Abstract
Oral supplementation with l-carnitine is a common therapeutic modality for mitochondrial disorders despite limited evidence of efficacy. Recently, a number of studies have demonstrated that a gut microbiota-dependent metabolite of l-carnitine, trimethylamine oxide (TMAO), is an independent and dose-dependent risk factor for cardiovascular disease (CVD). Given the limited data demonstrating efficacy with oral l-carnitine therapy and the newly raised questions of potential harm, we assessed plasma TMAO levels in patients with mitochondrial disease with and without oral l-carnitine supplementation. Nine subjects were recruited and completed the study. Eight out of 9 subjects at baseline had plasma TMAO concentrations <97.5th percentile (<15.5 μM). One subject with stage 3 renal disease, had marked elevation in plasma TMAO (pre 33.98 μm versus post 101.6 μm). Following at least 3 months of l-carnitine supplementation (1000 mg per day), plasma TMAO levels were markedly increased in 7out of 9 subjects; overall, plasma TMAO significantly increased 11.8-fold (p < 0.001) from a baseline median level of 3.54 μm (interquartile range (IQR) 2.55–8.72) to 43.26 (IQR 23.99–56.04) post supplementation. The results of this study demonstrate that chronic oral l-carnitine supplementation markedly increases plasma TMAO levels in subjects with mitochondrial disorders. Further studies to evaluate both the efficacy and long term safety of oral l-carnitine supplementation for the treatment of mitochondrial disorders are warranted.
Keywords: l-carnitine, Trimethylamine N-oxide, Mitochondrial disorders
1. Introduction
A variety of vitamins, cofactors and supplements are commonly used in the treatment of mitochondrial disorders. l-carnitine has been proposed as a treatment modality on the basis of its role in translocation of long chain fatty acids into the mitochondria for beta-oxidation and ATP production [1]. Studies have demonstrated that increasing muscle carnitine can enhance fatty acid oxidation while sparing glycogen [2] in healthy subjects and prevent fat gain during low intensity exercise [3,4]. However, other studies have failed to demonstrate an effect on substrate utilization [5,6]. Variance in study protocols may at least partially account for conflicting results as it has been suggested that co-ingestion of oral l-carnitine with carbohydrate is critical to increase muscle carnitine stores and protein can blunt the effect [7]. However, in a recent study of healthy subjects, no enhancement of exercise performance was demonstrated, despite documented increase in skeletal muscle carnitine, during a 24 week trial of high intensity interval training [8].
Studies of oral l-carnitine supplementation in patients with mitochondrial disorders are limited. A study of 12 patients with mitochondrial myopathy and chronic progressive external opthalmoplegia, found a modest increase in oxygen consumption and exercise tolerance during a standardized exercise test, after an 8 week trial of oral l-carnitine [9]. A limitation of this study was that there was no measurement of muscle carnitine. No long-term placebo-controlled studies have been completed to test the safety of oral l-carnitine therapy. Based on limited evidence to support efficacy, a recent consensus statement from the Mitochondrial Medicine Society, did not include oral l-carnitine as a therapeutic modality [10,11]. Yet in practice, and despite the recently published consensus statement, many mitochondrial patients remain on oral l-carnitine supplementation, and the United Mitochondrial disease foundation, a patient association, lists oral l-carnitine as a “first tier” supplement (https://www.umdf.org/what-is-mitochondrial-disease/treatments-therapies/).
l-carnitine, an abundant nutrient in red meat, is metabolized by intestinal bacteria to form a volatile metabolite, trimethylamine (TMA), which is converted to trimethylamine N-oxide (TMAO) by the hepatic enzyme, flavin monooxygenase 3 (FMO3) (Fig. 1). A potentially harmful effect of TMAO on cardiovascular health has been a focus of intense research in recent years. In 2011, metabolomics studies identified an association between TMAO and cardiovascular disease (CVD) in humans, and mechanistic studies indicated TMAO can accelerate atherosclerosis in animal models of disease [12]. Further in vivo studies established that dietary l-carnitine can serve as an alternative nutrient precursor for the generation of both TMA and TMAO in both humans and mice alike, and animal model studies showed that dietary supplementation with l-carnitine can accelerate atherosclerosis in atherosclerosis prone apolipoprotein E-/- mice [13]. Moreover, large scale clinical studies revealed that plasma levels of carnitine are dose dependently associated with incident risk for major adverse cardiovascular events (myocardial infarction (MI), stroke and death), and that the adverse risk associated with plasma carnitine levels is only observed amongst those who concomitantly have elevated TMAO levels [13]. The relationship between plasma TMAO and CVD was further studied in patients (n = 4007 subjects) undergoing elective coronary angiography. Compared to participants with TMAO in the lowest quartile, those in the highest quartile (>6.2 μm) had a significantly increased risk of incident CVD events such as MI, stroke or death independent of other cardiovascular risk factors [14]. Multiple studies have since confirmed an association between systemic TMAO levels and incident CVD event risk in various patient populations. Moreover, several meta-analyses have recently been published that systematically review the TMAO literature, all of which conclude that plasma TMAO is an independent and dose-dependent risk factor for CVD and mortality risks [[15], [16], [17]].
Fig. 1.
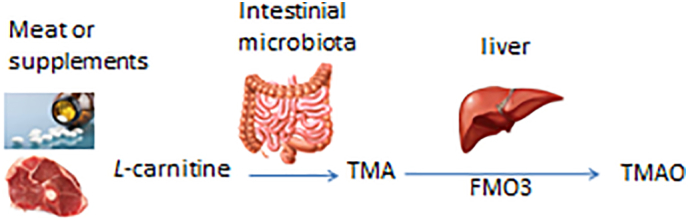
Dietary l-carnitine is converted by intestinal microbiota to trimethylamine (TMA), which is then converted to trimethylamine-oxide (TMAO) by the enzyme FMO3 in the liver.
A mechanistic role for TMAO in development of CVD has been supported by multiple additional studies. TMAO in animal model studies has been shown to promote aortic endothelial cell activation, and up regulation of inflammatory gene signatures [18]. Modulation of TMAO levels by suppression of FMO3 levels using antisense oligonucleotide targeted approaches has been shown to inhibit atherosclerosis in several animal model studies, including hypercholesterolemic LDL-R-/- models [19], and to impact tissue cholesterol homeostasis [18,20]. TMAO has also been linked to changes in visceral adipose tissue phenotype and metabolism [21]. TMAO has also been shown to directly interact with platelets, rapidly rendering them more responsive to agonists like ADP, thrombin and collagen, leading to changes in intracellular calcium signaling, hyper-responsiveness, and enhanced thrombosis potential in vivo [22]. Moreover, TMAO levels have been shown to dose-dependently be associated with incident thrombotic event risks in patients [22,23]. In recent human clinical intervention studies, supplemental choline provided to both omnivore and vegan/vegetarian alike was shown to result in elevation in plasma TMAO levels, and heightened platelet aggregometry responses in subjects, even in the presence of aspirin therapy [24]. Notably, in recent studies, use of a small molecule inhibitor that attenuates gut microbial production of TMA, and thus systemic TMAO levels, was shown to significantly reduce diet dependent atherosclerosis development in animal models [25].
Thus, a growing body of evidence is accruing indicating that the gut microbe-generated metabolite, TMAO, is linked to CVD and thrombosis risks. Given the limited evidence of benefit from oral l-carnitine supplementation in subjects with mitochondrial disorders, and the mounting evidence that TMAO both serves as a risk factor for CVD and adverse event risks in subjects, we sought to determine the degree to which oral l-carnitine supplementation increases TMAO levels in patients with mitochondrial disease.
2. Materials and methods
The Adult Metabolic clinic is the referral centre for patients suspected of mitochondrial disorders in British Columbia. Patients coded as having a highly probable or definite diagnosis of mitochondrial disease and undergoing standard treatment, were invited to participate in the study. Patient characteristics and diagnoses are summarized in Table 1. A diagnosis of mitochondrial disease was based on suggestive clinical features and either a pathogenic, or likely pathogenic nuclear gene or mtDNA mutation(s) as defined by ACMG criteria [26]. Ten patients were recruited into the study.
Table 1.
Patient characteristics.
| Case # | Age (years) | Sex (M/F) | Diagnosis | Creatinine (Cr) uM/eGFR⁎ (ml/min/1.73m2) | Diet |
|---|---|---|---|---|---|
| 1 | 48 | M | Predominant single mtDNA deletion 14 kb | Cr 43 eGFR- > 120 | Vegetarian |
| 2 | 42 | F | OPA c.2242C > T p.Arg748⁎ | No testing | Omnivore |
| 3 | 74 | F | Multiple mtDNA deletions | Cr 124 eGFR 37 | Omnivore |
| 4 | 63 | F | Multiple mtDNA deletions | Cr 46 eGFR 103 | Omnivore |
| 5 | 66 | F | ~ 25% mtDNA deletion 3.8 kb | Cr 62 eGFR 91 | Omnivore |
| 6 | 53 | F | m.8344 A > G (MERFF) | Cr 91 eGFR 62 | Omnivore |
| 7 | 59 | M | m.3243 A > G (MELAS) | Cr 64 eGFR 103 | Omnivore |
| 8 | 32 | F | m.3243 A > G (MELAS) | No testing | Omnivore |
| 9 | 74 | M | TWINKLE c.1105 T > C p.Ser369Pro | Cr 79 eGFR 84 | Omnivore |
| 10 | 38 | F | TWINKLE c.1105 T > C p.Ser369Pro | Cr 66 eGFR 103 | Omnivore |
Eight out of 10 patients received 1000 mg oral l-carnitine daily, either divided into 2 or 3 doses for >1 year prior to blood collection. Patients were off carnitine for at least 3 months before collection of the 2nd “off carnitine” blood sample. Subject 6 had a baseline sample collected off carnitine, and then placed on oral l-carnitine for 3 months prior to 2nd blood collection. Subject 2 was omitted from data analysis – incorrect timing of “on carnitine” sample – collected 1 week after resuming oral l-carnitine therapy. Patient 3 noted to have significant renal impairment.
eGFR calculated using the CKD-EPI (Chronic kidney disease epidemiology collaboration) equation. Levey et al. A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med. 2009;150:604–612.
Diet history: Patients completed a detailed diet history over the telephone with a Metabolic Dietitian. Each diet history was analyzed for carnitine content using the on-line diet analysis software by Carnipure TM. An average daily intake of carnitine was established for each patient.
Two blood samples were collected on each patient, on and off oral l-carnitine therapy. Nine out of 10 had been receiving oral l-carnitine (levocarnitine, 3-carboxy-2(R)-hydroxy-N,N,N-trimethyl-1-propanaminium, inner salt) 1000 mg daily, either divided in 2 or 3 doses, for >1 year, at the time of study recruitment. Blood samples were collected shortly after study recruitment and then again 3 months after discontinuing oral l-carnitine therapy. One patient was not receiving oral l-carnitine at the time of recruitment and was placed on oral l-carnitine for 3 months prior to the 2nd blood collection.
Methods: Plasma TMAO analysis was performed using stable isotope dilution tandem mass spectrometry on a Shimadzu 8050 triple quadrupole mass spectrometer, as previously described [27].
Statistical analysis: Paired t-test was used to compare the intra-individual difference in TMAO concentration on/off carnitine.
Clinical Research ethics Board (CREB) approval: Vancouver Coastal Health and Children's and Women's Health Centre of BC, University of British Columbia (UBC CREB #H14-01442)
3. Results
Patient characteristics (n = 10) are summarized in Table 1 along with plasma TMAO values on and off carnitine. Nine patients were omnivores and one patient was strictly vegetarian. One patient, subject 2, was omitted from data analysis due to incorrect timing of “on carnitine” sample.
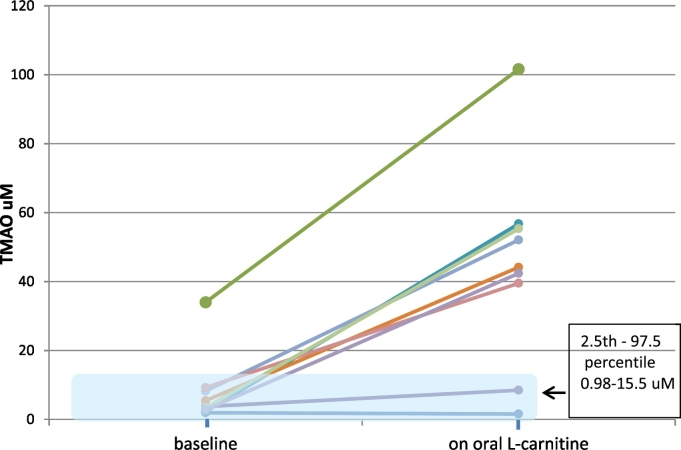
Subjects (n = 9) at baseline had a median plasma TMAO of 3.54 μm (interquartile range (IQR) 2.55-8.72; range 1.94-33.98) and on oral l-carnitine had a median plasma TMAO of 43.26 (IQR 23.99-56.04, range1.55-101.6). The median TMAO level on oral l-carnitine therapy increased 11.8 fold compared to baseline (p < 0.001), see Fig. 2.
Fig. 2.
Plasma TMAO levels at baseline and with oral l-carnitine (n = 9). Seven out of 9 patients had a marked elevation in plasma TMAO with oral l-carnitine supplementation, well above the reference interval.
One subject with stage 3 chronic kidney disease had marked elevation in plasma TMAO which increased 3-fold while on oral l-carnitine therapy (33.98 versus 101.6 μm). All subjects had a significant increase in plasma TMAO while receiving oral l-carnitine except subject 1, the only vegetarian in the study.
4. Discussion
The majority of patients in this study had marked elevation of plasma TMAO while receiving oral l-carnitine therapy. Given the strong evidence that TMAO is a risk factor for CVD, this raises concern regarding the safety of longterm oral l-carnitine use. Safety studies evaluating long-term exposure to oral l-carnitine in mitochondrial patients have not been performed. Oral l-carnitine is routinely prescribed for a number of inborn errors of metabolism including organic acidemias and primary carnitine uptake deficiency to remove build-up of harmful metabolites and/or replenish depleted carnitine stores [[28], [29], [30]]. Survival of patients with inborn errors of metabolism well into adulthood is now common yet long-term controlled safety and efficacy studies have not been done and have been recently been recommended [31].
A recently published consensus statement on the treatment of mitochondrial disorders does not include oral l-carnitine as a recommended treatment [10,11]. Although there is a lack of evidence of efficacy, this does not mean that carnitine is ineffective. Patients currently taking oral l-carnitine may be reluctant to discontinue treatment and physicians may be reluctant to change their current prescribing practice.
Alternative delivery models of carnitine have been suggested such as cyclic administration of oral antibiotic or periodic parenteral l-carnitine supplementation to reduce TMAO levels while maintaining sufficient carnitine [31]. It has been suggested that a vegetarian diet might protect against TMAO production from oral carnitine. Studies have demonstrated striking differences in the composition of gut microbiota in vegetarians and vegans compared with omnivores. Red meat, which is rich in carnitine, affects the composition of the gut microbial community promoting growth of bacterial species that metabolize l-carnitine to TMA. Vegetarians and vegans have reduced capacity to make TMA from dietary l-carnitine and have lower plasma TMAO levels compared to omnivores [32]. Only one patient in this study was a strict vegetarian and interestingly had no increase in TMAO after three months of recommended oral l-carnitine therapy. However, exposure to oral l-carnitine in animal models has demonstrated increased TMA synthetic capacity 10 fold with a concurrent shift in gut microbial composition. Patients with inborn errors of metabolism, on low protein/meat restricted diets have marked elevation of TMAO on chronic oral l-carnitine therapy [31]. Therefore, a vegetarian diet may not protect against TMAO production with longterm use of oral l-carnitine.
The only patient in this study with marked elevation in plasma TMAO at baseline, had chronic kidney disease (CKD stage 3). Plasma TMAO is cleared by the kidney and studies have demonstrated that TMAO levels are significantly elevated in CKD [33]. It has been suggested that TMAO contributes to cardiovascular disease in the context of CKD and intervention studies to reduce TMAO have been proposed to study this hypothesis [34].
There are several limitations of this study including small sample size and measurement of plasma TMAO only at a single time point on oral l-carnitine therapy. The findings in this study should be replicated in a larger study of patients with mitochondrial disease receiving oral l-carnitine.
In conclusion, the results of this study show that oral l-carnitine can markedly increase plasma TMAO in mitochondrial patients and therefore could increase risk of CVD over time. It is important to consider the potential harms of oral l-carnitine therapy especially given the limited evidence to date in support of health benefit in mitochondrial disorders. Further studies evaluating both the efficacy and long term safety of oral l-carnitine supplementation for the treatment of mitochondrial disorders are warranted.
Funding
This work was supported by a grant from the Canadian Rare Disease Foundation (12-15).
References
- 1.Stephens F.B., Constantin-Teodosiu D., Greenhaff P.L. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J. Physiol. 2007;581:431–444. doi: 10.1113/jphysiol.2006.125799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephens F.B., Constantin-Teodosiu D., Laithwaite D., Simpson E.J., Greenhaff P.L. An acute increase in skeletal muscle carnitine content alters fuel metabolism in resting human skeletal muscle. J. Clin. Endocrinol. Metabol. 2006;91:5013–5018. doi: 10.1210/jc.2006-1584. [DOI] [PubMed] [Google Scholar]
- 3.Stephens F.B., Wall B.J., Marimuthu K., Shannon C.E., Constantin-Teodosiu D., Macdonald I.A., Greenhaff P.L. Skeletal muscle carnitine loading increases energy expenditure, modulates fuel metabolism gene networks and prevents body fat accumulation in humans. J. Physiol. 2013;591(18):4655–4666. doi: 10.1113/jphysiol.2013.255364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wall B.T., Stephens F.B., Constantin-Teodosiu D., Marimuthu K., Macdonald I.A., Greenhaff P.L. Chronic oral ingestion of L-carnitine and carbohydrate increases muscle carnitine content and alters muscle fuel metabolism during exercise in humans. J. Physiol. 2011;589:963–973. doi: 10.1113/jphysiol.2010.201343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abramowicz W.N., Galloway S.D.R. Effects of acute versus chronic L-carnitine L-tartrate supplementation on metabolic responses to steady state exercise in males and females. Int. J. Sport Nutr. Exerc. Metab. 2005;15:386–400. doi: 10.1123/ijsnem.15.4.386. [DOI] [PubMed] [Google Scholar]
- 6.Broad E.M., Maughan R.J., Galloway S.D.R. Effects of four weeks L-carnitine L-tartrate ingestion on substrate utilization during prolonged exercise. Int. J. Sport Nutr. Exerc. Metab. 2005;15:665–679. doi: 10.1123/ijsnem.15.6.665. [DOI] [PubMed] [Google Scholar]
- 7.Stephens F.B., Constantin-Teodosiu D., Laithwaite D., Simpson E.J. Insulin stimulates L-carnitine accumulation in human skeletal muscle. FASEB J. 2006;20:377–379. doi: 10.1096/fj.05-4985fje. [DOI] [PubMed] [Google Scholar]
- 8.Shannon C.E., Ghasemi R., Greenhaff P.L., Stephens F.B. Increasing skeletal muscle carnitine availability does not alter the adaptations to high-intensity interval training. Scand J. Med. Sci. Sports. 2017 Mar 27 doi: 10.1111/sms.12885. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 9.Gimenes A.C., Bravo D.M., Napolis L.M., Mello M.T., Oliveira A.S.B., Neder J.A., Nery L.E. Efect of L-carnitine on exercise performance in patients with mitochondrial myopathy. Brazilian J. of Med. and Biol. Res. 2015;48(4):354–362. doi: 10.1590/1414-431X20143467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh S., Goldstein A., Koenig M.K., Scaglia F., Enns G.M., Saneto R., Anselm I., Cohen B.H., Falk M.J., Greene C., Gropman A.L., Haas R., Hirano M., Morgan P., Sims K., Tarnopolsky M., Van Hove J.L., Wolfe L., DiMauro S. Diagnosis and management of mitochondrial disease: a consensus statement from the Mitochondrial Medicine Society. Genet. Med. 2015 Sep;17(9):689–701. doi: 10.1038/gim.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parikh S., Goldstein A., Karaa A., Koenig M.K., Anselm I., Brunel-Guitton C., Christodoulou J., Cohen B.H., Dimmock D., Enns G.M., Falk M.J., Feigenbaum A., Frye R.E., Ganesh J., Griesemer D., Haas R., Horvath R., Korson M., Kruer M.C., Mancuso M., McCormack S., Raboisson M.J., Reimschisel T., Salvarinova R., Saneto R.P., Scaglia F., Shoffner J., Stacpoole P.W., Sue C.M., Tarnopolsky M., Van Karnebeek C., Wolfe L.A., Cunningham Z.Z., Rahman S., Chinnery P.F. Patient care standards for primary mitochondrial disease: a consensus statement from the mitochondrial medicine society. Genet Med. 2017 Jul:27. doi: 10.1038/gim.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z., Klipfell E., Bennett B.J., Koeth R., Levison B.S., Dugar B., Feldstein A.E., Britt E.B., Fu X., Chung Y.M., Wu Y., Schauer P., Smith J.D., Allayee H., Tang W.H., DiDonato J.A., Lusis A.J., Hazen S.L. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011 Apr 7;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koeth R.A., Wang Z., Levison B.S., Buffa J.A., Org E., Sheehy B.T., Britt E.B., Fu X., Wu Y., Li L., Smith J.D., DiDonato J.A., Chen J., Li H., Wu G.D., Lewis J.D., Warrier M., Brown J.M., Krauss R.M., Tang W.H., Bushman F.D., Lusis A.J., Hazen S.L. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013 May;19(5):576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang W.H., Wang Z., Levison B.S., Koeth R.A., Britt E.B., Fu X., Wu Y., Hazen S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013;368(17):1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heianza Y., Ma W., Manson J.E., Rexrode K.M., Qi LHeianza Y., Ma W., Manson J.I., Rexrode K.M. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: A systematic review and meta-analysis of prospective studies. J. Am. Heart Assoc. 2017 Jun 29;6(7) doi: 10.1161/JAHA.116.004947. (pii: e004947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qi J., You T., Li J., Pan T., Xiang L., Han Y., Zhu L. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. J. Cell. Mol. Med. 2017 Aug 7 doi: 10.1111/jcmm.13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiattarella G.G., Sannino A., Toscano E., Giugliano G., Gargiulo G., Franzone A., Trimarco B., Esposito G., Perrino C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. European Heart J. 2017 Oct 14;38(39):2948–2956. doi: 10.1093/eurheartj/ehx342. [DOI] [PubMed] [Google Scholar]
- 18.Seldin M.M., Meng Y., Qi H., Zhu W., Wang Z., Hazen S.L., Lusis A.J. Shih DM Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J. Am. Heart Assoc. 2016 Feb 22;5(2) doi: 10.1161/JAHA.115.002767. (pii: e002767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miao J., Ling A.V., Manthena P.V., Gearing M.E., Graham M.J., Crooke R.M., Croce K.J., Esquejo R.M., Clish C.B., Morbid Obesity Study Group, Vicent D., Biddinger S.B. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat. Commun. 2015 Apr 7;6:6498. doi: 10.1038/ncomms7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warrier M., Shih D.M., Burrows A.C., Ferguson D., Gromovsky A.D., Brown A.L., Marshall S., McDaniel A., Schugar R.C., Wang Z., Sacks J., Rong X., Vallim T.A., Chou J., Ivanova P.T., Myers D.S., Brown H.A., Lee R.G., Crooke R.M., Graham M.J., Liu X., Parini P., Tontonoz P., Lusis A.J., Hazen S.L., Temel R.E., Brown J.M. The TMAO-generating enzyme Flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep. 2015 Jan 14;12:036. doi: 10.1016/j.celrep.2014.12.036. (pii: S2211–1247(14)01065–1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schugar R.C., Shih D.M., Warrier M., Helsley R.N., Burrows A., Ferguson D., Brown A.L., Gromovsky A.D., Heine M., Chatterjee A., Li L., Li X.S., Wang Z., Willard B., Meng Y., Kim H., Che N., Pan C., Lee R.G., Crooke R.M., Graham M.J., Morton R.E., Langefeld C.D., Das S.K., Rudel L.L., Zein N., McCullough A.J., Dasarathy S., Tang W.H.W., Erokwu B.O., Flask C.A., Laakso M., Civelek M., Naga Prasad S.V., Heeren J., Lusis A.J., Hazen S.L., Brown J.M. The TMAO-producing enzyme Flavin-containing monooxygenase 3 regulates obesity and the Beiging of white adipose tissue. Cell Rep. 2017 Jun 20;19(12):2451–2461. doi: 10.1016/j.celrep.2017.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu W., Gregory J.C., Org E., Buffa J.A., Gupta N., Wang Z., Li L., Fu X., Wu Y., Mehrabian M., Sartor R.B., McIntyre T.M., Silverstein R.L., Tang W.H.W., DiDonato J.A., Brown J.M., Lusis A.J., Hazen S.L. Gut microbial metabolite TMAO enhances platelet Hyperreactivity and thrombosis risk. Cell. 2016 Mar 24;165(1):111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X.S., Obeid S., Klingenberg R., Gencer B., Mach F., Räber L., Windecker S., Rodondi N., Nanchen D., Muller O., Miranda M.X., Matter C.M., Wu Y., Li L., Wang Z., Alamri H.S., Gogonea V., Chung Y.M., Tang W.H., Hazen S.L., Lüscher T.F. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur. Heart J. 2017 Mar 14;38(11):814–824. doi: 10.1093/eurheartj/ehw582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu W., Wang Z., Tang W.H.W., Hazen S.L. Gut microbe-generated trimethylamine N-oxide from dietary choline is Prothrombotic in subjects. Circulation. 2017 Apr 25;135(17):1671–1673. doi: 10.1161/CIRCULATIONAHA.116.025338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z., Roberts A.B., Buffa J.A., Levison B.S., Zhu W., Org E., Gu X., Huang Y., Zamanian-Daryoush M., Culley M.K., DiDonato A.J., Fu X., Hazen J.E., Krajcik D., DiDonato J.A., Lusis A.J., Hazen S.L. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015 Dec 17;163(7):1585–1589. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., Voelkerding K., Rehm H.L. ACMG laboratory quality assurance committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the association for molecular pathology. Genet Med. 2015 May;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z., Levison B.S., Hazen J.E., Donahue L., Li X.M., Hazen S.L. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal. Biochem. 2014 Jun 15;455 doi: 10.1016/j.ab.2014.03.016. (35–4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walter J.H. L-carnitine in inborn errors of metabolism: what is the evidence? J. Inherit. Metab. Dis. 2003;26(2–3):181–188. doi: 10.1023/a:1024485117095. [DOI] [PubMed] [Google Scholar]
- 29.Winter S.C. Treatment of carnitine deficiency. J. Inherit. Metab. Dis. 2003;26(2–3):171–180. doi: 10.1023/a:1024433100257. [DOI] [PubMed] [Google Scholar]
- 30.Nasser M., Javaheri H., Fedorowicz A., Noorani Z. Carnitine supplementation for inborn errors of metabolism. Cochrane Database Syst. Rev. 2012;2 doi: 10.1002/14651858.CD006659.pub3. (CD006659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller M.J., Bostwick B.L., Kennedy A.D., Donti T.R., Sun Q., Sutton V.R., Elsea S.H. Chronic oral L-carnitine supplementation drives marked plasma TMAO elevations in patients with organic Acidemias despite dietary meat restrictions. JIMD Rep. 2016;30:39–44. doi: 10.1007/8904_2016_539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koeth R.A., Wang Z., Levison B.S., Buffa J.A., Org E., Sheehy B.T., Britt E.B., Fu X., Wu Y., Li L., Smith J.D., Donato J.A., Chen J., Li H., Wu G.D., Lewis J.D., Warrier M., Brown J.M., Krauss R.M., Wilson Tang W.H., Bushman F.D., Lusis A.J., Hazen S.L. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19(5):576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu K.Y., Xia G.H., Lu J.Q., Chen M.X., Zhen X., Wang S., You C., Nie J., Zhou H.W., Yin J. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci. Rep. 2017 May 3;7(1):1445. doi: 10.1038/s41598-017-01387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomlinson J.A.P., Wheeler D.C. The role of trimethylamine N-oxide as a mediator of cardiovascular complications in chronic kidney disease. Kidney Int. 2017 Oct;92(4):809–815. doi: 10.1016/j.kint.2017.03.053. (Epub 2017 Aug 12) [DOI] [PubMed] [Google Scholar]