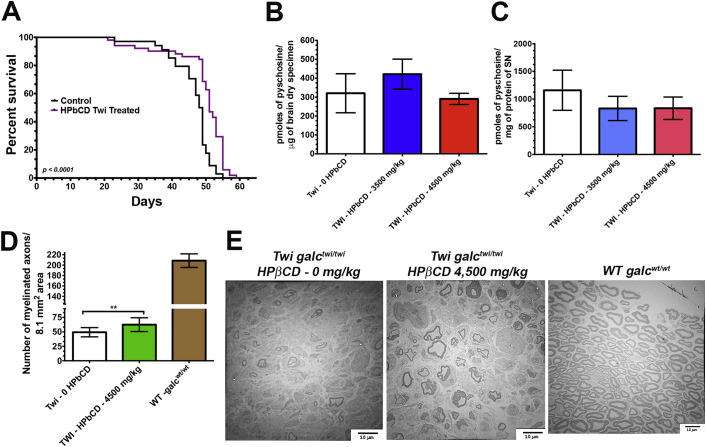
β-cyclodextrins are cyclic oligosaccharides assembled in a ring configuration containing a lipophilic central cavities and hydrophilic outer surfaces. Traditionally, cyclodextrins are used as excipients and absorption enhancers of hydrophobic molecules [1]. Among different cyclodextrin derivatives, 2-hydroxypropyl-β-cyclodextrin (HPβCD) showed to be an efficacious therapeutic agent for Niemann-Pick Disease Type C1 (NPC1), an autosomal-recessive and fatal neurodegenerative disorder [[2], [3], [4], [5], [6]], and it is currently being evaluated in clinical trials [7,8]. During in vivo experiments of small molecule candidates identified in a cell-based LC-MS/MS medium-throughput screening for psychosine-reducing molecules [9], we observed beneficial therapeutic effects of the HPβCD in the Twitcher mouse (Twi), C57BL/6 galctwi/twi, the murine model of globoid-cell leukodystrophy (GLD) or Krabbe disease, an inborn lysosomal disease caused by the deficiency of the lysosomal β-galactocerebrosidase (GALC) [10,11]. The Twi mouse model recapitulates the severe neurological course and demyelinating processes in both central (CNS) and peripheral (PNS) nervous systems due to the elevated cytotoxicity of psychosine in myelin-forming cells in the setting of GALC deficiency [[11], [12], [13], [14]]. To solubilize highly hydrophobic small molecule “hits” for murine experiments, HPβCD was used as a dissolvent as previously described [1]. In early experiments, the Twi mice group receiving HPβCD alone at 3500–4500 mg/kg subcutaneously from third day of life and then every two days showed slower progression of neurological symptoms and expanded lifespan (Fig.1A). No statistically significant differences in the levels of the cytotoxic psychosine were noticeable in the both brain and sciatic nerves of HPβCD-treated Twi mice (Fig.1B and 1C), indicating a distinct mechanism of action of HPβCD. To further investigate the effects of HPβCD, ultrastructural studies performed blindly in proximal and distal segments of sciatic nerves showed statistically significant preservation of myelinated axons in the cohort receiving HPβCD (Fig.1C). It is unlikely that HPβCD has any effect in the CNS given its inability to cross the blood-brain barrier (BBB) [2,15,16] and the unchanged psychosine levels in the brains of HPβCD-treated Twi mice (Fig.1B). Altogether, these serendipitous findings demonstrate the therapeutic potential of HPβCD in the demyelinating disease processes of GLD by yet unknown mechanisms. In addition, these results highlight the importance of careful selection of additives for dissolution of hydrophobic small molecules for animal studies.
Fig. 1.
The effects of 2-hydroxypropyl-β-cyclodextrin (HPβCD) in the Twitcher mouse model for Krabbe disease. (A) Survival analysis of Twitcher (Twi galctwi/twi) mice treated with HPβCD (n = 34) and controls (n = 51). Equal number of males and females of Twi galctwi/twi were used in both groups. From post-natal day 3 until their death, treated Twi mice group received 4500 mg/kg of HPβCD subcutaneously diluted in phosphate saline every 2 days. An initial group of 12 mice receiving 3500 mg/kg subcutaneously also in the same frequency and duration are included in this survival analysis. Psychosine levels measured by LC-MS/MS in the brain (B) and sciatic nerve (C) specimens showed no statistically significant differences between mice receiving HCβCD (n = 6) and those receiving only saline (n = 6). (D) The number of myelinated axonal bulbs were significantly increased in the mice receiving HCβCD (n = 6) in comparison to controls (n = 6) (p < 0.05). (E) The transmission electron microscopy images of sciatic nerves dissected post-mortem showed increased preservation of myelinated fibers in the group of Twi mice receiving HPβCD.
Acknowledgement
We are indebted with the assistance of the colleagues at University of Florida that indirectly assisted in the experiments of the studies here. We are thankful to Mariola Edelmann for proof-reading of the manuscript. We thank the assistance of Ernesto Bongarzone Ph.D. from University of Illinois, Chicago who kindly donated the breeders for our current Twitcher murine colony. We acknowledge the Center of Environmental Health and Toxicology (CEHT) at the University of Florida for the availability of the MS/MS instrumentation (NIH 1S10OD018141-01A1). The major work was funded by the grant 5R01NS079655-03 from National Institute of Neurological Disorders and Stroke (NINDS). We are also indebted with Animal Care Facility staff at the University of Florida for the support and assistance to the maintenance of our mouse colonies.
References
- 1.Vecsernyes M., Fenyvesi F., Bacskay I., Deli M.A., Szente L., Fenyvesi E. Cyclodextrins, blood-brain barrier, and treatment of neurological diseases. Arch. Med. Res. 2014;45:711–729. doi: 10.1016/j.arcmed.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 2.Camargo F., Erickson R.P., Garver W.S., Hossain G.S., Carbone P.N., Heidenreich R.A., Blanchard J. Cyclodextrins in the treatment of a mouse model of Niemann-Pick C disease. Life Sci. 2001;70:131–142. doi: 10.1016/s0024-3205(01)01384-4. [DOI] [PubMed] [Google Scholar]
- 3.Davidson C.D., Ali N.F., Micsenyi M.C., Stephney G., Renault S., Dobrenis K., Ory D.S., Vanier M.T., Walkley S.U. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yergey A.L., Blank P.S., Cologna S.M., Backlund P.S., Porter F.D., Darling A.J. Characterization of hydroxypropyl-beta-cyclodextrins used in the treatment of Niemann-Pick Disease type C1. PLoS One. 2017;12 doi: 10.1371/journal.pone.0175478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aqul A., Liu B., Ramirez C.M., Pieper A.A., Estill S.J., Burns D.K., Liu B., Repa J.J., Turley S.D., Dietschy J.M. Unesterified cholesterol accumulation in late endosomes/lysosomes causes neurodegeneration and is prevented by driving cholesterol export from this compartment. J. Neurosci. 2011;31:9404–9413. doi: 10.1523/JNEUROSCI.1317-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffin L.D., Gong W., Verot L., Mellon S.H. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat. Med. 2004;10:704–711. doi: 10.1038/nm1073. [DOI] [PubMed] [Google Scholar]
- 7.Berry-Kravis E., Chin J., Hoffmann A., Winston A., Stoner R., LaGorio L., Friedmann K., Hernandez M., Ory D.S., Porter F.D., O'Keefe J.A. Long-term treatment of Niemann-pick type C1 disease with intrathecal 2-Hydroxypropyl-Beta-Cyclodextrin. Pediatr. Neurol. Mar 2018;80:24–34. doi: 10.1016/j.pediatrneurol.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ory D.S., Ottinger E.A., Farhat N.Y., King K.A., Jiang X., Weissfeld L., Berry-Kravis E., Davidson C.D., Bianconi S., Keener L.A., Rao R., Soldatos A., Sidhu R., Walters K.A., Xu X., Thurm A., Solomon B., Pavan W.J., Machielse B.N., Kao M., Silber S.A., McKew J.C., Brewer C.C., Vite C.H., Walkley S.U., Austin C.P., Porter F.D. Intrathecal 2-hydroxypropyl-beta-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: a non-randomised, open-label, phase 1-2 trial. Lancet. 2017;390:1758–1768. doi: 10.1016/S0140-6736(17)31465-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribbens J.J., Moser A.B., Hubbard W.C., Bongarzone E.R., Maegawa G.H. Characterization and application of a disease-cell model for a neurodegenerative lysosomal disease. Mol. Genet. Metab. 2014;111:172–183. doi: 10.1016/j.ymgme.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki K. Globoid cell leukodystrophy (Krabbe's disease): update. J. Child Neurol. 2003;18:595–603. doi: 10.1177/08830738030180090201. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki K. My encounters with Krabbe disease: a personal recollection of a 40-Year journey with young colleagues. J. Neurosci. Res. 2016;94:965–972. doi: 10.1002/jnr.23735. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki K. Twenty five years of the "psychosine hypothesis": a personal perspective of its history and present status. Neurochem. Res. 1998;23:251–259. doi: 10.1023/a:1022436928925. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi T., Yamanaka T., Jacobs J.M., Teixeira F., Suzuki K. The Twitcher mouse: an enzymatically authentic model of human globoid cell leukodystrophy (Krabbe disease) Brain Res. 1980;202:479–483. doi: 10.1016/0006-8993(80)90159-6. [DOI] [PubMed] [Google Scholar]
- 14.Duchen L.W., Eicher E.M., Jacobs J.M., Scaravilli F., Teixeira F. Hereditary leucodystrophy in the mouse: the new mutant twitcher. Brain. 1980;103:695–710. doi: 10.1093/brain/103.3.695. [DOI] [PubMed] [Google Scholar]
- 15.Calias P. 2-hydroxypropyl-beta-cyclodextrins and the blood-brain barrier: considerations for Niemann-pick disease type C1. Curr. Pharm. Des. 2017;23(40):6231–6238. doi: 10.2174/1381612823666171019164220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walkley S.U., Davidson C.D., Jacoby J., Marella P.D., Ottinger E.A., Austin C.P., Porter F.D., Vite C.H., Ory D.S. Fostering collaborative research for rare genetic disease: the example of niemann-pick type C disease. Orphanet J. Rare Dis. 2016;11:161. doi: 10.1186/s13023-016-0540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]