Highlights
-
•
Disorders of calcium metabolism are frequently encountered in clinical practice.
-
•
Hypocalcemia accounted for 27.72% and hypercalcemia for 4.74% of inpatients.
-
•
Incidence of hypo- and hypercalcemia changed over time.
Keywords: Electrolytes, Hypercalcemia, Hypocalcemia, Inpatients, Elderly, Pediatrics
Abstract
Disorders of calcium metabolism are frequently encountered in routine clinical practice. However limited data are available on the epidemiology of hypocalcemia and hypercalcemia in hospitalized patients. Our aim was to evaluate the frequency of hypocalcemia and hypercalcemia in hospitalized patients.
This is a retrospective study based on the laboratory results of all hospitalized subjects (n = 12,334) whose calcemia was determined between January 1st, 2011 and December 31st, 2014. Measurements of serum calcium were carried out by a single centralized laboratory. Hypocalcemia was defined as serum calcium levels <8.2 mg/dl and hypercalcemia as serum calcium levels >10.4 mg/dl. Albumin correction was applied to adjust serum calcium values.
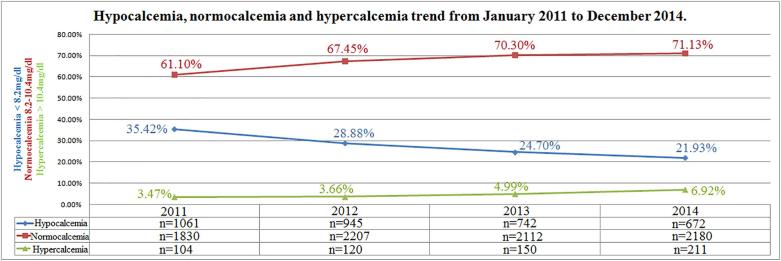
Overall, hypocalcemia accounted for 27.72% (n = 3420) and hypercalcemia for 4.74% (n = 585) of the 12,334 inpatients. The highest prevalence of hypocalcemia was found in patients over 65 yr. (n = 2097, 61.31%) vs. younger subjects, while the highest prevalence of hypercalcemia was observed in patients aged 0–18 yr. (n = 380, 64.95%). Hypocalcemia was more often encountered in males (n = 1952, 57.07%) while no gender differences were found regarding hypercalcemia. Incidence of hypocalcemia changed over time varying from 35.42% (n = 1061) in 2011 to 21.93% (n = 672) in 2014 (r = −0.98; p = 0.01). Differently, incidence of hypercalcemia did not significantly increase significantly from 3.47% (n = 104) in 2011 to 6.92% (n = 211) in 2014 (r = 0.94; p = 0.052).
Despite increased awareness about electrolytes disturbance, physicians should consider calcium levels because of life-threatening consequences associated to hypo- and hypercalcemia. Patient’s gender and age could be associated to a different risk of calcium disturbance in hospitalized patients.
Introduction
Calcium is the most abundant mineral in the body and participates with phosphorus to form calcium phosphate in bones and teeth. It is involved in many biological processes since it is essential for the normal functioning of nerves and muscles and plays a role in blood coagulation and in several enzymatic processes [1], [2].
As expected perturbation of calcium homeostasis may have a deep impact on human pathology [2]. Serum calcium levels are usually maintained within a normal range and ionized calcium is tightly regulated by the actions of parathyroid hormone (PTH) and 1,25-dihydroxyvitamin D (1,25[OH]2D) on the kidney, bone and gastrointestinal tract [3], [4].
PTH stimulates calcium release from bone and calcium resorption in the kidney, moreover it stimulates 1α-hydroxylation of 25-hydroxyvitamin D leading to the production of active 1,25-dihydroxyvitamin D (calcitriol) which modulates gastrointestinal calcium absorption [3].
In a clinical setting, either elevation (hypercalcemia) or reduction (hypocalcemia) of serum calcium concentrations could depend by several pathologies and could be associated with life-threatening consequences [2].
Increasing life expectancy and improvement of standard medical care have modified the epidemiology of some human diseases (e.g. renal failure, cancer). Thus, the incidence of calcium related disorders could be challenging over time [5], [6], [7], [8].
However, the prevalence of hypo- and hypercalcemia is dependent by the specific setting of patients [6], [7], [8].
Persistent hypercalcemia has been reported to occur in up to 1% of individuals in general population [9], [10] whereas the prevalence of hypocalcemia in hospital setting has been observed up to 3% [10]. Differently, hypocalcemia has been described to reach a prevalence of 18% in hospitalized patients and up to 85% in the intensive care units [5].
The main aim of our study was to update the incidence of hypocalcemia and hypercalcemia in a large cohort of inpatients subjects. The secondary aim was to explore whether age and gender could be associated with serum calcium alterations.
Materials and methods
This is a retrospective study considering all the patients whose calcium concentrations were determined during hospital recovery at the University Hospital of Messina, Messina, Italy, over the period from January 2011 to December 2014. Any age and gender were considered.
Total calcium levels were detected by a Centralized Laboratory of our hospital through an automated analyzer (Roche Modular Analytics P 800). All the measurements were corrected with serum albumin levels in accordance with the formula: corrected serum calcium = measured calcium + [(4.1-albumin) × 0.8] if albumin is <4.1 g/dl, or measured calcium − [(4.1-albumin) × 0.8] if albumin is >4.1 g/dl [11], [12].
Hypercalcemia was defined for calcium levels above 10.4 mg/dl, whereas hypocalcemia was defined for calcium levels under 8.2 mg/dl in accordance with the reference range of our Laboratory.
Recruited subjects came from multiple medical and surgical department clinics within our hospital.
The study was conducted in accordance with the ethical standards of our institutional research committee and with the 1964 Declaration of Helsinki and its later amendments. Written informed consent was not required. Statistical analyses were performed using MedCalc software (version 10.2.0.0; Mariakerke, 173 Belgium).
Statistical analyses were performed using MedCalc software (version 10.2.0.0; Mariakerke, 173 Belgium). The χ2 test was performed to calculate differences in the proportion of categorical variables. Linear regression was used to describe changes of incidences over time. Values of p < 0.05 were considered to indicate statistical significance.
Results
In the period from January 1st, 2011 to December 31st, 2014 we evaluated a sample of 12,334 internal patients whose calcium level was measured. We found 27.72% (n = 3420) of patients showing hypocalcemia and 4.74% (n = 585) showing hypercalcemia. Concerning patients with hypocalcemia we observed 42.93% of cases (n = 1468) in females and 57.07% (n = 1952) in males (χ2 = 136, p < 0.0001). Adult subjects were more likely to exhibit low calcium levels: in fact, hypocalcemia was found in 61.31% (n = 2097) of subjects over 65 yr., in 33.3% (n = 1139) of subjects aged 19–65 yr., finally in 5.38% (n = 184) of subjects aged 0–18 yr. (χ2 = 2407, p < 0.0001). At the same time, we detected 585 cases of hypercalcemia of whom 50.95% (n = 298) were observed in female and 49.05% (n = 287) in male patients (χ2 = 0.34, p = 0.55). The higher incidence of hypercalcemia was found in subjects aged 0–18 (64.95%, n = 380), whereas in the age range19–65 yr. was 16.92% (n = 99) and in patients over 65 yr. (n = 106) was 18.11% (χ2 = 395, p < 0.0001).
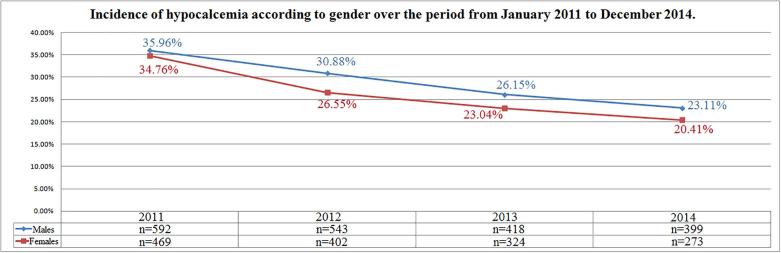
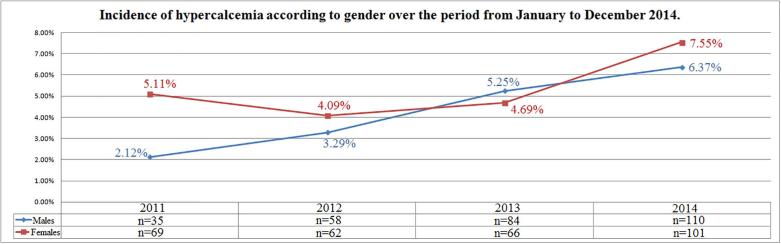
Incidence of hypocalcemia changed over time varying from 35.42% (n = 1061) in 2011 to 21.93% (n = 672) in 2014 (r = −0.98; p = 0.01); differently, incidence of hypercalcemia increased from 3.47% (n = 104) in 2011 to 6.42% (n = 211) in 2014 (r = 0.94; p = 0.052); normocalcemia cases increased from 61.10% (n = 1830) in 2011 to 71.13% (n = 2180) in 2014 (r = 0.93; p = 0.06) (Fig. 1). Incidence of hypocalcemia according to gender was reduced during the observation period in both genders (r = −0.96, p = 0.03 and r = −0.99, p = 0.006 in females and males respectively) (Fig. 2). Differently, incidence of hypercalcemia increased over time in males (r = 0.99, p = 0.006) but not in females (r = 0.67, p = 0.32) (Fig. 3).
Fig. 1.
Hypocalcemia, normocalcemia and hypercalcemia trend from January 2011 to December 2014.
Fig. 2.
Incidence of hypocalcemia according to gender over the period from January 2011 to December 2014.
Fig. 3.
Incidence of hypercalcemia according to gender over the period from January 2011 to December 2014.
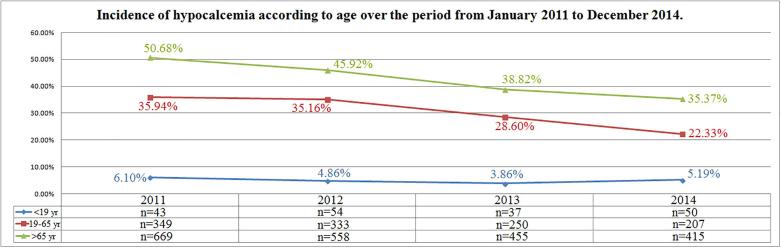
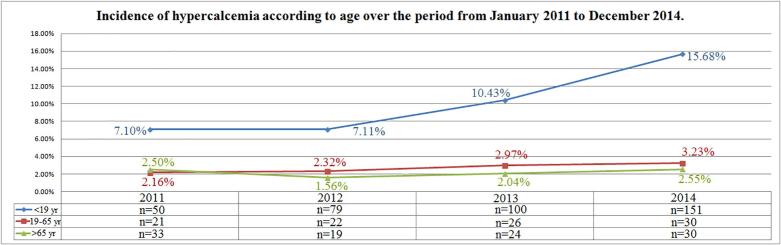
Distribution of cases of hypocalcemia according to age remained unchanged over time in the group of subjects in the age range 0–18 yr. (r = −0.52, p = 0.47) while in subjects between 19 and 65 yr. and in those ones over 65 yr. a reduction of cases was observed (r = −0.96, p = 0.04 and r = −0.99, p = 0.007, respectively) (Fig. 4). A tendency of increasing incidence of hypercalcemia was observed in the age ranges 0–18 yr. (r = 0.92, p = 0.07) and 19–65 yr. (r = 0.97, p = 0.02), while incidence of hypercalcemia remained unchanged in the subjects aged over 65 yr. (r = 0.17, p = 0.82) (Fig. 5).
Fig. 4.
Incidence of hypocalcemia according to age over the period from January 2011 to December 2014.
Fig. 5.
Incidence of hypercalcemia according to age over the period from January 2011 to December 2014.
Discussion
Our research study shows the temporal trend of serum calcium abnormalities observed in hospitalized patients. As known total calcium includes ionized calcium, protein-bound calcium and calcium complexed with inorganic and organic anions. Since about 80% of the protein-bound calcium is associated with albumin, albumin levels could influence calcemia. Thus we considered only patients whose calcium serum concentration was corrected taking in account albumin levels [11], [12], [13].
Hypo- and hypercalcemia are calcium disorders commonly observed in hospitalized patients, due to several causes as summarized in Table 1, Table 2 [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26].
Table 1.
Main causes of hypocalcemia.
|
|
|
Table 2.
Main causes of hypercalcemia.
|
|
|
Prevalence of hypocalcemia was previously reported to rank to 18% of all hospitalized patients and to be represented in 85% of patients in the intensive care units [5].
In our population the prevalence of hypocalcemia was 27.72%.
A common cause of hypocalcemia in primary care is vitamin D deficiency which is, irrespective of latitude, a frequent condition often resulting in secondary increased levels of PTH [3]. Mild and slowly developing hypocalcemia could be an asymptomatic laboratory finding, but hypocalcemia could be also a life-threatening metabolic disturbance and acute hypocalcemia can provoke severe symptoms which requires hospitalization [4], [5]. Hypocalcemia is frequently encountered in hospitalized patients and could be also empathized by low dietary calcium intake.
We noticed a tendency to a decreasing incidence of hypocalcemia over the observation period, with a reduction of cases of 13.49% from 2011 to 2014. Our findings are consistent with the wide attention recently attributed to vitamin D deficiency, especially in the elderly. As vitamin D is a key regulator of calcium absorption, adequate vitamin D status could contribute to maintain eucalcemia [27], [28], [29]. Incidence of hypocalcemia decreased of 14.35% in particular in females. This is at least in part due to more frequent prescriptions of vitamin D and/or calcium in accordance with the prevention of osteoporosis [30]. However a significant decreasing incidence of hypocalcemia was also observed in males as shown in Fig. 2.
The prevalence of hypercalcemia in our hospital was 4.74%. Our findings are consistent with previous data by Aishah et al. who found a smaller prevalence of 2.4% in hospitalized patients [31]. No relevant gender differences was found about prevalence of hypercalcemia, but an increased incidence of hypercalcemia in males and younger subjects was detected over the observation period. Although we did not investigate the causes of calcium abnormalities, we speculated that in the younger subjects an iatrogenic effect of multivitamins or calcium supplements could not be ruled out, in addition to the other causes of hypercalcemia reported in Table 2. Moreover, the high prevalence of hypercalcemia in young individuals is of concern and it might be important to warn about the consequences that this alteration may lead to: hypercalcemia could be associated with hypercalciuria and be responsible for the development of certain types of kidney stones (calcium oxalate dihydrate), the incidence of which is currently increasing in young individuals [32].
Despite increased awareness about electrolytes disturbances [33], physicians should consider calcium levels because of life-threatening consequences associated to hypo and hypercalcemia [2].
Gender and age of patient could be associated to a different risk of this electrolyte disorder in hospitalized patients.
A limitation of the present study is the impossibility of ruling out false values of calcemia due to some pre-analytical factors (e.g. venous occlusion, posture, alterations in protein binding, abnormal proteins, heparin, pH, drugs, temperature), but the large sample size is at the same time the main strength of this research. Although we did not investigated causes of calcium disturbances, we recognize as a limit of the study also the lack of data on PTH concentrations. PTH should be considered in fact as a “couple” along with calcium concentrations and should be included in the initial diagnostic work-up investigating calcium disturbances.
In conclusion our study focused on the incidence of serum calcium abnormalities in hospitalized patients, highlighting age and gender differences. Hypo- and hypercalcemia are common electrolyte disorders in hospitalized patients. Despite increased awareness about electrolytes disturbances, physicians should consider calcium levels because of life-threatening consequences associated to hypo- and hypercalcemia. Gender and age of patient could be associated to a different risk of this electrolyte disorder in hospitalized patients.
Declarations of interest
None.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jcte.2018.05.004.
Appendix A. Supplementary data
References
- 1.Espay A.J. Neurologic complications of electrolyte disturbances and acid-base balance. Handb Clin Neurol. 2014;119:365–382. doi: 10.1016/B978-0-7020-4086-3.00023-0. [DOI] [PubMed] [Google Scholar]
- 2.Akirov A., Gorshtein A., Shraga-Slutzky I., Shimon I. Calcium levels on admission and before discharge are associated with mortality risk in hospitalized patients. Endocrine. 2017 Aug;57(2):344–351. doi: 10.1007/s12020-017-1353-y. Epub 2017 Jun 30. [DOI] [PubMed] [Google Scholar]
- 3.Holick M.F. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 4.Body J.J., Bouillon R. Emergencies of calcium homeostasis. Rev Endocr Metab Disord. 2003;4(2):167–175. doi: 10.1023/a:1022994104070. [DOI] [PubMed] [Google Scholar]
- 5.Cooper M.S., Gittoes N.J. Diagnosis and management of hypocalcemia. BMJ. 2008;336(7656):1298–1302. doi: 10.1136/bmj.39582.589433.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ralston S.H., Gallacher S.J., Patel U., Campbell J., Boyle I.T. Cancer associated hypercalcemia: morbidity and mortality. Clinical experience in 126 treated patients. Ann Intern Med. 1990;112:499–504. doi: 10.7326/0003-4819-112-7-499. [DOI] [PubMed] [Google Scholar]
- 7.Egbuna O.I., Brown E.M. Hypercalcemia and hypocalcemia conditions due to calcium-sensing receptor mutations. Best Pract Res Clin Rheumatol. 2008;22(1):129–148. doi: 10.1016/j.berh.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liamis G., Milionis H.J., Elisaf M. A review of drug-induced hypocalcemia. J Bone Miner Metab. 2009;27(6):635–642. doi: 10.1007/s00774-009-0119-x. [DOI] [PubMed] [Google Scholar]
- 9.Palmér M., Jakobsson S., Akerström G., Ljunghall S. Prevalence of hypercalcaemia in a health survey: a 14-year follow-up study of serum calcium values. Eur J Clin Invest. 1988;18(1):39–46. doi: 10.1111/j.1365-2362.1988.tb01163.x. [DOI] [PubMed] [Google Scholar]
- 10.Frolich A. Prevalence of hypercalcemia in normal and in hospital populations. Dan Med Bull. 1998;45:436–439. [PubMed] [Google Scholar]
- 11.Catalano A., Basile G., Lasco A. Hypocalcemia: a sometimes overlooked cause of heart failure in the elderly. Aging Clin Exp Res. 2012;24(4):400–403. doi: 10.1007/BF03325272. [DOI] [PubMed] [Google Scholar]
- 12.Labriola L., Wallemacq P., Gulbis B., Jadoul M. The impact of the assay for measuring albumin on corrected calcium concentrations. Nephrol Dial Transplant. 2009;24:1834–1838. doi: 10.1093/ndt/gfn747. [DOI] [PubMed] [Google Scholar]
- 13.Riancho J.A., Arjona R., Sanz J., Olmos J.M., Valle R., Barcello J.R. Is the routine measurement of ionized calcium worthwhile in patients with cancer? Postgrad Med J. 1991;67:350–353. doi: 10.1136/pgmj.67.786.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bilezikian J.P., Khan A., Potts J.T., Jr, Ml Brandi., Clark B.L., Shoback D. Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res. 2011;26(10):2317–2337. doi: 10.1002/jbmr.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen C.J., Brown S. Severe hypocalcemia after intravenous bisphosphonate therapy in occult vitamin D deficiency. N Engl J Med. 2003;348(15):1503. doi: 10.1056/NEJM200304103481521. [DOI] [PubMed] [Google Scholar]
- 16.Mishra A., Wong L., Jonklaas J. Prolonged, symptomatic hypocalcemia with pamidronate administration and subclinical hypoparathyroidism. Endocrine. 2001;14(2):159–164. doi: 10.1385/ENDO:14:2:159. [DOI] [PubMed] [Google Scholar]
- 17.Tohem J.F., Bilezikian J.P. Diagnosis and treatment of hypocalcemic emergencies. Endocrinologist. 1996;6(1):10–18. [Google Scholar]
- 18.Lasco A., Catalano A., Morabito N. Are physicians ready to prevent osteoporotic fractures in hemodialysis patients? Int J Endocrinol Metab. 2012;10(4):634–635. doi: 10.5812/ijem.6239. Fall. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart A.F. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005;352:373–379. doi: 10.1056/NEJMcp042806. [DOI] [PubMed] [Google Scholar]
- 20.Brown E.M. Familial hypocalciuric hypercalcemia and other disorders with resistance to extracellular calcium. Endocrinol Metab Clin North Am. 2000;29:503–522. doi: 10.1016/s0889-8529(05)70148-1. [DOI] [PubMed] [Google Scholar]
- 21.Eftekhari F., Yousefzadeh D. Primary infantile hyperparathyroidism: clinical, laboratory and radiographic features in 21 cases. Skeletal Radiol. 1982;8:201–208. doi: 10.1007/BF00355507. [DOI] [PubMed] [Google Scholar]
- 22.Sargent J.T.S., Smith O.P. Haematological emergencies managing hypercalcemia in adults and children with haematological disorders. Br J Haematol. 2010;149:465–477. doi: 10.1111/j.1365-2141.2010.08173.x. [DOI] [PubMed] [Google Scholar]
- 23.Endelson G.W., Kleerekoper M. Hypercalcemic crisis. Med Clin North Am. 1995;79:79–92. doi: 10.1016/s0025-7125(16)30085-2. [DOI] [PubMed] [Google Scholar]
- 24.Nussbaum S.R., Gaz R.D., Arnold A. Hypercalcemia and ectopic secretion of parathyroid hormone by an ovarian carcinoma with rearrangement of the gene for parathyroid hormone. N Engl J Med. 1990;323:1324–1328. doi: 10.1056/NEJM199011083231907. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y.C., Sosnoski D.M., Mastro A.M. Breast cancer metastasis to the bone: mechanism of bone loss. Breast Cancer Res. 2010;12:215–226. doi: 10.1186/bcr2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clines G.A. Mechanisms and treatment of hypercalcemia of malignancy. Curr Opin Endocrinol Diabetes Obes. 2011;18:339–346. doi: 10.1097/MED.0b013e32834b4401. [DOI] [PubMed] [Google Scholar]
- 27.Catalano A., Morabito N., Di Stefano A., Morini E., Basile G., Faraci B. Vitamin D and bone mineral density changes in postmenopausal women treated with strontium ranelate. J Endocrinol Invest. 2015;38(8):859–863. doi: 10.1007/s40618-015-0299-2. [DOI] [PubMed] [Google Scholar]
- 28.Catalano A., Morabito N., Basile G., Cucinotta D., Lasco A. Calcifediol improves lipid profile in osteopenicatorvastatin-treated postmenopausal women. Eur J Clin Invest. 2015 Feb;45(2):144–149. doi: 10.1111/eci.12390. [DOI] [PubMed] [Google Scholar]
- 29.Catalano A., Morabito N., Atteritano M., Basile G., Cucinotta D., Lasco A. Vitamin D reduces musculoskeletal pain after infusion of zoledronic acid for postmenopausal osteoporosis. Calcif Tissue Int. 2012;90(4):279–285. doi: 10.1007/s00223-012-9577-6. [DOI] [PubMed] [Google Scholar]
- 30.Catalano A., Morabito N., Basile G., Fusco S., Castagna G., Reitano F. Fracture risk assessment in postmenopausal women referred to an Italian center for osteoporosis: a single day experience in Messina. Clin Cases Miner Bone Metab. 2013;10(3):191–194. [PMC free article] [PubMed] [Google Scholar]
- 31.Aishah A.B., Foo Y.N. A retrospective study of serum calcium levels in a hospital population in Malaysia. Med J Malaysia. 1995;50(3):246–249. [PubMed] [Google Scholar]
- 32.Tasian G.E., Ross M.E., Song L., Sas D.J., Keren R., Denburg M.R. Annual incidence of nephrolithiasis among children and adults in South Carolina from 1997 to 2012. Clin J Am Soc Nephrol. 2016;11(3):488–496. doi: 10.2215/CJN.07610715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Catalano A., Basile G., Ferro C., Bellone F., Scarcella C., Benvenga S. Hyponatremia as a leading sign of hypopituitarism. J Clin Transl Endocrinol: Case Rep. 2017;5:1–3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.