Abstract
Background
Transcranial magnetic stimulation (TMS) is a non-invasive brain stimulation technique that has shown promise as an adjunct treatment for the symptoms of Obsessive-Compulsive Disorder (OCD). Establishing a clear clinical role for TMS in the treatment of OCD is contingent upon evidence of significant efficacy and reliability in reducing symptoms.
Objectives
We present the basic principles supporting the effects of TMS on brain activity with a focus on network-based theories of brain function. We discuss the promises and pitfalls of this technique as a means of modulating brain activity and reducing OCD symptoms.
Methods
Synthesis of trends and critical perspective on the potential benefits and limitations of TMS interventions in OCD.
Findings
Our critical synthesis suggests the need to better quantify the role of TMS in a clinical setting. The context in which the stimulation is performed, the neural principles supporting the effects of local stimulation on brain networks, and the heterogeneity of neuroanatomy are often overlooked in the clinical application of TMS. The lack of consideration of these factors may partly explain the variable efficacy of TMS interventions for OCD symptoms.
Conclusions
Results from existing clinical studies and emerging knowledge about the effects of TMS on brain networks are encouraging but also highlight the need for further research into the use of TMS as a means of selectively normalising OCD brain network dynamics and reducing related symptoms. The combination of neuroimaging, computational modelling, and behavioural protocols known to engage brain networks affected by OCD has the potential to improve the precision and therapeutic efficacy of TMS interventions. The efficacy of this multimodal approach remains, however, to be established and its effective translation in clinical contexts presents technical and implementation challenges. Addressing these practical, scientific and technical issues is required to assess whether OCD can take its place alongside major depressive disorder as an indication for the use of TMS.
Keywords: TMS, Brain stimulation, Obsessive-compulsive disorder (OCD), Connectivity, Neuroimaging
Highlights
-
•
A clinical role for TMS in OCD has yet to be established.
-
•
Improving clinical efficacy of TMS relies on the mapping of network activity in OCD.
-
•
The state in which TMS is applied needs to be controlled.
-
•
Neuroimaging and computational modelling can help identify stimulation targets.
-
•
Neuronavigation can assist with the precise modulation of neural activity and connectivity.
1. Introduction
Obsessive-Compulsive Disorder (OCD) is a severe mental illness that affects 1–3% of the population (Stein et al., 1997; Ruscio et al., 2010). The phenomenology of OCD maps onto two main domains: obsessions (recurring and intrusive thoughts) and compulsions (ritualistic behaviours performed to reduce anxiety). OCD manifests as a heterogeneous clinical condition with the intensity and mix of obsessions and compulsions varying between patients (Harrison et al., 2013). Moreover, the clinical presentation of OCD is often associated with comorbid symptoms of general anxiety or depression (Overbeek et al., 2002). OCD may therefore represent a broad constellation of distinct syndromes and clinical phenotypes.
Similar to other psychiatric conditions, the diagnosis of OCD in primary care and psychiatric settings is often missed, late or erroneous (Glazier et al., 2013; Veldhuis et al., 2012). Moreover, data suggests that even when an appropriate diagnosis is made, <10% of patients receive a disorder-specific treatment that is evidence-based (Torres et al., 2007).
Current evidence supports the use of cognitive behavioural therapies (cognitive reappraisal and exposure interventions) and selective serotonin reuptake inhibitor (SSRIs) drugs as a first line treatment for OCD (Hirschtritt et al., 2017). Although pharmacological and behavioural interventions are effective treatments for OCD (Hirschtritt et al., 2017), over 40% of patients remain clinically symptomatic and highly disabled for decades after initial treatment (Bloch et al., 2006; Pallanti and Quercioli, 2006). Therefore, there is a pressing need to develop new efficacious, safe, and tolerable therapies to reduce the extreme burden of the disorder.
Transcranial magnetic stimulation (TMS) is an emerging non-invasive brain stimulation technique that has an established therapeutic role in major depressive disorder (MDD) (Kedzior et al., 2015; O'Reardon et al., 2007; Fitzgerald et al., 2003; Fitzgerald et al., 2006; Fitzgerald et al., 2009; George et al., 2010; George and Post, 2011). In particular, repetitive TMS (rTMS) has been approved by the American FDA regulatory authorities as a therapeutic intervention for symptoms of MDD in patients that failed to respond to at least one or two courses of pharmacological treatment (Horvath et al., 2010; Connolly et al., 2012). Specifically, rTMS has a role in the treatment of an acute depressive episode (O'Reardon et al., 2007; Fitzgerald et al., 2003) as well as in maintenance therapy (O'Reardon et al., 2005). Because of its efficacy [mean effect size of 0.55 of active vs placebo TMS, (Slotema et al., 2010)] and favourable safety profile (George and Post, 2011), TMS has obtained a definitive clinical role in both the private and public psychiatry sectors.
More recently, research has suggested that TMS holds potential to effectively complement existing behavioural and pharmacological therapies for OCD [for examples of existing studies see Table 1; for recent systematic reviews see (Blom et al., 2011; Berlim et al., 2013; Saba et al., 2015; Trevizol et al., 2016)]. In this perspective, we provide a brief introduction to the effects of TMS on brain activity, highlighting the promises and pitfalls of this technique as a viable therapeutic intervention for OCD. Currently, TMS is largely administered as a one size fits all therapy, without customising the choice of cortical stimulation according to a patient's specific clinical profile or imaging-based estimates of dysregulation of cortical network activity. The overarching aim of this paper is to consider the potential benefits of personalizing the administration of TMS interventions by means of each patient's symptom profile, neuroanatomical architecture and brain connectivity dysfunction across different mental states. We contend that the efficacy of TMS as a therapy for OCD can be improved by: i) understanding the mechanisms, timescales and network principles by which TMS modulates state dependent functional brain connectivity; and ii) personalizing TMS therapy by selecting cortical stimulation sites and other stimulation parameters according to each patient's symptom profile and state-specific brain connectivity dysfunction. We provide practical suggestions about how the use of TMS can be optimized in clinical settings.
Table 1.
| Stim. region | Study | Design | Target | No. of patients | TMS Coil | Type of stim./intensity/pulses per session/no. of sessions | Neuro-navigation | Context during TMS | Outcome measures | Main outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Dorsolateral prefrontal cortex | Studies reporting improvements in OCD symptoms | |||||||||
| Greenberg et al., 1997 | Single-blinded, randomised, non-sham-control | L + R DLPFC Control site = mid Occipital |
N = 12 OCD. Active 12, 6/12 depressed |
F8c | 20 Hz, 80%, 600 RMT, 1 | No. 5 cm anterior and 2 cm inferior to the optimal motor cortex site as detected by EMG (abductor pollicis brevis contraction) | NS |
|
OCD Symptoms: Compulsive urges significantly decreased after rDLPFC rTMS. No significant effect of rTMS to the occipital region. No change in obsessive thoughts. Depressive Symptoms: Modest increase in positive mood after rDLPFC rTMS only. |
|
| Elbeh et al., 2016 | Randomised, double blind, clinical trial | R DLPFC |
N = 45. Active 30 (1 Hz 15, 10 Hz 15), sham 15 |
F8c | 1 Hz, 100% RMT, 2000, 10; 10 Hz, 100% RMT, 2000, 10 |
No. 5 cm anterior to the optimal site for activating the right first dorsal interosseous muscle | NS |
|
OCD Symptoms: Significant interaction between time and group on YBOCS. 1 Hz showed a significant group × time interaction. No effect at 10 Hz. Anxiety Symptoms: HAMA scores showed a significant time × group interaction. 1 Hz: significant reduction in HAMA scores in active compared to sham over time. 10 Hz showed no interaction. CGI: Significant change in CGI-S scores over time for 1 Hz rTMS only. |
|
| Ho-Jun Seo et al., 2016 | Randomised control trial | R DLPFC |
N = 28. Active 14 sham 13, drop out 1 |
NS | 1 Hz 100% RMT, 1200, 15 | No. 5 cm anterior and in a parasagittal line from the point of maximum stimulation of the contralateral Abductor pollicis Brevis | NS |
|
OCD Symptoms: Active (vs. sham) showed a significant improvement on YBOCS over 3 weeks of rTMS. Anxiety Symptoms: No interaction for scores on HAMA. Depressive Symptoms: HAMD scores improved significantly for active vs sham over 3 weeks. No interaction for BDI. CGI: Group × time interaction for CGI scores over the 3 weeks of treatment. |
|
| Studies reporting no improvement in OCD symptoms | ||||||||||
| Alonso et al., 2001 | Randomised double blind, placebo control study | R DLPFC |
N = 18. Active 10, sham 8 |
70-mm circular coil | 1 Hz, 110% RMT, [Not Reported], 18 | Yes. MRI guided stimulation. Cortex superior to inferior frontal sulcus and anterior to precentral sulcus, centred over Brodmann areas 9 and 46, but also influencing areas 6, 8, 10, 44, and 45 |
NS |
|
OCD Symptoms: No significant difference between active vs sham over the 10 weeks post treatment. Depressive Symptoms: Non-significant group × time interaction on HAMD scores. |
|
| Prasko et al., 2006 | Randomised, double blind, sham-controlled | L DLPFC |
N = 33. Active 18, sham 12, drop out 3. |
F8c | 1 Hz, 110% RMT, 1800, 10 (5 weekly) | No. 5 cm anterior to the optimal motor cortex site (abductor pollicis brevis contraction) | NS |
|
OCD Symptoms: No significant difference between sham and active groups on YBOCS. Anxiety Symptoms: No differences between active and sham. |
|
| Sachdev et al., 2007 | Randomised, double blind, sham-controlled. Followed by open-label phase after week 2. |
L DLPFC | N = 18. Active 10, sham 8 |
F8c | 10 Hz, 110% RMT, 1500, 10 plus 20 optional where subjects were not blind | No. 5 cm anterior to the optimal site for activating the right first dorsal interosseous muscle |
NS |
|
OCD Symptoms: No significant difference in YBOCS scores between active and sham groups over 2 and 4 weeks (after controlling for depression). Six patients had a > 40% reduction in total YBOCS scores over 4 weeks. | |
| Sarkhel et al., 2010 | Randomised, participant blind study, sham-controlled | R prefrontal cortex |
N = 42. Active 21, sham 21 |
F8c | 10 Hz, 110% RMT, 800, 10 | No. 5 cm anterior to the optimal motor cortex site (abductor pollicis brevis contraction) | NS |
|
OCD Symptoms: No significant group × time interaction on YBOCS scores. Depressive Symptoms: Significant group x time interaction for HAMD scores. 76.2% of participants in active condition showed a 25% reduction in depressive symptoms compared to 66.7% in Sham condition. Anxiety Symptoms: Significant group × time interaction for HAMA scores. 52.4% of participants in the sham condition showed a 25% reduction in anxiety symptoms compared to active. CGI: No difference between groups over time. |
|
| Mansur et al., 2011 | Randomised, double blind, control trial. Followed by open label active phase for 10 sham rTMS participants | R DLPFC |
N = 30. Active 13, 14 sham, drop out 3 |
F8c | 10 Hz, 110% RMT, 2000, 30 (5 weekly) | No. 5 cm rostral to the point of optimal stimulation for the right abductor pollicis brevis muscle at a parasagittal plane | NS |
|
OCD Symptoms: No significant time x group interaction on YBOCS scores. Anxiety and Depressive Symptoms: No significant difference between groups on all scales. |
|
| Supplementary motor area | Studies reporting improvements in OCD symptoms | |||||||||
| Mantovani et al., 2006 | Open label pilot study | SMA |
N = 10. Active 8 (5 OCD, 3 TS), drop out 2. |
F8c | 1HZ 100%, RMT, 1200, 10 | No. SMA defined at 15% of the distance between inion and nasion anterior to CZ on the sagittal midline, according to the 10–20 EEG system | NS |
|
OCD Symptoms: 3/5 OCD showed reduction in YBOCS scores (>40%) at 3 months. The OCD group (5 OCD and 2 OCD + TS) showed significant reduction in YBOCS scores. Anxiety and Depressive Symptoms: Participants showed significant reductions in HAMA, HAMD, BDI and SAD scores over 2 weeks. CGI: All participants showed improvement on CGI scores from baseline to week 2. Effect present at 3 months. |
|
| Mantovani et al., 2010 | Double-blind, randomised, sham-controlled. Protocol was followed by optional open-label phase after 4 weeks. | Pre-SMA | N = 21. Active 9, sham 9, drop out 3. | F8c | 1 Hz, 100% RMT, 1200, 20 | Yes. Targeted using International 10–20 EEG system. Pre-SMA defined at 15% of the distance between inion and nasion, anterior to CZ (vertex) on sagittal midline. Coil handle positioned along sagittal midline, pointing toward occiput to stimulate the pre-SMA bilaterally. Stim site marked in Brain sight neuronav to monitor online coil positioning during stim. | NS |
|
OCD Symptoms: Active group showed a reduction in YBOCS-SR scores compared to sham after 4 weeks of rTMS. Effect remained significant after controlling for HAMD scores. CGI: Significant group x time interaction for CGI scores after 4 weeks. Open label study: Those that had previous rTMS showed significant further improvements on the YBOCS from week 4–8. Those previously receiving sham did not show any significant change in YBOCS scores from week 4–8. |
|
| Gomes et al., 2012 | Randomised, double blind trial | Bilateral Pre-SMA |
N = 22. Active 12, sham 10 |
F8c | 1 Hz, 100% RMT (based on visual detection of the thumb movement), 1200, 10 | No. Stimulation site was targeted using the International 10–20 EEG system. Pre-SMA was defined at 15% of the distance between inion and nasion anterior to CZ (vertex) on the sagittal midline. Coil handle positioned along the sagittal midline, pointing toward the occiput to stimulate the pre-SMA bilaterally. | NS |
|
OCD Symptoms: YBOCS scores showed a significant time × group interaction (at 2 weeks, until 14 weeks post treatment). Interaction significant after controlling for HAMD scores. Anxiety Symptoms: Significant difference between active and sham groups on HAMA and BAI scores at 2 weeks. No significant difference in HAMA scores between groups after 14 weeks. Depressive Symptoms: No significant difference on HAMD scores. CGI-S: Significant effect of time and treatment at 2 and 14 weeks. |
|
| Hawken et al., 2016 | Randomised double blind placebo controlled trail | SMA | N = 22. Active 10, Sham 12 | F8c | 1 Hz, 110% RMT, NS,25 | No. SMA defined at 15% of the distance between inion and nasion anterior to CZ (vertex). International 10–20 EEG system. | NS |
|
OCD Symptoms: Clinically significant decrease in YBOCS scores compared to both baseline and sham group (YBOCS reduction of ≥25%). This effect was maintained at six weeks. Depressive Symptoms and CGI: Active group showed significant reduction in HAMD and CGI scores compared to sham. |
|
| Pallanti et al., 2016 | Randomised open label trial | SMA | N = 42. 21 = Active, 21 = TAU (Anti-psychotics) | F8c | 1 Hz, 100% RMT, 1200, 15 | No. NS | NS |
|
OCD Symptoms: Significant time effect with 68% responders in active group and 24.0% responders in the antipsychotic augmentation group (YBOCS, reduction of ≥ 25%). Active group had 17.6% remission. Anxiety and Depressive symptoms: TMS group: HAMD and HAMA significantly decreased from pre to post. Antipsychotic group: Decrease in HAMD scores from pre and midway to post. HAMA scores were significantly lower between pre- and post-treatment. |
|
| Studies reporting no improvement in OCD symptoms | ||||||||||
| Pelissolo et al., 2016 | Randomised double blind | Pre-SMA | N = 40. 16 Active, 15 sham (9 dropout) | F8c | 1 Hz, 100% RMT, 1500, 20 | Yes. SMA defined as Sagittal midline, 2 cm anterior to the bi-commissural line at the anterior commissure. Stim site was marked using Brain sight neuronav program | NS |
|
OCD Symptoms: No difference between active vs sham groups on YBOCS scores. Anxiety and Depressive symptoms: No significant difference between groups on all scales. |
|
| Supplementary motor area and dorsolateral prefrontal cortex | Studies reporting no improvement in OCD symptoms | |||||||||
| Kang et al., 2009 | Double blind randomised control trial | SMA R DLPFC |
N = 20. Active 10, sham 10 |
F8c | R DLPFC = 1HZ, 110%, 1200, 10 SMA = 1HZ, 100%, 1200, 10 |
No. RDLPFC defined at 5 cm anterior to abductor pollicis brevis. SMA defined at 15% of distance between inion and nasion, anterior to CZ on sagittal midline, according to 10–20 EEG system. |
NS |
|
OCD Symptoms: No significant group × time interaction for YBOCS scores. Anxiety and Depressive Symptoms: No significant group × time interactions on all scales. |
|
| Prefrontal cortex | Studies reporting improvements in OCD symptoms | |||||||||
| Sachdev et al., 2001 | Single-blind, randomised, non-sham-control | L PFC or R PFC |
N = 12. Active 12; Active RDLPFC N = 6 Active LDLPFC N = 6 |
F8c | 10 Hz, 110% RMT, [Not reported], 10 | No. | NS |
|
OCD Symptoms: No significant difference between right- and left-sided rTMS. Improvements in obsessions, compulsions, and total YBOCS scores were shown after 2 weeks and at 1-month. These improvements were significant for obsessions and trended toward significant for total YBOCS scores after correcting for MADRS scores. | |
| Dunlop et al., 2016 | Non-randomised, control | dmPFC |
N = 60. Active 20, Healthy controls 40 (no rTMS) |
Butterfly Coil | 10 Hz, 120% RMT (visual inspection, activation of the extensor halluces longus), 3000 pulses per hemispheres (left then right) per session, 20 | Yes. Neuro-navigation was performed for anatomical land marking & co-registration of the brain to Talairach stereotaxic space, with the co-registered coil vertex coordinate (×0 y + 60 z + 60 in Talairach stereotaxic space) |
NS |
|
OCD Symptoms: YBOCS scores significantly decreased from baseline. A bimodal distribution was shown of responders and non-responder subpopulations (responder = 10 patients showed >50% improvement). Responders showed a 67.2% decrease in YBOCS scores. Non-responders showed a non-significant decrease of 11.4%. Responders and non-responders did not differ in baseline YBOCS scores. Anxiety and Depressive symptoms: Responders showed significant improvement on BAI, HAMD and BDI scores. Non-responders showed no significant change. |
|
| Orbitofrontal cortex | Studies reporting improvements in OCD symptoms | |||||||||
| Ruffini et al., 2009 | Randomised, blind, sham-controlled | L OFC |
N = 23. Active 16, sham 7 |
F8c | 1 Hz, 80% RMT, 600, 15 | No. The left OFC, which corresponds to Fp1 (International 10–20 EEG System). | NS |
|
OCD Symptoms: Significant group x time interaction in YBOCS scores until 10 weeks post stim. After week 10, statistical significance was lost. Anxiety Symptoms: No significant difference between active vs sham on HAMA scores after rTMS and 12 weeks post. Depressive Symptoms: HAMD scores did not significantly decrease after active rTMS and 12 weeks follow up. |
|
| Studies reporting no improvement in OCD symptoms | ||||||||||
| Nauczyciel et al., 2014 | Randomised, double blind, cross over (Sham and Active) | R OFC | N = 22 (3 not include in analyses due to comborbidities) | DB-80 butterfly double-cone coil | 1 HZ, 120% MT (based on visual detection of left abductor pollicus brevis muscle), 1200, 14 | No·Targeted using the International 10–20 EEG system. ROFC defined as the Fp2 electrode site. | NS |
|
OCD Symptoms: YBOCS scores decreased for both active and sham, no interaction. PET: Bilateral decrease in the metabolism of the OFC in active group compared to sham. |
|
Note: OCD = Obsessive Compulsive Disorder; TS = Tourette's Syndrome; Hz = Hertz; EEG = electroencephalogram; Stim = stimulation; RMT = Rest Motor Threshold; F8c = figure of eight coil; OFC = Orbital Frontal Cortex; PFC = prefrontal cortex; DLPFC = Dorsolateral prefrontal cortex; dmPFC = Dorsomedial prefrontal cortex; SMA = Supplementary motor area; SC = Sensorimotor Cortex; MNIMH = Modified National Institute of Mental Health Scale; YBOCS = Yale-Brown Obsessive Compulsive Scale; YBOCS-SR = Yale-Brown Obsessive Compulsive Scale-Self-report; CGI = Clinical Global Impression Scale; CGI-S = Clinical Global Impression-Severity subscale; HAMD = Hamilton Rating Scale for depression; HAMA = Hamilton Rating Scale for anxiety; MADRS = Montgomery–Åsberg Depression Rating Scale; MOCI = Maudsley Obsessive-Compulsive Inventory; YGTSS = Yale Global Tic Severity Scale; SAD = SAD persons scale; BDI = Beck Depression Inventory; BAI = Beck Anxiety Inventory; FS-36 = Medical Outcome study 36 item short form health survey; SASS = Social Adaptation Self-evaluation Scale; SCL-90 = Symptom Checklist-90; STAI = State Trait Anxiety Inventory; ZUNG-SAS = Zung Self-Rating Anxiety Scale; NS = Not specified; OTI = Obsessive Thoughts List; Global Assessment Functioning = GAF; TAU = Treatment As Usual; EMG = Electromyography.
2. Transcranial magnetic stimulation (TMS)
2.1. Principles and local effects of TMS
TMS is a non-invasive brain stimulation technique that alters the activity of neurons within a targeted cortical region (Hallett, 2000; Hallett, 2007). TMS is administered using a wire coil. During stimulation, a brief high-current is produced in the magnetic coil, generating a relatively strong magnetic field (up to several Tesla) that lasts for approximatively 100 microseconds (Hallett, 2007). This creates lines of magnetic flux that orientate perpendicularly to the plane of the wire coil. The flux then in turn induces an electric field perpendicular to the magnetic field, as determined by electromagnetic induction principles described by Michael Faraday in the nineteenth century. Rapidly changing magnetic fields induce electrical currents that can depolarise superficial axons and activate local neural circuits. The extent to which TMS generates these intra-cortical currents depends on multiple factors including the type and orientation of the coil, the frequency of stimulation, and the distance between the brain and the coil (Lefaucheur et al., 2014). Some of these factors can be controlled: For example, the shape of the magnetic coil can be changed to achieve strong but diffuse stimulation (i.e., round coils) or focal stimulation (i.e., figure-of-eight).
The local effects of TMS have been extensively studied, particularly in the motor system (Bohning et al., 1999; Huang et al., 2005; de Lara et al., 2017; Murphy et al., 2016). The motor system is easily accessible for TMS and also allows for a precise behavioural assessment of the effect of the stimulation through measuring the induced twitching of a muscle activated through stimulation of the contralateral motor cortex. In fact, the recording of motor evoked potentials (MEP) immediately following TMS allows one to assess both the location and effect of the stimulation on the motor cortex functional architecture (Wassermann et al., 1992). As we will discuss in the following paragraph, TMS can also be used to change cortical excitability. In this context, increases in the threshold of stimulation required to evoke a MEP after TMS are generally thought to indicate an inhibitory effect of stimulation whereas a lower stimulation threshold suggests an excitatory effect (Hallett, 2000; Huang et al., 2005). A common complementary measure to assess the neural effects of TMS is the change in the recruitment-curve slope [i.e., change in the MEP amplitude as a function of stimulus intensity, (Hallett, 2000)].
A number of stimulation protocols have been shown to increase or inhibit cortical activity for periods that outlast the duration of the stimulation (Lefaucheur et al., 2014). Due to this persisting effect, these paradigms have received the attention of clinical researchers interested in modulating the activity of a region, or network of regions, in disorders like MDD (Fox et al., 2012) and OCD (Berlim et al., 2013). High-frequency repetitive TMS (5–20 Hz) has been shown to increase cortical excitability whereas low frequency (1 Hz) repetitive TMS decreases cortical excitability (Chen et al., 1997; Pascual-Leone et al., 1994). More recently, shorter (~40–190 s) stimulation protocols have been developed to induce excitatory (intermittent theta burst stimulation, iTBS) and inhibitory (continuous theta burst stimulation, cTBS) effects which have been shown to last for over 60 min (see glossary, Huang et al., 2005). Several factors including BDNF polymorphism, the number of pulses, and the frequency of stimulation may influence the effect of TBS paradigms (Chung et al., 2016). Although further research is needed to improve the efficacy and reliability of TBS paradigms, compared to low and high frequency TMS protocols, TBS adopts a lower stimulation intensity (70% compared to 100% of resting motor threshold) and has a shorter duration (~1-3 min versus 15–20 min). Therefore, the application of TBS protocols is more easily tolerated by participants, particularly in the clinical setting, compared to more conventional repetitive TMS protocols. Importantly, a recent randomised, multicentre, non-inferiority clinical trial reported that the efficacy of iTBS to the left DLPFC in reducing symptoms of MDD is comparable to the one achieved by the longer 10 Hz rTMS protocol (Blumberger et al., 2018). This result suggests that high-frequency rTMS and iTBS have a similar effect on brain dynamics underpinning symptoms of depression.
Stimulation intensity for rTMS and TBS protocols is often titrated against the minimum intensity required to elicit a motor response when stimulating the motor cortex, known as the motor threshold. Determining the motor threshold for repetitive TMS can be performed visually or using electromyography (EMG). Visually, a common method is to define the motor threshold according to the lowest stimulation intensity needed to observe a movement on the targeted hand muscle in at least 5 out of 10 trials. Using EMG, the resting motor threshold can be defined as the lowest setting to generate ≥5 out of 10 MEPs (≥50 μV peak to peak). Given that safety guidelines for TMS have been developed using the EMG method and evidence suggests that the visual method overestimates the motor threshold (Westin et al., 2014), the use of EMG is advised for clinical interventions.
A quadripulse magnetic stimulation (QPS) protocol that involves delivering repetitive bursts of four monophasic TMS pulses applied at very short inter-pulse intervals has also been recently developed (Hamada et al., 2008; Hanajima et al., 2017). This recent stimulation protocol is based on theories of metaplasticity (see Section 3.2.) and has been proposed to improve the reliability of effects on MEP. However, these findings require replication. Moreover, its use outside the motor system has been limited and as such, this protocol has not yet been used for clinical research.
The use of TMS has not been confined to the sensorimotor system, but has also been used extensively to alter local brain activity and thus assess the contribution of targeted brain regions to various perceptual and cognitive functions (Walsh and Cowey, 2000; Polanía et al., 2018) including visual attention (Chambers et al., 2004). Unlike the motor cortex, stimulation of high-order associative brain regions such as the orbitofrontal cortex (OFC) does not have a direct response that can be easily validated in order to demonstrate the impact of stimulating OFC circuits. However, neuroimaging techniques, such as functional magnetic resonance imaging (fMRI) or electroencephalography (EEG), have been used to assess the impact of stimulation on brain activity (Ruff et al., 2006; Bestmann et al., 2005; Chung et al., 2016). In addition to characterising the effects of targeted TMS on local brain regions, functional neuroimaging data has highlighted the impact of local brain stimulation on the activity of widespread neural networks throughout the brain (Sale et al., 2015; Bortoletto et al., 2015).
2.2. Network effects of local TMS
The brain can be conceptualised as a complex network, with neurons connected locally into circuits and, through long-range anatomical projections, circuits connected into systems (Park and Friston, 2013; Sporns, 2011; Fornito et al., 2016). Structural networks are those constituted by direct anatomical connections, whereas functional connectivity denotes the correlated activity that these structural networks support (see glossary, Honey et al., 2007; Hermundstad et al., 2013; Cocchi et al., 2014). At the macroscopic scale, the organization of functional brain networks can be characterised using neuroimaging techniques such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). An increasing number of fMRI-based findings show that rTMS protocols can evoke changes in neural activity and functional connectivity between remote brain regions (Ruff et al., 2008; Ruff et al., 2006; Cocchi et al., 2016; Cocchi et al., 2015; Bestmann et al., 2005; Eldaief et al., 2011). For example, the stimulation of a frontal brain region involved in high-level visual processes (i.e., the frontal eye fields, FEF) can alter activity in, and connectivity between, early visual areas (Ruff et al., 2006; Cocchi et al., 2016). Importantly, this neurophysiological effect has been related to distinct changes in visual perception (Ruff et al., 2006). In the clinical setting, it has been shown that the effect of prefrontal stimulation to reduce symptoms of major depression may be related to the baseline (resting-state) level of functional connectivity between the targeted prefrontal region and the subgenual cingulate (Fox et al., 2012). These results highlight the importance of target selection when determining an appropriate TMS protocol. This emerging body of multimodal work, also emphasizes the significance of considering the effects of local TMS on whole-brain functional networks and supports the proposal that targeted TMS could redress abnormal patterns of neuronal network activity underpinning brain disorders (Fornito et al., 2015; Sale et al., 2015).
3. TMS as a potential intervention to alleviate symptoms of OCD
3.1. Dysfunctional brain networks in OCD
The application of TMS to therapeutically modulate brain network activity in OCD is contingent upon knowledge about the maladaptive brain activity that underlies the expression of OCD symptoms (Sale et al., 2015). Changes in “resting-state” (glossary) functional network activity in those with OCD provide useful goalposts. Analyzing resting-state activity has provided substantial utility in mapping the brain's functional network architecture and is not contingent on patient compliance in engaging a challenging task (Power et al., 2011). Moreover, resting-state brain activity has also been shown to predict task-induced neural activity (Cole et al., 2016), a broad range of behavioural performances (Smith et al., 2015), and psychopathology (Drysdale et al., 2017; Cocchi et al., 2012a; Lin et al., 2018).
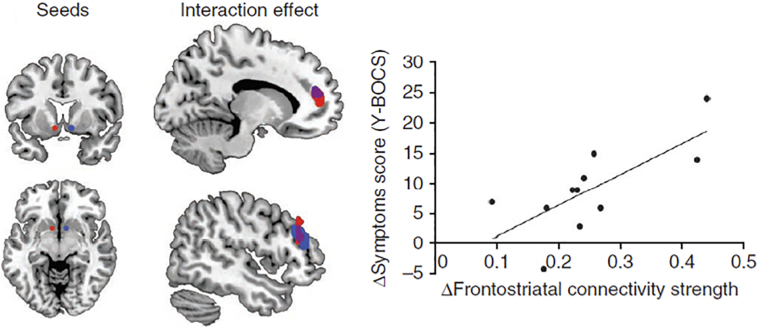
Increased resting-state functional connectivity between striatal and prefrontal brain regions —including the OFC and the frontal pole — is one of the most consistent findings in OCD [(Harrison et al., 2013; Harrison et al., 2009; Hou et al., 2014; Sakai et al., 2011; Figee et al., 2013), although see (Posner et al., 2014)]. Furthermore, the degree of hyper-connectivity between the striatum and the prefrontal cortex is linearly associated with symptom severity (Harrison et al., 2009; Figee et al., 2013) (Fig. 1).
Fig. 1.
Results from a study showing that invasive deep brain stimulation (DBS) of the left (red) and right (blue) ventral striatum (nucleus accumbens) modulated frontostriatal connectivity in the resting-state and while patients rated neutral or symptoms-provoking visual images (Figee et al., 2013). Resting-state functional neuroimaging results showed that DBS reduced functional connectivity between the ventral striatum and the prefrontal cortex (red and blue, with purple indicating overlap). Reductions in OCD symptoms (Y-BOCS) linearly correlated with DBS-induced changes in connectivity between the left ventral stiatum and the lateral prefrontal cortex (r = 0.72). Notably, DBS also modulated low frequency EEG oscillations in response to symptoms provoking stimuli (not shown). Reproduced with permission from (Figee et al., 2013).
While resting-state networks provide potential targets for TMS interventions in OCD, altered neural network activity during specific task performance may highlight state-dependent dysregulations that are specific to defined symptoms. For example, patients with OCD show increased task-induced connectivity in a brain network comprising the anterior insula-frontal operculum and the dorsal anterior cingulate when asked to engage in a cognitive task (Cocchi et al., 2012b) (Fig. 2). Importantly, this induced hyper-connectivity in the anterior insula-frontal operculum (salience network) was positively correlated with scores on the Beck Anxiety Inventory (r = 0.49, BAI). On the other hand, exposure to obsession-inducing images appears to exacerbate brain activity in the anterior cingulate and mid-orbitofrontal cortices (Morgiève et al., 2014). These different patterns of brain activity in OCD patients across different tasks highlight the importance of considering the context in which TMS is administered. In sum, brain network activity is not static but continuously changes as a function of internal and external demands (Cocchi et al., 2013). These dynamic patterns of activity may explain some of the response variability in previous clinical studies of TMS in OCD (e.g., see Table 1 in Lefaucheur et al., 2014). Constraining the mental state of participants using well-designed behavioural tasks will allow for a customised TMS intervention designed to target specific patterns of neural activity linked to distinct aspects of OCD pathophysiology.
Fig. 2.
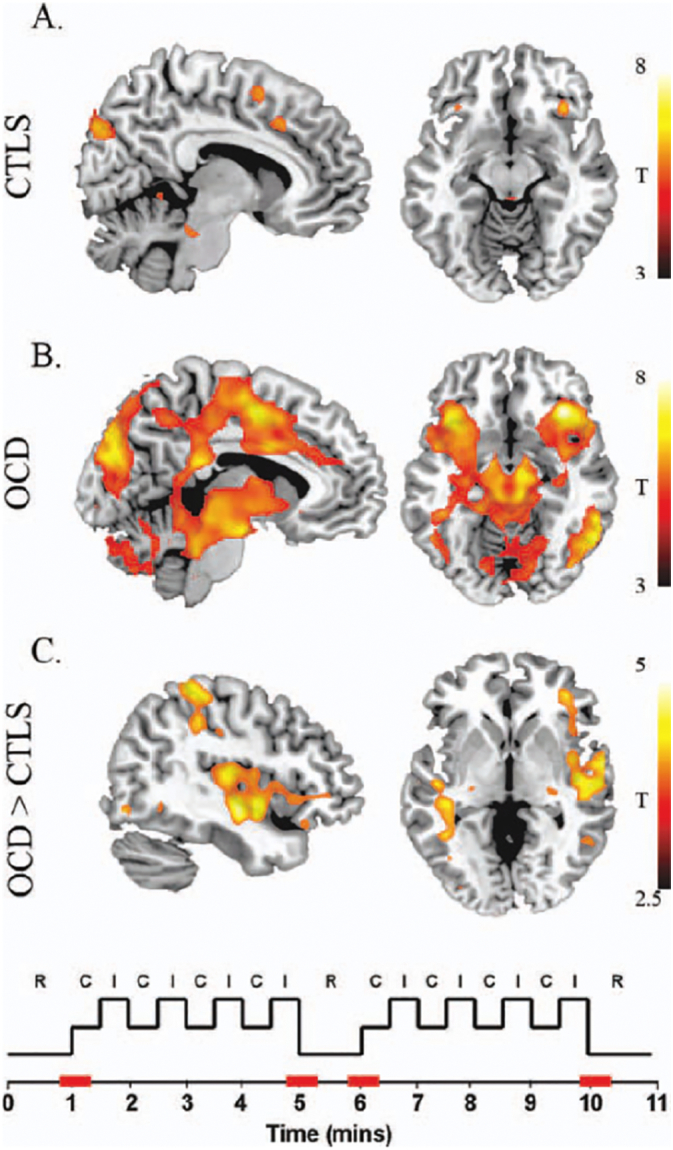
A Patterns of activity during transitions (highlighted in red in the bottom diagram) from resting-state (R) to external task performance [C = congruent trials and I = incongruent trials in the Multisource Interference Task, (Bush and Shin, 2006)]. In both healthy and OCD patient groups, transition periods were associated with activity in the dorsal anterior cingulate cortex, anterior insula, frontal operculum, substantia nigra, dorsal pons, left globus pallidus, occipital and parietal cortices. B. Patients with OCD showed additional activations in the thalamus, subthalamic nuclei, putamen, posterior insula, intraparietal, premotor/motor cortex and cerebellum. C. A contrast between the two groups showed greater activity in the right posterior insula, superior temporal gyrus and anterior insula-frontal operculum in OCD compared to controls. Reproduced with permission from (Cocchi et al., 2012b).
3.2. Toward an effective modulation of OCD networks
As outlined above, context dependent variability of network activity in OCD may play a significant role when determining an appropriate site for TMS interventions. We propose two key factors to consider when designing a symptom-directed TMS intervention; 1) whether the TMS is delivered at rest or during active engagement in a mental task; and 2) the customization of the optimal cortical region to target.
Stimulation in a state of rest draws support from the preceding resting-state fMRI studies that highlighted fronto-striatal hyper-connectivity in patients with OCD compared to matched healthy controls (e.g., Harrison et al., 2009). Therefore, there is an empirical rationale to selectively target the regions that compose resting-state fronto-striatal networks in patients with OCD. The observed hyper-activity of fronto-striatal resting-state networks in OCD also suggests that inhibitory stimulation may be appropriate to reduce the abnormal activity of OCD frontostriatal networks. Administering TMS in a state of rest also presents several practical advantages, including the simple instructions to patients (i.e., “please look at the fixation cross in front to you, relax, and avoid focusing on any particular thoughts”), and eschewing the need to learn a potentially challenging behavioural task. Furthermore, for the purposes of demonstrating a pre- vs post-TMS intervention analysis of brain activity, resting-state acquisitions are short and simple.
The majority of TMS trials in OCD have targeted frontal and prefrontal brain regions encompassing fronto-striatal networks (Sachdev et al., 2007; Gomes et al., 2012; Elbeh et al., 2016; Mantovani et al., 2013; Berlim et al., 2013; Pedapati et al., 2015; Sarkhel et al., 2010) (Table 1). However, the mental state of patients during application of TMS in these studies does not appear to have been carefully considered (Table 1). This lack of standardization may contribute to the large variability of the effects across studies and patients in the response to TMS interventions (Lefaucheur et al., 2014; Dunlop et al., 2016) (Table 1). Future studies adopting clear, standarised instructions for patients to undertake during the stimulation may reduce the response variability across subjects and trials, and may increase the clinical efficacy of the intervention.
Processing of an external stimulus is state-dependent – that is, the evoked brain activity depends on the pre-stimulus state of the neural system (Hanslmayr et al., 2007; Hipp et al., 2011; Cocchi et al., 2017). Evidence suggests that the brain spontaneously transitions between a number of dynamic spatiotemporal states, even at rest (Ghosh et al., 2008; Deco and Jirsa, 2012; Zalesky et al., 2014; Vidaurre et al., 2017; Cocchi et al., 2017). The precise state of the brain when TMS is delivered — particularly the target network — can therefore impact its efficacy, particularly for repetitive TMS protocols aiming to persistently modulate neural activity (Silvanto et al., 2008; Polanía et al., 2018). Administering TMS during constrained task conditions has therefore the potential to enhance the magnitude and precision of the desired effect. For example, bursts of high intensity TMS (vs low intensity) on the left resting motor cortex reduced the neural activity of the contralateral motor cortex. On the other hand, the same stimulation while participants performed an isometric contraction of the left hand caused an increase in neural activity in the right motor cortex (Bestmann et al., 2008). Task-based TMS (single pulse, short bursts, or repetitive) has therefore the potential to explicitly target symptom-specific neural deregulations, including circuits associated with hoarding symptoms (Tolin et al., 2012) and OCD symptom dimensions (Harrison et al., 2013). To date, the use of carefully designed behavioural tasks to selectively target dimensional or symptom-evoked brain network deregulations in OCD has been limited (e.g., Pedapati et al., 2015). Prospective clinical studies will benefit from a deeper understanding of the physiological and cognitive factors underpinning the selective response of task-evoked brain activity and connectivity to given TMS protocols (Bestmann et al., 2008; Bestmann et al., 2010; Feredoes et al., 2011). The combined use of TMS, particularly of repetitive protocols and behavioural tasks, will also require the assessment of the safety profile of such interventions in OCD.
In keeping with the aforementioned state effects, metaplastic principles can be used to predict and explain specific changes in local neural activity (for a review see Müller-Dahlhaus and Ziemann, 2015). Metaplasticity is a broad term encompassing a series of endogenous neural processes linked to activity-dependent synaptic plasticity. The influential Bienenstock-Cooper-Munro theory [BCM, (Bienenstock et al., 1982)], postulates that long term depression (LTD) is facilitated by high levels of preceding postsynaptic activity, whereas long term potentiation (LTP) is facilitated by low levels of recent postsynaptic activity. In agreement with this theory, Doeltgen and Ridding (2011) showed that priming cTBS with iTBS increases the LTD-like plasticity. These results have been replicated, and extended to iTBS, by Murakami et al. (2012). In addition to decreasing the variability of TMS effects, metaplasticity can facilitate the likelihood of obtaining synaptic changes in local circuits affected by OCD. Metaplasticity also refers to changes occurring on longer time scales (hours-to-weeks) possibly via subtle morphological synapto-dendritic changes. Such forms of metaplasticity may be relevant in planning the timing between TMS interventions and other therapies, including behavioural interventions (Tsagaris et al., 2016).
Patients with OCD undergoing TMS are frequently managed with concomitant pharmacotherapies. Many psychotropic medications, such as SSRIs, are known to modulate the activity of the central nervous system in OCD (Saxena et al., 2002; Nakao et al., 2005; Shin et al., 2014). It is therefore important to account for the effects of such psychotropics on brain activity, at rest and during active task engagement. Considering the effect of medication on given neural circuits can also offer the potential to effectively complement pharmacological interventions with TMS. Seminal work by Saxena et al. (2002) showed that 12 weeks of paroxetine hydrochloride treatment in OCD reduced glucose metabolism in the right caudate nucleus, right putamen, thalamus, right ventrolateral prefrontal cortex and bilateral orbitofrontal cortex. The reductions in the right putamen, caudate and thalamus occurred only in OCD patients that responded to the pharmacological intervention (i.e., 25% reduction in YBOCS scores as well as “very much” improved on a clinical scale). This finding supports the notion that OCD symptoms are linked to abnormal activity in the prefrontal-striatal-thalamic networks. Because higher doses of SSRIs (e.g., 60 mg/d of paroxetine hydrochloride) may not be tolerated, inhibitory TMS on the orbitofrontal cortex may facilitate the reduction of activity in the prefrontal-striatal-thalamic networks, enhancing the effect of medication in non-responders or reducing the dose required in those who do respond.
A second factor to consider when designing a TMS trial for OCD is the selection of the optimal cortical region to target. To date, the choice of stimulation site in OCD patients is predicated on approved protocols to treat medication-resistant MDD, resting-state neuroimaging work in OCD (Harrison et al., 2009; Harrison et al., 2013), and existing knowledge on fronto-striatal functional and anatomical connectivity (e.g., Sachdev et al., 2007). Accordingly, the large majority of existing studies have targeted the DLPFC (right, Gomes et al., 2012; left, Prasko et al., 2006), the supplementary motor area [SM, (Mantovani et al., 2010; Mantovani et al., 2013)], and the OFC (Ruffini et al., 2009; Nauczyciel et al., 2014). The results of these studies have been inconclusive for the DLPFC, with limited evidence supporting the stimulation of this region to alleviate symptoms of OCD (Slotema et al., 2010; Berlim et al., 2013; Lefaucheur et al., 2014). This outcome could be explained by the fact that whereas the DLPFC has been implicated in the cognitive effects of MDD, abnormalities in DLPFC-striatal connectivity have not been consistently reported in resting-state studies of OCD. However, context-dependent stimulation of the DLPFC in OCD may provide efficacy; namely, stimulation during the presentation of symptom-provoking stimuli. On the other hand, data from preliminary studies targeting the SMA or the prefrontal cortex are more encouraging [e.g, (Mantovani et al., 2010; Mantovani et al., 2013; Ruffini et al., 2009), Table 1], but these need to be replicated in well-powered, double-blind, clinical trials. In addition to their limited number and small sample size, existing TMS studies in OCD rarely use structural magnetic resonance imaging data to define the target region for stimulation personalized to the patient's own neuroanatomy. Compared to a commonly used scalp-based localization technique (localization of the best cortical site for activating a hand muscle, generally the abductor pollicis brevis, and then 5 cm anteriorly along the scalp surface), the use of neuronavigation to target a specific cortical site has been shown to significantly enhance the efficacy of TMS intervention in treatment resistant major depression at 4 weeks (p < 0.02) (Fitzgerald et al., 2009). Likewise, the optimal angle of stimulation [i.e., coil handle perpendicular to the crown of the targeted gyrus, (Thielscher et al., 2011; Richter et al., 2013)] is not adapted to each individual's neuroanatomy, increasing the variability in the response to stimulation (Thielscher et al., 2011; Opitz et al., 2013; Cocchi and Zalesky, 2018). Non-optimal angles of stimulation are less likely to effectively and directly modulate cortical activity and cortico-cortical connectivity (Thielscher et al., 2011; Opitz et al., 2013). Biophysical models of magnetic flux and current flow through skull, dura and cortex informed by patient-specific neuroimaging data provide a means to computationally simulate the impact of coil placement with respect to individual neuroanatomy. This approach could potentially be used to further personalize TMS delivery in OCD (Opitz et al., 2013).
New advances in psychiatric research suggest that the functional architecture of whole-brain networks can be used to parse patients' clinical heterogeneity and optimise the selection of TMS targets (Drysdale et al., 2017; Lin et al., 2018; Cocchi and Zalesky, 2018). In particular, a recent study including a large sample of patients with depression (n = 1188) has suggested that patients can be divided into four distinct clusters based on their distinct patterns of resting-state functional connectivity in limbic and frontostriatal networks (Drysdale et al., 2017). This study showcases the exciting new avenues to improve the efficacy of TMS interventions by personalizing interventions based on neurophysiological measures [see also (Lin et al., 2018)]. The use of neuroimaging-guided TMS presents several challenges in everyday clinical settings, including higher costs compared to standard interventions and expertise in neuroimaging and TMS neuronavigation. Ultimately, should future work confirm that the utility of TMS interventions for OCD relies upon such detailed pre-treatment assessments, then modelling by health economists will be required to determine whether it is cost effective in clinical practice.
4. Outstanding issues
Studies on the role of TMS in the treatment for OCD symptoms are so far limited. Moreover, the large majority of existing studies include small samples, use different stimulation parameters and cortical targets, do not standardise the context in which TMS is administered, and do not take advantage of imaging-guided TMS (neuronavigation). Therefore, there is a pressing need for new studies addressing the possible use of TMS as an efficacious and cost-effective therapeutic intervention for OCD. The use of TMS to effectively restore brain network dynamics and reduce symptoms of OCD may also benefit from a deeper understanding of how a focal perturbation of neural activity impacts activity in, and functional connectivity between, remote brain regions (Eldaief et al., 2011; Cocchi et al., 2015; Cocchi et al., 2016; Gollo et al., 2017; Ruff et al., 2006; Sale et al., 2015). Without this knowledge, the planning and outcome prediction of TMS interventions remain difficult and the development of new therapeutic protocols will be dependent on inefficient and costly trial and error approaches.
In the past five years, the modelling of altered brain network activity in OCD using neuroimaging data has grown substantially (Bandelow et al., 2016). Neuroimaging studies have shown a relationship between brain activity and symptoms in OCD but causality has yet to be determined. For example, the key role of activity within the prefrontal cortex for OCD symptoms has been highlighted by several resting-state studies (Harrison et al., 2009; Hou et al., 2014; Sakai et al., 2011; Figee et al., 2013; Morgiève et al., 2014). These studies suggest that a selective inhibition of the prefrontal (e.g., OFC) hyper-activity may reduce OCD symptom severity. However, these studies do not preclude the possibility that prefrontal hyper-activity may be linked to neurophysiological and/or cognitive compensatory mechanisms: Inhibiting this region could therefore worsen OCD symptoms. The link between symptoms and observed changes in brain activity and connectivity is not always straightforward and the potential modulating effect of TMS on this association requires careful consideration.
The neural principles underlying the effect of local TMS on distant brain regions are not fully understood. Addressing this is an important prelude to predicting how a local perturbation will impact upon the brain network dynamics implicated in OCD. For example, the OFC and the striatum, particularly the ventral striatum, are functionally interconnected at rest (Jaspers et al., 2017). Results from recent studies combining TMS and fMRI support the notion that local stimulation of a cortical region in the resting-state has greater impact on the functional connectivity between the target region and remote regions composing the same functional network (Eldaief et al., 2011; Cocchi et al., 2016). Therefore, inhibition of the OFC in a state of rest is expected to result in a significant reduction of functional connectivity within the striatum. However, while mainly circumscribed within a defined functional network, the impact of local TMS to the dynamic interplay between brain regions may “spill” over into regions belonging to brain networks not directly targeted by the stimulation (Cocchi et al., 2015; Gollo et al., 2017). The nature and implication of these “secondary” effects on brain dynamics to clinical interventions remain to be established.
In addition to the presence of functional connectivity changes, predicting the direction of changes in connectivity is also an active field of research. Recent work challenges the notion of a simple linear association between increases or reductions in local brain activity and changes in functional connectivity (Gollo et al., 2017; Cocchi et al., 2016). Studies have suggested that neural activity in highly interconnected brain regions [brain hubs, (van den Heuvel and Sporns, 2013)] is characterised by slower fluctuations in spiking activity (longer temporal receptive field) compared to peripheral regions such as the primary visual cortex (Baldassano et al., 2017; Murray et al., 2014; Gollo et al., 2015; Hasson et al., 2015; Hasson et al., 2008). In this sense, the intrinsic timescale of hub regions is slower than peripheral regions. Results from studies combining neuroimaging, TMS, and computational modelling support this temporal organization of the brain, showing that the impact of local stimulation to whole-brain patterns of communication between brain regions can be predicted by a cortical hierarchy of timescales (Gollo et al., 2017; Cocchi et al., 2016). Without valid predictive models, the targeted use of TMS to restore network dynamics in brain disorders relies solely on empirical work, which can be expensive and prone to ad hoc trial and error.
A further challenge to improving the likelihood of selectively modulating altered brain network activity in OCD is the inter- (Hamada et al., 2013; Dunlop et al., 2016) and intra-subject (Sale et al., 2007) variability in the local response to TMS. This variability has been linked to several factors including age (Rogasch et al., 2009), genetic influences (Antal et al., 2010; Kleim et al., 2006) and hormones (Sale et al., 2008). Studies have also highlighted the dynamic nature of functional brain connectivity in states of rest and external task (Cocchi et al., 2017; Hearne et al., 2017; Zalesky et al., 2014; Shine et al., 2016), suggesting that the time in which the stimulation is performed could impact the propagation of the local perturbation throughout the brain. Future research is needed to better understand the variability of TMS and allow effects that are more consistent across stimulation sessions and patients.
Finally, the translation of approaches combining computational modelling, advanced neuroimaging analysis, and behavioural tools that constrain mental states into the clinic presents significant practical challenges. In addition to technical advances streamlining data modelling, the effective implementation of the proposed multimodal approach will require multidisciplinary clinical teams. Such multidisciplinary planning of therapeutic interventions is common in other technologically-based medical disciplines (e.g., brain oncology).
5. Summary and recommendations
In concluding, we propose a summary of the state of research in this field and practical recommendations to improve the reliability of TMS interventions for OCD. While these recommendations are specific to OCD interventions, a number of them may be applicable to stimulation protocols used to treat symptoms of different brain disorders:
-
•
Whether TMS can effectively normalise brain activity underpinning OCD symptoms has yet to be established by clinical trials.
-
•
Theoretical and empirical advances in network neuroscience suggest that improving the efficacy of TMS for OCD is contingent on the mapping of altered whole-brain patterns of neural activity in OCD. Progress in the understanding of principles supporting how local TMS affects the activity within, and connectivity between, remote brain regions appears key to the development of new effective TMS interventions for OCD.
-
•
Neuroimaging, TMS, and computational modelling may help the identification of optimal stimulation targets to normalise the activity of defined neural networks underpinning OCD symptoms. The efficacy and practical implementation of this complex multimodal approach will need to be assessed by future clinical trials.
-
•
Altered brain network activity in OCD is state-dependent. In a state of rest, OCD symptoms have been linked to increased functional connectivity strength between the striatum and the prefrontal cortex [e.g., (Harrison et al., 2009)]. Therefore, if delivered at rest, inhibitory stimulation of the orbitofrontal and frontal pole regions holds the greatest potential to significantly improve symptoms. Stimulation of prefrontal regions can result in discomfort and thus, short protocols such as cTBS are preferred. While stimulation of DLPFC in OCD is not recommended in the resting-state, new evidence suggests that sessions of inhibitory TMS of this region during the viewing of symptom-provoking stimuli may facilitate the response to subsequent cognitive behavioural therapy (Olatunji et al., 2014). Overall, these preliminary findings support the notion that the effects of TMS are context dependent and affected by concomitant therapeutic interventions. We have presently discussed personalizing TMS according to an individual's functional connectivity and clinical profile, but in the future, it may be possible to further personalize TMS by administering stimulation during the presentation of personalized stimuli that elicit individually-specific responses.
-
•
The impact of psychotropic medication should be considered when planning TMS interventions. TMS could be used to facilitate the response to medication where dose increases for the patient are not possible. Such an approach is consistent with the broader notion that combination therapies may be more effective than either therapy in isolation.
-
•
The use of neuroimaging-guided neuronavigation can assist with the precise modulation of neural activity and connectivity underpinning symptoms of OCD. The consideration of anatomical individual differences is likely to improve the efficacy and reliability of TMS as a therapeutic intervention [e.g., (Fitzgerald et al., 2009)].
-
•
Individually tailored TMS that accounts for network mechanisms and state dependence may be a viable treatment modality for patients not suitable for pharmacological or behavioural therapies.
Funding
L.C., A.Z, P.F. and M.B. were supported by the Australian National Health Medical Research Council (L.C. APP1099082 and APP1138711, A.Z. APP1047648, M.B. APP1037196). This work was also supported by the Australian Research Council Centre of Excellence for Integrative Brain Function (M.B., ARC Centre Grant CE140100007). PBF is supported by a NHMRC Practitioner Fellowship (1078567).
Conflicting interests
PBF has received equipment for research from MagVenture A/S, Medtronic Ltd., Neuronetics and Brainsway Ltd. and funding for research from Neuronetics. He is on scientific advisory boards for Bionomics Ltd. and LivaNova and is a founder of TMS Clinics Australia.
Acknowledgments
The authors thank Bjorn Burgher, Saurabh Sonkusare and Caitlin Hall for helpful comments on a draft manuscript.
Glossary
- Electromagnetic induction: TMS use principles of electromagnetic induction to induce an electrical field sufficient to depolarize cortical axons.
- Functional connectivity: A measure of statistical dependence between neural signals recorded in different brain regions. Functional connectivity can be assessed using non-invasive neuroimaging techniques including fMRI. However, functional connectivity is agnostic regarding the causal interactions between brain regions and does not imply direct anatomical connections between them.
- Functional magnetic resonance imaging (fMRI): A common method to infer neural activity non-invasively. The blood‑oxygen-level dependent (BOLD) contrast signal measured using fMRI reflects changes in the proportion of oxy- and deoxygenated blood in a localised brain region (within a few millimeters) arising from neurovascular coupling. The temporal resolution of fMRI is in the order of seconds, capturing only a portion of neural dynamics.
- Hub: A region that possesses a large number of connections with other regions of the brain. Hubs are defined by measures of network centrality (e.g., degree).
- Long-term potentiation (LTP): Facilitation of neural transmission due to a long-lasting increase in the synaptic strength. Long-term depression (LTD) refers to the opposite phenomenon. The persistent effects of repetitive TMS and TBS protocols are thought to rely on LTP and LTD mechanisms.
- Motor evoked potentials (MEP): Electrical responses measured from superficial muscles in response to TMS of the brain motor cortex. MEPs are used to establish intensity of repetitive TMS and assess its effects when the motor system is targeted.
- Plasticity: Refers to the ability of the brain to change the properties of local neural circuits and larger brain networks. The mechanisms of action of several transcranial magnetic stimulation paradigms, including continuous theta burst stimulation (cTBS), rely on neural plasticity.
- Predictive models: Statistical and biophysical models to predict the likelihood of a given outcome. Predictive models can be used to optimise TMS parameters in order to achieve a precise modulation of neural network activity underpinning the symptoms of OCD.
- Resting-state: A common approach to assessing functional connectivity involves measuring brain activity while participants are in a state of rest. In a typical resting-state protocol, individuals are asked to remain awake and not engage in any particular task or mental activity. Correlations between low-frequency (< 0.1 Hz) resting-state BOLD signal across brain regions has been related to a fundamental property of brain organization, predicting neural dynamics and behaviours observed in task contexts (Smith et al., 2015; Cole et al., 2016). Resting-state studies have also highlighted associations between neural network activity at rest and symptoms of psychiatric disorders, including OCD (Harrison et al., 2009).
- Theta burst stimulation (TBS): Is a form of repetitive TMS that induces long-lasting changes in corticospinal excitability via long-term potentiation (LTP) and depression (LTD). TBS involves the delivery of 3 pulses of stimulation given at 50 Hz.
- Continuous theta burst stimulation (cTBS): Delivers an uninterrupted train of theta bursts. A common example is a 40 s train (600 pulses in total). This paradigm causes a reduction of local cortical excitability (Huang et al., 2005) and changes in functional connectivity between remote brain regions (Cocchi et al., 2015) that outlast the period of stimulation.
- Intermittent theta burst stimulation (iTBS): Is a two second train of theta bursts repeated every 10 s for 190 s (600 pulses) (Huang et al., 2005). This protocol has opposing effects to cTBS. The predicted after-effects of TBS are, however, variable (Hamada et al., 2013). TBS protocols have recently been validated to treat symptoms of major depression (Blumberger et al., 2018) but their efficacy in treating OCD symptoms remains unclear.
References
- Alonso P., Pujol J., Cardoner N. Right prefrontal repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: a double-blind, placebo-controlled study. Am. J. Psychiatr. 2001;158(7):1143–1145. doi: 10.1176/appi.ajp.158.7.1143. [DOI] [PubMed] [Google Scholar]
- Antal A., Chaieb L., Moliadze V. Brain-derived neurotrophic factor (BDNF) gene polymorphisms shape cortical plasticity in humans. Brain Stimul. 2010;3:230–237. doi: 10.1016/j.brs.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Baldassano C., Chen J., Zadbood A. Discovering event structure in continuous narrative perception and memory. Neuron. 2017;95:709–721. doi: 10.1016/j.neuron.2017.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelow B., Baldwin D., Abelli M. Biological markers for anxiety disorders, OCD and PTSD–a consensus statement. Part I: neuroimaging and genetics. World J. Biol. Psychiatry. 2016;17:321–365. doi: 10.1080/15622975.2016.1181783. [DOI] [PubMed] [Google Scholar]
- Berlim M.T., Neufeld N.H., Van den Eynde F. Repetitive transcranial magnetic stimulation (rTMS) for obsessive–compulsive disorder (OCD): an exploratory meta-analysis of randomized and sham-controlled trials. J. Psychiatr. Res. 2013;47:999–1006. doi: 10.1016/j.jpsychires.2013.03.022. [DOI] [PubMed] [Google Scholar]
- Bestmann S., Baudewig J., Siebner H.R. BOLD MRI responses to repetitive TMS over human dorsal premotor cortex. NeuroImage. 2005;28:22–29. doi: 10.1016/j.neuroimage.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Bestmann S., Swayne O., Blankenburg F. Dorsal premotor cortex exerts state-dependent causal influences on activity in contralateral primary motor and dorsal premotor cortex. Cereb. Cortex. 2008;18:1281–1291. doi: 10.1093/cercor/bhm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S., Swayne O., Blankenburg F. The role of contralesional dorsal premotor cortex after stroke as studied with concurrent TMS-fMRI. J. Neurosci. 2010;30:11926–11937. doi: 10.1523/JNEUROSCI.5642-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock E.L., Cooper L.N., Munro P.W. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J. Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M., Landeros-Weisenberger A., Kelmendi B. A systematic review: antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Mol. Psychiatry. 2006;11:622. doi: 10.1038/sj.mp.4001823. [DOI] [PubMed] [Google Scholar]
- Blom R.M., Figee M., Vulink N. Update on repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: different targets. Curr. Psychiatry Rep. 2011;13:289–294. doi: 10.1007/s11920-011-0205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberger D.M., Vila-Rodriguez F., Thorpe K.E. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391:1683–1692. doi: 10.1016/S0140-6736(18)30295-2. [DOI] [PubMed] [Google Scholar]
- Bohning D., Shastri A., Mcconnell K. A combined TMS/fMRI study of intensity-dependent TMS over motor cortex. Biol. Psychiatry. 1999;45:385–394. doi: 10.1016/s0006-3223(98)00368-0. [DOI] [PubMed] [Google Scholar]
- Bortoletto M., Veniero D., Thut G. The contribution of TMS–EEG coregistration in the exploration of the human cortical connectome. Neurosci. Biobehav. Rev. 2015;49:114–124. doi: 10.1016/j.neubiorev.2014.12.014. [DOI] [PubMed] [Google Scholar]
- Bush G., Shin L.M. The multi-source interference task: an fMRI task that reliably activates the cingulo-frontal-parietal cognitive/attention network. Nat. Protoc. 2006;1:308–313. doi: 10.1038/nprot.2006.48. [DOI] [PubMed] [Google Scholar]
- Chambers C.D., Payne J.M., Stokes M.G. Fast and slow parietal pathways mediate spatial attention. Nat. Neurosci. 2004;7:217–218. doi: 10.1038/nn1203. [DOI] [PubMed] [Google Scholar]
- Chen R., Classen J., Gerloff C. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Chung S.W., Hill A.T., Rogasch N.C. Use of theta-burst stimulation in changing excitability of motor cortex: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2016;63:43–64. doi: 10.1016/j.neubiorev.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Cocchi L., Zalesky A. Personalized transcranial magnetic stimulation in psychiatry. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2018 doi: 10.1016/j.bpsc.2018.01.008. https://www.biologicalpsychiatrycnni.org/article/S2451-9022(18)30022-3/pdf (In Press) [DOI] [PubMed] [Google Scholar]
- Cocchi L., Bramati I.E., Zalesky A. Altered functional brain connectivity in a non-clinical sample of young adults with attention-deficit/hyperactivity disorder. J. Neurosci. 2012;32:17753–17761. doi: 10.1523/JNEUROSCI.3272-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi L., Harrison B.J., Pujol J. Functional alterations of large-scale brain networks related to cognitive control in obsessive-compulsive disorder. Hum. Brain Mapp. 2012;33:1089–1106. doi: 10.1002/hbm.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi L., Zalesky A., Fornito A. Dynamic cooperation and competition between brain systems during cognitive control. Trends Cogn. Sci. 2013;17:493–501. doi: 10.1016/j.tics.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Cocchi L., Harding I.H., Lord A. Disruption of structure-function coupling in the schizophrenia connectome. Neurol. Clin. 2014;4:779–787. doi: 10.1016/j.nicl.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi L., Sale M.V., Lord A. Dissociable effects of local inhibitory and excitatory theta-burst stimulation on large-scale brain dynamics. J. Neurophysiol. 2015;113:3375–3385. doi: 10.1152/jn.00850.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi L., Sale M.V., Gollo L.L. A hierarchy of timescales explains distinct effects of local inhibition of primary visual cortex and frontal eye fields. elife. 2016;5 doi: 10.7554/eLife.15252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi L., Yang Z., Zalesky A. Neural decoding of visual stimuli varies with fluctuations in global network efficiency. Hum. Brain Mapp. 2017;38:3069–3080. doi: 10.1002/hbm.23574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M.W., Ito T., Bassett D.S. Activity flow over resting-state networks shapes cognitive task activations. Nat. Neurosci. 2016;19:1718–1726. doi: 10.1038/nn.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly K.R., Helmer A., Cristancho M.A. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. J. Cli. Psychiatry. 2012;73:e567–e573. doi: 10.4088/JCP.11m07413. [DOI] [PubMed] [Google Scholar]
- de Lara L.I.N., Tik M., Woletz M. High-sensitivity TMS/fMRI of the human motor cortex using a dedicated multichannel MR coil. NeuroImage. 2017;150:262–269. doi: 10.1016/j.neuroimage.2017.02.062. [DOI] [PubMed] [Google Scholar]
- Deco G., Jirsa V.K. Ongoing cortical activity at rest: criticality, multistability, and ghost attractors. J. Neurosci. 2012;32:3366–3375. doi: 10.1523/JNEUROSCI.2523-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeltgen S.H., Ridding M.C. Modulation of cortical motor networks following primed theta burst transcranial magnetic stimulation. Exp. Brain Res. 2011;215:199–206. doi: 10.1007/s00221-011-2886-6. [DOI] [PubMed] [Google Scholar]
- Drysdale A.T., Grosenick L., Downar J. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 2017;23:28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop K., Woodside B., Olmsted M. Reductions in Cortico-striatal Hyperconnectivity accompany successful treatment of obsessive-compulsive disorder with dorsomedial prefrontal rTMS. Neuropsychopharmacology. 2016;41:1395–1403. doi: 10.1038/npp.2015.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbeh K.A., Elserogy Y.M., Khalifa H.E. Repetitive transcranial magnetic stimulation in the treatment of obsessive-compulsive disorders: double blind randomized clinical trial. Psychiatry Res. 2016;238:264–269. doi: 10.1016/j.psychres.2016.02.031. [DOI] [PubMed] [Google Scholar]
- Eldaief M.C., Halko M.A., Buckner R.L. Transcranial magnetic stimulation modulates the brain's intrinsic activity in a frequency-dependent manner. Proc. Natl. Acad. Sci. 2011;108:21229–21234. doi: 10.1073/pnas.1113103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feredoes E., Heinen K., Weiskopf N. Causal evidence for frontal involvement in memory target maintenance by posterior brain areas during distracter interference of visual working memory. Proc. Natl. Acad. Sci. 2011;108:17510–17515. doi: 10.1073/pnas.1106439108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figee M., Luigjes J., Smolders R. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat. Neurosci. 2013;16:386–387. doi: 10.1038/nn.3344. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P.B., Brown T.L., Marston N.A. Transcranial magnetic stimulation in the treatment of depression: a double-blind, placebo-controlled trial. Arch. Gen. Psychiatry. 2003;60:1002–1008. doi: 10.1001/archpsyc.60.9.1002. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P.B., Benitez J., de Castella A. A randomized, controlled trial of sequential bilateral repetitive transcranial magnetic stimulation for treatment-resistant depression. Am. J. Psychiatr. 2006;163:88–94. doi: 10.1176/appi.ajp.163.1.88. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P.B., Hoy K., Mcqueen S. A randomized trial of rTMS targeted with MRI based neuro-navigation in treatment-resistant depression. Neuropsychopharmacology. 2009;34:1255–1262. doi: 10.1038/npp.2008.233. [DOI] [PubMed] [Google Scholar]
- Fornito A., Zalesky A., Breakspear M. The connectomics of brain disorders. Nat. Rev. Neurosci. 2015;16:159–172. doi: 10.1038/nrn3901. [DOI] [PubMed] [Google Scholar]
- Fornito A., Zalesky A., Bullmore E. Centrality and hubs. In: Fornito A., editor. Fundamentals of Brain Network Analysis. Academic Press; London: 2016. pp. 137–161. [Google Scholar]
- Fox M.D., Buckner R.L., White M.P. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol. Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George M.S., Post R.M. Daily left prefrontal repetitive transcranial magnetic stimulation for acute treatment of medication-resistant depression. Am. J. Psychiatr. 2011;168:356–364. doi: 10.1176/appi.ajp.2010.10060864. [DOI] [PubMed] [Google Scholar]
- George M.S., Lisanby S.H., Avery D. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch. Gen. Psychiatry. 2010;67:507–516. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Rho Y., Mcintosh A.R. Noise during rest enables the exploration of the brain's dynamic repertoire. PLoS Comput. Biol. 2008;4 doi: 10.1371/journal.pcbi.1000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazier K., Calixte R.M., Rothschild R. High rates of OCD symptom misidentification by mental health professionals. Ann. Clin. Psychiatry. 2013;25:201–209. [PubMed] [Google Scholar]
- Gollo L.L., Zalesky A., Hutchison R.M. Dwelling quietly in the rich club: brain network determinants of slow cortical fluctuations. Philos. Trans. R. Soc. B. 2015;370 doi: 10.1098/rstb.2014.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollo L.L., Roberts J.A., Cocchi L. Mapping how local perturbations influence systems-level brain dynamics. NeuroImage. 2017;160:97–112. doi: 10.1016/j.neuroimage.2017.01.057. [DOI] [PubMed] [Google Scholar]
- Gomes P.V., Brasil-Neto J.P., Allam N. A randomized, double-blind trial of repetitive transcranial magnetic stimulation in obsessive-compulsive disorder with three-month follow-up. J. Neuropsychiatry Clin. Neurosci. 2012;24:437–443. doi: 10.1176/appi.neuropsych.11100242. [DOI] [PubMed] [Google Scholar]
- Greenberg B.D., George M.S., Martin J.D. Effect of prefrontal repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: a preliminary study. Am. J. Psychiatry. 1997;154:867. doi: 10.1176/ajp.154.6.867. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406:147–150. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hamada M., Terao Y., Hanajima R. Bidirectional long-term motor cortical plasticity and metaplasticity induced by quadripulse transcranial magnetic stimulation. J. Physiol. 2008;586:3927–3947. doi: 10.1113/jphysiol.2008.152793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M., Murase N., Hasan A. The role of interneuron networks in driving human motor cortical plasticity. Cereb. Cortex. 2013;23:1593–1605. doi: 10.1093/cercor/bhs147. [DOI] [PubMed] [Google Scholar]
- Hanajima R., Tanaka N., Tsutsumi R. The effect of age on the homotopic motor cortical long-term potentiation-like effect induced by quadripulse stimulation. Exp. Brain Res. 2017:1–6. doi: 10.1007/s00221-017-4953-0. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S., Aslan A., Staudigl T. Prestimulus oscillations predict visual perception performance between and within subjects. NeuroImage. 2007;37:1465–1473. doi: 10.1016/j.neuroimage.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Harrison B.J., Soriano-Mas C., Pujol J. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch. Gen. Psychiatry. 2009;66:1189–1200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- Harrison B.J., Pujol J., Cardoner N. Brain corticostriatal systems and the major clinical symptom dimensions of obsessive-compulsive disorder. Biol. Psychiatry. 2013;73:321–328. doi: 10.1016/j.biopsych.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Hasson U., Yang E., Vallines I. A hierarchy of temporal receptive windows in human cortex. J. Neurosci. 2008;28:2539–2550. doi: 10.1523/JNEUROSCI.5487-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Chen J., Honey C.J. Hierarchical process memory: memory as an integral component of information processing. Trends Cogn. Sci. 2015;19:304–313. doi: 10.1016/j.tics.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawken E.R., Dilkov D., Kaludiev E. Transcranial magnetic stimulation of the supplementary motor area in the treatment of obsessive-compulsive disorder: a multi-site study. Int. J. Mol. Sci. 2016;17:420. doi: 10.3390/ijms17030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearne L.J., Cocchi L., Zalesky A. Reconfiguration of brain network architectures between resting-state and complexity-dependent cognitive reasoning. J. Neurosci. 2017;37:8399–8411. doi: 10.1523/JNEUROSCI.0485-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermundstad A.M., Bassett D.S., Brown K.S. Structural foundations of resting-state and task-based functional connectivity in the human brain. Proc. Natl. Acad. Sci. 2013;110:6169–6174. doi: 10.1073/pnas.1219562110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipp J.F., Engel A.K., Siegel M. Oscillatory synchronization in large-scale cortical networks predicts perception. Neuron. 2011;69:387–396. doi: 10.1016/j.neuron.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Hirschtritt M.E., Bloch M.H., Mathews C.A. Obsessive-compulsive disorder: advances in diagnosis and treatment. JAMA. 2017;317:1358–1367. doi: 10.1001/jama.2017.2200. [DOI] [PubMed] [Google Scholar]
- Honey C.J., Kotter R., Breakspear M. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc. Natl. Acad. Sci. 2007;104:10240–10245. doi: 10.1073/pnas.0701519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath J.C., Mathews J., Demitrack M.A. The NeuroStar TMS device: conducting the FDA approved protocol for treatment of depression. JoVE. 2010 doi: 10.3791/2345. (45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J.-M., Zhao M., Zhang W. Resting-state functional connectivity abnormalities in patients with obsessive-compulsive disorder and their healthy first-degree relatives. J. Psychiatry Neurosci. 2014;39:304. doi: 10.1503/jpn.130220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.Z., Edwards M.J., Rounis E. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Jaspers E., Balsters J.H., Kassraian Fard P. Corticostriatal connectivity fingerprints: probability maps based on resting-state functional connectivity. Hum. Brain Mapp. 2017;38:1478–1491. doi: 10.1002/hbm.23466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.I., Kim C.H., Namkoong K. A randomized controlled study of sequentially applied repetitive transcranial magnetic stimulation in obsessive-compulsive disorder. J. Cli. Psychiatry. 2009;70:1645–1651. doi: 10.4088/JCP.08m04500. [DOI] [PubMed] [Google Scholar]
- Kedzior K.K., Reitz S.K., Azorina V. Durability of the antidepressant effect of the high-frequency repetitive transcranial magnetic stimulation (rTMS) in the absence of maintenance treatment in major depression: a systematic review and meta-analysis of 16 double-blind, randomized, sham-controlled trials. Depress. Anxiety. 2015;32:193–203. doi: 10.1002/da.22339. [DOI] [PubMed] [Google Scholar]
- Kleim J.A., Chan S., Pringle E. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat. Neurosci. 2006;9:735. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- Lefaucheur J.-P., André-Obadia N., Antal A. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) Clin. Neurophysiol. 2014;125:2150–2206. doi: 10.1016/j.clinph.2014.05.021. [DOI] [PubMed] [Google Scholar]
- Lin H.Y., Cocchi L., Zalesky A. Brain-behavior patterns define a dimensional biotype in medication-naive adults with attention-deficit hyperactivity disorder. Psychol. Med. 2018;7:1–10. doi: 10.1017/S0033291718000028. [DOI] [PubMed] [Google Scholar]
- Mansur C.G., Myczkowki M.L., de Barros Cabral S. Placebo effect after prefrontal magnetic stimulation in the treatment of resistant obsessive-compulsive disorder: a randomized controlled trial. Int. J. Neuropsychopharmacol. 2011;14:1389–1397. doi: 10.1017/S1461145711000575. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Lisanby S.H., Pieraccini F., Ulivelli M., Castrogiovanni P., Rossi S. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive–compulsive disorder (OCD) and Tourette's syndrome (TS) Int. J. Neuropsychopharmacol. 2006;9(1):95–100. doi: 10.1017/S1461145705005729. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Simpson H.B., Fallon B.A. Randomized sham-controlled trial of repetitive transcranial magnetic stimulation in treatment-resistant obsessive–compulsive disorder. Int. J. Neuropsychopharmacol. 2010;13:217–227. doi: 10.1017/S1461145709990435. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Rossi S., Bassi B.D. Modulation of motor cortex excitability in obsessive-compulsive disorder: an exploratory study on the relations of neurophysiology measures with clinical outcome. Psychiatry Res. 2013;210:1026–1032. doi: 10.1016/j.psychres.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgiève M., N'Diaye K., Haynes W. Dynamics of psychotherapy-related cerebral haemodynamic changes in obsessive compulsive disorder using a personalized exposure task in functional magnetic resonance imaging. Psychol. Med. 2014;44:1461–1473. doi: 10.1017/S0033291713002237. [DOI] [PubMed] [Google Scholar]
- Müller-Dahlhaus F., Ziemann U. Metaplasticity in human cortex. Neuroscientist. 2015;21(2):185–202. doi: 10.1177/1073858414526645. [DOI] [PubMed] [Google Scholar]
- Murakami T., Müller-Dahlhaus F., Lu M.K. Homeostatic metaplasticity of corticospinal excitatory and intracortical inhibitory neural circuits in human motor cortex. J. Physiol. 2012;590:5765–5781. doi: 10.1113/jphysiol.2012.238519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S.C., Palmer L.M., Nyffeler T. Transcranial magnetic stimulation (TMS) inhibits cortical dendrites. elife. 2016;5 doi: 10.7554/eLife.13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J.D., Bernacchia A., Freedman D.J. A hierarchy of intrinsic timescales across primate cortex. Nat. Neurosci. 2014;17:1661–1663. doi: 10.1038/nn.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T., Nakagawa A., Yoshiura T. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: a functional magnetic resonance imaging study. Biol. Psychiatry. 2005;57:901–910. doi: 10.1016/j.biopsych.2004.12.039. [DOI] [PubMed] [Google Scholar]
- Nauczyciel C., Le Jeune F., Naudet F. Repetitive transcranial magnetic stimulation over the orbitofrontal cortex for obsessive-compulsive disorder: a double-blind, crossover study. Transl. Psychiatry. 2014;4 doi: 10.1038/tp.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olatunji B.O., Ferreira-Garcia R., Caseras X. Predicting response to cognitive behavioral therapy in contamination-based obsessive-compulsive disorder from functional magnetic resonance imaging. Psychol. Med. 2014;44:2125–2137. doi: 10.1017/S0033291713002766. [DOI] [PubMed] [Google Scholar]
- Opitz A., Legon W., Rowlands A. Physiological observations validate finite element models for estimating subject-specific electric field distributions induced by transcranial magnetic stimulation of the human motor cortex. NeuroImage. 2013;81:253–264. doi: 10.1016/j.neuroimage.2013.04.067. [DOI] [PubMed] [Google Scholar]
- O'Reardon J.P., Blumner K.H., Peshek A.D. Long-term maintenance therapy for major depressive disorder with rTMS. J. Cli. Psychiatry. 2005;66:1524–1528. doi: 10.4088/jcp.v66n1205. [DOI] [PubMed] [Google Scholar]
- O'Reardon J.P., Solvason H.B., Janicak P.G. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol. Psychiatry. 2007;62:1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Overbeek T., Schruers K., Vermetten E. Comorbidity of obsessive-compulsive disorder and depression: prevalence, symptom severity, and treatment effect. J. Cli. Psychiatry. 2002;63:1106–1112. doi: 10.4088/jcp.v63n1204. [DOI] [PubMed] [Google Scholar]
- Pallanti S., Quercioli L. Treatment-refractory obsessive-compulsive disorder: methodological issues, operational definitions and therapeutic lines. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2006;30:400–412. doi: 10.1016/j.pnpbp.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Pallanti S., Marras A., Salerno L., Makris N., Hollander E. Better than treated as usual: Transcranial magnetic stimulation augmentation in selective serotonin reuptake inhibitor-refractory obsessive–compulsive disorder, mini-review and pilot open-label trial. J. Psychopharmacol. 2016;30(6):568–578. doi: 10.1177/0269881116628427. [DOI] [PubMed] [Google Scholar]
- Park H.-J., Friston K. Structural and functional brain networks: from connections to cognition. Science. 2013;342 doi: 10.1126/science.1238411. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A., Valls-Solé J., Wassermann E.M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Pedapati E., Difrancesco M., Wu S. Neural correlates associated with symptom provocation in pediatric obsessive compulsive disorder after a single session of sham-controlled repetitive transcranial magnetic stimulation. Psychiatry Res. Neuroimaging. 2015;233:466–473. doi: 10.1016/j.pscychresns.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Pelissolo A., Harika-Germaneau G., Rachid F. Repetitive transcranial magnetic stimulation to supplementary motor area in refractory obsessive-compulsive disorder treatment: a sham-controlled trial. Int. J. Neuropsychopharmacol. 2016;12(8):19. doi: 10.1093/ijnp/pyw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanía R., Nitsche M.A., Ruff C.C. Studying and modifying brain function with non-invasive brain stimulation. Nat. Neurosci. 2018;21:174–187. doi: 10.1038/s41593-017-0054-4. [DOI] [PubMed] [Google Scholar]
- Posner J., Marsh R., Maia T.V. Reduced functional connectivity within the limbic cortico-striato-thalamo-cortical loop in unmedicated adults with obsessive-compulsive disorder. Hum. Brain Mapp. 2014;35:2852–2860. doi: 10.1002/hbm.22371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasko J., Paskova B., Záleský R. The effect of repetitive transcranial magnetic stimulation (rTMS) on symptoms in obsessive compulsive disorder. A randomized, double blind, sham controlled study. Neuro Endocrinol. Lett. 2006;27:327–332. [PubMed] [Google Scholar]
- Richter L., Neumann G., Oung S. Optimal coil orientation for transcranial magnetic stimulation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogasch N.C., Dartnall T.J., Cirillo J. Corticomotor plasticity and learning of a ballistic thumb training task are diminished in older adults. J. Appl. Physiol. 2009;107:1874–1883. doi: 10.1152/japplphysiol.00443.2009. [DOI] [PubMed] [Google Scholar]
- Ruff C.C., Blankenburg F., Bjoertomt O. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr. Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Ruff C.C., Bestmann S., Blankenburg F. Distinct causal influences of parietal versus frontal areas on human visual cortex: evidence from concurrent TMS-fMRI. Cereb. Cortex. 2008;18:817–827. doi: 10.1093/cercor/bhm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffini C., Locatelli M., Lucca A. Augmentation effect of repetitive transcranial magnetic stimulation over the orbitofrontal cortex in drug-resistant obsessive-compulsive disorder patients: a controlled investigation. Prim. Care Companion J. Clin. Psychiatry. 2009;11:226–230. doi: 10.4088/PCC.08m00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscio A., Stein D., Chiu W. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol. Psychiatry. 2010;15:53–63. doi: 10.1038/mp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba G., Moukheiber A., Pelissolo A. Transcranial cortical stimulation in the treatment of obsessive-compulsive disorders: efficacy studies. Curr. Psychiatry Rep. 2015;17:36. doi: 10.1007/s11920-015-0571-3. [DOI] [PubMed] [Google Scholar]
- Sachdev P.S., Mcbride R., Loo C.K. Right versus left prefrontal transcranial magnetic stimulation for obsessive-compulsive disorder: a preliminary investigation. J. Cli. Psychiatry. 2001;62:981–984. doi: 10.4088/jcp.v62n1211. [DOI] [PubMed] [Google Scholar]
- Sachdev P.S., Loo C.K., Mitchell P.B. Repetitive transcranial magnetic stimulation for the treatment of obsessive compulsive disorder: a double-blind controlled investigation. Psychol. Med. 2007;37:1645–1649. doi: 10.1017/S0033291707001092. [DOI] [PubMed] [Google Scholar]
- Sakai Y., Narumoto J., Nishida S. Corticostriatal functional connectivity in non-medicated patients with obsessive-compulsive disorder. Eur. Psychiatry. 2011;26:463–469. doi: 10.1016/j.eurpsy.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Sale M.V., Ridding M.C., Nordstrom M.A. Factors influencing the magnitude and reproducibility of corticomotor excitability changes induced by paired associative stimulation. Exp. Brain Res. 2007;181:615–626. doi: 10.1007/s00221-007-0960-x. [DOI] [PubMed] [Google Scholar]
- Sale M.V., Ridding M.C., Nordstrom M.A. Cortisol inhibits neuroplasticity induction in human motor cortex. J. Neurosci. 2008;28:8285–8293. doi: 10.1523/JNEUROSCI.1963-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale M.V., Mattingley J.B., Zalesky A. Imaging human brain networks to improve the clinical efficacy of non-invasive brain stimulation. Neurosci. Biobehav. Rev. 2015;57:187–198. doi: 10.1016/j.neubiorev.2015.09.010. [DOI] [PubMed] [Google Scholar]
- Sarkhel S., Sinha V.K., Praharaj S.K. Adjunctive high-frequency right prefrontal repetitive transcranial magnetic stimulation (rTMS) was not effective in obsessive–compulsive disorder but improved secondary depression. J. Anxiety Disord. 2010;24:535–539. doi: 10.1016/j.janxdis.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Saxena S., Brody A.L., Ho M.L. Differential cerebral metabolic changes with paroxetine treatment of obsessive-compulsive disorder vs major depression. Arch. Gen. Psychiatry. 2002;59:250–261. doi: 10.1001/archpsyc.59.3.250. [DOI] [PubMed] [Google Scholar]
- Seo H.-J., Jung Y.-E., Lim H.K. Adjunctive low-frequency repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex in patients with treatment-resistant obsessive-compulsive disorder: a randomized controlled trial. Clin. Psychopharmacol. Neurosci. 2016;14:153. doi: 10.9758/cpn.2016.14.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D.-J., Jung W.H., He Y. The effects of pharmacological treatment on functional brain connectome in obsessive-compulsive disorder. Biol. Psychiatry. 2014;75:606–614. doi: 10.1016/j.biopsych.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Shine J.M., Bissett P.G., Bell P.T. The dynamics of functional brain networks: integrated network states during cognitive task performance. Neuron. 2016;92:544–554. doi: 10.1016/j.neuron.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J., Muggleton N., Walsh V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn. Sci. 2008;12:447–454. doi: 10.1016/j.tics.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Slotema C.W., Dirk Blom J., Hoek H.W. Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J. Cli. Psychiatry. 2010;71:873. doi: 10.4088/JCP.08m04872gre. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E., Vidaurre D. A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat. Neurosci. 2015;18:1565–1567. doi: 10.1038/nn.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. Massachusetts Institute of Technology; Cambridge: 2011. Networks of the Brain. [Google Scholar]
- Stein M.B., Forde D.R., Anderson G. Obsessive-compulsive disorder in the community: an epidemiologic survey with clinical reappraisal. Am. J. Psychiatr. 1997;154:1120–1126. doi: 10.1176/ajp.154.8.1120. [DOI] [PubMed] [Google Scholar]
- Thielscher A., Opitz A., Windhoff M. Impact of the gyral geometry on the electric field induced by transcranial magnetic stimulation. NeuroImage. 2011;54:234–243. doi: 10.1016/j.neuroimage.2010.07.061. [DOI] [PubMed] [Google Scholar]
- Tolin D.F., Stevens M.C., Villavicencio A.L. Neural mechanisms of decision making in hoarding disorder. Arch. Gen. Psychiatry. 2012;69:832–841. doi: 10.1001/archgenpsychiatry.2011.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres A.R., Prince M.J., Bebbington P.E. Treatment seeking by individuals with obsessive-compulsive disorder from the British psychiatric morbidity survey of 2000. Psychiatr. Serv. 2007;58:977–982. doi: 10.1176/ps.2007.58.7.977. [DOI] [PubMed] [Google Scholar]
- Trevizol A.P., Shiozawa P., Cook I.A. Transcranial magnetic stimulation for obsessive-compulsive disorder: an updated systematic review and meta-analysis. J. ECT. 2016;32:262–266. doi: 10.1097/YCT.0000000000000335. [DOI] [PubMed] [Google Scholar]
- Tsagaris K.Z., Labar D.R., Edwards D.J. A framework for combining rTMS with behavioral therapy. Front. Syst. Neurosci. 2016;10:82. doi: 10.3389/fnsys.2016.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M.P., Sporns O. Network hubs in the human brain. Trends Cogn. Sci. 2013;17:683–696. doi: 10.1016/j.tics.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Veldhuis J., Dieleman J.P., Wohlfarth T. Incidence and prevalence of “diagnosed OCD” in a primary care, treatment seeking, population. Int. J. Psychiatry Clin. Pract. 2012;16:85–92. doi: 10.3109/13651501.2011.617454. [DOI] [PubMed] [Google Scholar]
- Vidaurre D., Smith S.M., Woolrich M.W. Brain network dynamics are hierarchically organized in time. Proc. Natl. Acad. Sci. 2017;114:12827–12832. doi: 10.1073/pnas.1705120114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh V., Cowey A. Transcranial magnetic stimulation and cognitive neuroscience. Nat. Rev. Neurosci. 2000;1:73–79. doi: 10.1038/35036239. [DOI] [PubMed] [Google Scholar]
- Wassermann E.M., Mcshane L.M., Hallett M. Noninvasive mapping of muscle representations in human motor cortex. Electroencephalogr. Clin. Neurophysiol. 1992;85:1–8. doi: 10.1016/0168-5597(92)90094-r. [DOI] [PubMed] [Google Scholar]
- Westin G.G., Bassi B.D., Lisanby S.H. Determination of motor threshold using visual observation overestimates transcranial magnetic stimulation dosage: safety implications. Clin. Neurophysiol. 2014;125:142–147. doi: 10.1016/j.clinph.2013.06.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Cocchi L. Time-resolved resting-state brain networks. Proc. Natl. Acad. Sci. 2014;111:10341–10346. doi: 10.1073/pnas.1400181111. [DOI] [PMC free article] [PubMed] [Google Scholar]