Abstract
Abiotrophia defectiva, also known as nutritionally variant streptococcus, is part of the normal flora of the oral cavity and urogenital and intestinal tracts and is a rare cause of infective endocarditis. It is fastidious or difficult to culture and associated with high rates of septic embolization, treatment failure and mortality. We describe an unusual presentation of infective endocarditis with severe mitral valve regurgitation due to Abiotrophia defectiva in an immunocompetent patient. After a complicated hospital course, surgical replacement of both the mitral and aortic valves was performed. We suggest that this patient likely had subacute infective endocarditis before diagnosis and treatment of her urinary tract infection, and following treatment failure, she developed life-threatening infective endocarditis. This case report highlights that patients with Abiotrophia defectiva infections are at high risk for infective endocarditis and that the clinical progression from this infection can be slow, with difficulty isolating the pathogen, which can significantly impact patient outcome.
Keywords: Cardiovascular, Abiotrophia defectiva, infective endocarditis, septic shock
Introduction
Abiotrophia defectiva (AD) infections are associated with high rates of septic embolization, treatment failure and mortality.1–3 We describe an unusual presentation of infective endocarditis (IE) due to AD, complicated by sepsis, severe valvulopathy and pulmonary hypertension, with characteristic septic embolization to the spleen and lower extremity. Surgical replacement of both the mitral and aortic valves was required. Intensivists and cardiac anesthesiologists, as well as other clinical providers and specialists involved in the management of these complicated cases, should be aware of this rare but potentially fatal infection and consider it as a cause of acute deterioration and organ failure in at-risk patients.
Case report
A 53-year-old immunocompetent female presented with a 1-week history of dizziness, worsening shortness of breath and increasing chest pressure. Prior to admission, she was diagnosed with a urinary tract infection, culture positive for AD. This was initially treated with sulfamethoxazole-trimethoprim 800/160 mg twice per day for a week followed by an intramuscular injection of ceftriaxone and oral ciprofloxacin 500 mg twice per day for 7 days.
On admission to hospital, she was lethargic, vital signs recorded were temperature of 36.7°C, blood pressure of 104/88 mmHg, heart rate of 175 beats per minute, respiratory rate of 21 breaths per minute and oxygen saturation of 98% on room air. Chest radiography was unremarkable and electrocardiogram showed atrial fibrillation with a rapid ventricular rate to 180 beats per minute. Brain natriuretic peptide was elevated at 819 pg/mL, initial troponin I value was 0.1 ng/mL with a repeat value of 0.34 ng/mL and a heparin infusion was started for presumed acute coronary syndrome as well as a diltiazem infusion, with improvement in heart rate control. Further workup revealed a lactic acidosis, acute kidney injury with creatinine 1.62 mg/dL (baseline 0.8 mg/dL) and thrombocytopenia to 29,000/mm3. Transthoracic echocardiogram showed a thickened and restricted mitral valve with a large vegetation (21 mm × 19 mm) on the posterior leaflet (Figure 1), with partial flail and severe regurgitation. The vegetation was seen to be obstructing inflow into the left ventricle with associated stenotic physiology. The aortic valve was also thickened with perforation of the non-coronary cusp and the ejection fraction was normal. Right ventricular systolic pressure was severely elevated at 70–80 mmHg with evidence of right ventricular overload.
Figure 1.
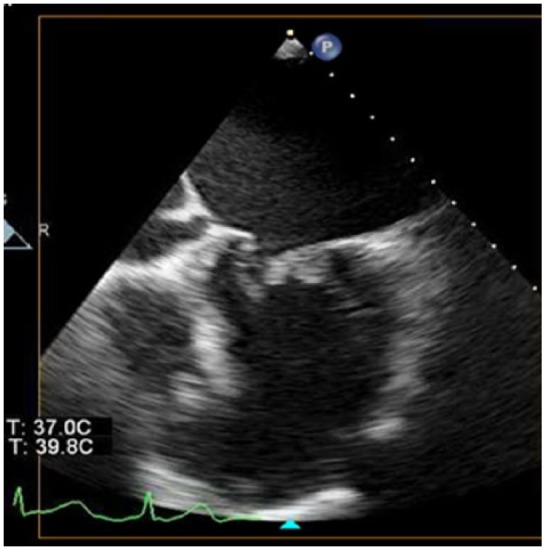
Transthoracic echocardiographic image with large vegetation attached to the posterior leaflet of the mitral valve.
Empiric vancomycin and piperacillin/tazobactam were started and subsequently changed within 24 h to ampicillin 2 g every 4 h and gentamycin 3 mg/kg every 24 h, in view of blood cultures growing gram-positive bacilli. Surgical intervention was deferred due to severe thrombocytopenia and bacteremia.
One week after admission, she developed acute heart failure, requiring emergent intubation and mechanical ventilation. Ampicillin was switched to ampicillin/sulbactam 3 g every 6 h with gentamycin and daptomycin 6 mg/kg, for better anerobic and resistant gram-positive coverage. This was followed by sudden onset septic embolization to the left lower limb causing critical ischemia, as well as new splenic infarcts (Figure 2), managed with left lower extremity thrombectomy and four-compartment fasciotomy. Blood cultures continued to show no definitive pathogen. Given the severe valvulopathy and metastatic septic embolization, surgical replacement of both the mitral and aortic valves was performed.
Figure 2.
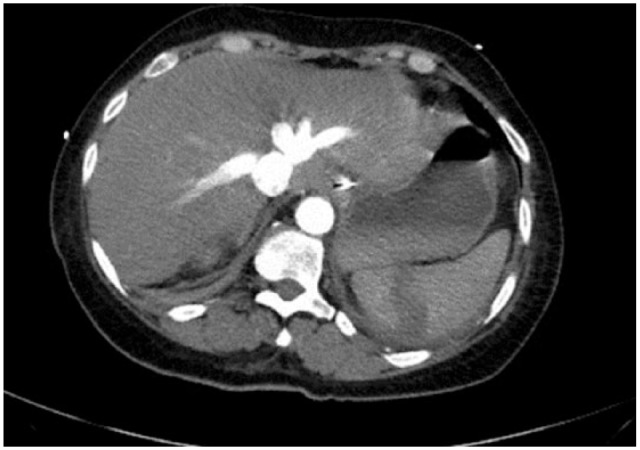
Computer tomographic image with splenic infarct due to septic embolization.
Intraoperative transesophageal echocardiogram showed an enlarged left atrium measuring 5.7 cm with thrombi present in both left and right atrial appendages (Figure 3). The aortic valve appeared thinned with evidence of severe aortic insufficiency. The posterior leaflet of the mitral valve was eroded by the large vegetation, and there were micro-abscesses in the posterior annulus and nodular extension up the endocardium into the left atrium. As pulmonary artery pressures remained elevated after mitral valve replacement, inhaled epoprostenol was initiated with good effect and weaned slowly.
Figure 3.
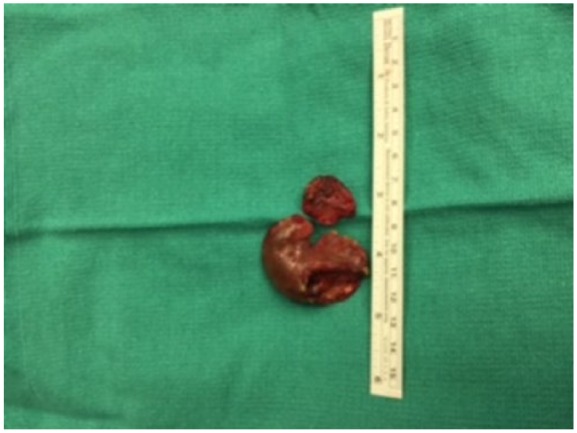
Photograph of thrombus removed from left atrial appendage.
Postoperative course was uncomplicated. Blood culture pathogen identification showed AD in addition to Gram-positive bacilli (cereus/thuringiensis, magaterium and subtilis) from initial blood cultures on admission. The patient was discharged from the intensive care unit after 2 weeks on broad spectrum antibiotics for a total of 6 weeks.
Discussion
Organisms of the genus Abiotrophia were first classified as nutritionally variant streptococci (NVS) in 1961.3 abiotrophia defectiva has been associated with life-threatening infections such as bacteremia, osteomyelitis, brain abscess, pancreatic abscess, septic arthritis and crystalline keratopathy.2,4
A 2001 study of 97 patients, by Christensen and Facklam,5 showed that IE was reported in 58% of patients, while in 26% of patients, septicemia or bacteremia was given as the clinical diagnosis. Endocarditis caused by NVS is implicated in 5%–6% of all streptococcal endocarditis cases, with <1% of all cases caused by abiotrophia defectiva.3,6
The reason that this pathogen has a higher affinity for the endocardium is due to its ability to secrete exopolysaccharide, enabling it to adhere to fibronectin in the extracellular matrix.7 In these cases, IE progresses slowly, but despite its sensitivity to antimicrobials, approximately 50% of patients need surgical management, as was the case with our patient.8
The identification of Abiotrophia is difficult because of its growth requirement and its confusing Gram stain appearance. It needs a high clinical suspicion and may be a diagnostic challenge that may require more advanced diagnostics modalities such as polymerase chain reaction (PCR) and/or matrix-assisted laser desorption ionization time-of-flight mass spectrophotometry (MALDI-TOF-MS). MALDI-TOF-MS is a relatively simple and inexpensive method of identification.2 At our institution, we do not offer these testing procedures, and therefore, this was a “send out” test to another institution which used MALDI-TOF-MS method of identification. Given that this identification was from two different blood samples, this was a hematogenous infection rather than merely colonization. If the identification had been from just one sample, it more likely would have been less significant and thus merely represented colonization. Also, the Bacillus cereus, Bacillus thuringiensis, Bacillus magaterium and Bacillus subtilis identification was from a single sample, and therefore, it was interpreted as probably caused by contamination from skin or perhaps an uncleaned sample bottle top.
Treatment failure rates as high as 41% occur despite the use of appropriate antibiotics.9 A mortality rate of up to 17% has been reported in the literature, which is high when compared to mortality rates from endocarditis caused by Viridans streptococci (0%–12%) and other pathogens.10 Most reported deaths are due to acute heart failure or major systemic embolization, both of which were manifested in our patient.
AD endocarditis predominantly occurs in the setting of pre-existing heart disease (90% of cases), and prosthetic heart valves are involved in 10% of patients.3,8 However, neither of these risk factors were present in our patient.
The American Heart Association guidelines recommend use of the same treatment as for Enterococcus endocarditis, that is, a combination of ampicillin or benzylpenicillin plus gentamycin for a period of 4–6 weeks.9 Alternatively, vancomycin can be used alone for 6 weeks in patients with a penicillin allergy. Ceftriaxone combined with gentamycin is an alternative option.10–12
Specific therapeutic dosing guidelines recommend 18–30 million units of penicillin per 24 h divided into six doses or 12 g of ampicillin per 24 h intravenously, divided into six doses, in addition to intravenous gentamycin at 3 mg/kg/24 h divided into three doses for 4–6 weeks. Vancomycin represents a good therapeutic choice in patients who have shown a poor response to penicillin/aminoglycoside combination therapy.13,14
The portal of entry for systemic bacteremia in our patient was most likely the genitourinary tract. Abiotrophia species are generally considered to be part of the normal flora of the urogenital and oropharyngeal tracts and can also be found in the gastrointestinal tract.11 Our patient was diagnosed with a urinary tract infection caused by abiotrophia defectiva in the weeks preceding presentation and acute deterioration with signs and symptoms of IE and cardiogenic and septic shock. Given that workup for her clinical deterioration showed hematogenous spread of her known Abiotrophia urinary tract infection and that IE was found to be the cause of her hemodynamic decompensation, it is a logical conclusion that the urinary presence of the bacterium was the primary cause of the IE. Although as stated previously, Abiotrophia species is also considered normal flora of the oropharyngeal tract, and there were no clinical signs or symptoms to suggest an oropharyngeal source (e.g. dental abscess, tonsillitis, upper respiratory tract infection). Also, this patient was not immunocompromised and did not have any history of congenital heart disease such as bicuspid aortic valve or prosthetic heart valve placement at the time of initial presentation. In our case, open heart surgery with complex anesthesia management due to sepsis and hemodynamic deterioration was required for definitive treatment, which was followed by an uncomplicated postoperative course.
Conclusion
This case report highlights that patients with known AD infections are at high risk for IE with severe cardiorespiratory compromise, even in the absence of known risk factors. Cardiac anesthesiologists and clinical specialists involved in the care of these patients, such as neurologists, hospitalists and infectious disease consultants, should be aware of this rare but potentially fatal infection, which may present a significant anesthetic challenge, and consider it as a cause of acute deterioration and organ failure in at-risk patients in the intensive care unit.
Acknowledgments
Presented at the 47th Annual Congress Society of Critical Care Medicine, Sepsis Research Snapshot Theater, Henry B Gonzalez Convention Center, San Antonio, Texas (February 2018). Published in abstract form as Foley ED, Castresana MR, et al. Abiotrophia defectiva a rare cause of infective endocarditis of the mitral valve. Crit Care Med 2018; 46(1): 716.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent: Written informed consent was obtained from the patient for their anonymized information to be published in this article
References
- 1. Ruoff KL. Nutritionally variant streptococci. Clin Microbiol Rev 1991; 4: 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holler JG, Pedersen LK, Calum H, et al. Using MALDI-TOF mass spectrometry as a rapid and accurate diagnostic tool in infective endocarditis: a case report of a patient with mitral valve infective endocarditis caused by Abiotrophia defective. Scand J Inf Dis 2011; 43: 234–237. [DOI] [PubMed] [Google Scholar]
- 3. Frenkel A, Hirsch W. Spontaneous development of L forms of streptococci requiring secretions of other bacteria or sulphydryl compounds for normal growth. Nature 1961; 191: 728–730. [DOI] [PubMed] [Google Scholar]
- 4. Roberts RB, Krieger AG, Schiller NL, et al. Viridans streptococcal endocarditis: The role of various species, including pyridoxal-dependent streptococci. Rev Infect Dis 1979; 1: 955–966. [DOI] [PubMed] [Google Scholar]
- 5. Christensen JJ, Facklam RR. Granulicatella and Abiotrophia species from human clinical specimens. J Clin Microbiol 2001; 39(10): 3520–3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heath CH, Bowen SF, McCarthy JS, et al. Vertebral osteomyelitis and discitis associated with Abiotrophia adiacens (nutritionally variant streptococcus) infection. Aust N Z J Med 1998; 28: 663. [DOI] [PubMed] [Google Scholar]
- 7. Carle MA, Giudice AD, Viglietti R, et al. Aortic valve endocarditis caused by Abiotrophia defectiva: case report and literature overview. In Vivo 2015; 29: 515–518. [PubMed] [Google Scholar]
- 8. Kiernan TJ, O’Flaherty N, Gilmore R, et al. Abiotrophia defective endocarditis and associated hemophagocytic syndrome—a first case report and review of the literature. Int J Infect Dis 2008; 12: 478–482. [DOI] [PubMed] [Google Scholar]
- 9. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15): 1435–1486. [DOI] [PubMed] [Google Scholar]
- 10. Tuazon CU, Gill V, Gill F. Streptococcal endocarditis: single vs combination antibiotic therapy and role of various species. Rev Infect Dis 1986; 8(1): 54–60. [DOI] [PubMed] [Google Scholar]
- 11. Alberti MO, Hindler JA, Humphries RM. Antimicrobial susceptibilities of Abiotrophia defectiva, Granulicatella adiacens, and Granulicatella elegans. Antimicrob Agents Chemother 2015; 1460(3): 1411–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zheng X, Freeman AF, Villafranca J, et al. Antimicrobial susceptibilities of invasive pediatric Abiotrophia and Granulicatella isolates. J Clin Microbiol 2004; 42(9): 4323–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bouvet A. Human endocarditis due to nutritionally variant streptococci: streptococcus adjacens and Streptococcus defectivus. Eur Heart J 1995; 16(Suppl. B): 24–27. [DOI] [PubMed] [Google Scholar]
- 14. Prasidthrathsint K, Fisher MA. Antimicrobial susceptibility patterns among a large, nationwide cohort of Abiotrophia and Granulicatella clinical isolates. J Clin Microbiol 2017; 55(4): 1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]


