Abstract

A small library of psoralen carboxylic acids and their corresponding benzenesulfonamide derivatives were designed and synthesized to evaluate their activity and selectivity toward tumor associated human carbonic anhydrase (hCA) isoforms IX and XII. Both psoralen acids and sulfonamides exhibited potent inhibition of IX and XII isozymes in the nanomolar concentration range. However, psoralen acids resulted as the most selective in comparison with the corresponding benzenesulfonamide derivatives. Our data indicate that the psoralen scaffold is a promising starting point for the design of highly selective tumor associated hCA inhibitors.
Keywords: hCA IX, hCA XII, inhibitors, tumor, coumarin, benzenesulfonamide
Coumarins are a class of heterocyclic compounds widely distributed in nature with a wide variety of biological activity such as antiviral,1,2 glucose-lowering agents,3,4 antimycotic,5 and antitumor.6−10
Calanolides A and B extracted from Calophyllum lanigerum show anti-HIV-1 activity.11 Furthermore, imperatorin has been reported to have anti-inflammatory, antibacterial, antifungal, antiviral, and anticancer activity and might have future clinical application.12 Noteworthy, other derivatives such as psoralen, bergapten, marmesin, and rutaretin are known antitubercular agents.13 In particular the 7H-furo[3,2-g]chromen-7-one derivatives, commonly known as psoralens, have been investigated for their ability to suppress proliferation and angiogenesis in human colon cancer cells by targeting HIF-1α via the mTOR/p70S6K/4E-BP1 and MAPK pathways7 and to reverse multidrug resistance in both lung cancer A549/D16 and breast cancer MCF-7/ADR cells.14,15
Moreover, the ability of coumarin derivatives to inhibit human carbonic anhydrase isozymes IX and XII (hCA IX and XII) has also been reported.16−19
Noteworthy, the chemical accessibility and the ease of functionalization of the coumarin nucleus allows for synthesizing diverse compound libraries.
The trans-membrane hCA IX and hCA XII isoforms have been associated with tumor progression and invasion.20−28 In normal conditions, CA IX is expressed in the stomach and few other tissues, while it is ectopically induced and highly overexpressed in many hypoxic solid tumors, by a direct transcriptional activation of the CA9 gene via HIF-1α. Therefore, the design of new and isozyme selective hCA inhibitors is an attractive challenge for medicinal chemists.
In this respect, the design of new psoralen derivatives as hCA IX and XII inhibitors could be advantageous and may lead to the identification of multitarget agents since other cancer related enzymes and pathways could also be hit by such derivatives.6,7,9,14,15,29,30
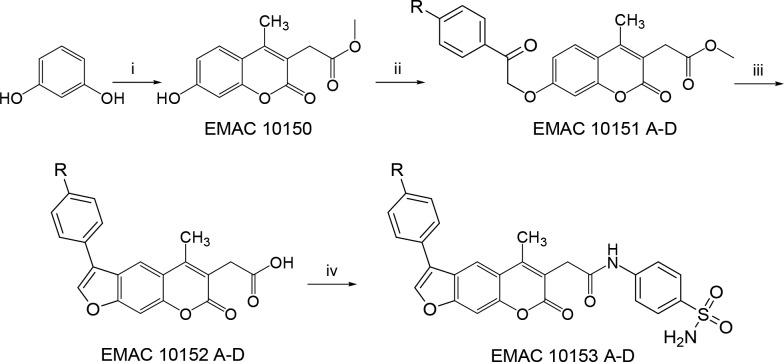
Pursuing on our efforts in the design and synthesis of hCA inhibitors,31,32 we have designed and synthesized a small library of coumarin (EMAC10151 A-D) and psoralen (EMAC10152 A-D) derivatives with the aim to target the hCA IX and XII isoforms. To achieve a deeper insight in the inhibitory potential of such derivatives, we have also synthesized EMAC10152 A-D and the corresponding benzenesulfonamide hybrids EMAC10153 A-D. The new compounds were synthesized by a versatile multistep synthetic approach (Scheme 1). Methyl 2-(7-hydroxy-4-methyl-2-oxo-2H-chromen-3-yl)acetate EMAC10150 was obtained by Pechmann condensation of resorcinol with dimethylacetylsuccinate in 98% sulfuric acid. EMAC10150 was converted into methyl 2-(4-methyl-2-oxo-7-aryloxy-2H-chromen-3-yl)acetate derivatives EMAC10151 A-D by Williamson reaction with the appropriate α-haloketone, in the presence of dry acetone and using potassium carbonate to generate the in situ alkoxide ion.
Scheme 1. Synthetic Pathway to Compounds EMAC10151 A-D, EMAC10152 A-D, and EMAC10153 A-D.
Reagents and conditions (i) dimethylacetylsuccinate, H2SO4 98%, RT; (ii) dry acetone, K2CO3, α-haloketone, reflux, 1–5 h.; (iii) propan-2-ol, NaOH 1 N, reflux, 4 h; (iv) SOCl2, RT, dry acetone, 4-aminobenzenesulfonamide, dry pyridine.
By heating EMAC10151 A-D in sodium hydroxide 1 M solution, the simultaneous intramolecular condensation and ester saponification took place to give 2-(5-methyl-7-oxo-3-aryl-7H-furo[3,2-g]chromen-6-yl)acetic acid EMAC10152 A-D.
The formation of the 2H-furo[2,3-h]chromen-2-one isomers was not observed, as confirmed by 1H NMR spectroscopy (Figure S1). Benzenesulfonamide hybrids EMAC10153 A-D were obtained via acyl chloride formation and subsequent reaction with 4-aminobenzenesulfonamide. All compounds were characterized by means of both analytical and spectroscopic methods (Figures S2–S25) and finally evaluated for the inhibition of four hCA isoforms I, II, IX, and XII (Table 1).
Table 1. Inhibition Data (Ki (nM)) Towards hCA I, II, IX, and XII of Compounds EMAC10151 A-D, EMAC10152 A-D, and EMAC10153 A-D.
| compound EMAC | R | hCAI | hCAII | hCAIX | hCAXII |
|---|---|---|---|---|---|
| 10151 A | Cl | >10000 | >10000 | 23.6 | 446.6 |
| 10151 B | CH3 | >10000 | >10000 | 122.8 | 56.6 |
| 10151 C | H | >10000 | >10000 | 89.7 | 72.5 |
| 10151 D | F | >10000 | >10000 | 84.7 | 250.0 |
| 10152 A | Cl | >10000 | >10000 | 94.7 | 9.3 |
| 10152 B | CH3 | >10000 | >10000 | 23.0 | 9.1 |
| 10152 C | H | >10000 | >10000 | 17.5 | 9.4 |
| 10152 D | F | >10000 | >10000 | 17.7 | 7.4 |
| 10153 A | Cl | 6829.7 | 55.1 | 17.8 | 2.4 |
| 10153 B | CH3 | 7069.0 | 560.0 | 91.6 | 3.4 |
| 10153 C | H | 7016.1 | 46.6 | 16.5 | 3.6 |
| 10153 D | F | 7148.1 | 79.5 | 108.4 | 49.9 |
| AAZ | // | 250 | 12.1 | 25.8 | 5.7 |
Most EMAC compounds possess, although with some distinction, selective activity toward the IX and XII isoforms. However, when the benzenesulfonamide moiety is present, a strong reduction of the selectivity becomes evident. In particular, in the case of compound EMAC10153 D, the complete loss of selectivity was observed.
On the contrary, both EMAC10151 A-D and EMAC10152 A-D are generally potent and selective inhibitors of the tumor associated hCA IX and XII isoforms. Nevertheless, some considerations should be reported. Regarding the methyl 2-(4-methyl-2-oxo-7-aryloxy-2H-chromen-3-yl)acetate derivatives, EMAC10151 A-D, a different activity profile could be observed, according to the substitution of the phenyl ring. Thus, the introduction of a halogen atom (Cl or F, EMAC10151 A and EMAC10151 D) in position 4 of the phenyl substituent oriented the activity and selectivity toward the IX isozyme. On the contrary, the unsubstituted phenyl or the 4-CH3-phenyl moieties led to an increase of the activity toward the hCA XII isoform. Indeed, a reduction of the activity toward hCA IX was observed for compound EMAC10151 B. However, although weaker (Ki = 122.8 nM), this derivative exhibited a high selectivity toward the hCA IX and XII isozymes. A similar behavior could be observed for all the psoralen carboxylic acid derivatives EMAC10152 A-D. All these compounds were potent and selective tumor associated isoforms IX and XII hCAIs. Their Ki values toward hCA IX and XII ranged from 94.7 to 7.4 nM, while no inhibition could be observed up to 10 000 nM concentration toward the off-target hCA I and hCA II isozymes. In more detail, it could be pointed out that, in the case of hCA IX inhibition, the biological activity is more influenced by the size of the substituent on the phenyl ring rather than by its nature. Thus, the introduction of a chlorine atom (EMAC10152 A) led to the less potent compound within the 10152 series. On the contrary, compounds EMAC10152 C and EMAC10152 D, bearing an unsubstituted phenyl and a fluoro phenyl moiety, respectively, resulted as the most potent inhibitors. Interestingly, hCA XII is inhibited at low nanomolar concentrations, regardless of the nature of the substitution. As mentioned above, in the case of the psoralen-benzenesulfonamide hybrids (EMAC10153 A-D), a potent activity toward the hCA XII was observed for most of the tested derivatives. The inhibition of the XII isozyme could still be observed at concentrations ranging from nanomolar to low nanomolar, but a lower selectivity is generally measured toward the off-target isozyme hCA II with respect to EMAC10151 and EMAC10152 series. Only in the case of compound EMAC10153 B, bearing a methylphenyl moiety on the furan ring, a good selectivity toward hCA IX and XII versus hCA I and II was found.
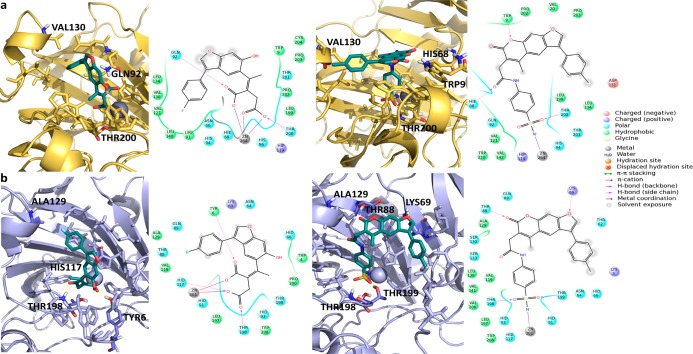
Computational methods have been applied in order to explain the selectivity of the synthesized coumarin derivatives toward hCA IX and hCA XII isoforms and their inability to inhibit the II isoform, which, in our intent, represents an off-target. To perform our studies, we chose the two compounds that showed the highest potency and selectivity toward hCA IX and XII: the acid derivative EMAC10152 D and the sulfonamide derivative EMAC10153 B. Indeed, these compounds have been docked inside the three crystal structures II (PDB 3f8e), IX (PDB 5fl4), and XII (PDB 4ww8).33,34 The previously validated QMPL35 protocol was applied.32 Both coumarin derivatives have been prepared considering the possibility that the coumarin moiety can be hydrolyzed by the Zn2+ activated water molecule of the enzyme cavity, which acts as a very potent nucleophile.33 Therefore, the compounds were ionized at pH 7.4. However, the sulfonamide group was considered both unionized, at pH 7.4, and ionized, taking into account the micro-basic pH inside the binding pocket (Figure S26). All molecules were subjected to conformational analysis, and the global minimum of each was selected for carrying out docking experiments. The putative binding mode suggested by docking simulations well explain the selectivity of our compounds. In fact, both compounds are not able to enter deeply in the active site of hCA II because of the steric hindrance of Phe 131 (Figures 1 and S27). On the contrary, they perfectly fit into the active site of hCA IX and hCA XII, where Phe 131 is substituted by Val 130 and Ala 129, respectively (Figure 1). In particular, the suggested binding mode shows that the coumarin moiety of compound EMAC10152 D can reach the catalytic site and be hydrolyzed. Conversely, like most sulfonamide derivatives, EMAC10153 B acts by interacting with the sulfonamide moiety, while the coumarin portion, oriented outside the pocket, cannot be hydrolyzed (Figure 1). According to the above suggested mechanism, only the complexes with hydrolyzed EMAC10152 D and closed EMAC10153 B were subjected to a postdocking procedure based on energy minimization in order to take into account the induced fit phenomena that occur upon ligand binding.36 The docking results are in agreement with experimental data regarding the isoform selectivity. In fact, EMAC compounds bound to hCA IX and XII showed lower G-score compared to the complex with isoform II (Table S1), indicating a major stability of the complexes with hCA IX and XII in agreement with the experimental results (Table 1).
Figure 1.

Three-dimensional representation of docking results of (a) EMAC10152 D in cyan and (b) EMAC10153 B in pink with the three hCA isoforms represented as surface: gray, hCA II; beige, hCA IX; light-blue, hCA XII. The active site is highlighted in pale yellow.
The analysis of the interaction mode with isoforms IX and XII (Figure 2) shows that EMAC10152 D and EMAC10153 B are able to establish a wide array of hydrogen bonds with crucial residues of the binding site and several hydrophobic interactions. In more detail, the carboxylic function of EMAC10152 D interacts as a bidentate chelator with Zn2+. Conversely, in the case of EMAC10153 A, the sulfonamide group plays a key role by interacting with Zn2+ and Thr residues, as it has been generally observed for this class of compounds.37,38
Figure 2.
Three-dimensional representation of the putative binding mode obtained by docking experiments of EMAC10152 D and EMAC10153 B into (a) hCA IX (beige) and (b) hCA XII (light blue), and the relative 2D representation of the complexes stabilizing interactions with the residues of the binding site.
Therefore, a putative binding mode seems to confirm the importance of the coumarin moiety in both closed and hydrolyzed conformations, although the different functionalization of EMAC compounds with respect to previously reported coumarins33 led to a different target recognition that involves the interaction with the zinc ion. Hence, this could represent an innovative mode of action. In addition, it was confirmed that a bulky moiety, linked to the catalytic site binding scaffold, allows selectivity over the off-target isoform II. In fact, in the case of isoform II, the steric hindrance of Phe131 prevents the ligand–target interaction.
According to these results, both psoralen and the hybrid compounds could be considered as promising scaffolds for the design of high selective inhibitors of the hCAs IX and XII isoforms. However, while very high selectivity was always achieved by coumarin (EMAC10151) and psoralen (EMAC10152) derivatives, the same behavior was not observed in the case of the hybrid compounds EMAC10153. Probably, this is mainly due to the presence of the strong Zn2+ binder benzenesulfonamide, which, on the one hand, may lead to very potent compounds but, on the other, could affect selectivity.
Conversely, hydrolyzed coumarin EMAC10151 and psoralen EMAC10152 most likely act as bidentate Zn2+ chelators, while hybrid compounds EMAC10153 may preferentially act as traditional Zn2+ binders. These results were very encouraging and pushed us to further investigate these derivatives in order to identify potential candidates for the treatment of hypoxic tumors.
Glossary
ABBREVIATIONS
- HIF-1α
hypoxia-inducible factor 1-alpha
- mTOR
mammalian target of rapamycin
- p70S6K
ribosomal protein S6 kinase beta-1
- 4E-BP1
eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1
- MAPK
mitogen-activated protein kinase
- hCA
human carbonic anhydrase
- AAZ
acetazolamide
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00170.
Experimental procedures and compounds characterization (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Ong E. B. B.; Watanabe N.; Saito A.; Futamura Y.; Abd El Galil K. H.; Koito A.; Najimudin N.; Osada H. Vipirinin, a coumarin-based HIV-1 VPR inhibitor, interacts with a hydrophobic region of VPR. J. Biol. Chem. 2011, 286 (16), 14049–14056. 10.1074/jbc.M110.185397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P.-H.; Ke Y.-Y.; Su C.-T.; Shiao H.-Y.; Hsieh H.-P.; Chao Y.-K.; Lee C.-N.; Kao C.-L.; Chao Y.-S.; Chang S.-Y. Inhibition of HIV-1 tat-mediated transcription by a coumarin derivative, BPRHIV001, through the Akt pathway. J. Virol. 2011, 85 (17), 9114–9126. 10.1128/JVI.00175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.; Sun L.; Huang X.; Li Z.; Zhang C.; Qian H.; Huang W. Novel coumarin modified GLP-1 derivatives with enhanced plasma stability and prolonged in vivo glucose-lowering ability. Br. J. Pharmacol. 2014, 171 (23), 5252–5264. 10.1111/bph.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.; Peng Z.; He D.; Yan C.; Liu W.. Coumarin-isatin type compound useful in treatment of diabetes mellitus and its preparation. CN105237521A, 2016.
- Mercer D. K.; Robertson J.; Wright K.; Miller L.; Smith S.; Stewart C. S.; O'Neil D. A. A prodrug approach to the use of coumarins as potential therapeutics for superficial mycoses. PLoS One 2013, 8 (11), e80760. 10.1371/journal.pone.0080760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa M. A.; Cooperwood J. S.; Khan M. O. F. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr. Med. Chem. 2008, 15 (26), 2664–2679. 10.2174/092986708786242877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi C.; Ma J.; Wang K. S.; Zuo H. X.; Wang Z.; Li M. Y.; Piao L. X.; Xu G. H.; Li X.; Quan Z. S.; Jin X. Imperatorin suppresses proliferation and angiogenesis of human colon cancer cell by targeting HIF-1α via the mTOR/p70S6K/4E-BP1 and MAPK pathways. J. Ethnopharmacol. 2017, 203, 27–38. 10.1016/j.jep.2017.03.033. [DOI] [PubMed] [Google Scholar]
- Xia W.; Gooden D.; Liu L.; Zhao S.; Soderblom E. J.; Toone E. J.; Beyer W. F.; Walder H.; Spector N. L. Photo-activated psoralen binds the ErbB2 catalytic kinase domain, blocking ErbB2 signaling and triggering tumor cell apoptosis. PLoS One 2014, 9 (2), e88983. 10.1371/journal.pone.0088983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leanza L.; Romio M.; Becker K. A.; Azzolini M.; Trentin L.; Manago A.; Venturini E.; Zaccagnino A.; Mattarei A.; Carraretto L.; Urbani A.; Kadow S.; Biasutto L.; Martini V.; Severin F.; Peruzzo R.; Trimarco V.; Egberts J.-H.; Hauser C.; Visentin A.; Semenzato G.; Kalthoff H.; Zoratti M.; Gulbins E.; Paradisi C.; Szabo I. Direct pharmacological targeting of a mitochondrial ion channel selectively kills tumor cells in vivo. Cancer Cell 2017, 31 (4), 516. 10.1016/j.ccell.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Min K.-J.; Um H. J.; Seo S. U.; Woo S. M.; Kim S.; Park J.-W.; Lee H.-S.; Kim S. H.; Choi Y. H.; Lee T.-J.; Kwon T. K. Angelicin potentiates TRAIL-induced apoptosis in renal carcinoma Caki cells through activation of caspase 3 and down-regulation of c-FLIP expression. Drug Dev. Res. 2018, 79 (1), 3–10. 10.1002/ddr.21414. [DOI] [PubMed] [Google Scholar]
- Gustafson K. R.; Fuller R. W.; Cardellina J. H. II; McMahon J. B.; Currens M. J.; Boyd M. R.; Kashman Y.; Buckheit R. W. Jr.; Hughes S. H.; Cragg G. M. The Calanolides, a novel HIV-inhibitory class of coumarin derivatives from the tropical rainforest tree, Calophyllum lanigerum. J. Med. Chem. 1992, 35 (15), 2735–2743. 10.1021/jm00093a004. [DOI] [PubMed] [Google Scholar]
- Kozioł E.; Skalicka-Woźniak K. Imperatorin–pharmacological meaning and analytical clues: profound investigation. Phytochem. Rev. 2016, 15 (4), 627–649. 10.1007/s11101-016-9456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C. C.; Cheng M. J.; Peng C. F.; Huang H. Y.; Chen I. S. A novel dimeric coumarin analog and antimycobacterial constituents from Fatoua pilosa. Chem. Biodiversity 2010, 7 (7), 1728–1736. 10.1002/cbdv.200900326. [DOI] [PubMed] [Google Scholar]
- Hsieh M.-J.; Chen M.-K.; Yu Y.-Y.; Sheu G.-T.; Chiou H.-L. Psoralen reverses docetaxel-induced multidrug resistance in A549/D16 human lung cancer cells lines. Phytomedicine 2014, 21 (7), 970–977. 10.1016/j.phymed.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Jiang J.; Wang X.; Cheng K.; Zhao W.; Hua Y.; Xu C.; Yang Z. Psoralen reverses the P-glycoprotein-mediated multidrug resistance in human breast cancer MCF-7/ADR cells. Mol. Med. Rep. 2016, 13 (6), 4745–4750. 10.3892/mmr.2016.5098. [DOI] [PubMed] [Google Scholar]
- Touisni N.; Maresca A.; McDonald P. C.; Lou Y.; Scozzafava A.; Dedhar S.; Winum J.-Y.; Supuran C. T. Glycosyl coumarin carbonic anhydrase IX and XII inhibitors strongly attenuate the growth of primary breast tumors. J. Med. Chem. 2011, 54 (24), 8271–8277. 10.1021/jm200983e. [DOI] [PubMed] [Google Scholar]
- Maresca A.; Supuran C. T. Coumarins incorporating hydroxy- and chloro-moieties selectively inhibit the transmembrane, tumor-associated carbonic anhydrase isoforms IX and XII over the cytosolic ones I and II. Bioorg. Med. Chem. Lett. 2010, 20 (15), 4511–4. 10.1016/j.bmcl.2010.06.040. [DOI] [PubMed] [Google Scholar]
- Nocentini A.; Carta F.; Ceruso M.; Bartolucci G.; Supuran C. T. Click-tailed coumarins with potent and selective inhibitory action against the tumor-associated carbonic anhydrases IX and XII. Bioorg. Med. Chem. 2015, 23 (21), 6955–6966. 10.1016/j.bmc.2015.09.041. [DOI] [PubMed] [Google Scholar]
- De Luca L.; Mancuso F.; Ferro S.; Buemi M. R.; Angeli A.; Del Prete S.; Capasso C.; Supuran C. T.; Gitto R. Inhibitory effects and structural insights for a novel series of coumarin-based compounds that selectively target human CA IX and CA XII carbonic anhydrases. Eur. J. Med. Chem. 2018, 143, 276–282. 10.1016/j.ejmech.2017.11.061. [DOI] [PubMed] [Google Scholar]
- Supuran C. T. Inhibition of carbonic anhydrase IX as a novel anticancer mechanism. World J. Clin. Oncol. 2012, 3 (7), 98–103. 10.5306/wjco.v3.i7.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winum J.-Y.; Rami M.; Scozzafava A.; Montero J.-L.; Supuran C. Carbonic anhydrase IX: a new druggable target for the design of antitumor agents. Med. Res. Rev. 2008, 28 (3), 445–463. 10.1002/med.20112. [DOI] [PubMed] [Google Scholar]
- Cecchi A.; Hulikova A.; Pastorek J.; Pastorekova S.; Scozzafava A.; Winum J. Y.; Montero J. L.; Supuran C. T. Carbonic anhydrase inhibitors. Design of fluorescent sulfonamides as probes of tumor-associated carbonic anhydrase IX that inhibit isozyme IX-mediated acidification of hypoxic tumors. J. Med. Chem. 2005, 48 (15), 4834–41. 10.1021/jm0501073. [DOI] [PubMed] [Google Scholar]
- Supuran C. T.; Winum J. Y. Carbonic anhydrase IX inhibitors in cancer therapy: an update. Future Med. Chem. 2015, 7 (11), 1407–14. 10.4155/fmc.15.71. [DOI] [PubMed] [Google Scholar]
- Araste F.; Ebrahimizadeh W.; Rasooli I.; Rajabibazl M.; Mousavi Gargari S. L. A novel VHH nanobody against the active site (the CA domain) of tumor-associated, carbonic anhydrase isoform IX and its usefulness for cancer diagnosis. Biotechnol. Lett. 2014, 36 (1), 21–28. 10.1007/s10529-013-1340-1. [DOI] [PubMed] [Google Scholar]
- De Simone G.; Supuran C. T. Carbonic anhydrase IX: Biochemical and crystallographic characterization of a novel antitumor target. Biochim. Biophys. Acta, Proteins Proteomics 2010, 1804 (2), 404–409. 10.1016/j.bbapap.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Horie K.; Kawakami K.; Fujita Y.; Sugaya M.; Kameyama K.; Mizutani K.; Deguchi T.; Ito M. Exosomes expressing carbonic anhydrase 9 promote angiogenesis. Biochem. Biophys. Res. Commun. 2017, 492 (3), 356–361. 10.1016/j.bbrc.2017.08.107. [DOI] [PubMed] [Google Scholar]
- Supuran C. T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473 (14), 2023–32. 10.1042/BCJ20160115. [DOI] [PubMed] [Google Scholar]
- Jiang J.; Zhao J. H.; Wang X. L.; Guo X. J.; Yang J.; Bai X.; Jin S. Y.; Ge R. L. Correlation between carbonic anhydrase IX (CA-9), XII (CA-12) and hypoxia inducible factor-2α (HIF-2α) in breast cancer. Neoplasma 2015, 62 (3), 456–463. 10.4149/neo_2015_054. [DOI] [PubMed] [Google Scholar]
- Rajabi M.; Hossaini Z.; Khalilzadeh M. A.; Datta S.; Halder M.; Mousa S. A. Synthesis of a new class of furo[3,2-c]coumarins and its anticancer activity. J. Photochem. Photobiol., B 2015, 148, 66–72. 10.1016/j.jphotobiol.2015.03.027. [DOI] [PubMed] [Google Scholar]
- Abdel Hafez O. M.; Amin K. M.; Abdel-Latif N. A.; Mohamed T. K.; Ahmed E. Y.; Maher T. Synthesis and antitumor activity of some new xanthotoxin derivatives. Eur. J. Med. Chem. 2009, 44 (7), 2967–74. 10.1016/j.ejmech.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Melis C.; Meleddu R.; Angeli A.; Distinto S.; Bianco G.; Capasso C.; Cottiglia F.; Angius R.; Supuran C. T.; Maccioni E. Isatin: a privileged scaffold for the design of carbonic anhydrase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32 (1), 68–73. 10.1080/14756366.2016.1235042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco G.; Meleddu R.; Distinto S.; Cottiglia F.; Gaspari M.; Melis C.; Corona A.; Angius R.; Angeli A.; Taverna D.; Alcaro S.; Leitans J.; Kazaks A.; Tars K.; Supuran C. T.; Maccioni E. N-Acylbenzenesulfonamide dihydro-1,3,4-oxadiazole hybrids: seeking selectivity toward carbonic anhydrase isoforms. ACS Med. Chem. Lett. 2017, 8 (8), 792–796. 10.1021/acsmedchemlett.7b00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca A.; Temperini C.; Vu H.; Pham N. B.; Poulsen S. A.; Scozzafava A.; Quinn R. J.; Supuran C. T. Non-zinc mediated inhibition of carbonic anhydrases: coumarins are a new class of suicide inhibitors. J. Am. Chem. Soc. 2009, 131 (8), 3057–62. 10.1021/ja809683v. [DOI] [PubMed] [Google Scholar]
- Leitans J.; Kazaks A.; Balode A.; Ivanova J.; Zalubovskis R.; Supuran C. T.; Tars K. Efficient expression and crystallization system of cancer-associated carbonic anhydrase isoform IX. J. Med. Chem. 2015, 58 (22), 9004–9. 10.1021/acs.jmedchem.5b01343. [DOI] [PubMed] [Google Scholar]
- Chung J. Y.; Hah J. M.; Cho A. E. Correlation between performance of QM/MM docking and simple classification of binding sites. J. Chem. Inf. Model. 2009, 49 (10), 2382–7. 10.1021/ci900231p. [DOI] [PubMed] [Google Scholar]
- Kollman P. A.; Massova I.; Reyes C.; Kuhn B.; Huo S.; Chong L.; Lee M.; Lee T.; Duan Y.; Wang W.; Donini O.; Cieplak P.; Srinivasan J.; Case D. A.; Cheatham T. E. Calculating structures and free energies of complex molecules: combining molecular mechanics and continuum models. Acc. Chem. Res. 2000, 33 (12), 889–897. 10.1021/ar000033j. [DOI] [PubMed] [Google Scholar]
- Meleddu R.; Maccioni E.; Distinto S.; Bianco G.; Melis C.; Alcaro S.; Cottiglia F.; Ceruso M.; Supuran C. T. New 4-[(3-cyclohexyl-4-aryl-2,3-dihydro-1,3-thiazol-2-ylidene)amino]benzene-1-sulfonamides, synthesis and inhibitory activity toward carbonic anhydrase I, II, IX, XII. Bioorg. Med. Chem. Lett. 2015, 25 (16), 3281–4. 10.1016/j.bmcl.2015.05.076. [DOI] [PubMed] [Google Scholar]
- Supuran C. T. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discovery 2008, 7 (2), 168. 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.