Abstract
We report the first high affinity neutral Bodipy fluorophores for selective imaging of mitochondria with notable sensitivity (∼100 nM) and insignificant cytotoxicity even at very high concentration (∼100 μM), when tested against HeLa cells. Further, these fluorophores are chemically robust and require no special conditions for storage.
Keywords: Bodipy, fluorescent probes, mitochondrial staining, cytotoxicity
Mitochondria are unusual intracellular organelles involved in many cellular processes and play a key role in cell survival and death. Their main function, however, is to provide cellular energy in the form of ATP for various cellular needs. An imbalance in mitochondrial bioenergetics leads to formation of excessive reactive oxygen species (ROS) that in turn cause oxidative stress-mediated cellular responses. A wide range of human diseases, such as metabolic disorders, cancers, and cardiovascular and neurological diseases are associated with abnormal functions in mitochondria.1 Thus, numerous therapeutic applications targeting the mitochondria have been developed and have been playing vital role in diagnosis and biomedical research.2
The morphological changes in mitochondria in living cells are typically discerned by fluorescence microscopy using mitochondria-selective dyes. A simple, yet efficient, approach to distinguish mitochondria from other organelles is to take the advantage of substantial negative electrochemical potential (130–170 mV, negative side) across the inner mitochondrial membrane. Based on this phenomenon, cationic lipophilic dyes tagged with triphenylphosphonium (TPP),3 5,5′,6,6′-tetrachloro 1,1′,3,3′-tetraethyl-benzimidazol carbocyanine iodide (JC-1),4 tetramethylrhodamine methyl ester (TMRM),5 and several others were developed for studying mitochondrial physiology. Majority of the commercial dyes used for mitochondrial imaging include rhodamine,6 and cyanine dyes7 suffer from various trade-offs such as low specificity, inhibiting mitochondrial respiration and high toxicity.8,9 However, most of the commercially available cationic probes are chemically unstable and thus require storage below −20 °C. It is a well-known fact that cationic molecules induce cytotoxicity by imposing mitochondrial membrane depolarization leading to unwarranted biological responses, thereby drastically limiting their applications. However, extensive studies on noncationic mitochondrial probes have shown that they have a poor fluorescence image profile pertinent to explore for alternate fluorescent mitochondrial probes.10−13 Neto et al. have succinctly discussed the relevance of neutral fluorophores by incorporating strong electron acceptor groups in the molecular skeleton. The low electron density (+δ) results in a specific regime of a neutral structure, enabling the fluorophore to localize in the mitochondria.14 Borondipyrromethene (Bodipy) derivatives show bright fluorescence, fair chemical stability, and structural features with magnificent photophysical properties. They are often used as biological probes, fluorescent stains, and protein labeling reagents.15 Also, various cationic-Bodipy dyes have been reported for staining mitochondria tagged with TPP,16−19cis-platin,20 phosphonium,21 and quarternized pyridine moieties.22,23
The present Letter reports the design and synthesis of three neutral Bodipy derivatives tethered with a phenoxymethylpyridine unit, namely BIP, CF-MONO, and CF-BI (Figure 1), which provide highly efficient molecules for targeting mitochondria. To the best of our knowledge, this is the first report on highly efficient neutral Bodipy-based specific mitochondrial staining.
Figure 1.
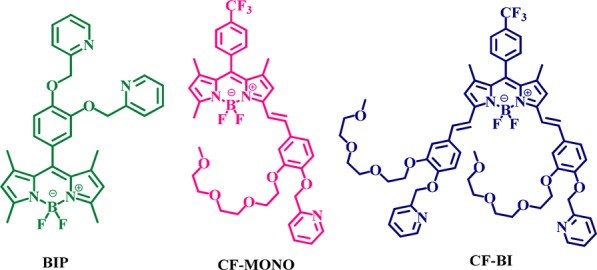
Molecular structures of Bodipy fluorophores.
The probes CF-MONO and CF-BI contain oligoethylene glycol moieties to maintain a hydrophilic–lipophilic balance and improved cell permeability. The electron accepting group −CF3 (trifluoromethyl group) has been incorporated in the molecular structures in order to result in +δ character over a specific region, facilitating the fluorophore localization in mitochondria.
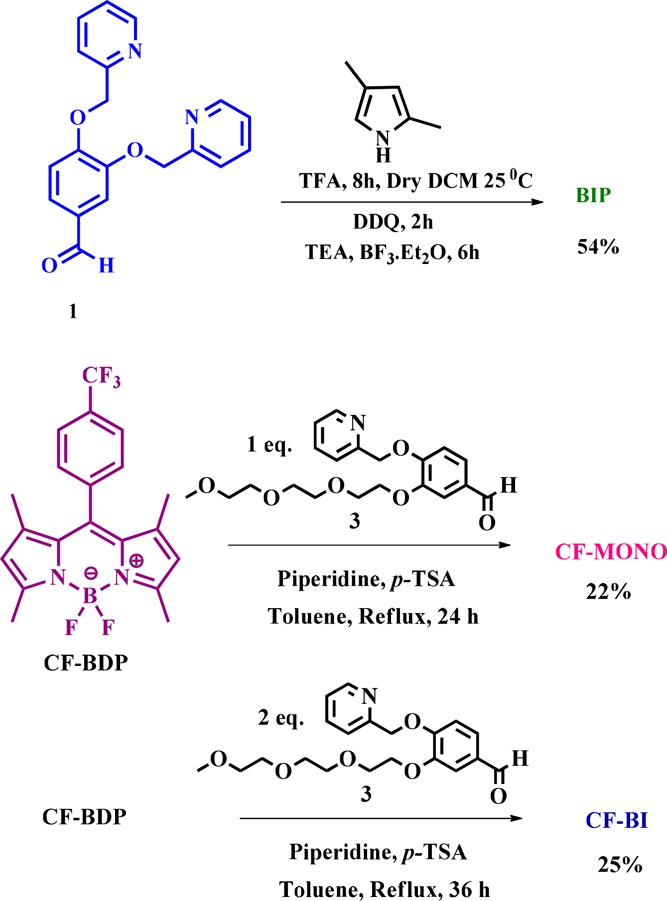
The choice of aldehydes 1 and 3 (Scheme 1) is the key for designing the present series of Bodipy based probes. The synthetic routes of compounds BIP, CF-MONO, and CF-BI were presented in Scheme 1. The compound BIP was synthesized by following classic one pot synthesis; by the acid catalyzed condensation of aldehyde 1 with 2,4 dimethyl pyrrole followed by the oxidation with DDQ to form dipyrrin, which in turn complexed with BF3·Et2O in the presence of triethylamine (see details in Supporting Information (SI)).24 The compounds CF-MONO and CF-BI were synthesized by Knoevenegal condensation of C3/C5 methyl groups of CF-BDP (for synthetic protocol, see SI) with aldehyde 3 in the presence of a catalytic amount of piperidine and p-TsOH in toluene under reflux conditions. The mono- and bi-condensations were controlled by adjusting the mole ratio of the reactants and monitoring the reaction time, resulting in CF-MONO and CF-BI derivatives of 22 and 25% yields, respectively (Scheme 1) (details are in SI). All the compounds were characterized by NMR (1H, 13C) and HRMS (see SI).
Scheme 1. Synthetic Route of Bodipy Fluorophores.
The photophysical properties of the Bodipy fluorophores were recorded in three solvents of varying polarity, including dichloromethane (Figure S1, SI), acetonitrile (ACN, Figure 2), and dimethylformamide (Figure S1, SI) at room temperature.
Figure 2.
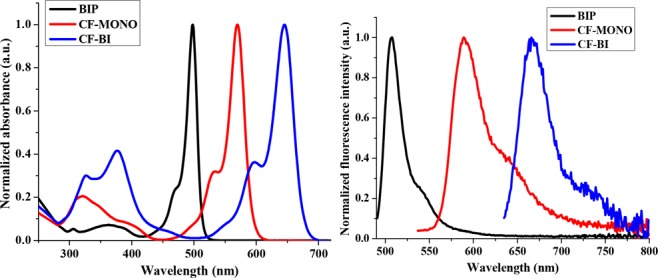
Normalized absorbance and emission spectra of Bodipy fluorophores in acetonitrile.
The normalized absorbance and emission spectra of these fluorophores in acetonitrile are shown in Figure 2. All these dyes possess small Stokes shifts (<25 nm) and maintain similar photophysical properties in different solvents. Fluorescence quantum yields were calculated using fluorescence emission spectra, obtained from low concentration solutions (0.045–0.05 OD), by a standard relative method. Bodipy-CO2H, rhodamine B, and zinc tert-butyl phthalocyanine were used as reference for BIP, CF-MONO, and CF-BI, respectively.25−27 The photophysical data are presented in Table1. Superior pH insensitivity and photostability was exhibited by the Bodipy fluorophores compared to the commercial Mitotracker Red CMXRos (Figures S2 and S3 in SI).
Table 1. Photophysical Properties of Bodipy Fluorophores.
| Λmax Absa (nm) |
Λmax Emsb (nm) |
εc (M−1 cm−1) |
Φd | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| dye | DCM | ACN | DMF | DCM | ACN | DMF | DCM | ACN | DMF | ACN |
| BIP | 502 | 498 | 501 | 514 | 510 | 515 | 1,37,671 | 89,562 | 81,707 | 0.51 |
| CF-MONO | 576 | 570 | 576 | 595 | 589 | 598 | 1,14,438 | 1,22,947 | 1,45,454 | 0.42 |
| CF-BI | 652 | 645 | 654 | 672 | 666 | 675 | 1,94,100 | 1,26,865 | 1,16,333 | 0.21 |
Absorption maximum.
Fluorescence emission maximum (obtained by exciting at the absorption maximum of the dye).
Extinction coefficient at absorption maximum.
Fluorescence quantum yield calculated by a standard relative method using Bodipy-CO2H, (ϕ = 0.57 in CH2Cl2), rhodamine B (ϕ = 0.70 in CH3OH), and zinc tert-butyl phthalocyanine (ϕ = 0.37 in benzene).
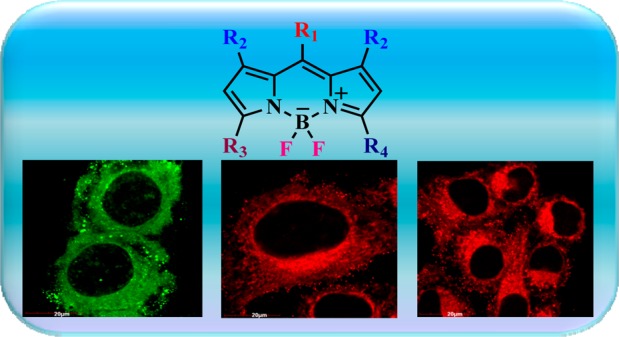
Fluorescence microscopy experiments were conducted to investigate the ability of Bodipy fluorophores to stain mitochondria in human cervical carcinoma (HeLa) cells. The intracellular distribution was observed through colocalization imaging experiments. HeLa cells were incubated with these Bodipy fluorophores (100 nM) at 37 °C for 30 min. Figure 3 displays the fluorescence microscopic images of cells and, after coincubation with both BIP and Mitotracker Red CMXRos, a commercially available mitochondria selective dye, from Thermofisher.
Figure 3.
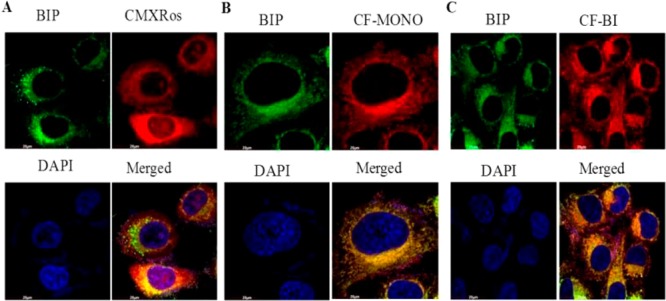
Mitochondrial staining by Bodipy fluorophores in HeLa cells: The cells were incubated with Bodipy fluorophores (100 nM) at 37 °C for 30 min. (A) Green fluorescence indicates mitochondrial staining of BIP compound by confocal microscopy using FITC filter. Red fluorescence indicates mitochondrial staining of Mitotracker Red CMXRos (Thermofisher) by confocal microscopy by using the rhodamine filter. Blue fluorescence indicates nuclear staining by DAPI. (B) Same as A, except that red fluorescence indicates mitochondrial staining of CF-MONO compound by confocal microscopy by the using rhodamine filter. (C) Same as A, except that red fluorescence indicates mitochondrial staining of CF-BI compound by confocal microscopy by using the rhodamine filter.
The results of confocal microscopy show that all the newly synthesized Bodipy fluorophores substantially stained the mitochondria with marginal retention in the cytoplasmic compartment (Figure 3). However, no fluorescence was noticed in the nuclear compartment. In contrast, slight fluorescence was observed in the nuclear compartment of the cells with the commercially available Mitotracker Red CMXRos (Figure 3), implying that these Bodipy fluorophores are very specific in staining mitochondria in living cells.
The accumulation of these Bodipy fluorophores in the mitochondrial fraction was further confirmed by high resolution mass spectrometry (HRMS). For HRMS studies, HeLa cells were treated with 10 μM Bodipy fluorophores for 24 h, and the cells were fractionated into cytosolic and mitochondrial fractions (see SI). From Table 2, it is evident that almost all (>99%) the Bodipy fluorophores are localized in the mitochondrial fraction. HRMS spectra of mitochondrial and cytosolic fractions are included in the SI.
Table 2. Cytosolic and Mitochondrial Uptake of Bodipy Fluorophores (from HRMS).
| dye | cytosolic fraction (N = 3) (nmol/μg protein) | mitochondrial fraction (N = 3) (nmol/μg protein) |
|---|---|---|
| BIP | 1.84 ± 0.91 | 6140.75 ± 300.94 |
| CF-MONO | 48.35 ± 9.2 | 6706.81 ± 163.09 |
| CF-BI | 0.0045 ± 0.81 | 4558.64 ± 229.0 |
We next studied the cytotoxicity of all these compounds in HeLa cells, treated at 100 μM for 24 h. None of the synthesized compounds caused any noticeable cell death. However, incubation of cells with 5 μM CMXRos resulted in significant cytotoxicity (Figure 4).
Figure 4.
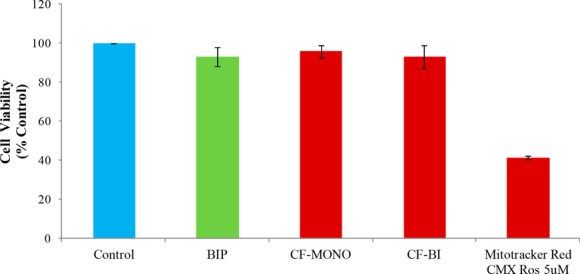
Effect of Bodipy fluorophores on cytotoxicity: HeLa cells were treated with Bodipy fluorophores (100 μM) and Thermofisher’s Mitotracker Red CMXRos (5 μM) for 24 h, and cell viability was measured by SRB assay. Cell viability data is an average of three independent experiments.
To examine whether the uptake of the dyes was dependent on the mitochondrial membrane potential,28 cells were treated with p-trifluoromethoxyphenylhydrazone (FCCP) prior to the staining procedure. FCCP is a mitochondrial uncoupler that causes rapid acidification of the mitochondria and dysfunction of ATP synthase and reduces the mitochondrial membrane potential (MMP or Ψm).29 All the probes showed considerable reduction in the mean fluorescence intensity upon FCCP-induced MMP depolarization (Figure 5). The reduction in fluorescence for BIP, CF-MONO, and CF-BI was 48%, 38%, and 38%, respectively (Figure 6). This result indicates that these fluorophores definitely localize in mitochondria and that its cellular uptake is sensitive to the mitochondrial membrane potential.
Figure 5.
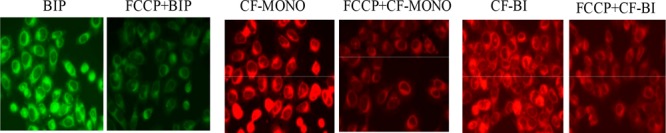
HeLa cells were pretreated with FCCP (20 μM) for 8 h before incubating with Bodipy fluorophores for 30 min (100 nM). Images were taken with a fluorescence microscope equipped with rhodamine and FITC filters.
Figure 6.
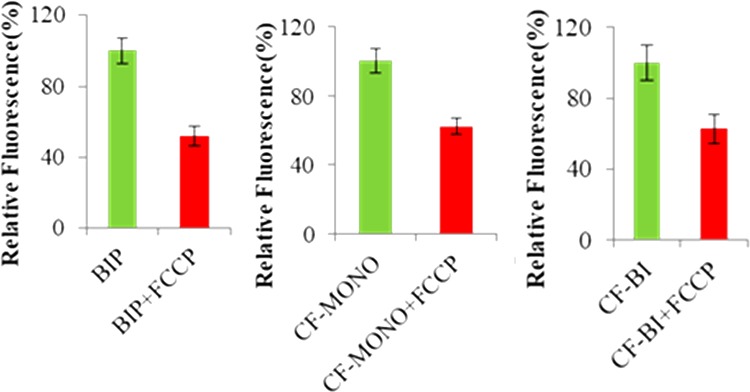
HeLa cells were pretreated with FCCP for 8 h before adding Bodipy fluorophores. Images were quantified with Image Proplus software.
In summary, highly specific neutral Bodipy-based fluorophores with exclusive affinity toward mitochondria were developed. The chemical robustness of these fluorophores requires no special conditions for storage. These Bodipy fluorophores present excellent mitochondrial targeting traits with exceptionally low cytotoxicity compared to commercially available mitochondrial probes. We believe exploring and commercialization of such fluorophores is highly needed for better understanding of mitochondrial diseases.
Acknowledgments
T.G. and S.Ka. thank UGC and ICMR, respectively, for providing the senior research fellowships. We thank Dr. B. Surendar Reddy for useful discussions. We sincerely thank Dr. A. C. Kunwar for his helpful suggestions.
Glossary
ABBREVIATIONS
- ACN
acetonitrile
- ATP
adenosine triphosphate
- Bodipy
borondipyrromethene
- BF3·Et2O
boron trifluoride diethyl etherate
- DAPI
4′,6-diamidino-2-phenylindole
- DCM
dichloromethane
- DDQ
2,3-dichloro-5,6-dicyanobenzoquinone
- DMF
dimethylformamide
- FCCP
p-trifluoromethoxy phenylhydrazone
- FITC
fluorescein isothiocyanate
- HeLa
human cervical carcinoma
- HRMS
high resolution mass spectrometry
- JC-1
5,5′,6,6′-tetrachloro-1,1′,3,3′tetraethyl-benzimidazolcarbocyanine iodide
- MMP
mitochondrial membrane potential
- NMR
nuclear magnetic resonance
- OD
optical density
- ROS
reactive oxygen species
- SRB
sulforhodamine B
- TFA
trifluoroacetic acid
- p-TsOH
p-toluenesulfonic acid
- TMRM
tetramethylrhodamine methylester
- TPP
triphenyl phosphonium
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00022.
Experimental procedures and characterization spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Zielonka J.; Joseph J.; Sikora A.; Hardy M.; Ouari O.; Vivar J. V.; Cheng G.; Lopez M.; Kalyanaraman B. Mitochondria-targeted triphenylphosphonium-based compounds: syntheses, mechanisms of action, and therapeutic and diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. 10.1021/acs.chemrev.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnari J.; Suomalainen A. Mitochondria: in sickness and in health. Cell 2012, 148, 1145–1159. 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. P. Targeting lipophilic cations to mitochondria. Biochim. Biophys. Acta, Bioenerg. 2008, 1777, 1028–1031. 10.1016/j.bbabio.2008.03.029. [DOI] [PubMed] [Google Scholar]
- Cossarizza A.; Contri M. B.; Kalashnikov G.; Franceschi C. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6’-tetrachloro-1,1’,3,3′-tetraethylbenzimidazol carbocyanine iodide (JC-1). Biochem. Biophys. Res. Commun. 1993, 1, 40–45. 10.1006/bbrc.1993.2438. [DOI] [PubMed] [Google Scholar]
- Floryk D.; Houstek J. Tetramethyl rhodamine methyl ester (TMRM) is suitable for cytofluorometric measurements of mitochondrial membrane potential in cells treated with digitonin. Biosci. Rep. 1999, 19, 27–34. 10.1023/A:1020193906974. [DOI] [PubMed] [Google Scholar]
- Norman J. P.; Perry S. W.; Barbieri J.; Brown E. B.; Gelbard H. A. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. BioTechniques 2011, 50, 98–115. 10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poot M.; Mao F.. Cyanine dyes that stain cells and mitochondria. US 6291203 B1.
- Nicholls D. G.; Ward M. W. Mitochondrial membrane potential and neuronal glutamate excitotoxicity: mortality and millivolts. Trends Neurosci. 2000, 23, 166–174. 10.1016/S0166-2236(99)01534-9. [DOI] [PubMed] [Google Scholar]
- Rimpelova S.; Briza T.; Kralova J.; Zaruba K.; Kejik Z.; Cisarova I.; Martasek P.; Ruml T.; Kral V. Rational design of chemical ligands for selective mitochondrial targeting. Bioconjugate Chem. 2013, 24, 1445–1454. 10.1021/bc400291f. [DOI] [PubMed] [Google Scholar]
- Lim S. H.; Thivierge C.; Sliwinska P. N.; Han J.; Bergh H. V.; Wagnieres G.; Burgess K.; Lee H. B. In Vitro and In vivo photocytotoxicity of boron dipyrromethene derivatives for photodynamic therapy. J. Med. Chem. 2010, 53, 2865–2874. 10.1021/jm901823u. [DOI] [PubMed] [Google Scholar]
- Jose J.; Loudet A.; Ueno Y.; Barhoumi R.; Burghardt R. C.; Burgess K. Intracellular imaging of organelles with new water-soluble benzophenoxazine dyes. Org. Biomol. Chem. 2010, 8, 2052–2059. 10.1039/b925845k. [DOI] [PubMed] [Google Scholar]
- Neto B. A. D.; Correa J. R.; Carvalho P. H. P. R.; Santos D. C. B. D.; Guido B. C.; Gatto C. C.; De Oliveira H. C. B.; Fasciotti M.; Eberlinb M. N.; da Silva E. N. Jr. Selective and efficient mitochondrial staining with designed 2,1,3-benzothiadiazole derivatives as live cell fluorescence imaging probes. J. Braz. Chem. Soc. 2012, 23, 770–781. 10.1590/S0103-50532012000400024. [DOI] [Google Scholar]
- Denora N.; Laquintana V.; Trapani A.; Suzuki H.; Sawada M.; Trapani G. New fluorescent probes targeting the mitochondriallocated translocator protein 18 kda (TSPO) as activated microglia imaging agents. Pharm. Res. 2011, 28, 2820–2832. 10.1007/s11095-011-0552-0. [DOI] [PubMed] [Google Scholar]
- Neto B. A. D.; Correa J. R.; Silva R. G. Selective mitochondrial staining with small fluorescent probes: importance, design, synthesis, challenges and trends for new markers. RSC Adv. 2013, 3, 5291–5301. 10.1039/c2ra21995f. [DOI] [Google Scholar]
- Kowada T.; Maeda H.; Kikuchi K. Bodipy-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. 10.1039/C5CS00030K. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Xiao Y.; Qi J.; Qu J.; Kim B.; Yue X.; Belfield K. D. Long-wavelength, photostable, two-photon excitable BODIPY fluorophores readily modifiable for molecular probes. J. Org. Chem. 2013, 78, 9153–9160. 10.1021/jo401379g. [DOI] [PubMed] [Google Scholar]
- Cheng G.; Fan J.; Sun W.; Sui K.; Jin X.; Wang J.; Peng X. A highly specific Bodipy-based probe localized in mitochondria for HClO imaging. Analyst 2013, 138, 6091–6096. 10.1039/c3an01152f. [DOI] [PubMed] [Google Scholar]
- Sui B.; Tang S.; Woodward A. W.; Kim B.; Belfield K. D. A Bodipy-based water-soluble fluorescent probe for mitochondria targeting. Eur. J. Org. Chem. 2016, 2016, 2851–2857. 10.1002/ejoc.201600238. [DOI] [Google Scholar]
- Dodani S. C.; Leary S. C.; Cobine P. A.; Winge D. R.; Chang C. J. A targetable fluorescent sensor reveals that copper-deficient SCO1 and SCO2 patient cells prioritize mitochondrial copper homeostasis. J. Am. Chem. Soc. 2011, 133, 8606–8616. 10.1021/ja2004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T.; Guan X.; Zheng M.; Jing X.; Xie Z. Mitochondria-localized fluorescent Bodipy-platinum conjugate. ACS Med. Chem. Lett. 2015, 6, 430–433. 10.1021/acsmedchemlett.5b00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam S.; Burke B. P.; Davies L. H.; Domarkas J.; Wallis J. F.; Waddell P. G.; Waby J. S.; Benoit D. M.; Seymour A. M.; Cawthorne C.; Higham J. L.; Archibald S. J. Structurally optimized Bodipy derivatives for imaging of mitochondrial dysfunction in cancer and heart cells. Chem. Commun. 2016, 52, 7114–7117. 10.1039/C5CC08325G. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Wu T.; Fan J.; Li Z.; Jiang N. J.; Wang B.; Dou S.; Song F.; Peng X. A Bodipy-based fluorescent dye for mitochondria in living cells, with low cytotoxicity and high photostability. Org. Biomol. Chem. 2013, 11, 555–558. 10.1039/C2OB26911B. [DOI] [PubMed] [Google Scholar]
- Jiang N.; Fan J.; Liu T.; Cao J.; Qiao B.; Wang J.; Gao P.; Peng X. A near-infrared dye based on BODIPY for tracking morphology changes in mitochondria. Chem. Commun. 2013, 49, 10620–10622. 10.1039/c3cc46143b. [DOI] [PubMed] [Google Scholar]
- Wang M.; Zhang Y.; Wang T.; Wang C.; Xue D.; Xiao J. Story of an age-old reagent: an electrophilic chlorination of arenes and heterocycles by 1-chloro-1,2-benziodoxol-3-one. Org. Lett. 2016, 18, 1976–1979. 10.1021/acs.orglett.6b00547. [DOI] [PubMed] [Google Scholar]
- Brouwer A. M. Standards for photoluminescence quantum yield measurements in solution. Pure Appl. Chem. 2011, 83, 2213–2228. 10.1351/PAC-REP-10-09-31. [DOI] [Google Scholar]
- Hart A. S.; Bikram K. C.; Subbaiyan N. K.; Karr P. A.; D’Souza F.; Hart A. S.; Bikram K. C.; Subbaiyan N. K.; Karr P. A.; D’Souza F. Phenothiazine-sensitized organic solar cells: effect of dye anchor group positioning on the cell performance. ACS Appl. Mater. Interfaces 2012, 4, 5813–5820. 10.1021/am3014407. [DOI] [PubMed] [Google Scholar]
- Lawrence D. S.; Whitten D. G. Photochemistry and photophysical properties of novel, unsymmetrically substituted metallophthalocyanines. Photochem. Photobiol. 1996, 64, 923–935. 10.1111/j.1751-1097.1996.tb01857.x. [DOI] [PubMed] [Google Scholar]
- Yuan H.; Cho H.; Chen H. H.; Panagia M.; Sosnovik D. E.; Josephson L. Fluorescent and radiolabeled triphenylphosphonium probes for imaging mitochondria. Chem. Commun. 2013, 49, 10361–10363. 10.1039/c3cc45802d. [DOI] [PubMed] [Google Scholar]
- To M. S.; Aromataris E. C.; Castro J.; Roberts M. L.; Barritt G. J.; Rychkov G. Y. Mitochondrial uncoupler FCCP activates proton conductance but does not block store-operated Ca(2+) current in liver cells. Arch. Biochem. Biophys. 2010, 495, 152–158. 10.1016/j.abb.2010.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


