Abstract
Carbon monoxide (CO) poisoning causes between 5,000−6,000 deaths per year in the US alone. The development of small molecule allosteric effectors of CO binding to hemoglobin (Hb) represents an important step toward making effective therapies for CO poisoning. To that end, we have found that the synthetic peptide IRL 2500 enhances CO release from COHb in air, but with concomitant hemolytic activity. We describe herein the design, synthesis, and biological evaluation of analogs of IRL 2500 that enhance the release of CO from COHb without hemolysis. These novel structures show improved aqueous solubility and reduced hemolytic activity and could lead the way to the development of small molecule therapeutics for the treatment of CO poisoning.
Keywords: Hemoglobin, CO poisoning, allosteric effector of hemoglobin
Hemoglobin (Hb), an α2β2 tetrameric globular protein found in red blood cells (RBCs),1,2 functions primarily to transport oxygen (O2) from the lungs to the outlying muscles and tissue and then to carry carbon dioxide from these tissues to the lungs for export.3 Hb has two main allosteric states, the relaxed (R) state and tense (T) state, that are conformationally different and exist in equilibrium. The R state Hb has a high O2 affinity and occurs when Hb is ligated at the heme, and the T state Hb has a low O2 affinity and occurs when Hb is unligated at the heme.1
There are numerous small molecules that bind to Hb, influencing the stability of the different allosteric states of Hb and thus the binding of O2, which can be measured by the shift in the O2 dissociation curve (ODC).1,3,4 2,3-Diphosphoglycerate (DPG)1 is an endogenous allosteric effector of human Hb in RBCs that shifts the allosteric equilibrium to the T state and right shifts the ODC, decreasing the oxygen affinity of Hb.5,6 Other nonendogenous right shifters include inositol hexaphosphate (IHP) and efaproxiral, the latter an uncharged right-shifter that was clinically studied as a radiation-enhancing compound for the treatment of hypoxic tumors.3 Conversely, there are a number of organic left-shifters, including vanillin and 5-hydroxymethylfurfural (5-HMF), that lead to an increase in Hb affinity for oxygen.3
Another molecule known to cause a left shift as well as a depression in the ODC is carbon monoxide (CO), a gas that has an approximately 200-fold greater affinity for Hb than O2.7−10 When CO binds to Hb to form carboxyhemoglobin (COHb), the oxygen transporting ability of Hb is impaired. This impairment, i.e., CO poisoning, is responsible for 40,000 emergency room visits and 5000−6000 deaths per year in the United States. Recent studies suggest targeting extra-hemoglobin effects due to the action of CO on a variety of heme-containing proteins,11 which increases nitric oxide (NO),11,12 and reactive oxygen species (ROS),13 and affects the function of myocardial ion channels.11,12 These effects could lead to myocardial injury14 and delayed development of central nervous system (CNS) impairment.11 One treatment for CO poisoning is the use of pulmonary oxygen.7 Azarov and co-workers recently reported the discovery of an engineered human neuroglobin (Ngb), a six-coordinate hemoprotein, that is able to scavenge CO from Hb. This Ngb has a higher affinity for CO than Hb, allowing it to out-compete COHb for CO and has been shown to rescue CO-poisoned mice.15
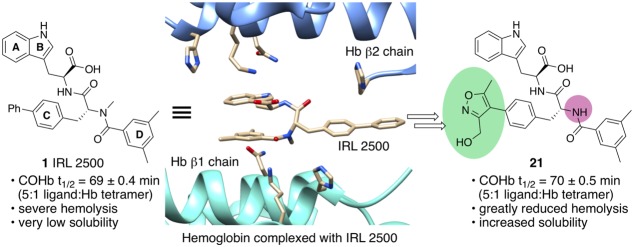
While the work of Azurov and co-workers represents a major accomplishment, we have taken a different approach to the development of a treatment for CO poisoning. We hypothesized that a small molecule able to bind to Hb and inhibit the binding of CO or enhance the release of CO from COHb could be used for the treatment of CO poisoning. Drug-like small molecule therapeutics could avoid the liabilities and delivery issues related to the use of protein therapeutics.16 We identified 427 compounds that bind to Hb from a screen of 38,700 small molecules.17 Only one of the 427 compounds, IRL 2500 1 (Figure 1a), decreased the affinity of Hb for O2. This decrease in affinity was accompanied by a slightly increased P50 (the partial oxygen pressure at which 50% of Hb is oxygenated) from 17 ± 0.3 mmHg (mean ± standard deviation, without IRL 2500) to 19 ± 0.1 mmHg at a 5-fold molar excess of the compound to Hb tetramer (5×). We hypothesized that binding of IRL 2500 to Hb could also affect the binding of CO or enhance the release of CO from COHb. Herein, we report the effect of IRL 2500 on the stability of COHb in air and on the design and synthesis of more drug-like derivatives of 1 that enhance the release of CO from COHb.
Figure 1.
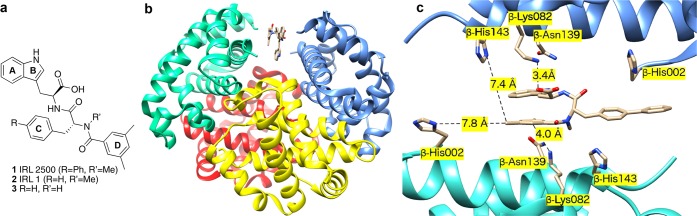
(a) Structures of 1 (IRL 2500), 2 (IRL 1), and secondary amide 3; (b) IRL 2500 1 (tan) bound in the β cleft of Hb (PDB ID 4L7Y). β chains teal and blue, α chains are shown as red and yellow. (c) Interactions between IRL 2500 1 and Hb at the β cleft with distances to important protein residues highlighted (see Supporting Information and Figure S1 for detailed description of the cobinding of IRL 2500 and DPG).
We have determined the crystal structure of deoxyHb complexed to IRL 2500 1. Like 2,3-DPG,3 IRL 2500 binds noncovalently to the β cleft of Hb in a 1:1 stoichiometry (Figure 1b,c and PDB ID 4L7Y). The structure was solved by molecular replacement using the native deoxyHb (PDB ID: 2DN2). Interestingly, we also observed DPG bound at the same place as IRL 2500 (see Supporting Information) in a 67% to 33% occupancy ratio. When compared to the crystal structure of 2DN2, the overall protein conformation, as well as the geometry of the β-cleft, is not changed by binding of IRL 2500 and DPG, except for slight movement of the side-chains of β-His143, β-Asn139, and β-Lys82 (Figure 1c). Cobinding of the two effectors is not required to reduce in vitro Hb affinity for oxygen. When IRL 2500 alone was added to purified Hb without DPG (IRL 2500/Hb tetramer = 5:1 mol/mol), the P50 was increased from 17 to 19 mmHg. However, under physiological conditions, reduction of Hb affinity for O2 in the presence of IRL 2500 may be due to cobinding of IRL 2500 and DPG at the β cleft.
The indole carboxyl group of IRL 2500 1 makes both water-mediated and direct hydrogen-bond interactions with the amine of β-Lys82, while the indole nitrogen and tryptophan amide nitrogen make direct and water-mediated interactions with the side-chain of β-Asn139, respectively (Figure 1c). There are hydrophobic interactions between IRL 2500 and the protein residues β-His2, β-Asn139, and β-His143. These provide additional interactions across the two β-subunit interfaces of deoxyHb that lead to further stabilization of the T state and decrease Hb affinity for O2 similar to IHP and efaproxiral.3,18
We found that IRL 2500 lowers the half-life of COHb in air. This half-life was determined by measuring the conversion of COHb to oxygenated Hb (oxyHb) in air. The half-life of COHb without IRL 2500 was 80 ± 0.7 min (mean ± SEM). In the presence of IRL 2500, the half-life of COHb was reduced to 69 ± 0.4 min (at 5× the concentration of IRL 2500 to Hb tetramer). We therefore propose that IRL 2500 shifts the allosteric equilibrium to the low O2 affinity T state Hb and enhances the release of CO from Hb. However, we also found that IRL 2500 has relatively low aqueous solubility and induces hemolysis on mixing with human red blood cells. These properties preclude the use of IRL 2500 to treat CO poisoning.
To increase aqueous solubility and reduce the hemolytic activity of IRL 2500 1, we prepared the less hydrophobic compound IRL 1 2 (Figure 1a), in which the biphenyl moiety is replaced with a single phenyl ring, and found that 2 has a modest CO half-life lowering activity (COHb half-life: 74 ± 0.5 min at 5× the concentration of 2 to Hb tetramer) relative to IRL 2500 but does not induce hemolysis.
We therefore initiated a structure–activity relationship (SAR) study around 2 to design derivatives with (1) increased reduction of CO half-life than 2; (2) greater aqueous solubility than 1; and (3) less hemolytic activity than 1. In this Letter, we describe the dramatic effect of changing the para substitution of the C-ring of 2, i.e., R (Figure 1a), on potency, aqueous solubility, and hemolysis.
In an attempt to both further reduce the hydrophobicity as well as to streamline the synthesis of the IRL analogs, we prepared 3 (Figure 1), the secondary amide (R′ = H) corresponding to tertiary amide 2 (R′ = Me). Compound 3 was synthesized in quantitative yield from the component amino acids in three chemical steps (see Supporting Information for details). As expected, the calculated cLogP of 3 using BioByte in Chemdraw was slightly reduced relative to that of 2 (4.6 for 3 vs 4.7 for 2). Most importantly, we observed that the COHb half-life for 3 was the same as that observed for 2 (Table 1), allowing us to prepare the IRL analogs as secondary amides.
Table 1. COHb Half-life, Hemolysis, cLogP, and Aqueous Solubility for IRL Analogs 1, 2, 3, and 15–22.
| COHb t1/2 (min)a |
|||||
|---|---|---|---|---|---|
| compound | compound/Hb 20:1 | compound/Hb 5:1 | hemolysisb | cLogPc | aqueous solubilityd |
| 1 (IRL 2500) | ND | 69 ± 0.4 | + | 6.6 | – |
| 2 (IRL 1) | 65 ± 0.5 | 74 ± 0.5 | – | 4.7 | – |
| 3 | 63 ± 0.5 | ND | – | 4.6 | – |
| 15 | 64 ± 0.4 | 69 ± 0.4*** | – | 6.5 | – |
| 16 | 60 ± 0.4*** | 71 ± 0.4*** | + | 5.9 | – |
| 17 | 77 ± 0.7 | 80 ± 0.7 | – | 4.1 | + |
| 18 | 57 ± 0.4*** | 72 ± 0.5** | – | 4.4 | – |
| 19 | 69 ± 0.9 | 74 ± 0.5 | – | 4.6 | + |
| 20 | 71 ± 0.4 | 78 ± 0.7 | – | 3.9 | + |
| 21 | 65 ± 0.4 | 70 ± 0.5*** | – | 3.1 | + |
| 22 | 67 ± 0.4 | 72 ± 0.5*** | – | 3.5 | – |
COHb half-life (t1/2, shown as mean ± standard error) was measured at 20:1 compound/Hb (compound 200 μM and Hb tetramer 10 μM) or 5:1 compound/Hb (compound 50 μM and Hb tetramer 10 μM) in DPBS with 5 vol % DMSO. COHb t1/2 with DMSO (as a control) was 80 ± 0.3 min. Significance relative to IRL 1 2: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. “ND” indicates half-life not determined.
Hemolysis measured using RBCs (50 μM Hb) and compound (1 mM) in 0.1 vol % DMSO. “–” indicates that the fraction of extracellular Hb was less than 1% of total Hb after incubating blood with the compound.
cLogP calculated with ChemDraw BioByte.
Solubility was evaluated by preparing 2.5 mM of the compound in DPBS with 1 vol % DMSO. “+” indicates that aggregates or crystals were not observed in the prepared solution. “–” indicates that aggregates or crystals were observed.
We next examined modification of the C-ring of 3 (Figure 1a). Specifically, we introduced different substituted arenes and heterocycles at the 4-position of the C-ring of 3 in an effort to improve aqueous solubility and reduce hydrophobicity, while maintaining the large hydrophobic surface area that is present in substituted biphenyl 1 (Figure 1). We used calculated cLogP as a predictive tool of aqueous solubility, which was then verified experimentally.
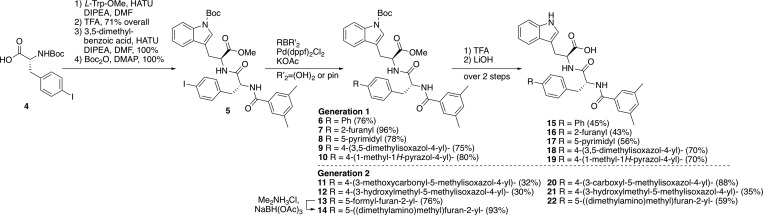
The desired compounds were accessed by Suzuki cross-coupling of the p-iodo IRL analog 5, the preparation of which is outlined in Scheme 1. Coupling of carbamate 4(19) with l-Trp amino ester, followed by Boc deprotection afforded an intermediate dipeptide (not shown), which was then coupled with 3,5-dimethylbenzoic acid, followed by Boc-indole formation, giving key intermediate 5 (61% overall yield in 6 steps from d-phenylalanine). After extensive screening of cross-coupling conditions, analogs 15–19 were prepared by Suzuki coupling of 5 with the requisite boronic acids to give 6–8 or the corresponding pinacolates to generate 9–10, followed by Boc deprotection (TFA) and ester hydrolysis (LiOH) (Scheme 1).
Scheme 1. Synthesis of C-ring IRL Analogs.
Compounds 15–19 reduced COHb half-life more than DMSO alone (Table 1). Additionally, furan 16 and the isoxazole derivative 18 reduced the COHb half-life more than 2 at 20:1 molar ratio of compound to Hb tetramer. Due to the increased activity of the compounds in this series, we also measured the COHb half-life at reduced concentration of compound (5:1), as indicated in Table 1, which is the highest concentration at which the highly hemolytic 1 can been examined. At this 5:1 ratio of compound/Hb, we found that 15 (biphenyl), 16 (furyl), and 18 (isoxazole) were all significantly more active than IRL 1 2. While biphenyl 15 did not cause hemolysis, it was tested for hemolysis at a much lower concentration (2:1 15/Hb) because of its poor aqueous solubility in the assay. Furan analog 16 caused slight hemolytic activity (1–2% of total Hb at 5:1 molar ratio of 16 to Hb tetramer), which tracks with the relatively high calculated cLogP value of 5.9 for 16. Isoxazole derivative 18 did not cause hemolysis. Testing the aqueous (1% DMSO/H2O) solubility of 15–19 revealed that furan 16, pyrimidine 17, and pyrazole 19 were each soluble at 2.5 mM. Based on these data, the two most promising analogs were furan 16 and isoxazole 18. However, there were drawbacks with each of these lead compounds; hemolytic activity with 16 and modest aqueous solubility with 18.
These results led us to design a second-generation of analogs with additional solubilizing functionalities attached to either the isoxazole or furan moieties (isoxazole acid 20, isoxazole alcohol 21, and dimethylaminomethylfuran 22, Scheme 1). Toward that end, we prepared 20–22 from the corresponding boronates and boronic acid for 20 and 21 and for 22, respectively (Scheme 1). Dimethylaminomethyl analog 22 was obtained by reductive amination of aldehyde 13, which was obtained using 5-formylfuran-2-boronic acid as a coupling partner.
Both hydroxymethyl isoxazole 21 and dimethylaminomethylfuran 22 were significantly more active at reducing COHb half-life than 2 at a 5:1 molar ratio of the compound to Hb tetramer. COHb half-life lowering activity with 21 was comparable to that of 1, but without hemolytic activity at up to five times the concentration of the compound to Hb tetramer. Furthermore, 21 exhibited superior aqueous solubility, making it an excellent candidate for further study.
Our results establish a delicate balance between hydrophilicity that is adequate to improve aqueous solubility and preclude hemolysis, and sufficient hydrophobicity for biological activity. We pursued compounds modified at the 4-position of the C ring, adding heteroaromatic rings that aid in solubility while retaining the hydrophobic surface area that appears necessary to reduce the half-life of COHb. Five of the biaryl analogs, biphenyl 15, furan 16, isoxazole 18, alcohol 21, and dimethyaminomethylfuran 22, led to reduced COHb half-life relative to that observed with 1. We were particularly gratified to find that COHb half-life was significantly reduced with 21 relative to 2, without hemolysis. These results demonstrate that it is possible to reduce the half-life of COHb with small molecules, a first step toward the use of small molecules for the treatment of CO poisoning. The combination of these IRL compounds and compounds targeting the extra-hemoglobin effects could have synergetic effects in treating myocardial injury and the delay of CNS impairment following CO poisoning. Further studies directed toward the expansion of the SAR of the IRL compounds and the effect of cLogP on hemolysis is underway in our laboratory, and our results will be reported in due course.
Acknowledgments
We would also like to acknowledge Dr. George Furst and Dr. Jun Gu for NMR facility support and Dr. Rakesh Kohli and Dr. Charles Ross for mass spectrometry services at the University of Pennsylvania.
Glossary
ABBREVIATIONS
- CO
carbon monoxide
- COHb
carboxyhemoglobin
- DPG
2,3-diphosphoglycerate
- Hb
hemoglobin
- O2
oxygen
- ODC
oxygen dissociation curve
- RBC
red blood cells
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00166.
Additional tables containing crystallography data, experimental details, and NMR spectra (PDF)
Accession Codes
PDB code for deoxyHb with bound 1 (IRL 2500) is 4L7Y.
Author Contributions
S.R.G., C.L., A.N., W.M.Z., and J.D.W. conceived the study and planned the experiments. S.R.G., C.L., and M.K.S. carried out the experiments and analyzed the data. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This work was supported by the fund from the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital (to W.M.Z.) and NIH/NIMHD grant MD009124 (to M.K.S). S.R.G. was supported by NIH grant T32 GM071339. Structural biology resources were provided in part by NIH grant CA16059 to the VCU Massey Cancer Center.
The authors declare no competing financial interest.
Notes
Previously published academic dissertation available online: Goldstein, Sara R, “The Design and Synthesis of Allosteric Effectors of Carbon Monoxide Binding to Hemoglobin” (2017). Dissertations available from ProQuest. AAI10255227. https://repository.upenn.edu/dissertations/AAI10255227
Supplementary Material
References
- Voet D.; Voet J. G.. Biochemistry; John Wiley & Sons: New York, 1995. [DOI] [PubMed] [Google Scholar]
- Textbook of Biochemistry with Clinical Correlations; Devlin T. M., Ed.; John Wiley & Sons, 2002. [Google Scholar]
- Safo M. K.; Bruno S. Allosteric Effectors of Hemoglobin: Past, Present and Future. Chemistry and Biochemistry of Oxygen Therapeutics: From Transfusion to Artificial Blood 2011, 285–300. 10.1002/9781119975427.ch21. [DOI] [Google Scholar]
- Bishop C.; Surgenor D. M.. The Red Blood Cell; Academic Press, 1964. [Google Scholar]
- Benesch R.; Benesch R. E. The Effect of Organic Phosphates from the Human Erythrocyte on the Allosteric Properties of Hemoglobin. Biochem. Biophys. Res. Commun. 1967, 26 (2), 162–167. 10.1016/0006-291X(67)90228-8. [DOI] [PubMed] [Google Scholar]
- Arnone A. X-Ray Diffraction Study of Binding of 2,3-Diphosphoglycerate to Human Deoxyhaemoglobin. Nature 1972, 237 (5351), 146–149. 10.1038/237146a0. [DOI] [PubMed] [Google Scholar]
- Clardy P. F.; Manaker S.; Perry H. Carbon monoxide poisoning https://www.uptodate.com/contents/carbon-monoxide-poisoning?search=Carbon%20monoxide%20poisoning&source=search_result&selectedTitle=1~71&usage_type=default@display_rank=1 (accessed April 2, 2015).
- Weaver L. K. Carbon Monoxide Poisoning. N. Engl. J. Med. 2009, 360, 1217–1225. 10.1056/NEJMcp0808891. [DOI] [PubMed] [Google Scholar]
- Weaver L. K. Carbon Monoxide Poisoning. Crit. Care Clin. 1999, 15 (2), 297–317. 10.1016/S0749-0704(05)70056-7. [DOI] [PubMed] [Google Scholar]
- Rose J. J.; Xu Q.; Wang L.; Gladwin M. T. Shining a Light on Carbon Monoxide Poisoning. Am. J. Respir. Crit. Care Med. 2015, 192 (10), 1145–1147. 10.1164/rccm.201508-1579ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raub J. A.; Benignus V. A. Carbon monoxide and the nervous system. Neurosci. Biobehav. Rev. 2002, 26 (8), 925–40. 10.1016/S0149-7634(03)00002-2. [DOI] [PubMed] [Google Scholar]
- Roderique J. D.; Josef C. S.; Feldman M. J.; Spiess B. D. A modern literature review of carbon monoxide poisoning theories, therapies, and potential targets for therapy advancement. Toxicology 2015, 334, 45–58. 10.1016/j.tox.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Akyol S.; Erdogan S.; Idiz N.; Celik S.; Kaya M.; Ucar F.; Dane S.; Akyol O. The role of reactive oxygen species and oxidative stress in carbon monoxide toxicity: An in-depth analysis. Redox Rep. 2014, 19 (5), 180–189. 10.1179/1351000214Y.0000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satran D.; Henry C. R.; Adkinson C.; Nicholson C. I.; Bracha Y.; Henry T. D. Cardiovascular manifestations of moderate to severe carbon monoxide poisoning. J. Am. Coll. Cardiol. 2005, 45 (9), 1513–1516. 10.1016/j.jacc.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Azarov I.; Wang L.; Rose J. J.; Xu Q.; Huang X. N.; Belanger A.; Wang Y.; Guo L.; Liu C.; Ucer K. B.; McTiernan C. F.; O’Donnell C. P.; Shiva S.; Tejero J.; Kim-Shapiro D. B.; Gladwin M. T. Five-Coordinate H64Q Neuroglobin as a Ligand-Trap Antidote for Carbon Monoxide Poisoning. Sci. Transl. Med. 2016, 8 (368), 368ra173. 10.1126/scitranslmed.aah6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno B. J.; Miller G. D.; Lim C. S. Basics and Recent Advances in Peptide and Protein Drug Delivery. Ther. Delivery 2013, 4 (11), 1443–1467. 10.4155/tde.13.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa A.; Lui F. E.; Wassaf D.; Yefidoff-Freedman R.; Casalena D.; Palmer M. A.; Meadows J.; Mozzarelli A.; Ronda L.; Abdulmalik O.; Bloch K. D.; Safo M. K.; Zapol W. M. Identification of a Small Molecule That Increases Hemoglobin Oxygen Affinity and Reduces SS Erythrocyte Sickling. ACS Chem. Biol. 2014, 9, 2318–2325. 10.1021/cb500230b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonetani T.; Park S. I.; Tsuneshige A.; Imai K.; Kanaori K. Global Allostery Model of Hemoglobin. Modulation of O2 Affinity, Cooperativity, and Bohr Effect by Heterotropic Allosteric Effectors. J. Biol. Chem. 2002, 277, 34508–34520. 10.1074/jbc.M203135200. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S.; Radke G.; Evans M.; Tomich J. Synth. Commun. 1996, 26, 1431–1440. 10.1080/00397919608003505. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.