Abstract
Background
The aim of this study was to assess whether venous occlusion plethysmography can be used to identify venous obstruction and predict clinical success of stenting.
Method
Receiver operated characteristic curves were used to determine the ability of venous occlusion plethysmography to discriminate between the presence and absence of obstruction, measured by duplex ultrasound and magnetic resonance venography, and to discriminate between successful and non-successful stenting, measured by VEINES-QOL/Sym.
Result
Two hundred thirty-seven limbs in 196 patients were included. Areas under the curve for post-thrombotic obstruction were one-second outflow volume 0.71, total venous volume 0.69 and outflow fraction 0.59. Stenting was performed in 45 limbs of 39 patients. Areas under the curve for identifying patients with successful treatment at one year after stenting were 0.57, 0.54 and 0.63, respectively.
Conclusion
Venous occlusion plethysmography cannot be used to identify venous obstruction proximal to the femoral confluence or to distinguish which patients will benefit from treatment.
Keywords: Hemodynamics, May–Thurner syndrome, postthrombotic syndrome, stents, thrombosis, treatment outcome
Introduction
Deep venous obstruction is a frequent cause of venous leg complaints and can be instigated by iliac vein compression or inadequate recanalisation after deep vein thrombosis.1,2 Both types of obstruction can be diagnosed using duplex ultrasound (DUS),3,4 computed tomography venography,5 magnetic resonance venography (MRV),4,5 intravascular ultrasound6 and conventional venography.7 If the obstructive component is proximally located to the femoral confluence, it can be adequately treated by percutaneous transluminal angioplasty and stenting.7–9 Both generic and disease specific quality of life (QoL) scores and venous scoring systems significantly improve,8–11 with patency rates ranging from 50% to 100%.8,9,11,12 Nonetheless, 15–20% of patients show little to no relief of complaints,13 often despite adequate recanalisation and patent stents that are free of stenosis. Moreover, most modalities used to identify obstruction are expensive, invasive and can be difficult to perform, especially in non-specialised centres. Therefore, an inexpensive, non-invasive test is needed to establish which patients warrant referral to a vascular surgeon and will ultimately benefit from treatment by stenting.
Air plethysmography (APG) is a non-invasive, functional test that can continuously assess real-time volumetric changes in the calf. This test uses an air-inflated cuff around the calf that detects pressure changes resulting from variation in calf circumference. Although APG was initially validated for deep vein incompetence,14,15 an outflow test for measuring obstruction was designed.16–18 However, use of this outflow test is debatable due to varying study results,17–24 with recent studies suggesting it mainly tests elastic recoil properties.21,22 Our own research has recently shown poor results in detecting chronic venous obstruction with this venous occlusion plethysmography (VOP).25 Nevertheless, little is known about the absolute volumetric changes or the predictive ability for success of treatment using the outflow test.
The aim of this study was to assess whether parameters obtained during VOP, including absolute volumetric changes, can be used to identify deep venous obstruction or predict clinical success of treatment by recanalisation and stenting.
Methods
Study design and patient selection
In this retrospective study, absolute volume changes and outflow fraction were measured using VOP. Their ability to discriminate between limbs with and without obstruction proximal to the femoral confluence, measured by DUS and MRV, was assessed. Additionally, the value of these parameters to discriminate between successful stenting and unsuccessful stenting, as measured by disease specific QoL, was evaluated.
Between the period from January 2011 until August 2013, patients referred to our tertiary, outpatient clinic with suspected deep venous obstruction were analysed using VOP at the time of presentation. In our clinic criteria for suspected outflow obstruction are the presence of venous claudication, history of deep vein thrombosis, the presence of an abdominal wall collateral vein, recurrence of varicosities <5 years after treatment, and C4–C6 disease according to the Clinical–Etiology–Anatomy–Pathophysiology (CEAP) classification. The control group consisted of patients who underwent VOP, yet did not suffer from deep venous obstruction. DUS and/or MRV demonstrated that control patients only suffered from superficial venous reflux or no venous disease at all.
Patients with a reliable VOP examination and sufficient imaging by DUS and/or MRV were included in this study. Patients with unreliable outflow test traces because of undefinable actions or evident air loss from the cuff or tubing were excluded from analysis. Patients with incomplete visualisation of all vessel segments from the popliteal vein to the inferior vena cava on DUS or MRV were also excluded. Additional analyses were performed for patients who also underwent stenting and had filled out QoL questionnaires before and one year after intervention.
Retrospective analysis of these data was approved by the Maastricht University Medical Centre institutional review board (METC 14-4-193, March 5, 2015). Individual patient consent was not obtained, as this is not required by Dutch law for retrospective studies. No patients registered an objection to anonymous use of data.
VOP
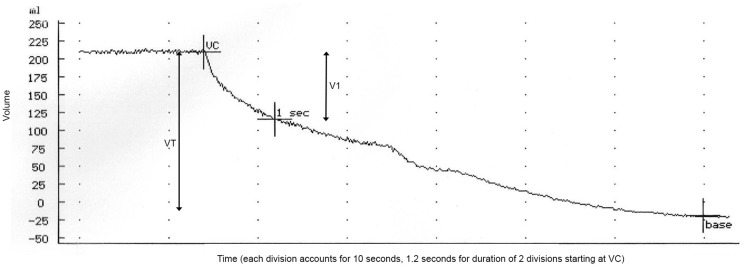
The outflow fraction test was performed by one of five dedicated physician researchers, using the APG Air Plethysmograph C-1000 (ACI Medical, San Marcos, CA). The procedure has been described in literature.16,26 In short, the affected lower limb was elevated above the level of the heart on a foam block while the patient was in the supine position. The plethysmography cuff was placed around the calf and calibrated by inflating and extracting 100 mL of air. Subsequently, a rapid deflatable cuff was placed above the knee and inflated to 80 mmHg, waiting until venous volume reached a stable plateau phase. Consecutively, the proximal cuff was rapidly deflated and outflow was measured, waiting until a stable phase was reached again. Total venous volume and outflow volume during the first second after deflation were measured. Outflow fraction represents the one-second outflow volume as the percentage of the total venous volume (Figure 1). Graphs were analysed and stored on the APG machine.
Figure 1.
Venous occlusion air plethysmography outflow test. A maximum amount of blood is accumulated in the calf (VC, accumulation phase not depicted in image), after which the proximal cuff is rapidly deflated. Outflow fraction equals volume expelled after one-second (V1)/total VT × 100%. VT: venous volume, VC: venous capacitance.
Imaging
DUS was performed with a Hitachi Aloka ProSound Alpha 7 Premiere machine (Aloka, Tokyo, Japan). The venous system was assessed from the suprarenal inferior vena cava down to the calf veins as described elsewhere.4 Post-thrombotic obstruction was defined as obstruction with intraluminal synechiae and flow division. Non-thrombotic iliac vein compression was defined as a >50% lumen reduction, compared with a healthy vein segment.
MRV was performed according to the protocol described in literature4 on a 1.5 T magnetic resonance imaging system (Intera; Philips Medical Systems, Best, the Netherlands). Post-thrombotic obstruction was defined as hypodense intraluminal changes indicative of trabeculation. Non-thrombotic iliac vein compression was defined as >50% lumen reduction, compared with a normal vein segment, and the presence of regional collateral veins.
Intervention
Patients with treatable obstruction and debilitating complaints were offered treatment by recanalisation and stenting. Those with post-thrombotic disease were treated under general anaesthesia or sedation, whereas patients with non-thrombotic iliac vein compression were treated under local anaesthesia. Access to the (common) femoral vein was obtained under ultrasound guidance, after which a guidewire was placed past the obstructed vein segments into a healthy segment. Subsequently, percutaneous transluminal angioplasty and stenting were performed, as has been described elsewhere.27 In cases where the femoral confluence was involved, a desobstruction of the common femoral vein and the orifices of its inflow vessels (endophlebectomy) was performed. A temporary arteriovenous fistula (AVF) was created to improve inflow into the stents and prevent early stent occlusion. This procedure was performed as described in literature.28
Statistical analysis
Continuous data are presented as mean with standard deviation or median with interquartile range, depending on normality of distribution. Categorical data are presented as absolute number and percentage. Linear regression analysis was performed to compare means of VOP parameters between control limbs and those with post-thrombotic and non-thrombotic obstruction. To account for correlation between observations, a clustered sandwich estimator was used to calculate the variance.29 Receiver operated characteristic (ROC) curves were constructed for VOP parameters and areas under the curve (AUC), with 95% confidence intervals (CI) were calculated to assess their abilities to discriminate between obstructed and non-obstructed limbs. AUCs were also calculated to evaluate the ability of these VOP parameters to discriminate between patients who show clinically relevant improvement in QoL after stenting and those who do not. Clinically relevant improvement was defined as 0.5 times the standard deviation of change in VEINES-QOL/Sym one year after stenting.30 Sub-analyses were performed for post-thrombotic obstruction and those with patent stents. We decided not to perform a sub-analysis for non-thrombotic iliac vein obstruction because of the low sample size of patients with QoL data and treatment for such an obstruction (n = 8). QoL before and after stenting were compared using a paired-samples t-test. AUC analysis and graphs were obtained using GraphPad Prism version 5.04 (GraphPad Software, San Diego, CA, USA). Other statistical analyses were performed using SPSS version 21.0 (IBM Corporation, Armonk, NY, USA) or Stata/IC version 13.1 (StataCorp, College Station, TX, USA).
Results
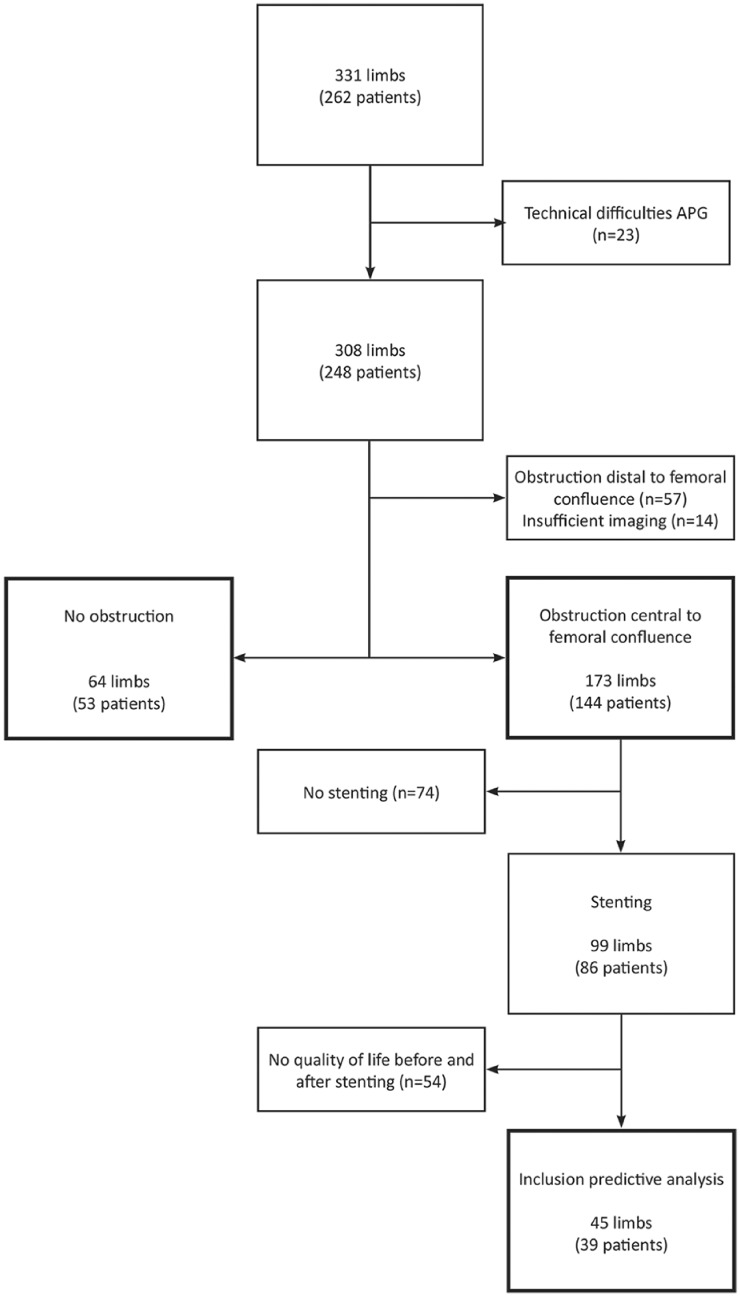
Using VOP, 331 limbs of 262 patients were analysed for deep venous obstruction at our outpatient clinic. As a result of technical difficulties, 23 limbs did not have proper VOP results. Of the remaining 308 limbs, 57 demonstrated post-thrombotic changes solely peripheral to the femoral confluence and 14 limbs had insufficient imaging data, leaving 237 limbs in 196 patients with either obstruction proximal to the femoral confluence or no obstruction at all (Figure 2). Mean age was 44.5 ± 14.2 years, and 64.8% were female. A total of 93.1% of patient were symptomatic with a median duration of 3 years (1–52), and 61.4% suffered from venous claudication. Post-thrombotic obstruction proximal to the femoral confluence was present in 62.0% limbs, non-thrombotic iliac vein compression in 11.0%, and no obstruction in 27.0% (Table 1). This latter group consisted of people suffering from no or only superficial venous disease and is defined as the control group.
Figure 2.
Patient inclusion flowchart. APG: air plethysmography.
Table 1.
Baseline patient characteristics.
| Total population | Patients undergoing stenting with QoL scores | |
|---|---|---|
| Patients | 196 | 39 |
| Limbs | 237 | 45 |
| Left | 109 (55.6) | 26 (66.7) |
| Right | 46 (23.5) | 7 (17.9) |
| Bilateral | 41 (20.9) | 6 (15.4) |
| Sex (M/F) | 69/127 (35.2/64.8) | 10/29 (25.6/74.4) |
| Age, years (mean ± SD) | 44.5 ± 14.2 | 43.3 ± 14.7 |
| Median duration of complaints, years (range) | 3 (1–52) | 5 (1–48) |
| Symptomatica | 224 (94.5) | 45 (100) |
| Venous claudicationb | 138 (58.2) | 31 (66.7) |
| History of venous interventions | 67 (34.4) | 9 (23.1) |
| Highest C of CEAPc | ||
| C0 | 24 (10.1) | 5 (11.1) |
| C1 | 18 (7.6) | 1 (2.2) |
| C2 | 44 (18.6) | 7 (15.6) |
| C3 | 66 (27.8) | 15 (33.3) |
| C4 | 55 (23.2) | 10 (22.2) |
| C5 | 21 (8.9) | 6 (13.3) |
| C6 | 7 (3.0) | 1 (2.2) |
| Post-thrombotic obstruction | 147 (62.0) | 37 (82.2) |
| Non-thrombotic iliac vein compression | 26 (11.0) | 8 (17.8) |
| No obstruction | 64 (27.0) | – |
QoL: quality of life; SD: standard deviation; CEAP: Clinical–Etiology–Anatomy–Pathophysiology.
2 missing.
14 missing, only for total population.
2 missing, only for total population.
Limbs without obstruction demonstrated a mean one-second outflow volume of 70.1 ± 33.3 mL. This was lower in limbs with post-thrombotic obstruction (48.7 ± 28.4 mL; P < .001) and limbs with non-thrombotic iliac vein compression (53.6 ± 26.0 mL, P = .014). Total venous volume was 142.6 ± 60.0 mL in healthy limbs, which was also lower in those with post-thrombotic obstruction (105.9 ± 49.6 mL; P < .001) and non-thrombotic iliac vein compression (108.8 ±41.8 mL, P = 0.003). Mean outflow fraction was 48.4 ± 9.3% in control limbs. In post-thrombotic limbs, this was only marginally lower (45.6 ± 13.1%; P = .089). Thus, mean outflow fraction was higher than the cut-off value for the presence of obstruction (<38%). For limbs with non-thrombotic iliac vein compression, outflow fraction was also not significantly different from control limbs (49.5 ± 13.1%; P = .704).
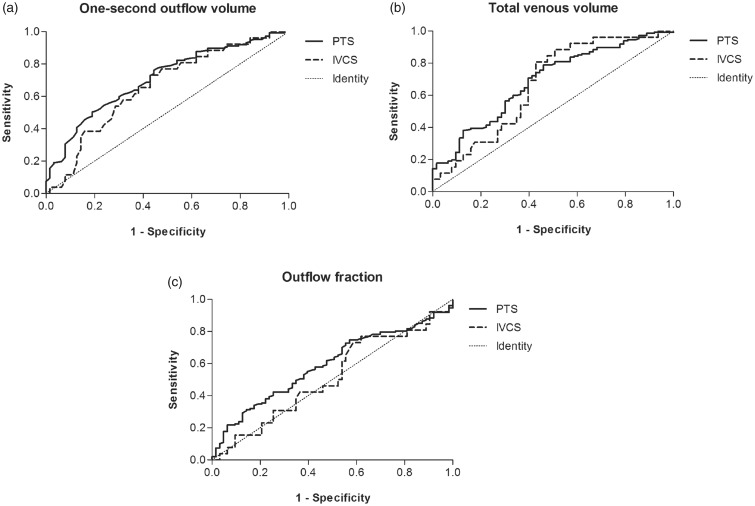
ROC curves were constructed to test the ability of the VOP parameters to discriminate between the presence and absence of deep venous obstruction proximal to the femoral confluence (Figure 3(a) to (c)). One-second outflow volume yielded an AUC of 0.71 (95% CI, 0.63–0.78) for post-thrombotic obstruction and 0.66 (95% CI, 0.54–0.78) for non-thrombotic iliac vein compression. Total venous volume demonstrated an AUC of 0.69 (95% CI, 0.61–0.77) for post-thrombotic and 0.67 (95% CI, 0.55–0.78) for non-thrombotic obstructions. The relative outflow fraction revealed lower AUCs: 0.59 (95% CI, 0.51–0.67) and 0.51 (95% CI, 0.38–0.65) for post-thrombotic and non-thrombotic obstruction, respectively.
Figure 3.
Receiver operated characteristic curves of the venous occlusion plethysmography parameters for the identification of obstruction. (a) One-second outflow volume, (b) total venous volume and (c) outflow fraction. IVCS: iliac vein compression syndrome; PTS: post-thrombotic syndrome.
Stenting was performed in 99 (41.8%) limbs of 86 patients, yet only 45 (45.5% of those treated) limbs in 39 patients had VEINES-QOL/Sym scores both before and one year after intervention. Eight limbs received stenting for non-thrombotic compression, whereas 37 limbs received stents for post-thrombotic syndrome. Fifteen of these also received an endophlebectomy and AVF. Baseline characteristics were similar to those of the complete group (Table 1). Mean VEINES-QOL was 49.8 ± 21.1 before intervention compared with 61.5 ± 25.0 one year after intervention (P = .031). VEINES-Sym improved from 46.9 ± 22.9 before stenting to 59.7 ± 27.6 one year after (P = .017).
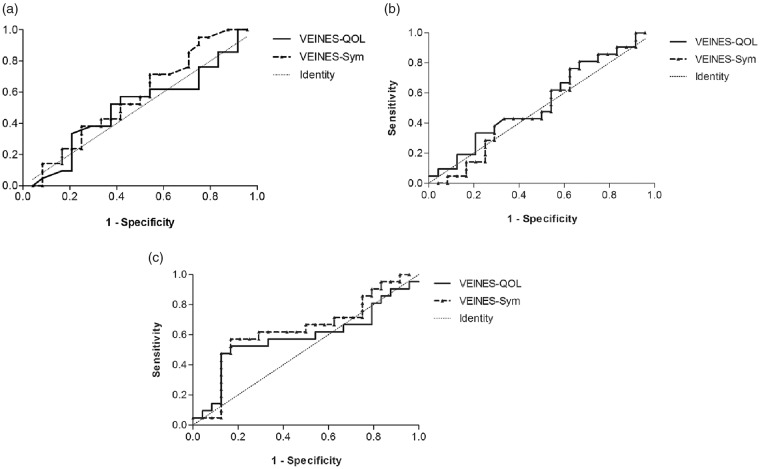
Employing 0.5 times the standard deviation of the change in QoL to define patients with successful treatment, 20 patients (21 limbs) had a successful treatment. Nineteen patients (24 limbs) had an unsuccessful treatment. One-second outflow volume yielded an AUC of 0.51 (95% CI, 0.34–0.69) for VEINES-QOL score and 0.57 (95% CI, 0.40–0.74) for VEINES-Sym score in identifying patients with a successful treatment. The AUC of total venous volume was 0.54 (95% CI, 0.37–0.72) for VEINES-QOL and 0.52 (95% CI, 0.35–0.69) for VEINES-Sym. Outflow fraction demonstrated an AUC of 0.58 (95% CI, 0.41–0.76) for VEINES-QOL and 0.63 (95% CI, 0.46–0.81) for VEINES-Sym (Table 2, Figure 4(a) to (c)).
Table 2.
Areas under the receiver operated characteristic curves for the different venous occlusion plethysmography parameters in identifying patients with clinically relevant quality of life improvement.
| One-second outflow volume |
Total venous volume |
Outflow fraction |
||||
|---|---|---|---|---|---|---|
| VEINES-QOL (95% CI) | VEINES-Sym (95% CI) | VEINES-QOL (95% CI) | VEINES-Sym (95% CI) | VEINES-QOL (95% CI) | VEINES-Sym (95% CI) | |
| Treated patients | 0.51 (0.34–0.69) | 0.57 (0.40–0.74) | 0.54 (0.37–0.72) | 0.52 (0.35–0.69) | 0.58 (0.41–0.76) | 0.63 (0.46–0.81) |
| Treated patients with patent stents | 0.53 (0.33–0.72) | 0.62 (0.43–0.80) | 0.51 (0.32–0.70) | 0.55 (0.36–0.74) | 0.59 (0.40–0.78) | 0.61 (0.42–0.80) |
| Patients treated for post-thrombotic obstruction | 0.52 (0.31–0.72) | 0.58 (0.39–0.77) | 0.55 (0.36–0.75) | 0.52 (0.33–0.71) | 0.58 (0.36–0.80) | 0.65 (0.45–0.85) |
CI: confidence interval.
Figure 4.
Receiver operated characteristic curves of the venous occlusion plethysmography parameters for the prediction of successful treatment, as measured by VEINES-QOL/Sym. (a) One-second outflow volume, (b) total venous volume and (c) outflow fraction.
At one year after intervention, loss of patency was present in seven limbs of five patients. Sub-analysis of patients with a patent stent at one year follow-up did not alter AUC results for one-second outflow volume (VEINES-QOL, 0.53; VEINES-Sym, 0.62), total venous volume (VEINES-QOL, 0.51; VEINES-Sym, 0.55), or outflow fraction (VEINES-QOL, 0.59; VEINES-Sym, 0.61). Type of obstruction also proved of little influence on the ability of the VOP parameters to predict clinical outcome, as one-second outflow volume (AUC, 0.52 for VEINES-QOL; 0.58 for VEINES-Sym), total venous volume (AUC, 0.55 for VEINES-QOL; 0.52 for VEINES-Sym), and outflow fraction (AUC, 0.58 for VEINES-QOL; 0.65 for VEINES-Sym) did not relevantly change (Table 2).
Discussion
This study demonstrates that absolute volumetric changes measured by VOP are not able to properly identify the presence of deep venous obstruction proximal to the femoral confluence. Previous research has already shown that the relative outflow fraction is not able to do this,25 though the hypothesis was that absolute volumetric changes might be more informative. We surmised that the level of pre-existent oedema should be of lesser influence on the total venous volume and absolute outflow volume after one second. Total venous volume has been found to significantly differ between obstructed and non-obstructed limbs before, yet with considerable overlap in range.17
One of the proposed explanations that outflow fraction yields poor results is the void left by the rapid deflatable cuff.31 This could initially lead to fair propulsion of blood, causing the outflow fraction to appear normal, which could also explain a higher outflow volume at one second. Another possible explanation is the distance of the iliac veins with respect to the plethysmography cuff. A significant obstruction proximal to the femoral confluence may not lead to abnormal values if expulsion into the popliteal and femoral veins is adequate. However, in the presence of post-thrombotic disease, these vein segments are often also affected to a certain extent. Finally, differences in venous anatomy and patient size will likely lead to a significant variation in volumetric reference values and thus an inferior ability of these absolute volumetric measurements to identify obstruction.
The ultimate goal of proper patient selection for interventional treatment of deep venous obstruction is that the appropriate patients are treated. Also, patients who will not benefit from treatment ought to be spared the possible complications and discomforts of such a treatment. Therefore, we evaluated whether the outflow parameters of VOP are able to identify those who will benefit from treatment the most. However, areas under the ROC curves for both the absolute volume parameters and the relative outflow fraction were too low for clinical use. Sub-analyses for aetiology of obstruction and patency of the stented tract did not yield better results. This lack of diagnostic ability might be influenced by post-thrombotic vessel damage distal to the treatable tract. The change in VEINES-QOL/Sym was striking as this appeared to be lower than previously reported in similar patient groups.10 As a result of the limited number of patients with both VOP results and valid QoL scores, we might have described a less representative group of patients. In addition, QoL may still change in some patients after one year.10
Given these negative results, the standard APG outflow test should be abandoned. However, a different plethysmography technique might be of more value in identifying patients with a clinically relevant obstruction. The venous drainage index is a parameter that can be obtained by postural changes of the patient using a tilt table. A small study describing three groups of participants (obstruction, primary reflux and controls) has shown promising results with respect to identification of obstructed patients, particularly given the lack of overlap in range between the obstructed patients and the other groups.32 Furthermore, another study measuring this parameter in healthy subjects undergoing various degrees of vascular compression demonstrates a correlation between the venous drainage index and degree of compression.33 Nonetheless, larger studies should prove its usefulness.
Several limitations of this study should be mentioned. Since control patients were also referred to our clinic with complaints that could be explained by deep venous obstruction, a selection bias may be present. Regarding the diagnostic abilities of obstruction, the lack of intravascular ultrasound use could have led to misidentification of some cases of iliac vein compression. However, this should not have influenced results in the post-thrombotic and treatment group. Finally, due to the retrospective nature of this study, missing data could have been of influence, especially with respect to analyses into the prediction of treatment effect, which resulted in a low sample size.
Conclusions
None of the measurements taken from the outflow curve of venous occlusion APG can be used to adequately identify deep venous obstruction proximal to the femoral confluence, nor can they be used to distinguish which patients will benefit from treatment and which will not. Therefore, use of this test is not warranted in daily clinical practice and future research should focus on other applications of this modality.
Acknowledgements
The authors would like to thank Rob Strijkers, MD, PhD, for his help in performing the APG examinations.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
Retrospective analysis of these data was approved by the Maastricht University Medical Centre institutional review board (METC 14-4-193, 5 March 2015). Individual patient consent was not obtained, as this is not required by Dutch law for retrospective studies. No patients registered an objection to anonymous use of data.
Guarantor
Cees HA Wittens
Contributorship
RLMK was responsible for conception and design of the study, study management, and data acquisition, analysis, and interpretation and wrote the article. FSC was involved in acquisition and interpretation of data, data analysis and critical revision of the manuscript. YLL, MAFW and IMT were involved in data acquisition and critical revision of the manuscript. CHAW was responsible for conception and design of the study, overall study supervision, and was involved in data acquisition and interpretation, and critical revision of the manuscript.
References
- 1.Lee BB, Nicolaides AN, Myers K, et al. Venous hemodynamic changes in lower limb venous disease: the UIP consensus according to scientific evidence. Int Angiol 2016; 35: 236–352. [PubMed] [Google Scholar]
- 2.Meissner MH, Eklof B, Smith PC, et al. Secondary chronic venous disorders. J Vasc Surg 2007; 46(Suppl S): 68S–83S. [DOI] [PubMed] [Google Scholar]
- 3.Labropoulos N, Borge M, Pierce K, et al. Criteria for defining significant central vein stenosis with duplex ultrasound. J Vasc Surg 2007; 46: 101–107. [DOI] [PubMed] [Google Scholar]
- 4.Arnoldussen CW, Toonder I, Wittens CH. A novel scoring system for lower-extremity venous pathology analysed using magnetic resonance venography and duplex ultrasound. Phlebology 2012; 27(Suppl 1): 163–170. [DOI] [PubMed] [Google Scholar]
- 5.Arnoldussen CW, de Graaf R, Wittens CH, et al. Value of magnetic resonance venography and computed tomographic venography in lower extremity chronic venous disease. Phlebology 2013; 28 Suppl 1: 169–175. [DOI] [PubMed] [Google Scholar]
- 6.Neglen P, Raju S. Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg 2002; 35: 694–700. [DOI] [PubMed] [Google Scholar]
- 7.Wittens C, Davies AH, Baekgaard N, et al. Editor's choice - Management of chronic venous disease: Clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2015; 49: 678–737. [DOI] [PubMed] [Google Scholar]
- 8.Neglen P, Hollis KC, Olivier J, et al. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg 2007; 46: 979–990. [DOI] [PubMed] [Google Scholar]
- 9.Seager MJ, Busuttil A, Dharmarajah B, et al. Editor's choice - A systematic review of endovenous stenting in chronic venous disease secondary to iliac vein obstruction. Eur J Vasc Endovasc Surg 2016; 51: 100–120. [DOI] [PubMed] [Google Scholar]
- 10.Catarinella FS, Nieman FH, de Wolf MA, et al. Quality-of-life in interventionally treated patients with post-thrombotic syndrome. Phlebology 2015; 30: 89–94. [DOI] [PubMed] [Google Scholar]
- 11.Yin M, Shi H, Ye K, et al. Clinical assessment of endovascular stenting compared with compression therapy alone in post-thrombotic patients with iliofemoral obstruction. Eur J Vasc Endovasc Surg 2015; 50: 101–107. [DOI] [PubMed] [Google Scholar]
- 12.de Wolf MA, de Graaf R, Kurstjens RL, et al. Short-term clinical experience with a dedicated venous nitinol stent: Initial results with the sinus-venous stent. Eur J Vasc Endovasc Surg 2015; 50: 518–526. [DOI] [PubMed] [Google Scholar]
- 13.de Wolf MAF, Arnoldussen CW, Grommes J, et al. Minimally invasive treatment of chronic iliofemoral venous occlusive disease. J Vasc Surg: Venous Lymphat Disord 2013; 1: 146–153. [DOI] [PubMed] [Google Scholar]
- 14.Gillespie DL, Cordts PR, Hartono C, et al. The role of air plethysmography in monitoring results of venous surgery. J Vasc Surg 1992; 16: 674–678. [PubMed] [Google Scholar]
- 15.Christopoulos D, Nicolaides AN, Szendro G. Venous reflux: quantification and correlation with the clinical severity of chronic venous disease. Br J Surg 1988; 75: 352–356. [DOI] [PubMed] [Google Scholar]
- 16.ACI Medical. APG air-plethysmograph models APG-1000C and APG-1000CP instruction and service manual, San Marcos, CA, 1990, 1990. [Google Scholar]
- 17.Kalodiki E, Calahoras LS, Delis KT, et al. Air plethysmography: the answer in detecting past deep venous thrombosis. J Vasc Surg 2001; 33: 715–720. [DOI] [PubMed] [Google Scholar]
- 18.Labropoulos N, Volteas N, Leon M, et al. The role of venous outflow obstruction in patients with chronic venous dysfunction. Arch Surg 1997; 132: 46–51. [DOI] [PubMed] [Google Scholar]
- 19.Delis KT, Bountouroglou D, Mansfield AO. Venous claudication in iliofemoral thrombosis: long-term effects on venous hemodynamics, clinical status, and quality of life. Ann Surg 2004; 239: 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurst DR, Forauer AR, Bloom JR, et al. Diagnosis and endovascular treatment of iliocaval compression syndrome. J Vasc Surg 2001; 34: 106–113. [DOI] [PubMed] [Google Scholar]
- 21.Lattimer CR, Geroulakos G, Kalodiki E. Calf volume changes with venous occlusion air plethysmography in assessment of patients after deep venous thrombosis. J Vasc Surg: Venous Lymphat Disord 2014; 2: 416–423. [DOI] [PubMed] [Google Scholar]
- 22.Lattimer CR, Kalodiki E, Kafeza M, et al. Quantifying the degree graduated elastic compression stockings enhance venous emptying. Eur J Vasc Endovasc Surg 2014; 47: 75–80. [DOI] [PubMed] [Google Scholar]
- 23.Locker T, Goodacre S, Sampson F, et al. Meta-analysis of plethysmography and rheography in the diagnosis of deep vein thrombosis. Emerg Med J 2006; 23: 630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raju S, Kirk O, Davis M, et al. Hemodynamics of “critical” venous stenosis and stent treatment. J Vasc Surg: Venous Lymphat Disord 2014; 2: 52–59. [DOI] [PubMed] [Google Scholar]
- 25.Kurstjens RL, de Wolf MA, Alsadah SA, et al. The value of hemodynamic measurements by air plethysmography in diagnosing venous obstruction of the lower limb. J Vasc Surg: Venous Lymphat Disord 2016; 4: 313–319. [DOI] [PubMed] [Google Scholar]
- 26.Nicolaides AN. Investigation of chronic venous insufficiency: A consensus statement (France, March 5–9, 1997). Circulation 2000; 102: E126–E163. [DOI] [PubMed] [Google Scholar]
- 27.de Graaf R, Wittens CH. Endovascular treatment options for chronic venous obstructions. Phlebology 2012; 27(Suppl 1): 171–177. [DOI] [PubMed] [Google Scholar]
- 28.Kurstjens RL, de Graaf R, Barbati ME, et al. Arteriovenous fistula geometry in hybrid recanalisation of post-thrombotic venous obstruction. Phlebology 2015; 30: 42–49. [DOI] [PubMed] [Google Scholar]
- 29.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics 2000; 56: 645–646. [DOI] [PubMed] [Google Scholar]
- 30.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 2003; 41: 582–592. [DOI] [PubMed] [Google Scholar]
- 31.Lattimer CR, Kalodiki E, Azzam M, et al. Pneumatic thigh compression reduces calf volume and augments the venous return. Phlebology 2015; 30: 316–322. [DOI] [PubMed] [Google Scholar]
- 32.Lattimer CR, Mendoza E. Simultaneous air-plethysmography and duplex scanning on a tilt-table in assessing gravitational venous drainage. J Vasc Surg: Venous Lymphat Disord 2016; 4: 151–152. [Google Scholar]
- 33.Lattimer CR, Doucet S, Geroulakos G, et al. Validation of the novel venous drainage index with stepwise increases in thigh compression pressure in the quantification of venous obstruction. J Vasc Surg: Venous Lymphat Disord 2017; 5: 88–95. [DOI] [PubMed] [Google Scholar]