2.5 | 2.1 | ||
| t1/2 (h) | 4.9 | 2.9 | 3.4 | 3.2 |
| F (%) | 82 | 76 | ||
Bolus administration i.v. and p.o. using 60% PEG400, 30% water, and 10% EtOH as vehicle. mpk = milligram per kilogram.
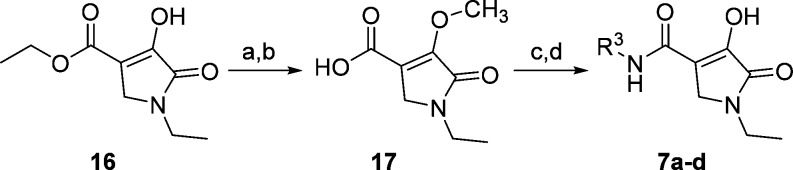
The synthesis of compounds 6a–g, 8–12, and 13a–d is reported in the Supporting Information. Synthesis of the in vivo candidate 7c and related compounds 7a, 7b, and 7d is shown in Scheme 1. Ethyl 1-ethyl-4-hydroxy-5-oxo-2,5-dihydro-1H-pyrrole-3- carboxylate (16)29,30 was first O-methylated with trimethylsilyldiazomethane in the presence of DIPEA,31 followed by saponification of the ester. The acid (17) was coupled with amines (R3-NH2) using the corresponding acid chloride generated with oxalyl chloride. The resulting amides were demethylated with boron trichloride to give the final products 7a and 7b. Compounds 7c and 7d were obtained by chiral HPLC separation of 7b. Compound 7c was also obtained from the homochiral amine synthesized using the method of Ellman.32 Excellent stereochemical induction was observed (dr = 99:1) when methylmagnesium bromide was combined with the (R)-N-tert-butanesulfinyl imine of 3-(3,4-dichlorophenyl)propanal at 60 °C leading to a high enantiomeric excess of the final product (see Supporting Information).
Scheme 1. Synthesis of Compounds 7a–d.
Reagents and conditions: TMSCH2N2, DIPEA, Et2O, rt, 48 h;
NaOH, MeOH/H2O (1:1), 70 °C, 1 h;
(i) oxalyl chloride, CH2Cl2, DMF(cat), (ii) R3-NH2, DIPEA, CH2Cl2, rt, 3 h;
BCl3, CH2Cl2, rt, 5 h. See Supporting Information for individual compound yield.
The compounds were evaluated using a PED-A1/DMPG mixed vesicle assay (EL assay) and an HDL assay using purified HDL particles as enzyme substrate with detection of the product, linoleoyl-lyso-phosphatidylcholine, by LCMS (ELHDL assay; see Supporting Information) in an effort to provide a bridging assay between in vitro and in vivo activity.
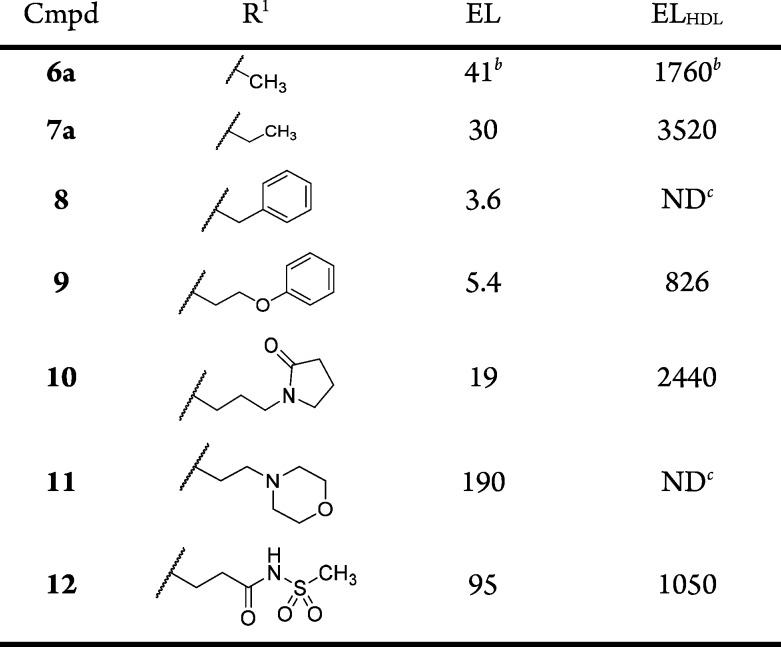
We first explored the SAR of the R1 position of the 1-methyl-5-oxo-2,5-dihydro-1H-pyrrole core found on compound 6a. It was observed that extending the R1 side chain with lipophilic or polar groups maintained or improved EL potency (see compounds 7a and 8–10, Table 2), ranging between 3.6 nM (compound 9, R1 = benzyl) and 30 nM (compound 7a, R1 = ethyl). Addition of a basic side chain (R1 = N-ethylmorpholine) reduced EL potency by ca. 5-fold, whereas the acidic acyl sulfonamide 12 had similar potency to compound 6a. When these compounds were tested using the ELHDL assay, a significant right-shift to reduced potency was observed giving ELHDL IC50/EL IC50 ratios between 11- and 150-fold.
Table 2. R1 Group SAR of 1-Methyl-5-oxo-2,5-dihydro-1H-pyrrole core (compounds 7a and 8–12).
IC50 values are an average of at least two independent determinations.
See Supporting Information for standard deviations.
ND = not determined.
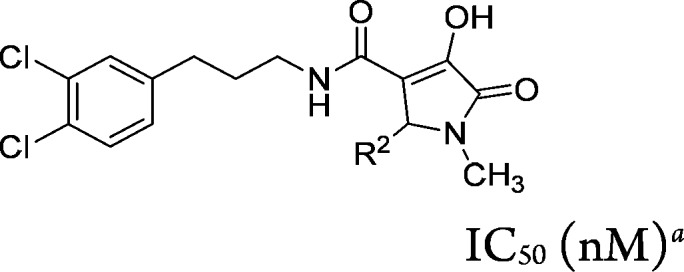
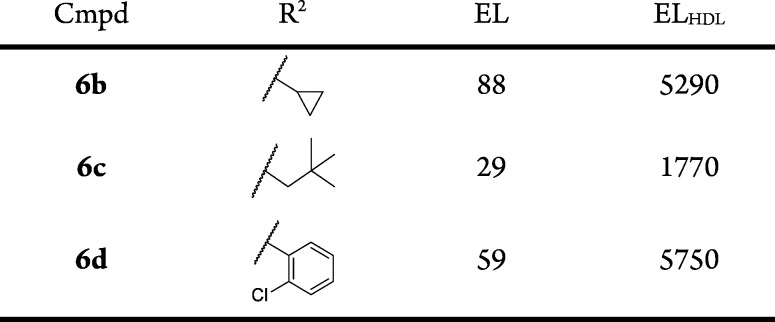
The SAR for substitution at the R2 position was examined within the context of R1 = CH3 (Table 3). A limited set of analogs was examined (compounds 6b–6d). All were of similar potency to the original hit. These analogs did not improve ELHDL potency and the ratio of ELHDL to EL potencies ranged from 60- to 100-fold. We concluded that further exploration of this position was not warranted.
Table 3. R2 Group SAR of 1-Methyl-5-oxo-2,5-dihydro-1H-pyrrole Core (Compounds 6b–d).
IC50 values are an average of at least two independent determinations.
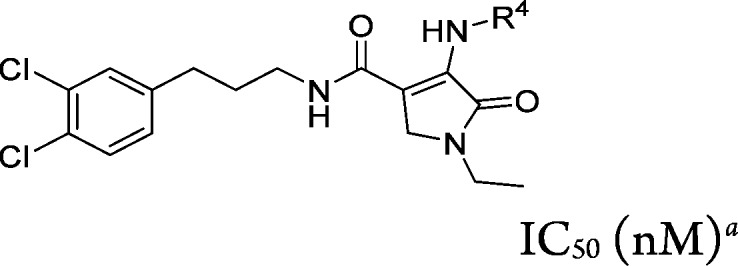
Replacing the C-4 hydroxyl substituent with amines (R4-NH2) in the context of R1 = ethyl and R3 = N-(3-(3,4-dichlorophenyl)prop-1-yl provided compounds 13a–d (Table 4). Modification at this position provided analogs that were slightly more potent or equipotent to 6a in the EL assay (IC50 range from <10 to 59 nM); however, a significant right-shift in potency in the ELHDL assay was observed, with ELHDL/EL ratios ranging from 50- to >300-fold.
Table 4. R4 Group SAR of 1-Methyl-5-oxo-2,5-dihydro-1H-pyrrole Core (Compounds 13a–d).
IC50 values are the average of at least two independent determinations.
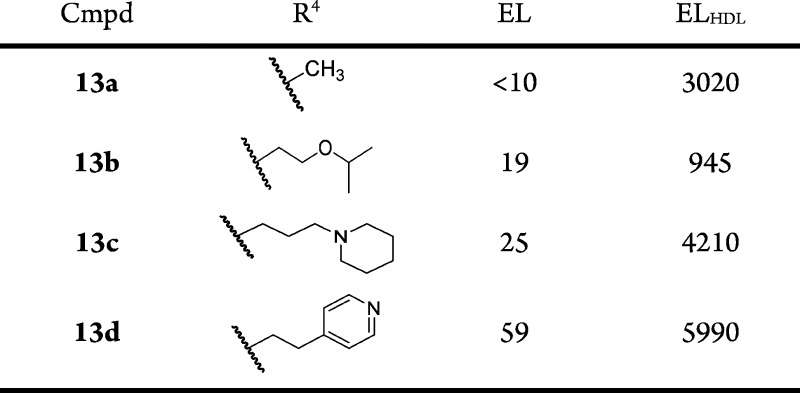
Exploration of the SAR of the amide side chain (R3) was accomplished in two series where R1 = CH3 or ethyl (Table 5). It was generally observed that the most potent compounds in the EL assay contained extended lipophilic R3 groups and two chlorine atoms located at C-3 and C-4 on the terminal phenyl. Thus, compound 6e (R3 = phenyethyl) was 10-fold less potent than the bis-chloro compound 6f, and compound 6a was ca. 140-fold more potent than the des-chloro analog compound 6g. Further SAR development of the R3 side chain identified a key modification that provided an analog with more balanced EL and ELHDL potency, compound 7b (EL IC50 = 44 nM, ELHDL IC50 = 264 nM). When the two enantiomers were obtained in homochiral form, one enantiomer (compound 7c; EL IC50 = 148 nM, ELHDL IC50 = 218 nM) was significantly more potent than the other (compound 7d) and was equally potent for mouse EL (mouse ELHDL IC50 = 100 nM). Importantly, the potency of compound 7c using human or mouse EL was relatively insensitive to increasing concentration of HDL (tested at 40, 100, 300, and 1000 μg/mL HDL; see Supporting Information) suggesting compound 7c is not competitive with HDL.
Table 5. R3 Group SAR of 1-Methyl-5-oxo-2,5-dihydro-1H-pyrrole Core (Compounds 6a, 6e–g, and 7b–d).
IC50 values are an average of at least two independent determinations.
See Supporting Information for standard deviations.
ND = not determined.
When tested against a panel of related enzymes, compound 7c was found to be >250-fold selective for EL versus LDL, monoacylglycerol lipase (MAGL), and pancreatic lipase (PL). Using the HDL-based assay, compound 7c was 12-fold selective for EL vs HL (see Supporting Information).
Pharmacokinetic evaluation of compound 7c using male CD1 mice at a dose of 1.0 mpk i.v. or p.o. (Table 6 and Chart 1) showed it to have excellent oral exposure (oral AUCtotal = 292 μM; 96% bioavailability) and Cmax (15 μM) with a longer half-life (t1/2 = 13 h) than compound 6a (t1/2 = 3.2 h).
Table 6. Pharmacokinetic Data for Compound 7c in CD1 Mice.
| parameter | i.v. | p.o. |
|---|---|---|
| dose (mpk)a | 1.0 | 1.0 |
| Cmax (μM) | 39 | 15 |
| Tmax (h) | 0.05 | 0.5 |
| AUCtotal (μM·h) | 304 | 292 |
| t1/2 (h) | 13 | 13 |
| F (%) | 96 | |
| C 24 h (nM) | 4310 | |
| Cfree 24 h (nM)b | 99 |
Dosing vehicle for i.v. and p.o. administration was 60% PEG400, 30% water, 10% ethanol. mpk = milligram per kilogram
Adjusted for mouse protein binding using 2.3% free fraction.
Chart 1. Mean Plasma Concentrations of Compound 7a in CD1 Micea.
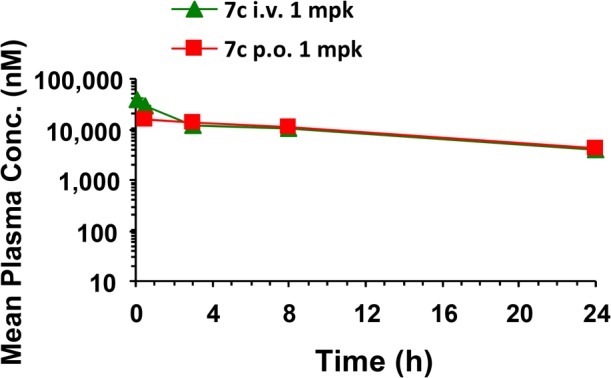
a Compound 7c dosed p.o. at 1 mpk in CD1 mice using 60% PEG400/30% water/10% ethanol as dosing solution.
When compound 7c was dosed at 10 and 50 mpk, systemic exposure was dose related but less than dose proportional for both (7.5-fold and 18-fold increase in AUCtotal, respectively, vs 1.0 mpk dose; see Supporting Information). Protein binding in mice was 97.7%.
When compound 7c was administered to C57BL/6J mice once a day p.o. for 5 days at 10 and 50 mpk, high plasma exposures were observed in the PK arm of the experiment (see Supporting Information). Thus, on day 5 at 50 mpk, Cmax = 238 μM (6 h postdose) and Ctrough = 57 μM (24 h postdose). After adjusting for protein binding, Cmax free (5.5 μM) and Ctrough free (1.3 μM) were 55- and 13-fold, respectively, above the mouse ELHDL IC50. Despite the high exposures achieved, there was no effect on plasma HDL-C levels at either dose (Chart 2). A follow-up study in normal hamsters dosed once a day for 7 days at 50 mpk gave the same outcome. In this study, drug exposures were comparable to mouse. Accounting for protein binding in hamster (98.4%), Cmax free = 5.1 μM (day 5, 6 h postdose), ca. 51-fold above the IC50 for mouse EL (see Supporting Information).
Chart 2. . Plasma HDL Levels in C57BL/6J Mice Dosed with Compound 7ca.
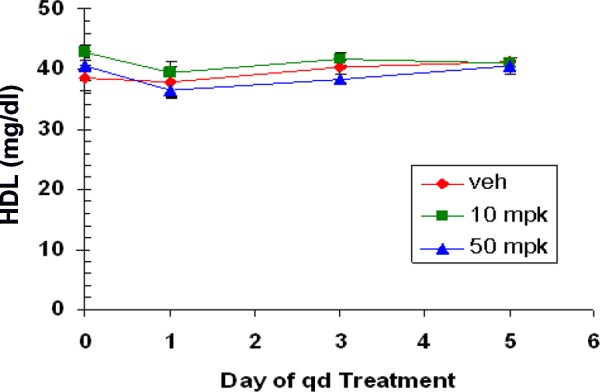
a Compound 7c was dosed at 10 and 50 mpk once daily p.o. using 60% PEG400/30% water/10% ethanol as dosing solution.
In summary, optimizing potency of screening hit 6a using the ELHDL assay resulted in the identification of compound 7c (ELHDL IC50 = 218 nM; mouse ELHDL IC50 = 100 nM). The lack of a pharmacodynamic effect in two animal models of increasing plasma HDL-C levels, despite achieving levels of compound exposure that would predict good inhibition of the target enzyme, suggests factors other than protein binding may be involved in the inability of compound 7c to inhibit plasma EL. In addition, these results point to the need to develop other, more physiologically relevant in vitro assays to guide compound progression for in vivo evaluation. The results of these investigations will be reported separately.
Acknowledgments
The authors would like to acknowledge Dr. Michael A. Walker for helpful chemistry discussions and for generously supplying large quantities of compound 15 and Mr. Robert A. Langish for acquiring HRMS data contained in the Supporting Information.
Glossary
Abbreviations
- DIPEA
diisopropylethylamine
- DMPG
1,2-dimyristoyl-sn-glycero-3-[phosphor-rac-(1-glycerol)] sodium salt;
- dr
diastereomeric ratio
- PED-A1
N-((6-(2,4-DNP)amino)-hexanoyl)-1-(BODIPY FL C5)-2-hexyl-sn-Gly-cero-3-phosphoethanolamine.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00138.
Reaction scheme, experimental procedures and characterization data for compounds 6a–g, 7a–d, 8–12, and 13a–d, standard deviations of EL and ELHDL IC50 data for compounds 6a and 7c, dependence of ELHDL potency on HDL concentration, mouse and hamster PK data, hamster PD data, biochemical assay protocols, and mouse in vivo protocol (PDF)
Author Contributions
The manuscript was written by J.J.H. and through contributions by all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Jaye M.; Lynch K. J.; Krawiec J.; Marchadier D.; Maugeais C.; Doan K.; South V.; Amin D.; Perrone M.; Rader D. J. A novel endothelial-derived lipase that modulates HDL metabolism. Nat. Genet. 1999, 21, 424–428. 10.1038/7766. [DOI] [PubMed] [Google Scholar]
- Hirata K.-i.; Dichek H. L.; Cioffi J. A.; Choi S. Y.; Leeper N. J.; Quintana L.; Kronmal G. S.; Cooper A. D.; Quertermous T. Cloning of a unique lipase from endothelial cells extends the lipase gene family. J. Biol. Chem. 1999, 274, 14170–14175. 10.1074/jbc.274.20.14170. [DOI] [PubMed] [Google Scholar]
- Maugeais C.; Tietge U. J. F.; Broedl U. C.; Marchadier D.; Cain W.; McCoy M. G.; Lund-Katz S.; Glick J. M.; Rader D. J. Dose dependent acceleration of high-density lipoprotien catabolism by endothelial lipase. Circulation 2003, 108, 2121–2126. 10.1161/01.CIR.0000092889.24713.DC. [DOI] [PubMed] [Google Scholar]
- Nijstad N.; Wiersma H.; Gautier T.; van der Giet M.; Maugeais C.; Tietge U. J. Scavenger receptor BI-mediated selective uptake is required for the remodeling of high density lipoprotien by endothelial lipase. J. Biol. Chem. 2009, 284, 6093–6100. 10.1074/jbc.M807683200. [DOI] [PubMed] [Google Scholar]
- Ma K.; Cilingiroglu M.; Otvos J. D.; Ballantyne C. M.; Marian A. J.; Chan L. Endothelial lipase is a major genetic determinant for high-density lipoportion concentration, structure, and metabolism. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 2748–2753. 10.1073/pnas.0438039100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T.; Choi S.; Kundu R. K.; Hirata K.-i.; Rubin E. M.; Cooper A. D.; Quertermous T. Endothelial lipase is a major determinant of HDL level. J. Clin. Invest. 2003, 111, 347. 10.1172/JCI16306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata K.-i.; Ishida T.; Matsushita H.; Tsao P. S.; Quertermous T. Regulated expression of endothelial cell-derived lipase. Biochem. Biophys. Res. Commun. 2000, 272, 90–93. 10.1006/bbrc.2000.2747. [DOI] [PubMed] [Google Scholar]
- Jin W.; Sun G.-S.; Marchadier D.; Octtaviani E.; Glick J. M.; Rader D. J. Endothelial cell secrete triglyceride lipase and phospholipase activities in response to cytokines as a result of endothelial lipase. Circ. Res. 2003, 92, 644–650. 10.1161/01.RES.0000064502.47539.6D. [DOI] [PubMed] [Google Scholar]
- Badellino K. O.; Wolfe M. L.; Reilly M. P.; Rader D. J. Endothelial lipase is increase in vivo by inflammation in humans. Circulation 2008, 117, 678–685. 10.1161/CIRCULATIONAHA.107.707349. [DOI] [PubMed] [Google Scholar]
- Badellino K. O.; Wolfe M. L.; Reilly M. P.; Rader D. J. Endothelial lipase concentrations are increased in metabolic syndrome and associated with coronary atherosclerosis. PloS Medicine 2006, 3, 0245–0252. 10.1371/journal.pmed.0030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu S. W. M.; Tan K. C. B.; Huang Y.; Wong Y. Type 2 diabetes mellitus and endothelial lipase. Atherosclerosis 2008, 198, 441–447. 10.1016/j.atherosclerosis.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Riederer M.; Lechleitner M.; Hrzenjak A.; Koefeler H.; Desoye G.; Heinemann A.; Frank S. Endothelial lipase (EL) and EL-generated lysophosphatidylcholines promote IL-8 expression in endothelial cells. Atherosclerosis 2011, 214, 338–344. 10.1016/j.atherosclerosis.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azumi H.; Hirata K.-i.; Ishida T.; Kojima Y.; Rikitake Y.; Takeuchi S.; Inoue N.; Kawashima S.; Hayashi Y.; Itoh H.; Quertermous T.; Yokoyama M. Immunohistochemical localization of endothelial cell-derived lipase in atherosclerotic human coronary arteries. Cardiovasc. Res. 2003, 58, 647–654. 10.1016/S0008-6363(03)00287-6. [DOI] [PubMed] [Google Scholar]
- Bartels E. D.; Nielsen J. E.; Lindegaard M. L. S.; Hulten L. M.; Schroeder T. V.; Nielsen L. B. Endothelial lipase is highly expressed in macrophages in advanced human atherosclerotic lesions. Atherosclerosis 2007, 195, e42–e49. 10.1016/j.atherosclerosis.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Kojma Y.; Hirata K.-i.; Ishida T.; Shimokawa Y.; Inoue N.; Kawashima S.; Quertermous T.; Yokoyama M. Endothelial lipase modulates monocyte adhesion to the vessel wall. J. Biol. Chem. 2004, 279, 54032–54038. 10.1074/jbc.M411112200. [DOI] [PubMed] [Google Scholar]
- Qiu G.; Hill J. S. Endothelial lipase promotes apolipo-protein AI-mediated cholesterol efflux in THP-1 macrophages. Arterioscler., Thromb., Vasc. Biol. 2009, 29, 84–91. 10.1161/ATVBAHA.108.176487. [DOI] [PubMed] [Google Scholar]
- Strauss J. G.; Zimmermann R.; Hrzenjak A.; Zhou Y.; Kratky D.; Levak-Frank S.; Kostner G. M.; Zechner R.; Frank S. Endothelial cell-derived lipase mediates uptake and binding of high-density lipoprotein (HDL) particles and the selective uptake of HDL-associated cholesterol esters independent of its enzymic activity. Biochem. J. 2002, 368, 69–79. 10.1042/bj20020306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersma H.; Gatti A.; Nijstad N.; Kuipers F.; Tietge U. J. F. Hepatic SR-BI, not endothelial lipase, expression determines biliary cholesterol secretion in mice. J. Lipid Res. 2009, 50, 1571–1580. 10.1194/jlr.M800434-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. J.; Lagor W. R.; Sankaranaravanan S.; Yasuda T.; Quertermous T.; Rothblat G. H.; Rader D. J. Impact of combined deficiency of hepatic lipase and endothelial lipase on the metabolism of both high-density lipoproteins and apolipoprotein B-containing lipoprotiens. Circ. Res. 2010, 107, 357–364. 10.1161/CIRCRESAHA.110.219188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W.; Millar J. S.; Broedl U.; Glick J. M.; Rader D. J. Inhibition of endothelial lipase causes increased HDL-cholesterol levels in vivo. J. Clin. Invest. 2003, 111, 357–362. 10.1172/JCI16146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S.; Dean R.; Jia Q.; Zenova A.; Zhong J.; Grayson C.; Xie C.; Lindgren A.; Samra P.; Sojo L.; van Heek M.; Lin L.; Percival D.; Fu J.-m.; Winther M. D.; Zhang Z. Discovery of XEN445: a potent and selective endothelial lipase inhibitor raises plasma HDL-cholesterol concentration in mice. Bioorg. Med. Chem. 2013, 21, 7724–7734. 10.1016/j.bmc.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Greco M. N.; Connelly M. A.; Leo G. C.; Olson M. W.; Powell E.; Huang Z.; Hawkins M.; Smith C.; Schalk-Hihi C.; Darrow A. L.; Xin H.; Lang W.; Damiano B. P.; Hlasta D. J. A thiocarbamate inhibitor of endothelial lipase raises HDL cholesterol levels in mice. Bioorg. Med. Chem. Lett. 2013, 23, 2595–2597. 10.1016/j.bmcl.2013.02.113. [DOI] [PubMed] [Google Scholar]
- Edmondson A. C.; Brown R. J.; Kathiresan S.; Cupples L. A.; Demissie S.; Manning A. K.; Jensen M. K.; Rimm E. B.; Wang J.; Rodrigues A.; Bamba V.; Khetarpal S. A.; Wolfe M. L.; DerOhannessian S.; Li M.; Reilly M. P.; Aberle J.; Evans D.; Hegele R. A.; Rader D. J. Loss of function variants in endothelial lipase are a cause of elevated HDL cholesterol in humans. J. Clin. Invest. 2009, 119, 1042–1050. 10.1172/JCI37176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voight B. F.; Peloso G. M.; Orho-Melander M.; Frikke-Schmidt R.; Barbalic M.; Jensen M. K.; Hindy G.; Hólm H.; Ding E. L.; Johnson T.; Schunkert H.; Samani N. J.; Clarke R.; Hopewell J. C.; Thompson J. F.; Li M.; Thorleifsson G.; Newton-Cheh C.; Musunuru K.; Pirruccello J. P.; Saleheen D.; Chen L.; Stewart A. F. R.; Schillert A.; Thorsteinsdottir U.; Thorgeirsson G.; Anand S.; Engert J. C.; Morgan T.; Spertus J.; Stoll M.; Berger K.; Martinelli N.; Girelli D.; McKeown P. P.; Patterson C. C.; Epstein S. E.; Devaney J.; Burnett M.-S.; Mooser V.; Ripatti S.; Surakka I.; Nieminen M. S.; Sinisalo J.; Lokki M.-L.; Perola M.; Havulinna A.; de Faire U.; Gigante B.; Ingelsson E.; Zeller T.; Wild P.; de Bakker P. I. W.; Klungel O. H.; Maitland-van der Zee A.-H.; Peters B. J. M.; de Boer A.; Grobbee D. E.; Kamphuisen P. W.; Deneer V. H. M.; Elbers C. C.; Onland-Moret N. C.; Hofker M. H.; Wijmenga C.; Verschuren W. M. M.; Boer J. M. A.; van der Schouw Y. T.; Rasheed A.; Frossard P.; Demissie S.; Willer C.; Do R.; Ordovas J. M.; Abecasis G. R.; Boehnke M.; Mohlke K. L.; Daly M. J.; Guiducci C.; Burtt N. P.; Surti A.; Gonzalez E.; Purcell S.; Gabriel S.; Marrugat J.; Peden J.; Erdmann J.; Diemert P.; Willenborg C.; König I. R.; Fischer M.; Hengstenberg C.; Ziegler A.; Buysschaert I.; Lambrechts D.; Van de Werf F.; Fox K. A.; El Mokhtari N. E.; Rubin D.; Schrezenmeir J.; Schreiber S.; Schäfer A.; Danesh J.; Blankenberg S.; Roberts R.; McPherson R.; Watkins H.; Hall A. S.; Overvad K.; Rimm E.; Boerwinkle E.; Tybjaerg-Hansen A.; Cupples L. A.; Reilly M. P.; Melander O.; Mannucci P. M.; Ardissino D.; Siscovick D.; Elosua R.; Stefansson K.; O’Donnell C. J.; Salomaa V.; Rader D. J.; Peltonen L.; Schwartz S. M.; Altshuler D.; Kathiresan S. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet 2012, 380, 572–580. 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T.; Ishida T.; Rader D. J. Update on the role of endothelial lipase in high-density lipoprotein metabolism, reverse cholesterol transport and atherosclerosis. Circ. J. 2010, 74, 2263–2270. 10.1253/circj.CJ-10-0934. [DOI] [PubMed] [Google Scholar]
- Goodman K. B.; Bury M. J.; Cheung M.; Cichy-Knight M. A.; Dowdell S. E.; Dunn A. K.; Lee D.; Lieby J. A.; Moore M. L.; Scherzer D. A.; Sha D.; Suarez D. P.; Murphy D. J.; Harpel M. R.; Manas E. S.; McNulty D. E.; Annan R. S.; Matico R. E.; Schwartz B. K.; Trill J. J.; Sweitzer T. D.; Wang D.-y.; Keller P. M.; Krawiec J. A.; Jaye M. C. Discovery of potent, selective sulfonylfuran urea endothelial lipase inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 27. 10.1016/j.bmcl.2008.11.033. [DOI] [PubMed] [Google Scholar]
- Keller P. M.; Rust T.; Murphy D. J.; Matico R.; Trill J. J.; Krawiec J. a.; Jurewicz A.; Jaye M.; Harpel M.; Thrall S.; Schwartz B. A high-throughput screen for endothelial lipase using HDL as substrate. J. Biomol. Screening 2008, 13, 468–475. 10.1177/1087057108319738. [DOI] [PubMed] [Google Scholar]
- O’Connell D. P.; LeBlanc D. F.; Cromley D.; Billheimer J.; Rader D. J.; Bachovchin W. W. Design and synthesis of boronic acid inhibitors of endothelial lipase. Bioorg. Med. Chem. Lett. 2012, 22, 1397–1401. 10.1016/j.bmcl.2011.12.043. [DOI] [PubMed] [Google Scholar]
- Southwick P. L.; Previc E. P.; Casanova J. Jr.; Carlson E. H. A study of some 2,3-dioxopyrrolidines and derived bipyrrolidines. J. Org. Chem. 1956, 21, 1087–1095. 10.1021/jo01116a009. [DOI] [Google Scholar]
- Reid S. T.; De Silva D. Photocyclization of ethyl 2,3-dioxopyrrolidine-4-carboxylates to alkenes; the synthesis of ethyl 2,3-dioxohexahydroazepine-6-carboxylates. Tetrahedron Lett. 1983, 24, 1949–1950. 10.1016/S0040-4039(00)81813-4. [DOI] [Google Scholar]
- Aoyama T.; Terasawa S.; Sudo K.; Shioiri T. New methods and reagents in organic synthesis. 46. Trimethylsilyldiazomethane: a convenient reagent for the O-methylation of phenols and enols. Chem. Pharm. Bull. 1984, 32, 3759–3760. 10.1248/cpb.32.3759. [DOI] [Google Scholar]
- Cogan D. A.; Liu G.; Ellman J. Assymetric synthesis of chiral amines by highly diastereoselective 1,2- additions of organometallic reagents to N-tert-butanesulfinyl imines. Tetrahedron 1999, 55, 8883–8904. 10.1016/S0040-4020(99)00451-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.