Abstract
Primary Ciliary Dyskinesia (PCD) is a genetic disorder causing chronic oto-sino-pulmonary disease. No single diagnostic test will detect all PCD cases. Transmission electron microscopy (TEM) of respiratory cilia was previously considered the gold standard diagnostic test for PCD, but 30% of all PCD cases have either normal ciliary ultrastructure or subtle changes which are non-diagnostic. These cases are identified through alternate diagnostic tests, including nasal nitric oxide measurement, high speed videomicroscopy analysis, immunofluorescent staining of axonemal proteins, and/or mutation analysis of various PCD causing genes. Autosomal recessive mutations in DNAH11 and HYDIN produce normal TEM ciliary ultrastructure, while mutations in genes encoding for radial spoke head proteins result in some cross-sections with non-diagnostic alterations in the central apparatus interspersed with normal ciliary cross-sections. Mutations in nexin link and dynein regulatory complex genes lead to a collection of different ciliary ultrastructures; mutations in CCDC65, CCDC164, and GAS8 produce normal ciliary ultrastructure, while mutations in CCDC39 and CCDC40 cause absent inner dynein arms and microtubule disorganization in some ciliary cross-sections. Mutations in CCNO and MCIDAS cause near complete absence of respiratory cilia due to defects in generation of multiple cellular basal bodies; however, the scant cilia generated may have normal ultrastructure. Lastly, a syndromic form of PCD with retinal degeneration results in normal ciliary ultrastructure through mutations in the RPGR gene. Clinicians must be aware of these genetic causes of PCD resulting in non-diagnostic TEM ciliary ultrastructure and refrain from using TEM of respiratory cilia as a test to rule out PCD.
INTRODUCTION
Primary Ciliary Dyskinesia (PCD) is a rare genetic disease affecting motile cilia biogenesis, structure, and function, resulting in chronic oto-sino-pulmonary infections, infertility, and often organ laterality defects. Diagnosing PCD remains difficult, as no single test detects all known forms of this disease, and frequently, several distinct tests are required to confirm a PCD diagnosis. Historically, Transmission Electron Microscopy (TEM) was the mainstay of PCD diagnosis, surveying for disease-causing ultrastructural defects within individual ciliary axonemes. However, with advancement in PCD diagnostics, well-phenotyped cases of PCD, with normal or non-diagnostic ciliary ultrastructure, emerged frequently in diagnostic cohorts. These cases were detected through alternative PCD diagnostic tests, including abnormal high speed videomicroscopy analysis (HSVA), low levels of nasal nitric oxide, abnormal ciliary protein pattern on immunofluorescent staining, and/or pathogenic variants in PCD-associated genes. [Table 1] Advances in molecular genetic techniques, including whole exome sequencing, have allowed discovery of novel PCD-associated genes in cases of PCD with normal or non-diagnostic ciliary ultrastructure on TEM.
TABLE 1. Diagnostic value of other PCD tests with normal ciliary ultrastructure on TEM.
| Genetic defect | TEM | Nasal nitric oxide* | HSVA | Immunofluorescence |
|---|---|---|---|---|
| DNAH11 | 100% normal | Low | Abnormal | Sometimes abnormal |
| HYDIN | 100% normal | Low | Non-diagnostic | Normal |
| CCNO/MCIDAS | No cilia | Low | Inadequate cilia for analysis | Normal in CCNO, Abnormal in MCIDAS |
| Nexin Link/DRC Genes - CCDC65, CCDC164, GAS8 | Mostly normal | Low | Abnormal in CCDC65, CCDC164, Non-diagnostic in GAS8 | Normal |
| Nexin Link/DRC Genes - CCDC39, CCDC40 | Most cilia show IDA defects, 50% cilia show microtubule disorganization | Low | Abnormal | Abnormal |
| RSPH4A, RSPH9, RSPH3 | Up to 50% normal | Low | Abnormal | Abnormal |
| RPSPH1 | Up to 80% normal | Low or normal | Non-diagnostic | Abnormal |
| RPGR | Mostly normal | Low or normal | Abnormal | Normal |
Low nasal nitric oxide value is <77 nL/minute in cooperative patients performing velum closure maneuvers31
Accurate interpretation of TEM analysis requires appreciation of the limitations of this test. Current estimates suggest that approximately 30% of PCD cases have normal or non-diagnostic ciliary ultrastructure,1,2 and a recent meta-analysis reports at least 26% of PCD, diagnosed through genetics, HSVA, or nasal nitric oxide measurement, lack detectable ultrastructural changes on TEM.3 Therefore, clinicians cannot conclude that a normal TEM analysis excludes PCD. Respiratory viral infections, environmental pollutants and other airway insults can induce secondary changes in ciliary ultrastructure that may overlap with some of the subtle changes seen in PCD; therefore, care must be taken to avoid misinterpretation of these secondary changes as “consistent with PCD”. The goals of this paper are to outline the normal changes which can occur in TEM samples from healthy individuals and the pathologic changes that can appear in various forms of PCD where TEM changes are subtle, sporadic, or non-diagnostic.
“NORMAL” CILIARY ULTRASTRUCTURE ON TEM
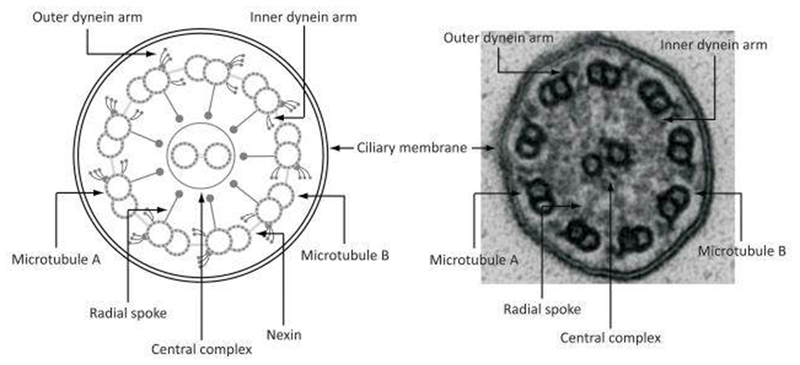
Defining the range of normal ciliary ultrastructure findings is key to understanding the forms of PCD resulting in normal TEM or only subtle TEM changes. Most experts recommend at least twenty to fifty individual ciliary cross sections, obtained from several different cell clusters in the biopsy material, as an adequate diagnostic sample.4–7 The presence of microvilli surrounding the axonemes suggests the cross sections were obtained at an appropriate ciliary level and not too close to the ciliary tip, where normal structures can be altered. Axonemal cross-sections consist of a circular array of nine microtubule doublets surrounding a central apparatus housing a microtubule pair (classic 9+2 configuration). Outer dynein arms (ODA) and inner dynein arms (IDA) are studded along each doublet; radial spokes link each doublet (at the level of the IDA) to the central apparatus; and nexin links join each doublet with adjacent doublets [Figure 1].
Figure 1.
Illustration and TEM image of normal ciliary ultrastructure
Normal 9+2 ciliary arrangement, with 9 outer doublet microtubules surrounding a central pair and normal outer and inner dynein arms. Even in this normal sample, electron dense material inside the ciliary membrane makes the inner dynein arms, radial spokes, and nexin links appear indistinct, as is often the case in clinical samples. Not that only one to two radial spokes are visible in this normal sample. Reproduced with permission from 61.
On clinical TEM studies, the visible numbers of ODA are often less than expected due to processing artifact, with normal cilia demonstrating only 6 to 7 visible and full-length ODA.7 Inner dynein arm numbers can also be less than ideal on clinical TEM samples, with only 3 to 4 IDA seen in a normal cross section, due to variable spacing of IDA isoforms along the ciliary axoneme.7–9 Isolated IDA defects, without any other ultrastructural anomalies, can occur secondary to TEM sampling issues or processing artifact, and this isolated defect resolves in 25% of cases upon repeat biopsy sampling.10 Additionally, none of the 38 known PCD-causing genes result in isolated IDA defects11, and while extensive genotyping has revealed genetic causes of IDA defects in animal models and in human sperm12–14, similar efforts have failed to show causative genetic variants in human respiratory cilia. Thus, some PCD experts dispute the existence of a true form of PCD where only isolated IDA defects are seen.
Nexin links and radial spokes can often be indistinct due to background electron dense material encountered in sample processing [Figure 1], and often no more than one of these structures are visible per ciliary cross-section in healthy individuals.15 In TEM images with a large amount of secondary artifact (samples obtained during acute illness or crushed from biopsy with forceps, presence of many smudged cells, or frequently interrupted ciliary membranes), the number of visible structures decreases significantly, including the central apparatus and peripheral doublets, which can be missing intermittently on poorly preserved samples.16 Ciliary disorientation, where an axis drawn through the central apparatus varies by more than 20 degrees amongst individual cilia in the same study, is no longer considered a primary ciliary defect and is often seen when patients are recovering from viral respiratory infection or other secondary insults.17 Additionally, TEM studies of healthy controls suggest sporadic ciliary abnormalities can be present in up to 10% of ciliary cross sections.9,18 In subjects with PCD, TEM ultrastructural defects are often present more frequently within the sample, and some authors suggest anomalies be present in at least 20% of all cross sections to be considered diagnostic of PCD.4 However, this notion has notable limitations for several recently discovered PCD causing gene mutations, as discussed in the following sections. Thus, even normal ciliary ultrastructure can be difficult to judge, and only experienced clinicians should be interpreting ciliary TEM biopsies. As a result, some academic centers elect to have their ciliary TEM studies processed and interpreted by third party services that are adept with these challenging studies.
HALLMARK TEM DEFECTS IN PCD
Several hallmark TEM defects are recognized as disease-causing in PCD. Absence of the ODA is the most common defect in PCD cases diagnosed by TEM. Outer dynein arm defects can occur in isolation or in combination with absent IDA, depending on the affected gene. In some cases, the ODA is consistently truncated with only short ODA “stubs” apparent.19
Isolated ODA defects have been associated with numerous PCD genes, including DNAH5 and DNAI1 (which account for approximately 30% of all PCD cases) as well as DNAI2, DNAL1, NME8, CCDC114, CCDC151, ARMC4, and TTC25.11 ODA+IDA defects have been associated with multiple PCD genes (DNAAF1, DNAAF2, DNAAF3, LRRC6, C21orf59, HEATR2, ZMYND10, DYX1C1, SPAG1, CCDC103, and PIH1D3) that encode cytoplasmic proteins involved in pre-assembly of ciliary axonemes.15 Despite vigorous investigations, genetic causes of some ODA and ODA+IDA defects remain unknown.
Inner dynein arm defect with microtubule disorganization (IDA+MTD) is also a hallmark diagnostic ciliary defect reflecting structural changes in the dynein regulatory complex.16–19 While a majority of ciliary cross sections with this defect are missing their inner dynein arms, the accompanying microtubule disorganization occurs more sporadically and usually in less than 50% of the axonemes.20–23 Genetic mutations in CCDC39 and CCDC40 are frequently associated with IDA+MTD defects, but the genetic cause for some IDA+MTD defects has not yet been discovered. This defect is discussed further in the section on nexin links and dynein regulatory complex defects.
SPECIFIC FORMS OF PCD WITH NORMAL TEM ULTRASTRUCTURE
DNAH11 Mutations
Initially described in 2008, autosomal recessive mutations in DNAH11 (Chr 7) cause PCD with completely normal ciliary ultrastructure on TEM. In a well-phenotyped patient cohort, with classic PCD symptoms (chronic oto-sino-pulmonary disease, neonatal respiratory distress, bronchiectasis, infertility, and situs inversus totalis), Schwabe and colleagues demonstrated low nasal nitric oxide levels and abnormal, hyperkinetic beat on HSVA.24 Through genetic linkage analysis, DNAH11 mutations were identified in this group. Although DNAH11 encodes one of the ODA heavy chain proteins, mutated DNAH11 does not affect placement of other ODA proteins (DNAH5, DNAH9) found along the ciliary axoneme, and the ODA appears overall normal on TEM.25 Further analysis reveals some DNAH11 mutations can affect ODA structures in proximal areas of the ciliary axoneme, causing limited ODA abnormalities visible on detailed immunofluorescent staining.26 However, these immunofluorescent changes in ODA proteins are not detected in all PCD cases with pathogenic variants in DNAH11. High resolution electron tomography also demonstrates subtle ODA changes in the proximal axoneme, which are not visible on conventional TEM analysis.26
Mutations in DNAH11 are estimated to account for 6–9% of all PCD cases11, and 22–72% of PCD cases with normal ciliary ultrastructure.1,27 Further clinical study has demonstrated a variety of organ laterality defects resulting from DNAH11 mutations, including situs ambiguus in murine models and in humans.28,29 Organ laterality defects are seen with all forms of PCD arising from defects in dynein arm genes, through identical loss of dynein arm proteins in embryonic nodal cilia, which must function normally to guide organ placement in developing embryos.30 PCD cases with DNAH11 mutations may be identified through abnormally low nasal nitric oxide testing31 and abnormal, hyperkinetic ciliary beat pattern on HSVA.27 Mutations in DNAH11 may be detected on commercial PCD genetic panels.
HYDIN Mutations
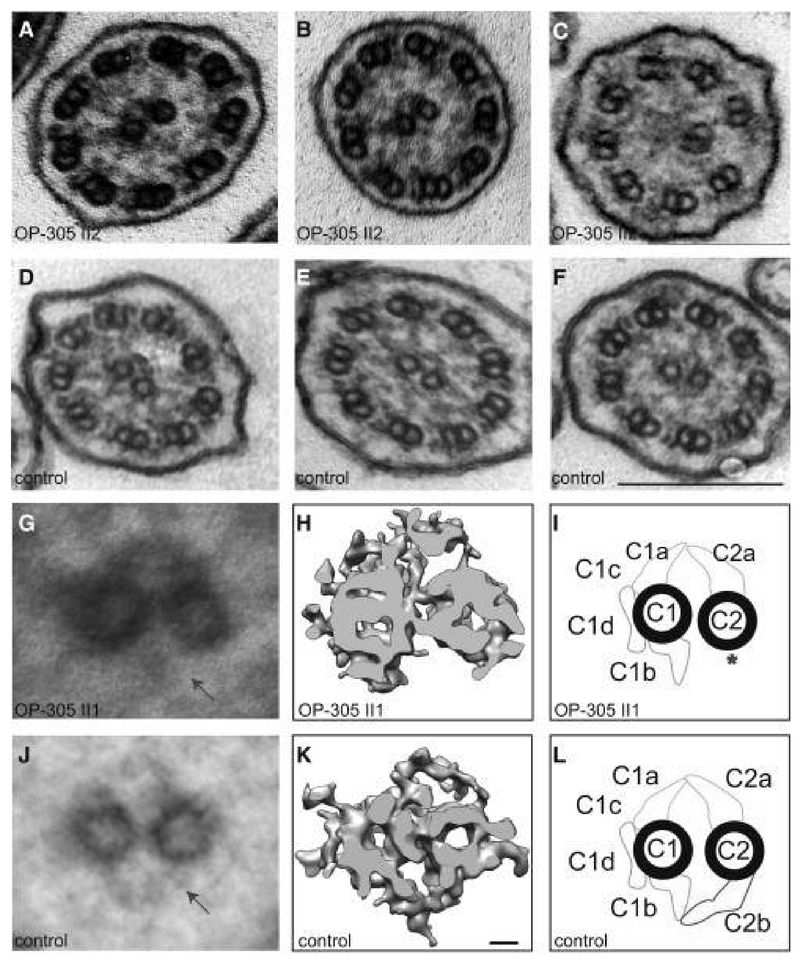
Autosomal recessive mutations in the HYDIN gene (Chr 16, with a nearly identical paralogous HYDIN2 segment on Chr 1) cause PCD with completely normal TEM ciliary ultrastructure. Originally discovered as a gene causing hydrocephalus32, HYDIN encodes for a central apparatus ciliary protein, yet the central apparatus appears normal on conventional TEM. However, subjects with HYDIN mutations lack the C2b projection of the central apparatus on specialized electron tomography.33 [Figure 2]
Figure 2.
TEM and electron tomography images with disease causing HYDIN mutations
TEM cross sections of HYDIN-mutant individual, showing normal ciliary ultrastructure (A & B). Rarely, ciliary transposition defects can be seen (C). Healthy control TEM images (D-F) demonstrate normal ciliary ultrastructure. Composite image averages of six affected (G) and 6 control (J) TEM images show loss of the C2b appendage from the central apparatus (arrow). A detailed analysis of the CP apparatus by electron microscopic tomography (H and I) identified the absence of the C2b projection in HYDIN-mutant cilia from one individual compared to control samples (K and L). Each rendered image (H and K) represents six cross-sections averaged from a dual-axis tomogram made up of 140 images at 2 degree tilts. The scale bars represent 10 nm (rendered picture) and 200 nm (TEM pictures). Reproduced with permission from 33.
Although quite rare (<1% of all PCD), HYDIN mutations are found in small consanguineous cohorts from Germany and the Faroe Islands (through a prominent founder effect). HYDIN mutations result in classic PCD oto-sino-pulmonary symptoms, yet they do not cause organ laterality defects (since dynein arm proteins are unaffected, allowing embryonic nodal cilia to function normally). Immunofluorescence studies also reveal normal dynein arm proteins and fail to detect PCD cases caused by HYDIN mutations. High speed videomicroscopy analysis of HYDIN mutations rarely shows rotatory movements and mainly demonstrates a mild reduction of the ciliary bending capacity. These subtle functional changes make it difficult to establish a HYDIN diagnosis with HSVA alone.34 However, nasal nitric oxide values are low in patients with HYDIN mutations. Unfortunately, even genetic testing for HYDIN is exceedingly complicated, due to the paralogous HYDIN2 segment, which can easily confuse results. Thus, HYDIN is not routinely assessed on commercial PCD genetic panels.
CCNO & MCIDAS Mutations
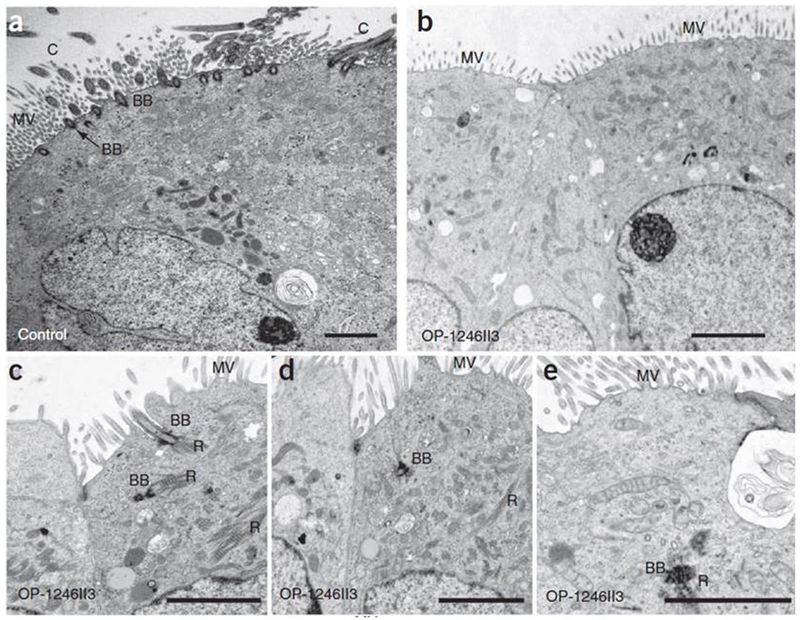
Since their recent discovery in 2014, mutations in CCNO and MCIDAS (both on chr 5) have been implicated as autosomal recessive causes of reduced generation of multiple motile cilia, formerly known as ciliary aplasia. While some experts classify this disease as distinct from PCD35, the clinical presentations of chronic oto-sino-pulmonary disease, bronchiectasis, neonatal respiratory distress, and infertility are nearly identical to PCD. Transmission electron microscopy studies of affected individuals often show a complete lack of cilia on the apical cell surfaces. However, meticulous research TEM studies demonstrate one to two cilia per epithelial cell, instead of the expected 200 cilia per cell in healthy controls.35 [Figure 3] Additionally, overall decreased numbers of basal bodies are abnormally trapped in the cytoplasm, supporting a centriolar amplification and localization problem as the cause of decreased motile cilia, as opposed to an axonemal ciliation problem. The residual cilia show normal presence of axonemal motor proteins and ciliary motility in those with CCNO mutations, while the ciliary motility proteins (DNAH5 and CCDC39) are absent, and consequently cilia are immotile in those with mutations in MCIDAS mutations.36
Figure 3.
TEM images in PCD caused by CCNO mutations
TEM photographs of respiratory epithelial cells of individuals with CCNO mutations show severe reduction in the numbers of motile cilia and basal bodies at the apical cell region and mislocalization of basal bodies and rootlets within the cytoplasm. (a) Respiratory epithelial cell from a healthy control individual showing several basal bodies (BB) on the apical membrane nucleating multiple motile cilia (C) and microvilli (MV) at the apical cell region. (b–e) Respiratory epithelial cells of individuals with CCNO mutations. Occasionally, one can see basal bodies abnormally located inside the cytoplasm, with ciliary rootlets (R). Scale bars, 2 μm. Mutations in MCIDAS produce similar TEM images. Reproduced with permission from 35.
Patients with CCNO or MCIDAS mutations have not been linked to organ laterality defects, as the proteins encoded by these genes do not affect formation or function of embryonic nodal cilia, which are normally present as a single cilium per embryonic cell. However, since MCIDAS mutations do result in abnormal axonemal motor proteins through lack of downstream cellular signaling, clinical cases with organ laterality defects remain possible, though unlikely. Nasal nitric oxide values are low in patients with CCNO or MCIDAS mutations.35–37 High speed videomicroscopy analysis is normally unfeasible in these patients, as locating the few residual cilia per epithelial cell proves difficult, and reviewers often mistakenly conclude that the lack of cilia stems from a secondary process.
Mutations in CCNO and MCIDAS are both estimated to account for <1% of PCD cases.11 However in one Israeli cohort with mucociliary clearance disorders, 11 out of 171 families (6.5%) had biallelic mutations in CCNO (n=10) or MICDAS (n=1) causing their disease.37 An increased prevalence for PCD in the Irish Traveler population exists with the CCNO gene.38 Mutations in CCNO and MCIDAS are detected on commercial PCD genetic panels.
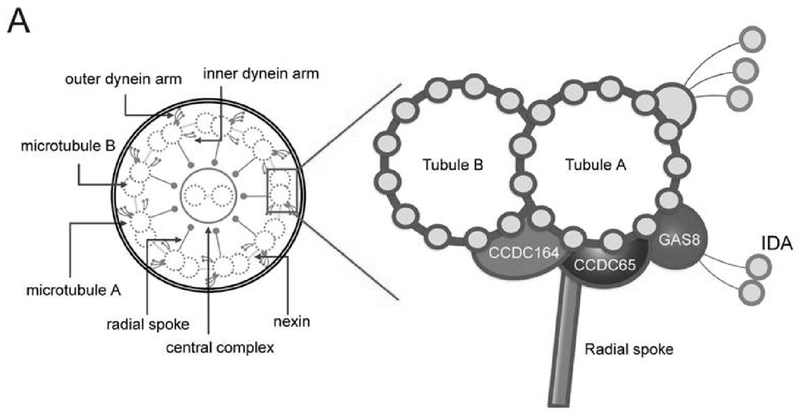
Nexin Link/Dynein Regulatory Complex Genes
The nexin links and dynein regulatory complex (N-DRC) serve as a connection between the IDA, radial spokes, and outer doublets, allowing these structures to communicate with each other through poorly understood mechanisms. [Figure 4] The N-DRC also creates resistance to microtubule sliding, providing alignment and stabilization to the functional cilium.39 Autosomal recessive mutations in CCDC164 (chr 2) and GAS8 (chr 16), both encoding for proteins in the N-DRC, cause PCD with normal ciliary ultrastructure on TEM analysis.40,41 Healthy patients have at least one nexin link visible per ciliary cross-section15, but those with mutations in CCDC164 and GAS8 genes usually do not have any visible nexin links per cross section. Mutations in GAS8 can also show subtle misalignment of peripheral doublets in up to 25% of ciliary cross sections, which can be dismissed as normal variation. By comparison, healthy controls have 4 to 11% of peripheral doublets misaligned.41 However, this misalignment anomaly is usually not obvious enough to provide diagnostic certainty of PCD through TEM images alone. Residual axonemal motor proteins in the ODA and IDA are normal on immunofluorescent studies.40,41
Figure 4.
Illustration of the proposed ciliary structure for the inner dynein arm and dynein regulatory complex
Reproduced with permission from 42.
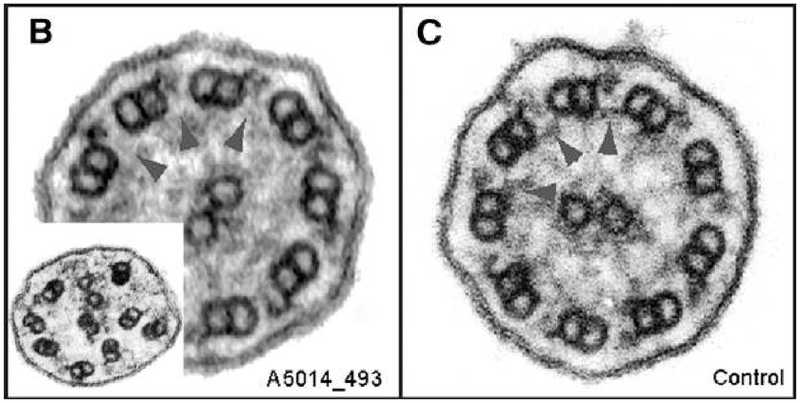
Careful analysis of TEM images in another mutated N-DRC gene causing PCD, named CCDC65 (Chr 12), reveals normal ciliary ultrastructure.42 However, in one PCD cohort with CCDC65 mutations, approximately 5 to 15% of ciliary cross sections also show microtubule disorganization, which is more conspicuous as a PCD causing anomaly, but can still be dismissed as artifact and non-diagnostic of PCD, particularly when present without any other ultrastructural defects.43 [Figure 5] On immunofluorescent staining, dynein motor proteins along the ciliary axoneme are normal in PCD from CCDC65 mutations.43
Figure 5.
TEM images of cilia with disease causing CCDC65 mutations
(B) TEM of a patient with PCD from CCDC65 mutations, showing normal dynein arms and central pairs. Note that upon close examination, N-DRC links are missing (arrowheads). Microtubule disorganization (B, inset) was observed in 5%–15% of PCD individual cilia sections with CCDC65 mutations. (C) TEM from a healthy control, showing normal dynein inner arms and nexin links (arrowheads). Reproduced with permission from 43.
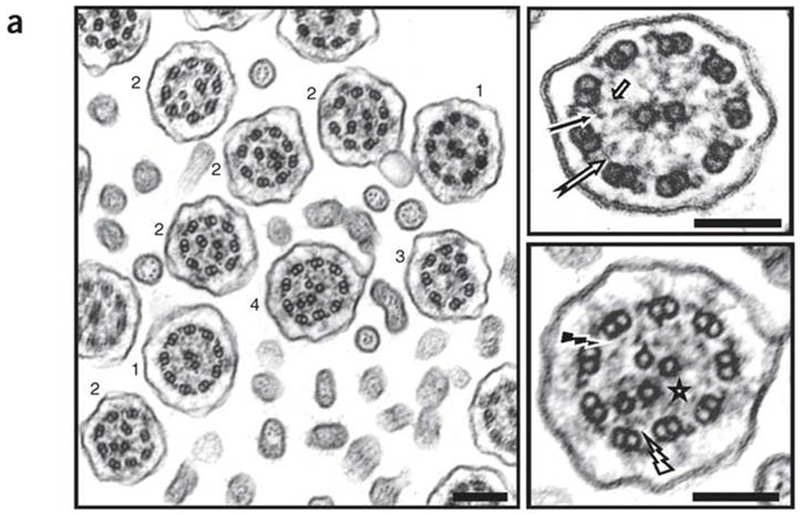
Mutations in two other N-DRC genes, CCDC39 (chr 3) and CCDC40 (chr 17), cause reduced or absent IDA in the majority of cilia, with variable expression of microtubule disorganization in up to 50% of ciliary cross sections.20–22 [Figure 6] With high electron density in the background of ciliary samples, the IDA loss from CCDC39 and CCDC40 mutations may be interpreted as non-diagnostic of PCD, especially in cases where the microtubule disorganization occurs in a minority of cross sections. In microtubule disorganization, the nine peripheral microtubules are retained but are mislocalised, often becoming more centralised, while the central microtubule pair may be variably lost (9+0), eccentrically positioned towards the periphery (9+2), or increased in number with a supernumerary central pair present (9+4).23 These disorganized microtubules are often seen best on low power images allowing comparison of ciliary arrangement between dozens of cross sections in the same photomicrograph field.
Figure 6.
TEM images of cilia with disease causing CCDC39 mutations
Transmission electron microscopy of respiratory cilia from an individual who is homozygous for CCDC39 mutations, showing absence of inner dynein arms in all ciliary sections, associated with a range of other, heterogeneous defects: isolated absence of the nine inner dynein arms (1), axonemal disorganization with mislocalized peripheral doublet associated with either a displacement of the central pair (2), an absence of the central pair (3), or supernumerary central pairs (4). Magnification of the axoneme from a normal cilium is shown in the upper right panel with presence of inner dynein arms (black arrow), nexin links (white arrow) and radial spokes (short arrow). The axonemal disorganization found in cases is associated with defects of inner dynein arms (black flash), nexin links (white flash) and radial spokes (star) that are better seen after magnification (lower right panel). Scale bar, 0.2 μm. Reproduced with permission from 22.
Patients with PCD from any N-DRC mutation (all autosomal recessive inheritance pattern) have typical symptoms of PCD; however, only CCDC39 and CCDC40 mutations result in organ laterality defects. PCD patients with mutations in CCDC39 and CCDC40 may also have worse clinical outcomes versus PCD patients with other dynein arm defects.44 Nasal nitric oxide levels are low for all N-DRC defects, regardless of the causative gene. High speed videomicroscopy analysis shows somewhat stiff and dyskinetic waveforms in CCDC6543 and CCDC16440 mutations, but ciliary waveforms are grossly normal with GAS8 mutations, making HSVA a poor test for detecting PCD cases caused by GAS8.41 Mutations in CCDC39 and CCDC40 produce much more abnormal HSVA waveforms, with greatly decreased beat amplitude and severely rigid cilia.34 Mutations in CCDC164, CCDC65, and GAS8 are each estimated to account for <1% of PCD, while mutations in CCDC39 and CCDC40 account for 4–9% and 3–4% of PCD, respectively.11 The prevalence of CCDC65 gene is increased within the Ashkenazi Jewish population.11 Mutations in N-DRC genes are detected on most commercial PCD genetic panels.
Radial Spoke Head Genes
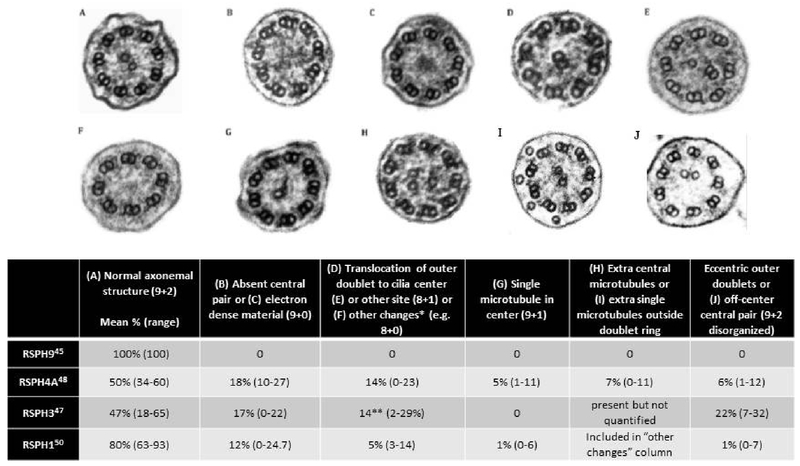
Four genes cause PCD through radial spoke defects - RSPH9 (Chr 6), RSPH4A (Chr 6), RSPH3 (Chr 6), and RSPH1 (Chr 21). These mutations produce various defects at the junction of the radial spoke heads and the central apparatus, resulting in diverse ciliary ultrastructure changes on TEM. Mutations in RSPH9, identified in several consanguineous Arab/Bedouin families, result in completely normal ciliary ultrastructure.45,46 Mutations in RSPH4A and RSPH3 result in approximately 50% of cilia with normal ultrastructure, while the remainder have varying defects, including roughly 20% missing the central apparatus altogether and 10% with “8+1” ciliary translocation arrangements (one peripheral microtubule shifted to the center to compensate for the missing central pair).45,47,48 [Figure 7] Detailed imaging studies of transposition defects with TEM and electron tomography explain this varied pattern as a twisting of the ciliary axoneme, resulting in a normal central apparatus (9+2 arrangement) at the ciliary base, with loss of the central pair in the mid-section of the axoneme (9+0 arrangement), and reappearance of a transposed peripheral doublet to the ciliary center (8+1 arrangement) in the distal portion of the axoneme.49 [Figure 8] Finally, mutations in RSPH1 result in overall normal TEM images, but meticulous inspection reveals only 80% of the axonemes are normal, with the remaining 20% showing various anomalies, such as absence of the central pair or translocation of an outer doublet to the center.50 [Figure 7]
Figure 7.
Ciliary ultrastructural defects seen on TEM in various radial spoke head gene mutations
* Other changes consists of the following: 8+0 arrangement, 7 outer doublets with central pair and 2 single microtubules in the center, 8 outer doublets with 2 central pairs, 8 outer doublets with 4 central single microtubules, 8 outer doublets without a central pair and 1 doublet outside of the outer ring.
** Only accounting for 8+1 transposition defects
Reprinted and adapted with permission of the American Thoracic Society. Copyright © 2017 American Thoracic Society. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society. 50
Figure 8.
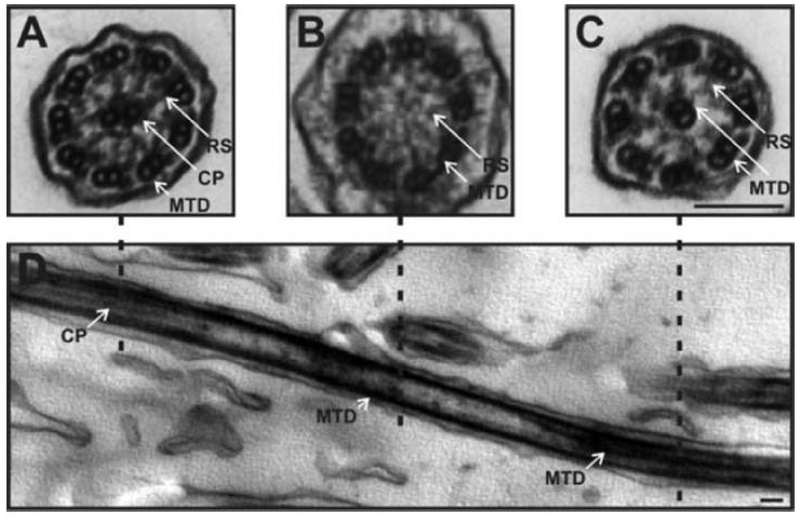
TEM images of cilia with transposition defects
The TEM ultrastructural arrangement of the axoneme as seen in cilia with transposition defects. (A) A transverse view showing a normal appearing 9+2 ciliary arrangement that changes distally to (B) a 9+0 arrangement where the central pair disappears, and towards the tip of the cilium (C) where one of the microtubule doublets moves into the center to give an 8+1 arrangement. This particular pattern indicates a transposition defect. The change in arrangement can be seen in the longitudinal section (D). White arrows indicate microtubule doublets (MTD), radial spokes (RS) and the central pair (CP). Scale bar 100 nm. Reproduced with permission from 49.
None of the radial spoke head genes result in organ laterality defects; normal embryonic nodal cilia lack radial spokes, and thus are not dependent upon these structures for normal function and guidance of organ placement. Most patients with PCD from radial spoke head gene mutations present with classic PCD oto-sino-pulmonary symptoms. However, those with RSPH1 mutations can have a milder clinical phenotype, with only 50% displaying neonatal respiratory distress, a delayed onset of daily wet cough sometimes (occurring after the first year of life which is quite atypical for classic PCD), and better lung function than PCD from mutations in other genes.50
All radial spoke head gene mutations causing PCD result in low nasal nitric oxide, except for RSPH1, which can sometimes produce nasal nitric oxide levels above the accepted PCD diagnostic cutoff value of 77 nanoliters/minute.31,50 Thus, nasal nitric oxide measurement can miss PCD caused by RSPH1 mutations. Immunofluorescent studies show that mutations in RSPH4A cause absence of RSHP4A, RSHP9, and RSPH1 proteins in affected cilia, thereby establishing RSPH4A as the core protein of the radial head. Ciliary axonemes with mutations in RSPH1 lack RSPH1 and RSPH9 proteins on immunofluorescent staining, while axonemes affected by RSPH9 mutations only lack RSPH9 proteins in the axoneme.51 Mutations in RSPH3 also produce abnormal immunofluorescent staining of RSPH3 protein.47 All radial spoke head gene mutations show abnormal HSVA, mostly with an abnormal rotational beat pattern when viewed from above. However, RSPH1 mutations may result in sections of ciliary biopsies with completely normal ciliary beat frequency and pattern.34,52,53
Disease causing mutations in RSPH4A, RSPH1, and RSPH9 are estimated to account for PCD in 1–2%, 2%, and <1% of cases, respectively.11 Mutations in RSPH3 account for at least 10% of PCD cases with central apparatus/radial spoke defects.47 The prevalence of the RSPH4A gene is increased in the Irish Traveler, United Kingdom Pakistani, and Puerto Rican populations.38,45,48 Mutations in radial spoke head genes are detected on most commercial PCD genetic panels.
Syndromic Forms of PCD
X-linked recessive mutations in the RPGR gene can cause PCD in males, with a form of retinal degeneration and progressive visual impairment called Retinitis Pigmentosa. Recent reports of RPGR causing PCD describe TEM images as grossly normal54, yet some early cases reported 30% normal cilia, with the remaining cross sections showing various dynein arm anomalies, accompanied by less frequent central apparatus and nexin link changes.55 Ciliary disorientation, usually considered a secondary defect that is not diagnostic of PCD, was greatly increased after cellular regrowth in one PCD patient with RPGR mutations.54 Immunofluorescence of ODA and IDA proteins was normal in two RPGR-mutated subjects, and nasal nitric oxide levels were borderline low in one of these subjects, while normal in the other subject.54 In one of these subjects, HSVA revealed an increase in uncoordinated ciliary activity. Unfortunately, several other reports of RPGR causing PCD did not include TEM analysis.56–58
With PCD due to RPGR mutations, the classic symptoms of PCD are present very early in life, and retinal degeneration can similarly present in early childhood or remain silent until the third decade of life. These two diseases only rarely coexist, with many more people having RPGR mutations resulting in retinal disease but not in PCD.59 Organ situs defects have not been reported in PCD cases due to RPGR. Mutations in RPGR likely account for <1% of PCD cases.11 One other syndromic form of PCD coexists with the OFD1 gene, resulting in a genetic syndrome of mental retardation and macrocephaly, yet TEM analysis was never performed in the single case report of this association.60 Mutations in both the RPGR and OFD1 genes are detected on commercial PCD genetic panels.
Conclusion
Primary Ciliary Dyskinesia is a heterogenic disorder, and no single test can reliably diagnose all cases of this disease. Approximately 30% of PCD cases (confirmed by genetic testing) have normal or near normal ciliary ultrastructure on TEM. Interpretation of TEM studies remains somewhat subjective, and therefore, requires extensive expertise because of the wide range of normal ultrastructure that can overlap with PCD-associated defects. While ciliary TEM remains an integral part of PCD diagnostic testing, its future role in PCD diagnosis will likely be as a second tier test, reserved for cases when other more standardized and objective diagnostic tests, such as nasal nitric oxide and genetic testing, are inconclusive.
Footnotes
Declaration: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- 1.Boon M, Smits A, Cuppens H, et al. Primary ciliary dyskinesia: critical evaluation of clinical symptoms and diagnosis in patients with normal and abnormal ultrastructure. Orphanet J Rare Dis. 2014;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knowles MR, Daniels LA, Davis SD, Zariwala MA, Leigh MW. Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med. 2013;188(8):913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kouis P, Yiallouros PK, Middleton N, Evans JS, Kyriacou K, Papatheodorou SI. Prevalence of primary ciliary dyskinesia in consecutive referrals of suspect cases and the transmission electron microscopy detection rate: a systematic review and meta-analysis. Pediatr Res. 2017;81(3):398–405. [DOI] [PubMed] [Google Scholar]
- 4.Papon JF, Coste A, Roudot-Thoraval F, et al. A 20-year experience of electron microscopy in the diagnosis of primary ciliary dyskinesia. Eur Respir J. 2010;35(5):1057–1063. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro AJ, Zariwala MA, Ferkol T, et al. Diagnosis, monitoring, and treatment of primary ciliary dyskinesia: PCD foundation consensus recommendations based on state of the art review. Pediatr Pulmonol. 2016;51(2):115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bush A, Cole P, Hariri M, et al. Primary ciliary dyskinesia: diagnosis and standards of care. Eur Respir J. 1998;12(4):982–988. [DOI] [PubMed] [Google Scholar]
- 7.Leigh MW, O’Callaghan C, Knowles MR. The challenges of diagnosing primary ciliary dyskinesia. Proc. 2011;8(5):434–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porter ME. Axonemal dyneins: assembly, organization, and regulation. Curr Opin Cell Biol. 1996;8(1):10–17. [DOI] [PubMed] [Google Scholar]
- 9.de Iongh RU, Rutland J. Ciliary defects in healthy subjects, bronchiectasis, and primary ciliary dyskinesia. Am J Respir Crit Care Med. 1995;151(5):1559–1567. [DOI] [PubMed] [Google Scholar]
- 10.O’Callaghan C, Rutman A, Williams GM, Hirst RA. Inner dynein arm defects causing primary ciliary dyskinesia: repeat testing required. Eur Respir J. 2011;38(3):603–607. [DOI] [PubMed] [Google Scholar]
- 11.Zariwala MA, Knowles MR, Leigh MW. Primary Ciliary Dyskinesia In: Pagon RA, Adam MP, Ardinger HH, et al. , eds. GeneReviews(R). Seattle (WA): University of Washington, Seattle University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved.; 1993. [PubMed] [Google Scholar]
- 12.Tanaka H, Iguchi N, Toyama Y, et al. Mice deficient in the axonemal protein Tektin-t exhibit male infertility and immotile-cilium syndrome due to impaired inner arm dynein function. Mol Cell Biol. 2004;24(18):7958–7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben Khelifa M, Coutton C, Zouari R, et al. Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am J Hum Genet. 2014;94(1):95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neesen J, Kirschner R, Ochs M, et al. Disruption of an inner arm dynein heavy chain gene results in asthenozoospermia and reduced ciliary beat frequency. Hum Mol Genet. 2001;10(11):1117–1128. [DOI] [PubMed] [Google Scholar]
- 15.Carlen B, Lindberg S, Stenram U. Absence of nexin links as a possible cause of primary ciliary dyskinesia. Ultrastruct Pathol. 2003;27(2):123–126. [DOI] [PubMed] [Google Scholar]
- 16.Jorissen M, Willems T, Van der Schueren B, Verbeken E. Secondary ciliary dyskinesia is absent after ciliogenesis in culture. Acta Otorhinolaryngol Belg. 2000;54(3):333–342. [PubMed] [Google Scholar]
- 17.Jorissen M, Willems T. The secondary nature of ciliary (dis)orientation in secondary and primary ciliary dyskinesia. Acta Otolaryngol. 2004;124(4):527–531. [DOI] [PubMed] [Google Scholar]
- 18.Rossman CM, Lee RM, Forrest JB, Newhouse MT. Nasal ciliary ultrastructure and function in patients with primary ciliary dyskinesia compared with that in normal subjects and in subjects with various respiratory diseases. Am Rev Respir Dis. 1984;129(1):161–167. [DOI] [PubMed] [Google Scholar]
- 19.Knowles MR, Ostrowski LE, Loges NT, et al. Mutations in SPAG1 cause primary ciliary dyskinesia associated with defective outer and inner dynein arms. Am J Hum Genet. 2013;93(4):711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanchon S, Legendre M, Copin B, et al. Delineation of CCDC39/CCDC40 mutation spectrum and associated phenotypes in primary ciliary dyskinesia. J Med Genet. 2012;49(6):410–416. [DOI] [PubMed] [Google Scholar]
- 21.Becker-Heck A, Zohn IE, Okabe N, et al. The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation. Nat Genet. 2011;43(1):79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merveille AC, Davis EE, Becker-Heck A, et al. CCDC39 is required for assembly of inner dynein arms and the dynein regulatory complex and for normal ciliary motility in humans and dogs. Nat Genet. 2011;43(1):72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antony D, Becker-Heck A, Zariwala MA, et al. Mutations in CCDC39 and CCDC40 are the major cause of primary ciliary dyskinesia with axonemal disorganization and absent inner dynein arms. Hum Mutat. 2013;34(3):462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwabe GC, Hoffmann K, Loges NT, et al. Primary ciliary dyskinesia associated with normal axoneme ultrastructure is caused by DNAH11 mutations. Hum Mutat. 2008;29(2):289–298. [DOI] [PubMed] [Google Scholar]
- 25.Fliegauf M, Olbrich H, Horvath J, et al. Mislocalization of DNAH5 and DNAH9 in respiratory cells from patients with primary ciliary dyskinesia. Am J Respir Crit Care Med. 2005;171(12):1343–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dougherty GW, Loges NT, Klinkenbusch JA, et al. DNAH11 Localization in the Proximal Region of Respiratory Cilia Defines Distinct Outer Dynein Arm Complexes. Am J Respir Cell Mol Biol. 2016;55(2):213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knowles MR, Leigh MW, Carson JL, et al. Mutations of DNAH11 in patients with primary ciliary dyskinesia with normal ciliary ultrastructure. Thorax. 2012;67(5):433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucas JS, Adam EC, Goggin PM, et al. Static respiratory cilia associated with mutations in Dnahc11/DNAH11: a mouse model of PCD. Hum Mutat. 2012;33(3):495–503. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro AJ, Davis SD, Ferkol T, et al. Laterality defects other than situs inversus totalis in primary ciliary dyskinesia: insights into situs ambiguus and heterotaxy. Chest. 2014;146(5):1176–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olbrich H, Haffner K, Kispert A, et al. Mutations in DNAH5 cause primary ciliary dyskinesia and randomization of left-right asymmetry. Nat Genet. 2002;30(2):143–144. [DOI] [PubMed] [Google Scholar]
- 31.Leigh MW, Hazucha MJ, Chawla KK, et al. Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia. Ann Am Thorac Soc. 2013;10(6):574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dawe HR, Shaw MK, Farr H, Gull K. The hydrocephalus inducing gene product, Hydin, positions axonemal central pair microtubules. BMC Biol. 2007;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olbrich H, Schmidts M, Werner C, et al. Recessive HYDIN mutations cause primary ciliary dyskinesia without randomization of left-right body asymmetry. Am J Hum Genet. 2012;91(4):672–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raidt J, Wallmeier J, Hjeij R, et al. Ciliary beat pattern and frequency in genetic variants of primary ciliary dyskinesia. Eur Respir J. 2014;44(6):1579–1588. [DOI] [PubMed] [Google Scholar]
- 35.Wallmeier J, Al-Mutairi DA, Chen CT, et al. Mutations in CCNO result in congenital mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat Genet. 2014;46(6):646–651. [DOI] [PubMed] [Google Scholar]
- 36.Boon M, Wallmeier J, Ma L, et al. MCIDAS mutations result in a mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat Commun. 2014;5:4418. [DOI] [PubMed] [Google Scholar]
- 37.Amirav I, Wallmeier J, Loges NT, et al. Systematic Analysis of CCNO Variants in a Defined Population: Implications for Clinical Phenotype and Differential Diagnosis. Hum Mutat. 2016;37(4):396–405. [DOI] [PubMed] [Google Scholar]
- 38.Casey JP, McGettigan PA, Healy F, et al. Unexpected genetic heterogeneity for primary ciliary dyskinesia in the Irish Traveller population. Eur J Hum Genet. 2015;23(2):210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bower R, Tritschler D, Vanderwaal K, et al. The N-DRC forms a conserved biochemical complex that maintains outer doublet alignment and limits microtubule sliding in motile axonemes. Mol Biol Cell. 2013;24(8):1134–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wirschell M, Olbrich H, Werner C, et al. The nexin-dynein regulatory complex subunit DRC1 is essential for motile cilia function in algae and humans. Nat Genet. 2013;45(3):262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olbrich H, Cremers C, Loges NT, et al. Loss-of-Function GAS8 Mutations Cause Primary Ciliary Dyskinesia and Disrupt the Nexin-Dynein Regulatory Complex. Am J Hum Genet. 2015;97(4):546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horani A, Brody SL, Ferkol TW, et al. CCDC65 mutation causes primary ciliary dyskinesia with normal ultrastructure and hyperkinetic cilia. PLoS ONE. 2013;8(8):e72299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Austin-Tse C, Halbritter J, Zariwala MA, et al. Zebrafish Ciliopathy Screen Plus Human Mutational Analysis Identifies C21orf59 and CCDC65 Defects as Causing Primary Ciliary Dyskinesia. Am J Hum Genet. 2013;93(4):672–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis SD, Ferkol TW, Rosenfeld M, et al. Clinical features of childhood primary ciliary dyskinesia by genotype and ultrastructural phenotype. Am J Respir Crit Care Med. 2015;191(3):316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castleman VH, Romio L, Chodhari R, et al. Mutations in radial spoke head protein genes RSPH9 and RSPH4A cause primary ciliary dyskinesia with central-microtubular-pair abnormalities. Am J Hum Genet. 2009;84(2):197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alsaadi MM, Gaunt TR, Boustred CR, et al. From a single whole exome read to notions of clinical screening: primary ciliary dyskinesia and RSPH9 p.Lys268del in the Arabian Peninsula. Ann Hum Genet. 2012;76(3):211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeanson L, Copin B, Papon JF, et al. RSPH3 Mutations Cause Primary Ciliary Dyskinesia with Central-Complex Defects and a Near Absence of Radial Spokes. Am J Hum Genet. 2015;97(1):153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daniels ML, Leigh MW, Davis SD, et al. Founder mutation in RSPH4A identified in patients of Hispanic descent with primary ciliary dyskinesia. Hum Mutat. 2013;34(10):1352–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burgoyne T, Lewis A, Dewar A, et al. Characterizing the ultrastructure of primary ciliary dyskinesia transposition defect using electron tomography. Cytoskeleton. 2014;71(5):294–301. [DOI] [PubMed] [Google Scholar]
- 50.Knowles MR, Ostrowski LE, Leigh MW, et al. Mutations in RSPH1 cause primary ciliary dyskinesia with a unique clinical and ciliary phenotype. Am J Respir Crit Care Med. 2014;189(6):707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frommer A, Hjeij R, Loges NT, et al. Immunofluorescence Analysis and Diagnosis of Primary Ciliary Dyskinesia with Radial Spoke Defects. Am J Respir Cell Mol Biol. 2015;53(4):563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Onoufriadis A, Shoemark A, Schmidts M, et al. Targeted NGS gene panel identifies mutations in RSPH1 causing primary ciliary dyskinesia and a common mechanism for ciliary central pair agenesis due to radial spoke defects. Hum Mol Genet. 2014;23(13):3362–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kott E, Legendre M, Copin B, et al. Loss-of-function mutations in RSPH1 cause primary ciliary dyskinesia with central-complex and radial-spoke defects. Am J Hum Genet. 2013;93(3):561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bukowy-Bieryllo Z, Zietkiewicz E, Loges NT, et al. RPGR mutations might cause reduced orientation of respiratory cilia. Pediatr Pulmonol. 2013;48(4):352–363. [DOI] [PubMed] [Google Scholar]
- 55.Moore A, Escudier E, Roger G, et al. RPGR is mutated in patients with a complex X linked phenotype combining primary ciliary dyskinesia and retinitis pigmentosa. J Med Genet. 2006;43(4):326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zito I, Downes SM, Patel RJ, et al. RPGR mutation associated with retinitis pigmentosa, impaired hearing, and sinorespiratory infections. J Med Genet. 2003;40(8):609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohga H, Suzuki T, Fujiwara H, Furutani A, Koga H. [A case of immotile cilia syndrome accompanied by retinitis pigmentosa]. Nippon Ganka Gakkai Zasshi. 1991;95(8):795–801. [PubMed] [Google Scholar]
- 58.van Dorp DB, Wright AF, Carothers AD, Bleeker-Wagemakers EM. A family with RP3 type of X-linked retinitis pigmentosa: an association with ciliary abnormalities. Hum Genet. 1992;88(3):331–334. [DOI] [PubMed] [Google Scholar]
- 59.Tee JJ, Smith AJ, Hardcastle AJ, Michaelides M. RPGR-associated retinopathy: clinical features, molecular genetics, animal models and therapeutic options. Br J Ophthalmol. 2016;100(8):1022–1027. [DOI] [PubMed] [Google Scholar]
- 60.Budny B, Chen W, Omran H, et al. A novel X-linked recessive mental retardation syndrome comprising macrocephaly and ciliary dysfunction is allelic to oral-facial-digital type I syndrome. Hum Genet. 2006;120(2):171–178. [DOI] [PubMed] [Google Scholar]
- 61.Horani A, Brody SL, Ferkol TW. Picking up speed: advances in the genetics of primary ciliary dyskinesia. Pediatr Res. 2014;75(1–2):158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]