Introduction
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, also known as drug-induced hypersensitivity syndrome, is a rare, potentially life-threatening adverse drug reaction with an estimated mortality rate up to 10%.1 We report a case of DRESS syndrome caused by ivermectin, which, to our knowledge, has not been reported previously.
Case report
A 36-year-old woman was admitted to our hospital with a 4-week history of a pruritic generalized maculopapular rash. She had been in good health and was not taking any regular medication. Her general practitioner had prescribed a dose of oral ivermectin (200 μg/kg), which was repeated 1 week later, for suspected scabies 2 weeks before rash onset. A physical examination found a widespread erythematous, edematous, and exfoliative eruption; facial edema; and lymphadenopathy involving the cervical, axillary, and inguinal regions (Fig 1). She was subfebrile (37.5°C), and no pustules or mucosal involvement was observed. There were no signs of scabies, and skin scraping was negative. Laboratory studies found leukocytosis (white cell count, 14.1 × 109/L), isolated eosinophilia (5.6 × 109/L), and elevated liver enzymes (aspartate aminotransferase level, 41 U/L; alanine aminotransferase level, 62 U/L). Serology results for hepatitis A, B, and C; HIV; Trichinella; and Toxocara canis were negative. Serologic and polymerase chain reaction tests for Epstein-Barr virus, cytomegalovirus, parvovirus B19, and human herpesvirus-6 were compatible with past infection, without evidence of viral reactivation. Blood and urine cultures were negative. A skin biopsy specimen obtained from the abdomen found moderate spongiosis and superficial dermal perivascular lymphocytic infiltrate with numerous eosinophils, consistent with a drug hypersensitivity reaction (Fig 2). All findings were consistent with a diagnosis of DRESS syndrome caused by ivermectin based on the European Registry of Severe Cutaneous Adverse Reactions (RegiSCAR) criteria, with a RegiSCAR score of 6, which indicates a definite case of DRESS syndrome. Supportive care was initiated using emollients and H1-antihistamines. Skin involvement, lymphadenopathy, liver dysfunction and hypereosinophilia progressively resolved over the subsequent 3 weeks.
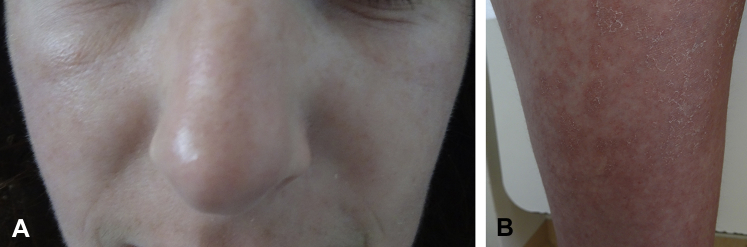
Fig 1.
DRESS syndrome 2 weeks after starting ivermectin treatment. A, Facial edema. B, Close-up view of erythematous, edematous, and exfoliative dermatitis on the thigh.
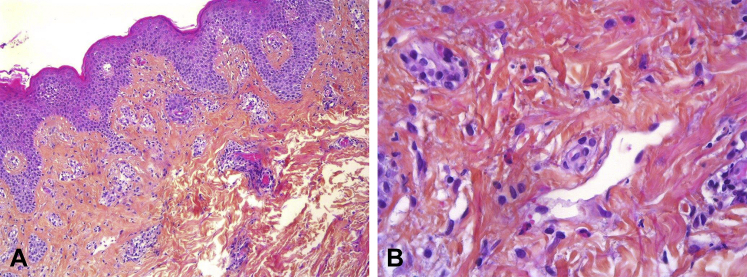
Fig 2.
Histopathology of lesional abdominal skin. A, Low power. Consistent with drug hypersensitivity reaction, this biopsy shows mild spongiosis with a vesicle and superficial perivascular lymphocytic infiltrate with eosinophils. B, High power. Numerous eosinophils. (Hematoxylin-eosin stain; original magnifications: A, ×100; B, ×400.)
Discussion
DRESS syndrome is a severe adverse drug reaction that typically occurs 2 to 6 weeks after initiation of the offending drug. DRESS syndrome is characterized by generalized exanthema with facial swelling, fever, lymphadenopathy, leukocytosis with eosinophilia, and internal organ involvement with liver or renal dysfunction.2 Diagnosis of DRESS syndrome is established by applying the RegiSCAR scoring system shown in Table I.3, 4 The most common causative drugs include antiepileptics, allopurinol, antimicrobial sulfonamides, minocycline, and vancomycin.2 An association with reactivation of viruses of the herpes family (human herpesvirus-6, Epstein-Barr virus, and cytomegalovirus) has also been reported.5 To our knowledge, this is the first reported case adding ivermectin to the list of drugs causing DRESS syndrome.
Table I.
| No | Yes | Unknown | |
|---|---|---|---|
| Fever ≥ 38.5°C | −1 | 0 | −1 |
| Enlarged lymph nodes (≥2 sites, >1 cm) | 0 | 1 | 0 |
| Atypical lymphocytes | 0 | 1 | 0 |
| Eosinophilia | 0 | — | 0 |
| 0.7-1.499 × 109/L or 10%-19.9% | — | 1 | — |
| ≥1.5 × 109/L or ≥20% | — | 2 | — |
| Skin rash | 0 | — | 0 |
| Extent >50% | 0 | 1 | 0 |
| At least 2 of edema, infiltration, purpura, scaling | −1 | 1 | 0 |
| Biopsy suggesting DRESS | −1 | 0 | 0 |
| Internal organ involved | 0 | — | 0 |
| 1 | — | 1 | — |
| 2 or more | — | 2 | — |
| Resolution in >15 d | −1 | 0 | −1 |
| At least 3 biological investigations done and negative to exclude alternative diagnosis | 0 | 1 | 0 |
Final score: <2, no cases; 2-3, possible case; 4-5, probable case; >5, definite case.
The therapeutic management of DRESS syndrome is challenging and should always be discussed and adjusted based on the severity of organ involvement.6 Recently, a consensus group of the French Society of Dermatology published recommendations for the management of DRESS syndrome.7 Withdrawal of the culprit drug and supportive care with emollients and H1-antihistamines are recommended for all cases and may be sufficient to obtain remission in some nonsevere DRESS cases with mild hepatitis, as shown in our patient. In the absence of signs of severity, patients can be treated with topical corticosteroids in addition to supportive therapy. In the presence of signs of severity (transaminase levels >4 times normal, renal involvement), treatment with systemic corticosteroids (0.5-1 mg/kg/d) is warranted. In patients with life-threatening systemic disease such as fulminant hepatitis or hemophagocytosis, antiviral treatments including ganciclovir and intravenous immunoglobulins have been considered as alternative treatments, but their efficacy has yet to be demonstrated, and they may even be deleterious.1, 8, 9
Ivermectin is a semisynthetic derivative of a family of macrocyclic lactones called avermectins. Ivermectin binds to invertebrate-specific glutamate and γ-aminobutyric acid-gated chloride channels and interrupts neurotransmission.10 Ivermectin exhibits a good safety profile because of its inability to cross the blood-brain barrier in humans and other mammals. Ivermectin is recommended for the treatment of onchocerciasis, strongyloidiasis, filariasis, and cutaneous larva migrans in humans. It is also routinely used for the treatment of scabies.11 Two other cases of ivermectin-induced severe cutaneous adverse reactions (SCARs) were reported recently.12, 13 One case of Stevens-Johnson syndrome (SJS) occurred in a 38-year-old Cameroonian man infected with HIV who received a single dose of ivermectin during a nationwide antifilarial campaign, and 1 case of toxic epidermal necrolysis (TEN) occurred in a 45-year-old man after taking a single dose of ivermectin for suspected body louse. Interestingly, in both cases, the drug reaction occurred 3 days after taking ivermectin, which is faster than usual for SJS/TEN. Indeed, SJS/TEN typically begins 1 to 3 weeks after the onset of drug intake.1 In addition, similar to our case, both patients were treated with supportive care and made an unremarkable recovery.
When ivermectin is used to treat filarial parasites, adverse reactions (Mazzotti reactions) occasionally occur, including fever, urticaria, swollen and tender lymph nodes, myalgia, tachycardia, and hypotension.14 These adverse reactions occur within 7 days of the administration of ivermectin and are likely related to the intensity of the filarial infection and the release of parasite antigen.15 The Mazzotti reaction can be distinguished from SCAR, similar to DRESS syndrome, based on characteristic cutaneous findings, the onset of symptoms, and visceral involvement.
Oral ivermectin exhibits a high margin of safety, and millions of patients worldwide use ivermectin with good tolerance.11 However, caution is warranted after these reports of SCARs associated with ivermectin. We present this case of ivermectin-induced DRESS syndrome to alert clinicians about this rare, but potentially life-threatening, complication of a commonly prescribed antiparasitic drug. A dermatologic consultation may be useful in atypical scabies cases before initiating empirical treatment with oral ivermectin to assist in the differentiation of skin rashes and confirm the diagnosis of scabies.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Husain Z., Reddy B.Y., Schwartz R.A. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol. 2013;68:693. doi: 10.1016/j.jaad.2013.01.033. e1-e14. [DOI] [PubMed] [Google Scholar]
- 2.Kardaun S.H., Sekula P., Valeyrie-Allanore L. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071–1080. doi: 10.1111/bjd.12501. [DOI] [PubMed] [Google Scholar]
- 3.Kardaun S.H., Sidoroff A., Valeyrie-Allanore L. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;155:609–611. doi: 10.1111/j.1365-2133.2006.07704.x. [DOI] [PubMed] [Google Scholar]
- 4.Roujeau J.C., Allanore L., Liss Y., Mockenhaupt M. Severe cutaneous adverse reactions to drugs (SCAR): definitions, diagnostic criteria, genetic predisposition. Dermatol Sinica. 2009;27:203–209. [Google Scholar]
- 5.Seishima M., Yamanaka S., Fujisawa T., Tohyama M., Hashimoto K. Reactivation of human herpesvirus (HHV) family members other than HHV-6 in drug-induced hypersensitivity syndrome. Br J Dermatol. 2006;155:344–349. doi: 10.1111/j.1365-2133.2006.07332.x. [DOI] [PubMed] [Google Scholar]
- 6.Funck-Brentano E., Duong T.A., Bouvresse S. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246–252. doi: 10.1016/j.jaad.2014.10.032. [DOI] [PubMed] [Google Scholar]
- 7.Ingen-Housz-Oro S., Duong T.A., de Prost N. Treatment of severe cutaneous adverse drug reactions. Ann Dermatol Venereol. 2018 doi: 10.1016/j.annder.2018.02.014. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Descamps V., Collot S., Mahe E., Houhou N., Crickx B., Ranger-Rogez S. Active human herpesvirus 6 infection in a patient with drug rash with eosinophilia and systemic symptoms. J Invest Dermatol. 2003;121:215–216. doi: 10.1046/j.1523-1747.2003.12333.x. [DOI] [PubMed] [Google Scholar]
- 9.Joly P., Janela B., Tetart F. Poor benefit/risk balance of intravenous immunoglobulins in DRESS. Arch Dermatol. 2012;148:543–544. doi: 10.1001/archderm.148.4.dlt120002-c. [DOI] [PubMed] [Google Scholar]
- 10.del Giudice P., Marty P. Ivermectin: a new therapeutic weapon in dermatology? Arch Dermatol. 1999;135:705–706. doi: 10.1001/archderm.135.6.705. [DOI] [PubMed] [Google Scholar]
- 11.Kircik L.H., Del Rosso J.Q., Layton A.M., Schauber J. Over 25 years of clinical experience with ivermectin: an overview of safety for an increasing number of indications. J Drugs Dermatol. 2016;15:325–332. [PubMed] [Google Scholar]
- 12.Aroke D., Tchouakam D.N., Awungla A.T., Mapoh S.Y., Ngassa S.N., Kadia B.M. Ivermectin induced Stevens-Johnson syndrome: case report. BMC Res Notes. 2017;10:179. doi: 10.1186/s13104-017-2500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seegobin K., Bueno E., Maharaj S., Ashby T., Brown M., Jones L. Toxic epidermal necrolysis after ivermectin. Am J Emerg Med. 2017 doi: 10.1016/j.ajem.2017.09.021. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Twum-Danso N.A. Serious adverse events following treatment with ivermectin for onchocerciasis control: a review of reported cases. Filaria J. 2003;(2 Suppl 1):S3. doi: 10.1186/1475-2883-2-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Currie B.J., McCarthy J.S. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717–725. doi: 10.1056/NEJMct0910329. [DOI] [PubMed] [Google Scholar]