Abstract
In order to assess the potential of sPLA2-X as a therapeutic target for atherosclerosis, novel sPLA2 inhibitors with improved type X selectivity are required. To achieve the objective of identifying such compounds, we embarked on a lead generation effort that resulted in the identification of a novel series of indole-2-carboxamides as selective sPLA2-X inhibitors with excellent potential for further optimization.
Keywords: Secreted phospholipase A2 type X, inhibitor, X-ray crystallography, atherosclerosis, coronary artery disease
Secreted phospholipases A2 (sPLA2s) are structurally conserved enzymes catalyzing the hydrolysis of glycerophospholipids at the sn-2 position, leading to the production of free fatty acids and lysophospholipids.1 Accumulating evidence indicates that several sPLA2 isoforms, namely, IIA (sPLA2-IIA), sPLA2-III, sPLA2-V, and sPLA2-X play significant, distinct, or overlapping roles in one or several steps of atherogenesis.2 This led to the clinical testing of A-002 (varespladib methyl) (Figure 1), a nonselective inhibitor of sPLA2-IIA, sPLA2-V, and X isoforms, for the treatment of coronary artery disease.3 Group X sPLA2 (sPLA2-X) is structurally related to other sPLA2 isoforms, and among these, it is the most potent at hydrolyzing phosphatidylcholine, a highly abundant phospholipid at the cell surface and on lipoproteins.4,5 This enzyme is expressed in atherosclerotic lesions and peripheral blood cells.6In vitro and in vivo studies have reported pro-atherogenic properties of sPLA2-X comprising lipoprotein modifications leading to foam cell formation, reduced cell cholesterol efflux, altered immune cells maturation, and vascular cells proliferation.7−10 These effects indicate that sPLA2-X is a potential novel therapeutic target for the treatment of cardiovascular diseases.2 However, an in vivo study in a mouse model of atherosclerosis has shown that expression of sPLA2-X in bone marrow cells limits the development of atherosclerosis.11 Additionally, the VISTA-16 (Vascular Inflammation Suppression to Treat Acute Coronary Syndrome) phase III trial showed that administration of varespladib methyl did not reduce the occurrence of acute coronary syndrome (ACS) events but significantly increased the risk of nonfatal myocardial infarction.12 These results could indicate that a broad spectrum sPLA2 inhibitor may be detrimental in atherosclerotic cardiovascular disease. Alternatively, novel sPLA2 inhibitors with improved potency, safety, and selectivity would be required to demonstrate a therapeutic benefit in atherosclerotic conditions. The availability of isoform-selective sPLA2 inhibitors could help dissecting the individual contribution of different sPLA2 enzymes in pro- and antiatherogenic mechanisms. We therefore embarked on a discovery effort aiming at generating sPLA2-X inhibitors13 possessing 30-fold selectivity over other sPLA2 subtypes and suitable for oral administration. The lead generation part of this project will be described here.
Figure 1.
sPLA2 inhibitor varespladib methyl, its active metabolite varespladib, and initial hit 1.
A combination of NMR screening and high throughput screening (HTS) of the AstraZeneca compound collection was devised to identify chemical starting points with the potential to deliver selective inhibitors of sPLA2-X (see Supporting Information for a more detailed description). This resulted in the identification of indole carboxamide 1 as a low micromolar inhibitor of sPLA2-X (Figure 1).
A crystal structure of 1 bound to sPLA2-X was solved and showed that the carboxamide group of 1 established three hydrogen bonds and one coordination bond with the protein and the catalytic calcium ion, respectively (Figure 2), similar to analogous interactions reported in the literature,14 highlighting the importance of primary amides as warheads for sPLA2 inhibition. The indole core of 1 occupies a hydrophobic pocket lined by residues Leu5, Val9, Pro17, Tyr20, Cys43, Ile94, and Leu98. Interestingly, this lipophilic pocket differs across sPLA2 isoforms. In sPLA2-IIa, the most closely related isoform to sPLA2-X, Leu5, Val9, and Leu98 are replaced by Phe, Ile, and Phe, respectively (Figure 2). We hypothesized that these differences in side chain sizes could effectively result in a difference in pocket sizes, thus potentially offering a design manifold to improve sPLA2 isoform selectivity by modifying/substituting the phenyl part of the indole core. The meta-trifluoromethyl phenyl group of 1 is involved in hydrophobic interactions with Ile2, Leu5, Gly28, Leu29, Tyr50, and Lys61.
Figure 2.

Crystal structure of sPLA2-X in complex with compound 1 (orange). The amide of 1 forms three hydrogen bonds to the protein (2.7–3.1 Å) and one coordination bond to the calcium ion (2.5 Å), all marked by dashed lines.
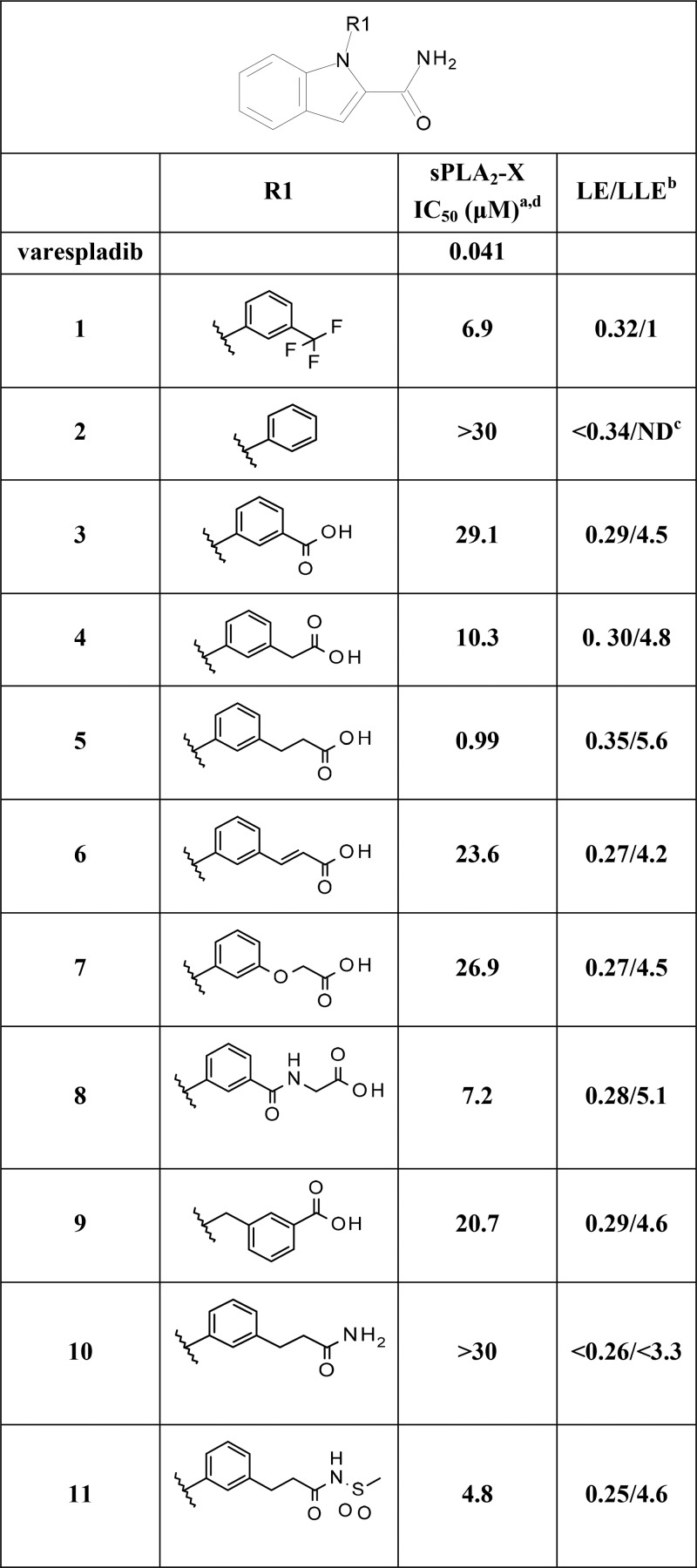
Based on this structural framework, we set out to improve both the inhibitory potency and the selectivity versus the most widely abundant sPLA2 isoforms IIa and V. Ligand efficiency (LE) and ligand lipophilicity efficiency (LLE) were carefully monitored during this process to ensure that, despite the lipophilic nature of the binding pocket, lipophilicity and molecular properties would be kept in line with oral bioavailability potential. As a first step, we embarked on derivatizing the terminal N-aryl ring substituting the indole nitrogen atom of 1 with a view to establishing a second ligand interaction with the calcium ion. We reasoned this design hypothesis could have resulted in improved sPLA2 inhibition while affording a more balanced physical chemical profile compatible with adequate solubility and metabolic stability by virtue of the envisioned polar chelating groups. Here, based on available data,14−18 special emphasis was placed on carboxylic acids/bioisosteres, as summarized in Table 1. The unsubstituted N-phenyl-containing derivative (2) was essentially inactive. We noticed that, in a number of sPLA2-X crystal structures available to our team, a water molecule or component of the crystallization buffer was found to coordinate the calcium ion in close proximity to the area occupied by the trifluoromethyl group of 1. We therefore decided to focus on this position for introducing structural elements compatible with a second calcium interaction. Introduction of a carboxylic acid group in the meta position resulted in measurable inhibition of sPLA2-X (3, Table 1). Atomic spacers between the aryl and carboxylic acid group improved the activity 3-fold for methylene (4) and 30-fold for the ethylene resulting in the first submicromolar sPLA2-X inhibitor, endowed with high ligand and ligand lipophilicity efficiencies (5, Table 1). Based on this result and to further validate our modeling results, modification of the ethyl spacer by rigidification through unsaturation (6) or variation of the phenyl anchoring moiety using ether (7) or amide (8) groups was attempted but turned out to be poorly tolerated with seven to 27-fold loss of sPLA2-X inhibitory activity. An initial attempt of functionalizing the indole core by N-benzylation instead of N-arylation proved counterproductive as well (9, Table 1). Replacement of the carboxylic acid group by a primary amide (10) yielded a complete loss of inhibition, while an acylsulfonamide bioisosteric element (11) was tolerated albeit with a 5-fold reduction in potency, indicating specific spatial and electronic restraints for the coordination of the calcium ion. Having identified 5 as a promising sPLA2-X inhibitor, we assessed the importance of the indole system (see Table S1 in the Supporting Information). Truncation of the indole to a pyrrole ring (12) highlighted the requirement of a bicyclic system for sPLA2-X inhibition. Introduction of polarity using 7-azaindole (13) or imidazo[1,2-a] pyridine (14) scaffolds was not tolerated. Conservative changes using thienopyrrole cores (15, 16) also resulted in total loss of inhibitory activity. Partial reduction of the indole core by saturation of the phenyl ring (17) was tolerated, although this reduced sPLA2-X inhibition 6-fold. Based on these results, we resorted to using the indole scaffold of 5 as the basis for further molecular designs to explore the impact of indole substituents on both potency and selectivity. Systematic introduction of methyl groups (18–21, Table 2) indicated position 6 on the indole core as the most favorable for further optimization, due to the 4-fold sPLA2-X inhibition improvement over 5 and the structural data available. Here, improvement of potency via introduction of electron withdrawing and lipophilic substituents such as chloride (23) and trifluoromethyl (24) yielded encouraging sPLA2-X inhibitory activity when using high density lipoprotein (HDL) as a more disease-relevant sPLA2-X substrate (Table 2). Further biological screening indicated that more voluminous substituents of electron donating nature (i.e., ethyl (25), cyclopropyl (26), and methoxy (27)) did not offer any advantages over methyl. Introduction of polarity via hydroxymethyl (28) and cyano (29) substituents did not result in any further potency gains. Based on these emerging structure–activity relationships, we targeted bulkier substituents with electron withdrawing character. Specifically, trifluoromethoxy (31) proved superior to difluoromethoxy (30) and 2,2,2-trifluoroethoxy (32) alternatives (Table 3).
Table 1. sPLA2-X Potency and Ligand Efficiencies for Compounds 1–11.
Results are mean of at least two experiments. Experimental errors within 20% of value [enzyme]. Substrate composition: 50 mg of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (Avanti Polar Lipids 850457P) in 1 mL of 4% NonidetP40 (USB) and 2% deoxycholic acid (Sigma D6750).
Ligand efficiency (LE) is expressed in units of kcal·mol–1 per non-hydrogen atom and calculated as −RT pIC50(sPLA2-X)/2.303 × heavy atom count using R = 0.001987 kcal·mol–1·K–1 and T = 298 K. Ligand lipophilicity efficiency (LLE) calculated as −pIC50(sPLA2-X) – measured logD7.4.
Not determined.
Top concentration tested: 30 μM.
Table 2. sPLA2-X Potency and Ligand Efficiencies for Compounds 18–32.

| R | sPLA2-X (HDL) IC50 (μM)a | sPLA2-IIa IC50 (μM)a | sPLA2-V IC50 (μM)a | LE/LLEb | |
|---|---|---|---|---|---|
| varespladib | 0.041 (0.15) | 0.012 | 0.124 | 0.36/6.6 | |
| 5 | H | 0.99 | NAc | NAc | 0.37/5.9 |
| 18 | 4-Me | 0.83 | 0.35/5.3 | ||
| 19 | 5-Me | 2.7 | 0.32/4.8 | ||
| 20 | 6-Me | 0.23 | NAc | NAc | 0.38/5.8 |
| 21 | 7-Me | 1.9 | NAc | NAc | 0.33/5.2 |
| 22 | 6-F | 0.26 | NAc | NAc | 0.36/6.0 |
| 23 | 6-Cl | 0.09 (1.1) | 5.7 | NAc | 0.4/6.0 |
| 24 | 6-CF3 | 0.043 (0.48) | 0.89 | 3.7 | 0.37/5.9 |
| 25 | 6-Et | 0.17 | 4.4 | NAc | 0.37/5.5 |
| 26 | 6-cPr | 0.16 (1.9) | 2.3 | >10 | 0.36/5.5 |
| 27 | 6-OMe | 0.62 | NAc | NAc | 0.33/5.9 |
| 28 | 6-CH2OH | 2.8 | NAc | NAc | 0.3/5.6 |
| 29 | 6-CN | 0.11 (0.99) | 3.9 | NAc | 0.37/6.7 |
| 30 | 6-OCF2H | 0.068 (0.37) | 3.6 | NAc | 0.36/6.2 |
| 31 | 6-OCF3 | 0.026 (0.27) | 0.31 | 2.23 | 0.36/6.0 |
| 32 | 6-OCH2CF3 | 0.093 (0.74) | >10 | NAc | 0.33/5.6 |
Results are mean of at least two experiments. Experimental errors within 20% of value [enzyme]. Substrates compositions (i) PC: 50 mg of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (Avanti Polar Lipids 850457P) in 1 mL of 4% NonidetP40 (USB) and 2% deoxycholic acid (Sigma D6750). (ii) HDL: HDL isolated from human plasma by ultracentrifugation was used as substrate.
Ligand efficiency (LE) is expressed in units of kcal·mol–1 per non-hydrogen atom and calculated as −RT pIC50(sPLA2-X)/2.303 × heavy atom count using R = 0.001987 kcal·mol–1·K–1 and T = 298 K. Ligand lipophilicity efficiency (LLE) calculated as −pIC50(sPLA2-X) – measured logD7.4.
Not active at highest tested concentration (30 μM).
Table 3. Profiling of Compound 31a.
| solubility (pH = 7.4) (μM) | 98 | |
| Caco-2 Papp (10–6 cm/s) | 18.8 | |
| human hepatocytes Clint (μL/min/10–6 cells) | 1.7 | |
| sPLA2-X IC50 (μM) | 0.026 | |
| sPLA2-X mouse IC50 (μM) | 0.096 | |
| sPLA2-IIa IC50 (μM) | 0.31 | |
| sPLA2-V IC50 (μM) | 2.23 | |
| pharmacokinetics | rat | dog |
| dose i.v./p.o. (μmol/kg) | 1/4 | 1/– |
| CL (mL/min/kg) | 9.1 | 0.75 |
| AUC (μM·h) | 1.84/11 | 8.78/– |
| Vdss (L/kg) | 2.54 | 0.44 |
| F (%) | 100 | |
See Supporting Information for experimental details
The crystal structure of sPLA2-X and 31 confirmed the bidentate calcium interaction by the carboxamide and carboxylic groups (Figure 3). The carboxylic acid of 31 that also forms an additional hydrogen bond to the main chain nitrogen of Gly30. The ethylene linker utilizes a narrow space defined by the side chains of Asp47, Tyr50, and Lys61. One side of the phenyl substituent is stacking against the main chains of Gly28 and Leu29. The trifluoromethoxy substituent is accommodated in a hydrophobic pocket with extensive van der Waals contacts (distances 3.6–4.0 Å) to the side chains of Ile2, Ala6, Pro17, Ile18, and Met21. The binding mode of the indole core was unaltered compared to 1, and as hypothesized, sequence comparison between sPLA2-X, -IIa, and -V suggests that residues in the indole binding pocket are likely to be determinants for isoform selectivity (Figure 3A) by preventing the indole core from adopting the same binding mode, as reflected by the lower affinity of 31 for these isoforms (11- and 85-fold reduced potency to sPLA2-X, respectively, Table 3).
Figure 3.
(A) Sequence alignment of mature sPLA2-X (top), -IIa (middle), and -V (bottom). Black arrows mark residues that interact with the indole core. Blue arrows indicate residues make up the hydrophobic pocket that harbors the trifluoromethoxy moiety. Red arrows highlight residues predicted to be determinants of sPLA2 isoform selectivity. (B) Superposition of crystal structures of sPLA2-X in complex with 1 (orange) and 31 (green). The structure shows that the carboxyl group of 31 forms the second interaction to the calcium ion. Three residues are marked in red (Leu5, Val9, and Ile94), as they are predicted to be responsible for isoform selectivity. (C) Crystal structure of sPLA2-X in complex with 31. Residues lining the lipophilic pocket that harbors the trifluoromethoxy moiety are labeled.
While some close derivatives possessed better selectivity profiles (e.g., 30 and 32), 31 demonstrated improved lipolytic activity of sPLA2-X on HDL (IC50: 270 nM) and was therefore profiled further. Compound 31 did not display any significant binding to a large panel of biological targets and no significant inhibition of ion channels, enzymes, and transporters that might be related to toxicity (Table 3). While the aqueous solubility and passive permeability of 31 were adequate, its in vitro metabolic stability was suboptimal, and this readily translated in sufficient oral bioavailability but high unbound clearance. These properties made 31 inadequate as a tool compound for in vivo evaluation due to too high doses required to cover IC50 and IC90 but offered clear avenues for further optimization.
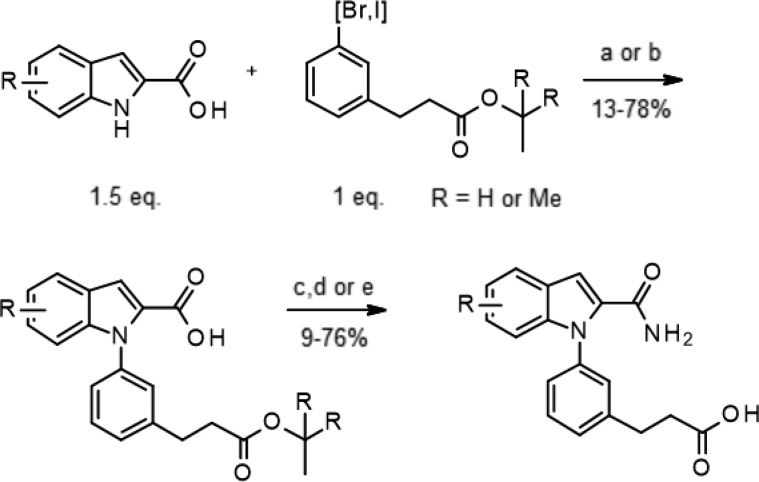
The indole 2-carboxamides derivatives were efficiently prepared by liquid phase parallel synthesis using the approach described in Scheme 1. Starting from commercially available or readily accessible19 indole-2-carboxylic acids, the N-arylation was performed using two different copper based methods.20,21 The intermediate acids were then converted to primary amides before final removal of the tert-butyl ester protecting group. This synthetic sequence produced only modest to good overall yields but proved robust enough for the rapid generation of arrays of diverse derivatives presented here.
Scheme 1. General Synthesis of Indole-2 Carboxamides Derivatives.
Reagents and conditions: (a) Cu(OAc)2 (1 equiv), DBU (3 equiv), DMSO, μw, 140–150 °C; (b) CuI (0.2 equiv), piperidine-2-carboxylic (0.4 equiv), K2CO3 (3 equiv), DMF, 110 °C; (c) NH4Cl (3 equiv), TBTU (1.5 equiv), DIEA (9 equiv), DMF, rt; (d) 4 M HCl in 1,4-dioxane, rt; (e) LiOH, THF, water, rt.
In summary, starting from the indole carboxamide hit 1, a series of structure-based, medicinal chemistry explorations resulted in the identification of derivative 31, a novel sPLA2-X inhibitor with favorable properties for further investigation. The optimization of its selectivity and in vivo pharmacokinetic profile toward sPLA2-X-centric, preclinical proof of concept studies in animals is detailed in the Companion paper.
Acknowledgments
The authors would like to acknowledge the support of the Structure Analysis and Separation Science group of AstraZeneca Gothenburg.
Glossary
Abbreviations Used
- sPLA2
secreted phospholipase 2
- VISTA
Vascular Inflammation Suppression to Treat Acute Coronary Syndrome
- HTS
high throughput screening
- ACS
acute coronary syndrome
- PC
phosphatidylcholine
- HDL
high density lipoprotein
- LE
ligand efficiency
- LLE
lipophilicity ligand efficiency
- Papp
apparent permeability
- Clint
intrinsic clearance
- i.v.
intravenous
- p.o.
per oral
- CL
clearance
- AUC
area under curve
- Vdss
steady state volume of distribution
- F
oral bioavailability
- DBU
2,3,4,6,7,8,9,10-octahydropyrimido[1,2-a]azepine
- DMSO
dimethyl sulfoxide
- DMF
dimethylformamide
- TBTU
2-(1H-benzo[d][1,2,3]triazol-1-yl)-1,1,3,3-tetramethylisouronium tetrafluoroborate
- DIEA
diisopropylethylamine
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.7b00505.
Experimental details, synthesis, assay protocols, and X-ray crystallographic statistics (PDF)
Accession Codes
PDB code for the X-ray crystal structure described in this study has been deposited in the Protein Data Bank under the accession codes 5OW8 and 5OWC.
Author Present Address
○ (F.G.) D.E. Shaw Research, 120 W 45th Street, New York, New York 10036, United States.
Author Present Address
◆ (H.-G.B.) Medivir AB, SE-141 22 Huddinge, Sweden.
Author Present Address
□ (H.d.l.M.) RISE Research Institutes of Sweden Division Bioeconomy, SE-417 56 Gothenburg, Sweden.
Author Present Address
△ (T.O.) Gothenburg University, SE-405 30 Gothenburg, Sweden.
Author Present Address
▼ (Å.M.) Alfa Laval Lund AB, SE-226 55 Lund, Sweden.
Author Present Address
⬡ (G.S.) SCA Hygiene products AB, SE-851 88 Stockholm, Sweden.
Author Present Address
¶ (F.K.) SciLifeLab, Drug Discovery & Development Platform, SE-171 21 Solna, Sweden.
Author Present Address
■ (K.H.) SARomics Biostructures, SE-223 63 Lund, Sweden.
The authors declare no competing financial interest.
Supplementary Material
References
- Murakami M.; Taketomi Y.; Miki Y.; Sato H.; Yamamoto K.; Lambeau G. Emerging roles of secreted phospholipase A2 enzymes: the 3rd edition. Biochimie 2014, 107, 105–113. 10.1016/j.biochi.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Rosenson R. S.; Hurt-Camejo E. Phospholipase A2 enzymes and the risk of atherosclerosis. Eur. Heart J. 2012, 23, 2899–2909. 10.1093/eurheartj/ehs148. [DOI] [PubMed] [Google Scholar]
- Rosenson R. S.; Fraser H.; Trias J.; Hislop C. Varespladib methyl in cardiovascular disease. Expert Opin. Invest. Drugs 2010, 10, 1245–1255. 10.1517/13543784.2010.517193. [DOI] [PubMed] [Google Scholar]
- Kokotou M. G.; Limnios D.; Nikolaou A.; Psarra A.; Kokotos G. Inhibitors of phospholipase A2 and their therapeutic potential: an update on patents (2012–2016). Expert Opin. Ther. Pat. 2017, 27, 217–225. 10.1080/13543776.2017.1246540. [DOI] [PubMed] [Google Scholar]
- Pan Y. H.; Yu B. Z.; Singer A. G.; Ghomashchi F.; Lambeau G.; Gelb M. H.; Jain M. K.; Bahnson B. J. Crystal structure of human group X secreted phospholipase A2. Electrostatically neutral interfacial surface targets zwitterionic membranes. J. Biol. Chem. 2002, 9, 29086–29093. 10.1074/jbc.M202531200. [DOI] [PubMed] [Google Scholar]
- Pruzanski W.; Lambeau G.; Lazdunski M.; Cho W.; Kopilov J.; Kuksis A. Hydrolysis of minor glycerophospholipids of plasma lipoproteins by human group IIA, V and X secretory phospholipases A2. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids 2007, 1736, 5–19. 10.1016/j.bbalip.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Jönsson-Rylander A. C.; Lundin S.; Rosengren B.; Pettersson C.; Hurt-Camejo E. Role of secretory phospholipases in atherogenesis. Curr. Atheroscler. Rep. 2008, 10, 252–259. 10.1007/s11883-008-0039-6. [DOI] [PubMed] [Google Scholar]
- Karabina S. A.; Brochériou I.; Le Naour G.; Agrapart M.; Durand H.; Gelb M.; Lambeau G.; Ninio E. Atherogenic properties of LDL particles modified by human group X secreted phospholipase A2 on human endothelial cell function. FASEB J. 2006, 20, 2547–2549. 10.1096/fj.06-6018fje. [DOI] [PubMed] [Google Scholar]
- Ishimoto Y.; Yamada K.; Yamamoto S.; Ono T.; Notoya M.; Hanasaki K. Group V and X secretory phospholipase A(2)s-induced modification of high-density lipoprotein linked to the reduction of its antiatherogenic functions. Biochim. Biophys. Acta, Mol. Cell Res. 2003, 1642, 129–138. 10.1016/S0167-4889(03)00120-4. [DOI] [PubMed] [Google Scholar]
- Atout R.; Karabina S. A.; Dollet S.; Carreras M.; Payré C.; André P.; Lambeau G.; Lotteau V.; Ninio E.; Perrin-Cocon L. Human group X secreted phospholipase A2 induces dendritic cell maturation through lipoprotein-dependent and -independent mechanisms. Atherosclerosis 2012, 222, 367–374. 10.1016/j.atherosclerosis.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Pruzanski W.; Kopilov J.; Kuksis A. Hydrolysis of lipoproteins by sPLA2’s enhances mitogenesis and eicosanoid release from vascular smooth muscle cells: Diverse activity of sPLA2’s IIA, V and X. Pruzanski W, Kopilov J, Kuksis A. Prostaglandins Other Lipid Mediators 2016, 122, 64–68. 10.1016/j.prostaglandins.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Nicholls S. J.; Kastelein J. J.; Schwartz G. G.; Bash D.; Rosenson R. S.; Cavender M. A.; Brennan D. M.; Koenig W.; Jukema J. W.; Nambi V.; Wright R. S.; Menon V.; Lincoff A. M.; Nissen S. E. VISTA-16 Investigators. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA 2014, 311, 252–262. 10.1001/jama.2013.282836. [DOI] [PubMed] [Google Scholar]
- Dennis E. A.; Cao J.; Hsu Y. H.; Magrioti V.; Kokotos G. Phospholipase A2 Enzymes: Physical Structure, Biological Function, Disease Implication, Chemical Inhibition, and Therapeutic Intervention. Chem. Rev. 2011, 111, 6130–6185. 10.1021/cr200085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevitz R. W.; Bach N. J.; Carlson D. G.; Chirgadze N. Y.; Clawson D. K.; Dillard R. D.; Draheim S. E.; Hartley L. W.; Jones N. D.; Mihelich E. D.; Olkowski J. L.; Snyder D. W.; Sommers C.; Wery J. P. Structure-based design of the first potent and selective inhibitor of human non-pancreatic secreted phospholipase A2. Nat. Struct. Mol. Biol. 1995, 2, 458–465. 10.1038/nsb0695-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillard R. D.; Bach N. J.; Draheim S. E.; Berry D. R.; Carlson D. G.; Chirgadze N. Y.; Clawson D. K.; Hartley L. W.; Johnson L. M.; Jones N. D.; McKinney E. R.; Mihelich E. D.; Olkowski J. L.; Schevitz R. W.; Smith A. C.; Snyder D. W.; Sommers C. D.; Wery J.-P. Indole inhibitors of human nonpancreatic secretory phospholipase A2. 1.Indole-3-acetamides. J. Med. Chem. 1996, 39, 5119–5136. 10.1021/jm960485v. [DOI] [PubMed] [Google Scholar]
- Dillard R. D.; Bach N. J.; Draheim S. E.; Berry D. R.; Carlson D. G.; Chirgadze N. Y.; Clawson D. K.; Hartley L. W.; Johnson L. M.; Jones N. D.; McKinney E. R.; Mihelich E. D.; Olkowski J. L.; Schevitz R. W.; Smith A. C.; Snyder D. W.; Sommers C. D.; Wery J.-P. Indole inhibitors of human nonpancreatic secretory phospholipase A2. 2.Indole-3-acetamides with additional functionalities. J. Med. Chem. 1996, 39, 5137–5158. 10.1021/jm960486n. [DOI] [PubMed] [Google Scholar]
- Dillard R. D.; Bach N. J.; Draheim S. E.; Berry D. R.; Carlson D. G.; Chirgadze N. Y.; Clawson D. K.; Hartley L. W.; Johnson L. M.; Jones N. D.; McKinney E. R.; Mihelich E. D.; Olkowski J. L.; Schevitz R. W.; Smith A. C.; Snyder D. W.; Sommers C. D.; Wery J.-P. Indole inhibitors of human nonpancreatic secretory phospholipase A2. 2.Indole-3-glyoxamides. J. Med. Chem. 1996, 39, 5159–5175. 10.1021/jm960487f. [DOI] [PubMed] [Google Scholar]
- Smart B. P.; Oslund R. C.; Walsh L. A.; Gelb M. H. The first potent inhibitor of mammalian group X secreted phospholipase A2: elucidation of sites for enhanced binding. J. Med. Chem. 2006, 49, 2858–2860. 10.1021/jm060136t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazaré M.; Schneider C.; Lindenschmidt A.; Will D. W. A Flexible, Palladium-catalyzed indole and azaindole synthesis by direct annulation of chloroanilines and chloroaminopyridines with ketones. Angew. Chem., Int. Ed. 2004, 43, 4526–4528. 10.1002/anie.200460122. [DOI] [PubMed] [Google Scholar]
- Guo X.; Rao H.; Fu H.; Jiang Y.; Zhao Y. An inexpensive and efficient copper catalyst for N-arylation of amines, amides and nitrogen-containing heterocycles. Adv. Synth. Catal. 2006, 348, 2197–2202. 10.1002/adsc.200606198. [DOI] [Google Scholar]
- Huang H.; Yan X.; Zhu W.; Liu H.; Jiang H.; Chen K. Efficient copper-promoted N-arylations of aryl halides with amines. J. Comb. Chem. 2008, 10, 617–619. 10.1021/cc800048p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.