Abstract
Introduction
Phenylketonuria (PKU) is an inborn error of metabolism associated with an increased risk of behavioural and mood disorders. There are currently no reliable markers for monitoring mood in PKU. The purpose of this study was to evaluate salivary serotonin as a possible non-invasive marker of long-term mood symptoms and central serotonin activity in patients with PKU.
Methods
20 patients were recruited from our Adult Metabolic Diseases Clinic. Age, sex, plasma phenylalanine (Phe) level, DASS (Depression Anxiety Stress Scales) depression score, DASS anxiety score, BMI, salivary serotonin, salivary cortisol, 2-year average Phe, 2-year average tyrosine (Tyr), and 2-year average Phe:Tyr ratio were collected for each patient. Spearman's ρ correlation analysis was used to determine if there was any relationship between any of the parameters.
Results
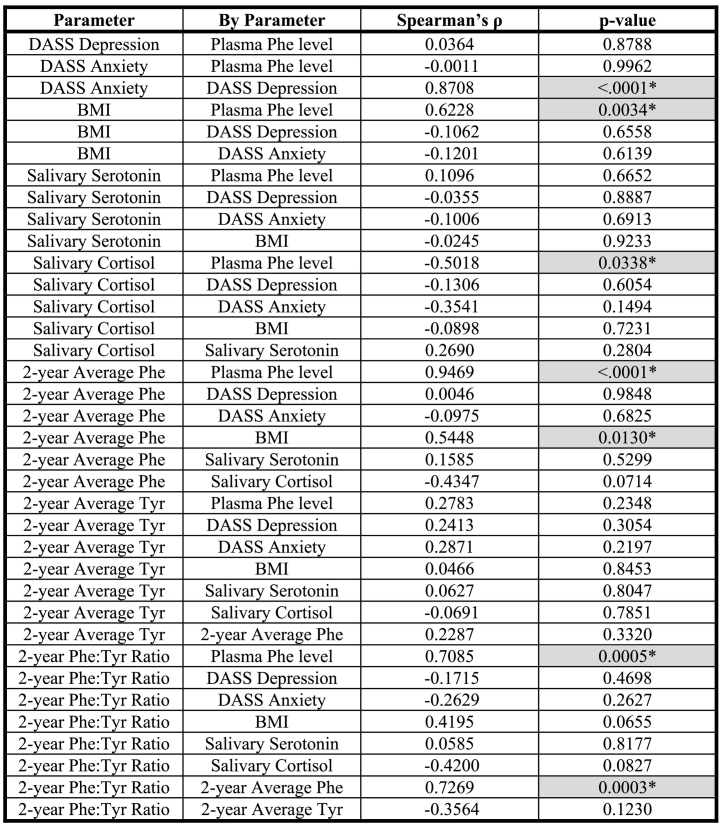
There were positive correlations between DASS anxiety and DASS depression scores (Spearman's ρ = 0.8708, p-value < 0.0001), BMI and plasma Phe level (Spearman's ρ = 0.6228, p-value = .0034), and 2-year average Phe and BMI (Spearman's ρ = 0.5448, p-value = .0130). There was also a negative correlation between salivary cortisol and plasma Phe level (Spearman's ρ = −0.5018, p-value = .0338). All other correlations were not statistically significant.
Conclusion
Salivary serotonin does not correlate with peripheral phenylalanine levels, DASS depression scale scores, or DASS anxiety scale scores, implying that salivary serotonin does not reflect central serotonin turnover. Additionally, this study suggests that salivary serotonin is not a suitable marker for monitoring dietary control, mood, or anxiety in PKU.
Synopsis
Salivary serotonin does not correlate with peripheral phenylalanine levels, DASS depression scale scores, or DASS anxiety scale scores, suggesting that salivary serotonin is not a suitable marker for monitoring dietary control, mood, or anxiety in PKU.
Keywords: Phenylketonuria (PKU), Salivary serotonin, Salivary cortisol, Depression, Anxiety, Mood disorders
1. Introduction
Since the discovery of Phenylketonuria (PKU) in 1934 by Ivar Asbjørn Følling, much research has been invested in the management of this inborn error of metabolism. While the mainstay of treatment is centered on protein-restricted diets, other therapies have been researched and utilized, such as BH4 supplementation, large neutral amino acids (LNAA), and glycomacropeptides (GMP). Recent developments include gene therapy and enzyme substitution or replacement [1].
Continued research is necessary because of suboptimal cognitive and executive function, as well as increased risk of behavioural and mood disorders in those with poor dietary management. Even in early-treated children and adolescents, psychological and psychiatric complications such as depression, anxiety, stress experience, and reduced self-esteem along with attentional impairment, restricted scholastic performance and achievement, executive functioning limitations, and concomitant restricted autonomy are commonplace. In adulthood, conditions such as generalized anxiety, phobias, depressed mood, social maturity deficits, and social isolation are common barriers to functioning and quality of life [13,40].
From a biological standpoint, elevations in phenylalanine (Phe) levels probably do not directly cause the psychiatric symptoms. There are three likely mechanisms through which hyperphenylalaninemia affects brain function indirectly:
-
1.
Elevated Phe concentrations have a neurotoxic effect that in turn contributes to structural brain damage and inhibition of myelin development in poorly-treated children and dysmyelination in adults who discontinue the Phe-restricted diet [36]. Even children with early-treated PKU can have white matter changes on MRI [3]. Neuroimaging studies demonstrate high-signal intensity in the periventricular white matter, which can extend into subcortical and frontal regions in more severe cases. The histopathology of these lesions reveals that diffuse white matter pathology in untreated patients reflects hypomyelination, while in the early-treated patients is more likely due to intramyelinic edema, or intracellular accumulation of a hydrophilic metabolite [23]. This pathology is associated with metabolic control and may be reversed if patients adhere to a strict Phe-restricted diet [4].
-
2.
Increased concentrations of Phe overwhelm the common amino acid transport system into the brain, which cause the failure of import of several amino acids, such as tyrosine and tryptophan. [22]. It is well known that large neutral amino acids (LNAA), including phenylalanine, compete for transport across the blood-brain-barrier via the L-type amino acid carrier. The direct effects of the elevated brain Phe and depleted LNAA are probably the cause of the abnormalities in brain development and function seen in untreated PKU. When the plasma LNAAs are increased, the influx of Phe into the brain may be lowered [37].
-
3.
Reductions of brain tyrosine and tryptophan lead to reductions in brain dopamine and serotonin, since the former are precursors of the latter. In turn, this neurotransmitter deficiency is associated with depression (serotonin deficiency) and behavioural disturbances (especially frontal lobe dysfunction in dopamine deficiency) [35]. Sleep disturbances in PKU patients have been also well documented [11]. Bioavailability of tyrosine due to changes in intestinal microbiota and altered metabolism of tryptophan via the kynurenine pathway in patients who are ingesting medical foods also contribute to the bioamine defects [32]. Finally, animal studies have been extremely useful to investigate and demonstrate the mechanisms by which elevated Phe affects the brain, discussing the importance of changes in protein synthesis, transport of LNAA, synthesis of monoamine neurotransmitters and activity of glutamate receptors amongst other mechanisms [28].
Currently, there is no reliable biochemical marker that can be used as surrogate for chronic symptoms of depression and anxiety in patients with PKU. Rather, we use self-report assessment tools for psychiatric conditions to identify these symptoms. A few previous studies assessed the utility of CSF dopamine and serotonin in PKU patients [6,20], but the invasiveness of this test renders it unsuitable for routine monitoring. Thus, we sought to determine the relationship between salivary serotonin levels and psychological symptoms associated with depression and anxiety in young adults with PKU. Our hypothesis was that salivary serotonin would correlate with these psychological entities and could be used as a non-invasive marker of central serotonin activity in individuals with PKU. Positive correlation between platelet and CSF serotonin has been demonstrated before in non-PKU patients and rats, but no studies looking for correlation between salivary and CSF serotonin levels were done [5].
2. Methods
2.1. Ethics and recruitment
Institutional research ethics board (UBC Ethics Board, ID# H12–01687) approval was obtained, after which we recruited and obtained informed consent from 20 adults with early-treated PKU who had been treated in our Adult Metabolic Diseases Clinic. Exclusion criteria included individuals on Selective Serotonin Reuptake Inhibitors (SSRIs), considering the effect of these drugs of lowering peripheral serotonin levels [2].
2.2. Phe level and salivary sampling
At a regular follow-up clinic visit, a blood spot Phe level was drawn and a clean pipette sample of approximately 0.2–0.5 mL of saliva was drawn from the mouth the morning of that clinic visit, between 8 and 11 AM, which is the peak salivary serotonin secretion [39]. Phenylalanine analysis was performed on a Biochrome amino acid analyzer (with ion exchange column). Patients were instructed to avoid heavy exercise, sexual intercourse, alcohol, caffeine, and cheese on the sampling day. They were also required to brush their teeth without toothpaste and to rinse their mouth with water 10 min before sampling. We have also ran 3 saliva samples as control from 3 non-PKU adults.
2.3. Average Phe, Average Tyr, and BMI
Average Phe levels over the previous two years were calculated for each individual to ensure representative Phe levels. Similarly, average Tyrosine (Tyr) levels for the previous two years was calculated to exclude nutritional tyrosine deficiency. Height and weight were used to calculated body mass index (BMI).
2.4. DASS: Depression, Anxiety and Stress Scale
Study participants completed a short psychometric instrument that evaluates psychological symptoms commonly experienced by PKU populations. The DASS is a validated 42-item self-report instrument developed as a clinical and research tool to measure core symptoms as conventionally defined within three negative clinically significant emotional states (typically described as depression, anxiety, and stress) [10,16,25]. Respondents were asked to rate each item according to a 4-point scale with regard to the extent over the previous week that each statement applied to them. Subscale scores for each of the three symptom constellations were calculated by summation. Scores between 0 and 9 for depression and between 0 and 7 for anxiety are considered to be normal, whereas scores above the respective cutoffs are categorized in the scale manual as mild, moderate, severe or extremely severe.
2.5. Salivary serotonin and cortisol
Salivary serotonin levels were determined by Serotonin Ultrasensitive ELISA using the Eagle Biosciences Kit (Catalog#: SEU39-K01, lot SES 109). Three replicates were conducted for each sample. Salivary cortisol levels were determined by Salivary Cortisol ELISA using the Eagle Biosciences Kit (Catalog#: OR32-K01, lot 3809). One replicate was conducted for each sample. The Eagle Biosciences Program (elisaanalysis.com) was used to plot photometric data and extrapolate salivary serotonin and cortisol levels.
Statistical analysis involved calculation of the means and standard deviations for the measured parameters. SAS JMP 13.1.0 was used for all statistical analyses.
Normality of the data was determined using the Shapiro-Wilks test. Student's t-test for normally distributed data (plasma Phe levels and age) and Wilcoxon's rank sum test for non-normally distributed data (the remaining 8 variables) were used to determine if there were statistically significant differences between males and females. Non-parametric Spearman's ρ correlation analysis was applied given the majority of the data were not normally distributed between the 10 continuous variables (age, plasma Phe level, DASS depression score, DASS anxiety score, BMI, salivary serotonin, salivary cortisol, 2-year average Phe, 2-year average Tyr, 2-year average Phe:Tyr ratio).
3. Results
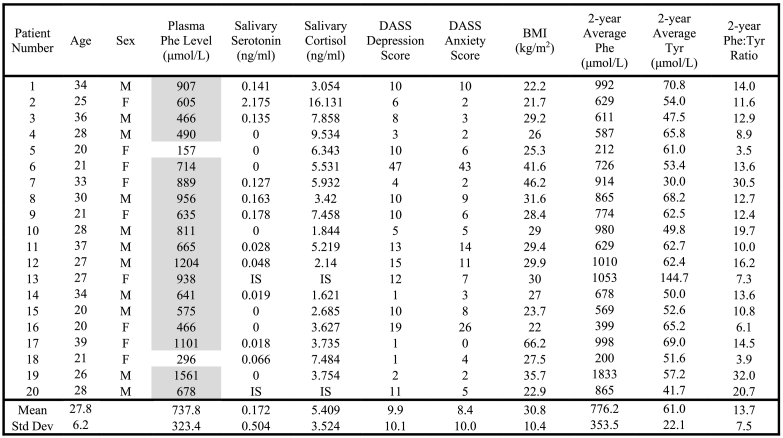
Raw values, means, and standard deviations of age, sex, plasma Phe level, salivary serotonin, salivary cortisol, DASS depression scale, DASS anxiety scale, BMI, 2-year average Phe, 2-year average Tyr, and 2-year average Phe:Tyr ratio are presented in Table 1. Salivary serotonin and cortisol values could not be reported for two individuals because of insufficient saliva samples. There were no statistically significant differences between males and females for any of the variables (data not shown).
Table 1.
Results of plasma phe level, salivary serotonin, salivary cortisol, DASS depression score, DASS anxiety score, gender, BMI, 2-year Average Phe, and 2-year Average Tyr.
Plasma Phe Levels in grey shading are above the target range (target range 120–360 μmol/L). Phe = Phenylalanine; Tyr = Tyrosine; BMI = Body Mass Index (kg/m2); IS = Insufficient Sample; Std Dev = Standard Deviation.
The results of the Spearman's ρ correlation analysis are shown in Table 2. There were statistically significant positive correlations between DASS anxiety and DASS depression scores (Spearman's ρ = 0.8708, p-value < 0.0001), BMI and plasma Phe level (Spearman's ρ = 0.6228, p-value = .0034), and 2-year average Phe and BMI (Spearman's ρ = 0.5448, p-value = 0.0130). There was also a statistically significant negative correlation between salivary cortisol and plasma Phe level (Spearman's ρ = −0.5018, p-value = 0.0338). All other correlations, including all correlations involving age (data not shown) and all correlations involving salivary serotonin were not statistically significant.
Table 2.
Correlation between Plasma Phe level, DASS Depression Score, DASS Anxiety Score, BMI, Salivary Serotonin, Salivary Cortisol, 2-year Average Phe, 2-year Average Tyr, and 2-year Average Phe:Tyr Ratio.
For these 9 parameters, the 36 possible correlation interactions are shown. Plasma Phe level was the only parameter that was normally distributed. Thus, the non-parametric Spearman's ρ correlation coefficient was determined for each of the correlation interactions. Phe = Phenylalanine; Tyr = Tyrosine; BMI = Body Mass Index (kg/m2); * (with grey shading) = statistically significant correlation (two-sided p-value <.05).
4. Discussion
4.1. Salivary serotonin, depression, and anxiety
This study sought to determine the utility of salivary serotonin as a measure of central serotonin activity. We did not find statistically significant associations between salivary serotonin and peripheral phenylalanine levels or between salivary serotonin and measures of depression and anxiety. This is in contrast with the findings of Matsunaga, who found negative correlation between salivary serotonin levels and happiness rating scores in healthy young adults. This may be due to the fact that serotonin measurements were at a single point, rather than multiple, throughout the day, reflecting a circadian rhythm. It may also be related to the uniqueness of the PKU patient population [30]. There is no evidence that salivary serotonin is derived from blood, as it has not been shown that the serotonergic system directly projects from the brain to salivary glands [18]. Our findings suggest that salivary serotonin does not reflect central serotonin turnover, but rather peripheral serotonin levels and like plasma serotonin it does not reflect central serotonin functioning.
Serotonin (5-hydroxytryptamine or 5-HT) is a monoamine neurotransmitter synthesized in the neurons of the midbrain Raphe nuclei in the CNS and in the enterochromaffin cells of the gut. The rate-limiting step in serotonin synthesis is catalyzed by two genetically distinct isoforms of tryptophan hydroxylase: TPH1, which is expressed peripherally and in the pineal gland, and TPH2, which is preferentially expressed in the brain. Serotonin is stored intracellularly in vesicles and granules [45].
Re-uptake of neuronal serotonin across presynaptic plasma membranes is mediated by the serotonin transporter (SERT). SERT is highly expressed in platelets, which do not synthesize serotonin but store serotonin produced by the enterochromaffin cells. Serotonin measurement in blood measures platelet serotonin level, which is equivalent to peripheral serotonin stores. Brain serotonin levels must be measured in cerebrospinal fluid (CSF). Salivary serotonin, measured in patients with major depressive disorders has been shown to have diurnal variation like melatonin, and was previously suspected to correlate more with the central, than peripheral, serotonin levels [39]. Whereas salivary prolactin has demonstrated consistent association with CSF levels of 5-hydroxy-indoleacetic-acid (5HIAA), a serotonin metabolite, in primates, that relationship has not been validated in humans, and such it is not a suitable marker for central serotonin turnover studies in humans [24,42]. Salivary cortisol levels have also been shown to correlate with central serotonergic activity after waking in depressed patients [43].
Other indices of serotonin activity have been explored in patients with PKU. Platelet serotonin concentrations correlated well with PKU dietary treatment in one study, although they do not reflect central serotonergic activity [33]. Yano et al. [46] showed that urinary 6-sulfatoxymelatonin and dopamine are good biological markers for monitoring dietary therapy in PKU. They also demonstrated that supplementation with Large Neutral Amino Acids (LNAA) led to increased serum melatonin and urine dopamine levels in PKU [47]. However, since melatonin is synthesized from serotonin in the pineal body, it could be a marker for the peripheral serotonin synthesized through TPH1, which is expressed in the periphery and pineal gland and such, not a marker for central serotonin turnover.
4.2. Plasma Phe, depression, and anxiety
Although some authors have found that short-term high Phe levels have significant direct negative effects on mood and were predictive of anxiety-related symptoms [41], we did not find an association between Phe level and DASS test findings for depression and anxiety, consistent with findings by other authors [12,27]. While it is clear that adults with PKU have a higher incidence of depression and anxiety [13,35], PKU-related depression and anxiety symptoms appear to be more strongly related to measures of PKU control early in life [12]. There is also a significant positive correlation shown between Phe levels and psychiatric symptom severity in adults with PKU [7,8]. Moreover, sustained reduction of Phe levels in adult PKU patients who discontinued their diet, can lead to improvement of the neuropsychological abnormalities and emotional wellbeing [9].
4.3. Secondary correlations
Despite the lack of correlation as discussed above, we were able to document statistically significant correlations in some of the secondary parameters that we evaluated. The strong positive correlation between DASS depression and DASS anxiety scores is not surprising, since depression and anxiety are co-morbid conditions in PKU. Clacy et al. [15] showed that DASS depression, DASS anxiety, and DASS stress scores were all elevated in 8 patients aged 15–25 with lifelong elevated Phe:Tyr ratios when compared to a normative general population. In our study, DASS depression and DASS anxiety scores did not correlate with Phe:Tyr ratios. This may be due to the fact that our data included only 2-year Phe:Tyr ratios rather than lifelong records. Additionally, the mean age of patients in that study was 19.37 years, whereas it was 27.8 years in our study.
The positive correlation between BMI and plasma Phe level has previously been reported in the literature. Robertson et al. [38] described a positive association between BMI and Phe concentrations in a British sample of 236 individuals with PKU. As one explanation for that finding, it is possible that a low Phe diet encourages higher carbohydrate intake. Other potential explanations for this relationship include poor diet adherence in older patients who move away from home and start college or work. They often chose more fast foods that are higher in fat and protein, being also under pressure from their peers to eat “normal”. Higher Phe levels can affect organizational skills and executive functioning, which in turn will affect decision making around food choices, but also prevent them from organizing themselves to do regular exercise.
Our data showed a negative correlation between salivary cortisol and plasma Phe level, consistent with a physiological mechanism previously uncovered in a number of rat studies [14,21,31,34]. Greengard and Delvalle [19] showed that cortisol is a positive regulator of phenylalanine hydroxylase (PAH) and increases the enzyme activity in immature rat liver, but has no effect in adult ones. Mutant Reuber H4 hepatoma cells express very little PAH and contain <5% of the parental mRNA, but PAH gene transcription is stimulated when incubated with hydrocortisone [17]. Our data in PKU individuals suggest that elevated glucocorticoid levels can stimulate PAH levels and/or activity, lowering plasma Phe levels, possibly explaining the negative correlation we observed. Further clinical studies in human PKU populations are necessary to more fully characterize this potential regulatory effect of glucocorticoids on PAH. Higher salivary cortisol levels have been associated with depression and anxiety in several studies [26,44]. Our finding of negative correlation between Phe levels and salivary cortisol suggests that depression in PKU patients may not be related to abnormalities in the hypothalamic-pituitary-adrenocortical axis.
4.4. Limitations
One limitation of this study is the small number of participants, although we do not believe that the lack of association between salivary serotonin with the other parameters is due to sample size limitations. Another limitation is the sparse number of reports documenting a normal range of salivary serotonin; where reports exist, there is disagreement in this normal range. For example, Marukawa et al. [29] found that the range of salivary serotonin in healthy controls was 450 ± 405 ng/mL using reversed-phase high-performance liquid chromatography with electrochemical detection, while Tan et al. [39] found the range to be 1.09 ± 0.40 ng/mL using a commercially available competitive radioimmunoassay. We presume that the difference in measurement technique accounted for the marked discrepancy in these healthy populations. Our immunoassay (ELISA) technique yielded non-PKU controls with salivary serotonin levels of 0–0.277 ng/mL, most consistent with the radioimmunoassay study. The fact that we had not compared non-PKU controls' salivary serotonin levels with self-reported psychiatric evaluation is another limitation of the study.
5. Conclusion
This study suggests that salivary serotonin is not a suitable marker for monitoring mood and anxiety, and hence, central serotonin turnover in PKU. However, it was able to replicate the previously-reported positive correlation between BMI and plasma Phe levels. Additionally, we showed a negative correlation between salivary cortisol and plasma Phe levels, for which there may be a physiologic mechanism that has yet to be fully characterized in humans. Further studies are needed to identify a suitable peripheral marker of central serotonin turnover in PKU patients and to explore the regulatory function of glucocorticoids on phenylalanine hydroxylase.
Acknowledgments
Acknowledgements
We would like to thank all the patients who participated in this research.
Details of the contributions of individual authors
Joseph Leung: Processed salivary samples, analyzed data, wrote manuscript.
Caroline Selvage: Recruited patients, collected salivary samples, reviewed manuscript.
Taryn Bosdet: Recruited patients, collected salivary samples, reviewed manuscript.
Jennifer Branov: Recruited patients, collected salivary samples, reviewed manuscript.
Annie Rosen-Heath: Recruited patients, collected salivary samples, reviewed manuscript.
Carole Bishop: Recruited patients, collected salivary samples, reviewed manuscript.
Sandra Sirrs: Conceived of project, reviewed manuscript.
Gabriella Horvath: Conceived of project, wrote manuscript.
Name of author who serves as guarantor
Gabriella Horvath.
Competing interests
Joseph Leung: No declarations relevant to this manuscript.
Caroline Selvage: No declarations relevant to this manuscript.
Taryn Bosdet: No declarations relevant to this manuscript.
Jennifer Branov: No declarations relevant to this manuscript.
Annie Rosen-Heath: No declarations relevant to this manuscript.
Carole Bishop: No declarations relevant to this manuscript.
Sandra Sirrs: No declarations relevant to this manuscript.
Gabriella Horvath: No declarations relevant to this manuscript.
Details of ethics approval
Ethics approval was obtained from the UBC Clinical Research Ethics Board and the Vancouver Coastal Health Research Ethics Board (ID# H12–01687).
Patient consent
Informed patient consent was obtained from each study subject according to the Declaration of Helsinki.
References
- 1.Al Hafid N., Christodoulou J. Phenylketonuria: a review of current and future treatments. Transl Pediatr. 2015;4:304–317. doi: 10.3978/j.issn.2224-4336.2015.10.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson G.M. Peripheral and central neurochemical effects of the selective serotonin reuptake inhibitors (SSRIs) in humans and nonhuman primates: assessing bioeffect and mechanism of action. Dev. Neurosci. 2004;22:359–404. doi: 10.1016/j.ijdevneu.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Anderson P.J., Wood S.J., Francis D.E., Coleman L., Andersen V., Boneh A. Are neuropsychological impairments in children with early-treated Phenylketonuria (PKU) related white matter abnormalities or elevated Phenylalanine levels? Dev. Neuropsychol. 2007;32:645–668. doi: 10.1080/87565640701375963. [DOI] [PubMed] [Google Scholar]
- 4.Anderson P.J., Leuzzi V. White matter pathology in phenylketonuria. Mol. Genet. Metab. 2010;99:S3–S9. doi: 10.1016/j.ymgme.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Audhya T., Adams J.B., Johansen L. Correlation of serotonine levels in CSF, platelets, plasma and urine. Biochim. Biophys. Acta. 2012;1820:1496–1501. doi: 10.1016/j.bbagen.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Bieber Nielsen J., Lou H.C., Guttler F. Effects of diet discontinuation and dietary tryptophan supplementation on neurotransmitter metabolism in phenylketonuria. Brain Dysf. 1988;1:51–56. [Google Scholar]
- 7.Bilder D.A., Burton B.K., Coon H., Leviton L., Ashworth J., Lundy B.D., Vespa H., Bakian A.V., Longo N. Mol. Genet. Metab. 2013;108:155–160. doi: 10.1016/j.ymgme.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Bilder D.A., Kobori J.A., Cohen-Pfeffer J.L., Johnson E.M., Jurecki E.R., Grant M.L. Mol. Genet. Metab. 2017;121:1–8. doi: 10.1016/j.ymgme.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Bik-Multanowski M., Didycz B., Mozrzymas R., Nowacka M., Kaluzny L., Cichy W., Schneiberg B., Amilkiewicz J., Bilar A., Gizewska M., Lange A., Starostecka E., Chrobot A., Wojcicka-Bartlomiejczyk B.I., Milanowski A. JIMD. 2008;31:S415–S418. doi: 10.1007/s10545-008-0978-7. [DOI] [PubMed] [Google Scholar]
- 10.Brown T.A., Chorpita B.F., Korotitsch W., Barlow D.H. Psychometric properties of the depression anxiety stress scales (DASS) in clinical samples. Behav. Res. Ther. 1997;35:79–89. doi: 10.1016/s0005-7967(96)00068-x. [DOI] [PubMed] [Google Scholar]
- 11.Bruinenberg V.M., Gordijn M.C.M., MacDonald A., van Spronson F.J., Van der Zee E.A. Front. Neurol. 2017;8:167. doi: 10.3389/fneur.2017.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brumm V.L., Azen C., Moats R.A. Neuropsychological outcome of subjects participating in the PKU adult collaborative study: a preliminary review. J. Inherit. Metab. Dis. 2004;27:549–566. doi: 10.1023/b:boli.0000042985.02049.ff. [DOI] [PubMed] [Google Scholar]
- 13.Brumm V.L., Bilder D., Waisbren S.E. Psychiatric symptoms and disorders in phenylketonuria. Mol. Genet. Metab. 2010;99:S59–63. doi: 10.1016/j.ymgme.2009.10.182. [DOI] [PubMed] [Google Scholar]
- 14.Chiappelli F., Haggerty D.F., Lynch M., Popják G. Translation of phenylalanine hydroxylase-specific mRNA in vitro: evidence for pretranslational control by glucocorticoids. Proc. Natl. Acad. Sci. 1981;78:2105–2109. doi: 10.1073/pnas.78.4.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clacy A., Sharman R., McGill J. Depression, anxiety, and stress in young adults with phenylketonuria: associations with biochemistry. J. Dev. Behav. Pediatr. 2014;35:388–391. doi: 10.1097/DBP.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 16.Crawford J.R., Henry J.D. The depression anxiety stress scales (DASS): normative data and latent structure in a large non-clinical sample. Br J. Clin. Psychol. 2003;42:111–131. doi: 10.1348/014466503321903544. [DOI] [PubMed] [Google Scholar]
- 17.Dahl H.H.M., Mercer J.F.B. Isolation and sequence of a cDNA clone which contains the complete coding region of rat phenylalanine hydroxylase. J. Biol. Chem. 1986;261:4148–4153. [PubMed] [Google Scholar]
- 18.Ferreira J.N., Hoffman M.P. Interactions between developing merves and salivary glands. Organ. 2013;9:199–205. doi: 10.4161/org.25224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greengard O., Delvalle J.A. The regulation of phenylalanine hydroxylase in rat tissue in vivo. Biochem. J. 1976;154:619–624. doi: 10.1042/bj1540619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guttler F., Lou H. Dietary problems of phenylketonuria: effect on CNS transmitters and their possible role in behavior and neuropsychological function. J. Inherit. Metab. Dis. 1986;9:S169–177. doi: 10.1007/BF01799701. [DOI] [PubMed] [Google Scholar]
- 21.Haggerty D.F., Chiappelli F., Kern R., Scully S., Lynch M. Regulation by glucocorticoids of rat-liver phenylalanine hydroxylase in vivo. Biochem. Biophys. Res. Commun. 1983;115:965–970. doi: 10.1016/s0006-291x(83)80029-1. [DOI] [PubMed] [Google Scholar]
- 22.Hargreaves K.M., Pardridge W.M. Neutral amino acid transport at the human blood-brain barrier. J. Biol. Chem. 1988;263:19392–19397. [PubMed] [Google Scholar]
- 23.Leuzzi V., Tosetti M., Montanaro D., Carducci C., Artiola C., Antonozzi I., Burroni M., Carnevale F., Chiarotti F., Popolizio T., Giannatempo G.M., D'Alesio V., Scarabino T. The pathogenesis of white matter abnormalities in phenylketonuria. A multimodal 3.0 tesla MRI and magnetic resonance spectroscopy (1H MRS) study. JIMD. 2007;30:209–216. doi: 10.1007/s10545-006-0399-4. [DOI] [PubMed] [Google Scholar]
- 24.Lindell S.G., Suomi S.J., Shoaf S., Linnoila M., Higley J.D. Salivary prolactin as a marker for central serotonin turnover. Biol. Psychiatry. 1999;46:568–572. doi: 10.1016/s0006-3223(99)00037-2. [DOI] [PubMed] [Google Scholar]
- 25.Lovibond S.H., Lovibond P.F. 2nd ed. Psychology Foundation; Sydney: 1995. Manual for the Depression Anxiety Stress Scales. (ISBN 7334-1423-0) [Google Scholar]
- 26.Mannie Z.N., Harmer C.J., Cowen P.J. Increased waking salivary cortisol levels in young people at familial risk of depression. Am. J. Psychiatry. 2007;164:617–621. doi: 10.1176/ajp.2007.164.4.617. [DOI] [PubMed] [Google Scholar]
- 27.Manti F., Nardecchia F., Chiarotti F., Carducci C., Carducci C., Leuzzi V. Psychiatric disorders in adolescents and young adult patients with phenylketonuria. Mol. Genet. Metab. 2016;117:12–18. doi: 10.1016/j.ymgme.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Martynyuk A.E., van Spronson F.J., Van der Zee E.A. Animal models of brain dysfunction in phenylketonuria. Mol. Genet. Metab. 2010;99:S100–S105. doi: 10.1016/j.ymgme.2009.10.181. [DOI] [PubMed] [Google Scholar]
- 29.Marukawa H., Shimomura T., Takahashi K. Salivary substance P, 5-hydroxytryptamine, and gamma-aminobutyric acid levels in migraine and tension-type headache. Headache. 1996;36:100–104. doi: 10.1046/j.1526-4610.1996.3602101.x. [DOI] [PubMed] [Google Scholar]
- 30.Matsunaga M., Ishii K., Ohtsubo Y., Noguchi Y., Ochi M., Yamasue H. Association between salivary serotonin and the social sharing of happiness. PLoS One. 2017;12 doi: 10.1371/journal.pone.0180391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGee M.M., Greengard O., Knox W.E. The quantitative determination of phenylalanine hydroxylase in rat tissues. Biochem. J. 1972;127:669–674. doi: 10.1042/bj1270669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ney D.M., Murali S.G., Stroup B.M., Nair N., Sawin E.A., Rohr F., Levy H.L. Metabolomic changes demonstrate reduced bioavailability of tyrosine and altered metabolism of tryptophan via the kynurenine pathway with ingestion of medical foods in phenylketonuria. Mol. Genet. Metab. 2017;121:96–103. doi: 10.1016/j.ymgme.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ormazabal A., Vilaseca M.A., Pérez-Dueñas B. Platelet serotonin concentrations in PKU patients under dietary control and tetrahydrobiopterin treatment. J. Inherit. Metab. Dis. 2005;28:863–870. doi: 10.1007/s10545-005-0153-3. [DOI] [PubMed] [Google Scholar]
- 34.Parniak M., Pilkington J. Glucocorticoid stimulation of tetrahydrobiopterin levels and phenylalanine hydroxylase activity in rat hepatoma cells. Biochem. Cell Biol. 1989;67:293–296. doi: 10.1139/o89-044. [DOI] [PubMed] [Google Scholar]
- 35.Pietz J., Fätkenheuer B., Burgard P., Armbruster M., Esser G., Schmidt H. Psychiatric disorders in adult patients with early-treated phenylketonuria. Pediatrics. 1997;99:345–350. doi: 10.1542/peds.99.3.345. [DOI] [PubMed] [Google Scholar]
- 36.Pietz J., Dunckelmann R., Rupp A. Neurological outcome in adult patients with early-treated phenylketonuria. Eur. J. Pediatr. 1998;157:824–830. doi: 10.1007/s004310050945. [DOI] [PubMed] [Google Scholar]
- 37.Pietz J., Kreis R., Rupp A., Mayatepek E., Rating D., Boesch C., Bremer H.J. Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria. J. Clin. Invest. 1999;103:1169–1178. doi: 10.1172/JCI5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robertson L.V., McStravick N., Ripley S. Body mass index in adult patients with diet-treated phenylketonuria. J. Hum. Nutr. Diet. 2013;26:S1–S6. doi: 10.1111/jhn.12054. [DOI] [PubMed] [Google Scholar]
- 39.Tan Z.L., Bao A.M., Tao M., Liu Y.J., Zhou J.N. Circadian rhythm of salivary serotonin in patients with major depressive disorder. Neuro Endocrinol. Lett. 2007;28:395–400. [PubMed] [Google Scholar]
- 40.Targum S.D., Lang W. Neurobehavioural problems associated with phenylketonuria. Psychiatry (Edgmont). 2010;7:29–32. [PMC free article] [PubMed] [Google Scholar]
- 41.ten Hoedt A.E., de Sonneville L.M., Francois B. High phenylalanine levels directly affect mood and sustained attention in adults with phenylketonuria: a randomized, double-blind, placebo-controlled, crossover trial. J. Inherit. Metab. Dis. 2011;34:165–171. doi: 10.1007/s10545-010-9253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thijssen J.H.H., SHM Van Goozen, Van England H., Matute L.M.P., Blankenstein M.A. None of four commercially available assays detects prolactin in human saliva. Clin. Chem. 2000;46:1409–1410. [PubMed] [Google Scholar]
- 43.Uhl I., Norra C., Pirkl P.A. Central serotonergic activity correlates with salivary cortisol after waking in depressed patients. Psychopharmacology. 2011;217:605–607. doi: 10.1007/s00213-011-2325-1. [DOI] [PubMed] [Google Scholar]
- 44.Vreeburg S.A., Hoogendijk W.J.G., DeRijk R.H., van Dyck R., Smit J.H., Zitman F.G., Penninx B.W.J.H. Salivary cortisol levels and the 2-year course of depressive and anxiety disorders. Psychoendocrinology. 2013;38:1494–1502. doi: 10.1016/j.psyneuen.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 45.Walther D.J., Bader M. A unique central tryptophan hydroxylase isoform. Biochem. Pharmacol. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- 46.Yano S., Moseley K., Azen C. Large neutral amino acid supplementation increases melatonin synthesis in phenylketonuria: a new biomarker. J. Pediatr. 2013;162:999–1003. doi: 10.1016/j.jpeds.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yano S., Moseley K., Fu X., Azen C. Evaluation of Tetrahydribiopterin therapy with large neutral amino acid supplementation in phenylketonuria: effects on potential peripheral biomarkers, melatonin and dopamine, for brain monoamine neurotransmitters. PLoS One. 2016;11 doi: 10.1371/journal.pone.0160892. [DOI] [PMC free article] [PubMed] [Google Scholar]