Abstract
Sialidosis is an autosomal recessive lysosomal storage disease caused by pathogenic variants in NEU1 which encodes lysosomal sialidase (neuraminidase 1). Lysosomal neuraminidase catalyzes the removal of terminal sialic acid molecules from glycolipids, glycoproteins and oligosaccharides. Sialidosis is classified into two types, based on phenotype and age of onset. Patients with the milder type 1 typically present late, usually in the second or third decade, with myoclonus, ataxia and visual defects. Type 2 is more severe and presents earlier with coarse facial features, developmental delay, hepatosplenomegaly and dysostosis multiplex. Presentation and severity of the disease are related to whether lysosomal sialidase is inactive or there is some residual activity. Diagnosis is suspected based on clinical features and increased urinary bound sialic acid excretion and confirmed by genetic testing showing pathogenic variants in NEU1. We report a patient with type 1 sialidosis who presented mainly with ataxia and both generalized and myoclonic seizures but no visual involvement. Whole exome sequencing of the proband detected compound heterozygous likely pathogenic variants (S182G and G227R) in NEU1.
Abbreviations: WES, Whole exome sequencing
Keywords: Sialidosis, Ataxia, Myoclonus, Neurodeaminiase, NEU1
1. Introduction
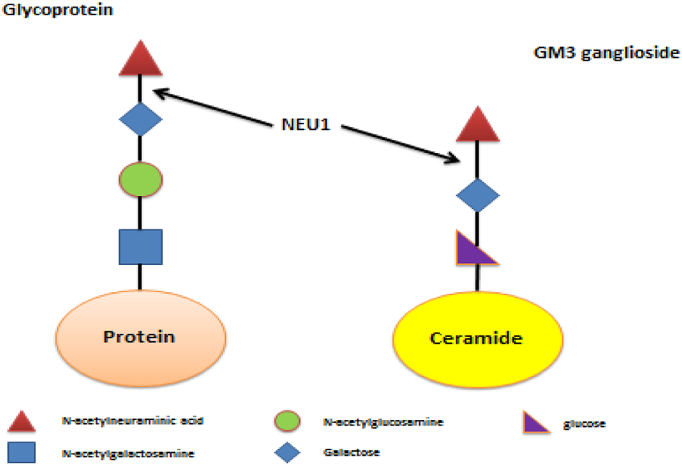
Four neuraminidases have been identified in humans to date: NEU1, NEU2, NEU3 and NEU4, each with a different subcellular localization, substrate preference and enzymatic properties. Lysosomal neuraminidase (NEU1) is the most clinically relevant neuraminidase due to its involvement in genetic disorders of metabolism such as sialidosis [1], [2]. Sialidosis is an autosomal recessive lysosomal storage disease caused by pathogenic variants in NEU1 which encodes lysosomal sialidase (neuraminidase 1). Lysosomal neuraminidase catalyzes the removal of terminal sialic acid molecules (N-acetylneuraminic acid or NANA) from glycolipids, glycoproteins and oligosaccharides (Fig. 1) [3]. Sialidase is a component of a multi-enzyme lysosomal complex containing other enzymes such as β-galactosidase and cathepsin A. Mutations that affect the activity or stability of sialidase or disrupts its association with the multi-enzyme complex, can cause the disorder [4]. Sialidosis is classified into two types, based on phenotype and age of onset. Patients with the milder type 1 typically present later, usually in the second or third decade, with myoclonus, ataxia and visual defects [5], [6]. Type 2 is more severe and presents younger with coarse facial features, developmental delay, hepatosplenomegaly and dysostosis multiplex. A congenital form has also been described which manifests prenatally and is associated with ascites and hydrops fetalis [7], [8]. Presentation and severity of the disease are related to whether lysosomal sialidase is inactive or if there is some residual activity [9]. Diagnosis is suspected based on clinical features and increased urinary bound sialic acid excretion and confirmed by genetic testing showing pathogenic variants in NEU1[10].
Fig. 1.
Pathways showing NEU1 conversion of N-acetylneuraminic to acid galactose and N-acetylneuraminic to galactose.
2. Case report
Our patient is an East-Asian 20 year-old male who presents with slowly progressive ataxia causing gait difficulty and multiple falls, as well as dysarthria. His symptoms first started when he was 12 year-old, when he developed an episode of vertigo followed by loss of consciousness and subsequent vomiting. His vertigo lasted for approximately one week and then had resolved. Subsequently, the patient started having seizures which were generalized spells, as described by observers, that lasted for a few seconds and were followed by loss of consciousness, headache and vomiting. He also developed jerking movements that began symmetrically in his upper and lower extremities. The patient denies any visual symptoms such as double or blurry vision. The patient's prenatal history and birth were uncomplicated and he achieved developmental milestones normally. Parental consanguinity was denied and there was no family history of seizures, ataxia or other neurologic conditions.
On physical exam, his cranium was normocephalic and atraumatic. Pupils were equal, round and reactive to light and accommodation. His funduscopic examination was normal with no retinal abnormalities such as macular cherry-red spot. He was awake, alert and oriented to person, place, and time. His memory and concentration were intact, but his speech was slow and dysarthric. Nystagmus was not present in vertical or horizontal directions; however there was saccadic hypometria in all planes. The patient had abnormal rapid alternating movements and finger-to-nose tests. He had jerky myoclonic movements of the proximal musculature. Motor examination revealed increased tone and brisk deep tendon reflexes. The patient's seizures ceased after he started taking levetiracetam.
The patient's paraneoplastic panel was normal, as well as his electroencephalogram (EEG) and magnetic resonance imaging (MRI) of the brain (Fig. 2, Fig. 3). He underwent ‘ataxia comprehensive evaluation testing’ via Athena commercial labs (Table 1), which included triplet repeat and sequencing of common ataxia related genes (ADCK3, AFG3L2, ANO10, APTX, ATM, ATN1, ATXN1, ATXN10, ATXN2, ATXN3, ATXN7, ATXN8OS, CACNA1A, CACNB4, EEF2, FGF14, FLVCR1, FXN, GRM1, ITPR1, KCNA1, KCNC3, KCND3, MRE11A, MTPAP, PDYN, POLG, PPP2R2B, PRKCG, SACS, SETX, SIL1, SLC1A3, SPTBN2, SYNE1, SYT14, TBP, TDP1, TGM6, TTBK2, TTPA, VAMP1), which revealed variants of uncertain significance in ADCK3, CACNA1A and SPTBN2, none of which we felt to be causal. Subsequently, whole exome sequencing (WES) was performed which detected compound heterozygous likely pathogenic variants S182G and G227R in NEU1 (Table 2).
Fig. 2.
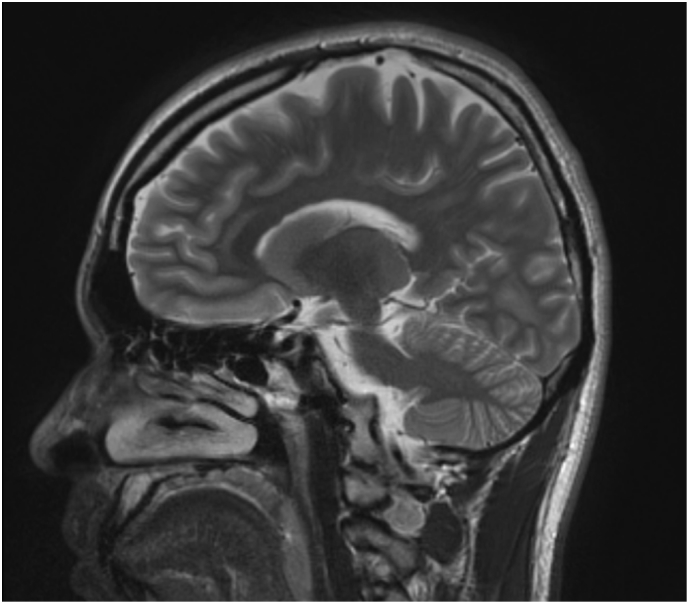
MRI brain w/o contrast, sagittal view. The cerebellum and brainstem appear normal in size, morphology and signal characteristics. Normal SWI signal characteristics of the substantia nigra and red nucleus. Hippocampal formations have normal size, morphology and signal characteristics. No diffusion restriction or significant SWI susceptibility. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
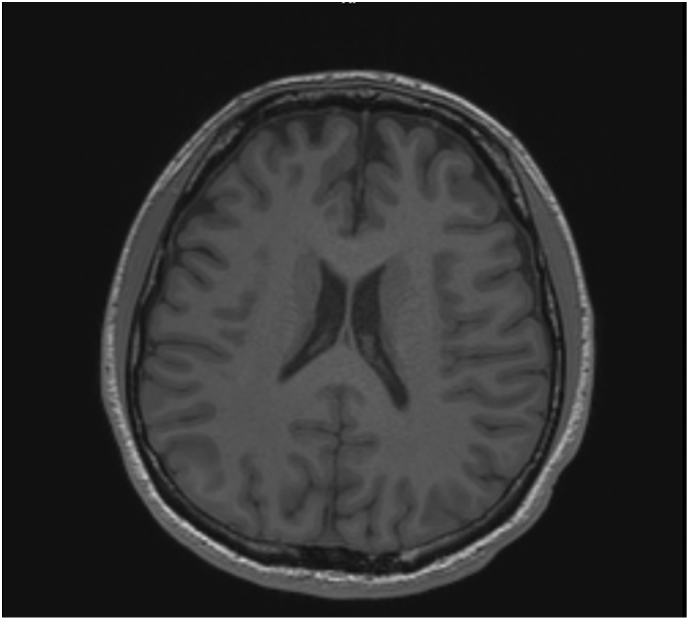
MRI brain w/o contrast, axial view. No abnormal cortical signal. No hydrocephalus, mass effect, midline shift or abnormal extra-axial fluid.
Table 1.
Ataxia, comprehensive evaluation.
| Gene/test | Technical result | Mutation type | Inheritance | Clinical relevance | Reference |
|---|---|---|---|---|---|
| ADCK3 | c.1773 T > C | Heterozygous synonymous | Autosomal recessive | Unknown | NM_020247.4 |
| CACNA1A | c.2283 (Isoform1) G > C | Heterozygous synonymous | Autosomal dominant | Unknown | NM_000068.2 |
| SPTBN2 | c.%75 + 8C > T | Heterozygous intronic | Autosomal dominant | Unknown | NM_006946.2 |
All other analyzed segments were negative.
Table 2.
Whole exome sequence analysis.
| Gene | Disease | Mode of inheritance | Variant | Coding DNA | Inherited form | Classification | Reference |
|---|---|---|---|---|---|---|---|
| NEU1 | NEU1-related disorder | Autosomal recessive | p.S182G | c.544 A < G | Mother | Likely pathogenic variant | NM_000434.3 |
| NEU1 | NEU1-related disorder | Autosomal recessive | p.G227R | c.679 G > A | Father | Likely pathogenic variant | NM_000434.3 |
3. Results
Our proband underwent a test entitled ‘comprehensive ataxia evaluation’ which identified three variants of unknown significance (VUS). The variants detected in this analysis have not been definitively demonstrated to be associated with hereditary ataxia. Subsequently WES was performed; the patient's mother was heterozygous for the S182G variant in NEU1 and his father was heterozygous for the G227R variant in NEU1, confirming bi-allelic inheritance. The S182G variant has been reported previously in the homozygous state in an individual with sialidosis type 1 [4]. The G227R variant has also been reported previously in homozygous and compound heterozygous state in individuals with NEU1-related disorders [10], [11], [12]. These variants are non-conservative amino acid substitution, which are likely to impact secondary protein structure as these residues differ in polarity, charge, size and/or other properties.
A custom-developed analysis tool (Xome Analyzer), capillary sequencing or another appropriate method was used to confirm all potentially pathogenic variants identified in this individual and relative samples. Sequence alterations were reported according to the Human Genome Variation Society (HGVS) nomenclature guidelines.
4. Discussion
Although macular cherry-red spot is a key finding in both types of sialidosis, its absence, especially when other neurological signs are present, does not rule out these disorders [10], [13]. Individuals presenting with myoclonus in the absence of the classic cherry-red spot finding have been reported before [10], [11]. This diversity of clinical phenotypes is thought to be attributed to the molecular heterogeneity of the sialidase pathogenic variants, and subsequent residual enzyme activity [6]. Also, the ongoing detection of new pathogenic variants in NEU1 may help in genotype-phenotype correlations in the future, which may aid in natural history genetic counselling of those who are affected and their families [9], [14].
We did not perform a urinary sialic acid test on our patient due to the ambiguity of testing, since having a negative urinary sialic acid test does not rule out sialidase deficiency [12], [15]. His molecular and phenotypic data were consistent with sialidosis. We recommend that a genetic evaluation be done without the need for a preceding urinary sialic acid levels test, as false-negatives are reported in the medical literature, particularly for older patients presenting with features suggestive of sialidosis including ataxia and myoclonus. Finally, we demonstrate the complexity and confusion that can arise with clinical genetic testing. His initial ataxia test was labelled as a ‘comprehensive ataxia evaluation’ yet did not include genes known to cause ataxia, such as the causal NEU1 in this case. To the less astute clinician, the reassuring ‘comprehensive evaluation’ can be misleading as it suggests this is the only test that is needed. This point highlights the need for medical genetics input in these complex cases and reminds us genetic testing can be complex and confusing [16], [17], [18], [19], [20], [21], [22], [23], [24].
In summary, we describe an adult male with ataxia and myoclonus, absent occular findings such as macular cherry red spot, and bi-allelic variants in NEU1 resulting in the very rare diagnosis of sialidosis type 1.
Disclosure
None.
Contribution statement
Ahmed Mohammad – organization, manuscript preparation of first draft.
Katelyn Bruno – research scientist, manuscript review and critique.
Stephanie Hines – clinician, manuscript review and critique.
Paldeep Atwal – medical geneticist, project execution, design, review and critique.
Acknowledgments
The authors would like to thank the patient for his permission to publish this manuscript.
References
- 1.Bonten E. Characterization of human lysosomal neuraminidase defines the molecular basis of the metabolic storage disorder sialidosis. Genes Dev. 1996;10(24):3156–3169. doi: 10.1101/gad.10.24.3156. [DOI] [PubMed] [Google Scholar]
- 2.Miyagi T., Yamaguchi K. Mammalian sialidases: physiological and pathological roles in cellular functions. Glycobiology. 2012;22(7):880–896. doi: 10.1093/glycob/cws057. [DOI] [PubMed] [Google Scholar]
- 3.Pattison S. Five novel mutations in the lysosomal sialidase gene (NEU1) in type II sialidosis patients and assessment of their impact on enzyme activity and intracellular targeting using adenovirus-mediated expression. Hum. Mutat. 2004;23(1):32–39. doi: 10.1002/humu.10278. [DOI] [PubMed] [Google Scholar]
- 4.Lukong K.E. Characterization of the sialidase molecular defects in sialidosis patients suggests the structural organization of the lysosomal multienzyme complex. Hum. Mol. Genet. 2000;9(7):1075–1085. doi: 10.1093/hmg/9.7.1075. [DOI] [PubMed] [Google Scholar]
- 5.Franceschetti S., Canafoglia L. Sialidoses. Epileptic Disord. 2016;18(S2):89–93. doi: 10.1684/epd.2016.0845. [DOI] [PubMed] [Google Scholar]
- 6.Seyrantepe V. Molecular pathology of NEU1 gene in sialidosis. Hum. Mutat. 2003;22(5):343–352. doi: 10.1002/humu.10268. [DOI] [PubMed] [Google Scholar]
- 7.Aylsworth A.S. A severe infantile sialidosis: clinical, biochemical, and microscopic features. J. Pediatr. 1980;96(4):662–668. doi: 10.1016/s0022-3476(80)80734-7. [DOI] [PubMed] [Google Scholar]
- 8.Beck M. Neuraminidase deficiency presenting as non-immune hydrops fetalis. Eur. J. Pediatr. 1984;143(2):135–139. doi: 10.1007/BF00445802. [DOI] [PubMed] [Google Scholar]
- 9.Bonten E.J. Novel mutations in lysosomal neuraminidase identify functional domains and determine clinical severity in sialidosis. Hum. Mol. Genet. 2000;9(18):2715–2725. doi: 10.1093/hmg/9.18.2715. [DOI] [PubMed] [Google Scholar]
- 10.Canafoglia L. Expanding sialidosis spectrum by genome-wide screening: NEU1 mutations in adult-onset myoclonus. Neurology. 2014;82(22):2003–2006. doi: 10.1212/WNL.0000000000000482. [DOI] [PubMed] [Google Scholar]
- 11.Muona M. A recurrent de novo mutation in KCNC1 causes progressive myoclonus epilepsy. Nat. Genet. 2015;47(1):39–46. doi: 10.1038/ng.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schene I.F. Pitfalls in diagnosing neuraminidase deficiency: psychosomatics and normal sialic acid excretion. JIMD Rep. 2016;25:9–13. doi: 10.1007/8904_2015_472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kivlin J.D., Sanborn G.E., Myers G.G. The cherry-red spot in Tay-Sachs and other storage diseases. Ann. Neurol. 1985;17(4):356–360. doi: 10.1002/ana.410170409. [DOI] [PubMed] [Google Scholar]
- 14.Coutinho M.F. Lysosomal multienzymatic complex-related diseases: a genetic study among Portuguese patients. Clin. Genet. 2012;81(4):379–393. doi: 10.1111/j.1399-0004.2011.01625.x. [DOI] [PubMed] [Google Scholar]
- 15.van der Ham M. Quantification of free and total sialic acid excretion by LC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007;848(2):251–257. doi: 10.1016/j.jchromb.2006.10.066. [DOI] [PubMed] [Google Scholar]
- 16.Atwal P.S. Mutations in the complex III assembly factor tetratricopeptide 19 gene TTC19 are a rare cause of leigh syndrome. JIMD Rep. 2014;14:43–45. doi: 10.1007/8904_2013_282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunde K. Case report: 5 year follow-up of adult late-onset mitochondrial encephalomyopathy with lactic acid and stroke-like episodes (MELAS) Mol. Gen. Metab. Rep. 2016;9:94–97. doi: 10.1016/j.ymgmr.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackburn P.R. Silent tyrosinemia type I without elevated tyrosine or succinylacetone associated with liver cirrhosis and hepatocellular carcinoma. Hum. Mutat. 2016;37(10):1097–1105. doi: 10.1002/humu.23047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donti T.R., Blackburn P.R., Atwal P.S. Holocarboxylase synthetase deficiency pre and post newborn screening. Mol. Gen. Metab. Rep. 2016;7:40–44. doi: 10.1016/j.ymgmr.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atwal P.S. Novel X-linked syndrome of cardiac valvulopathy, keloid scarring, and reduced joint mobility due to filamin A substitution G1576R. Am. J. Med. Gen. Part A. 2016;170A(4):891–895. doi: 10.1002/ajmg.a.37491. [DOI] [PubMed] [Google Scholar]
- 21.Blackburn P.R. Maple syrup urine disease: mechanisms and management. Appl. Clin. Genet. 2017;10:57–66. doi: 10.2147/TACG.S125962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blackburn P.R. Whole exome sequencing of a patient with suspected mitochondrial myopathy reveals novel compound heterozygous variants in RYR1. Mol. Gen. Genomic Med. 2017;5(3):295–302. doi: 10.1002/mgg3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gass J. Whole exome sequencing identifies atypical welander distal myopathy in patient. J. Clin. Neuromuscul. Dis. 2017;18(3):152–156. doi: 10.1097/CND.0000000000000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atwal P.S. Clinical whole-exome sequencing: are we there yet? Genitourin. Med. 2014;16(9):717–719. doi: 10.1038/gim.2014.10. [DOI] [PubMed] [Google Scholar]