Short abstract
Background
Cetirizine has been shown to be effective for relief of seasonal allergic rhinitis (SAR) symptoms. Allergic rhinitis symptoms have been reported to have circadian variations, with symptoms tending to be most bothersome overnight and in the morning.
Objective
To evaluate the effects of different cetirizine dosing schedules in comparison to twice daily (BID) chlorpheniramine and placebo on SAR symptoms at 12 and 24 hours postdose.
Methods
Study 1 subjects received cetirizine 10-mg once daily in the morning (QAM), cetirizine 10-mg once daily at bedtime (QHS), cetirizine 5-mg twice daily, or placebo. Study 2 subjects received cetirizine 5-mg QAM, cetirizine 10-mg QHS, chlorpheniramine 8-mg BID, or placebo. The primary end point was total symptom severity complex (TSSC); TSSC was the sum of symptom severity ratings averaged over the 2-week study period. Post hoc analyses of reflective symptom severity assessed in the morning (TSSCAM) and in the evening (TSSCPM) were conducted to evaluate cetirizine’s effects at 12 and 24 hours postdose.
Results
In study 1, subject- and investigator-assessed TSSC was significantly lower in all cetirizine groups versus placebo (P ≤ .003). In study 2, subject-assessed TSSC was significantly lower in all cetirizine groups versus placebo (P ≤ .04) and was numerically lower for investigator-assessed TSSC. Post hoc analyses demonstrated that cetirizine significantly improved TSSCAM at 12 and 24 hours postdose versus placebo in both studies regardless of dosing schedule. TSSCPM significantly improved at 12 and 24 hours postdose in all study 1 cetirizine groups versus placebo. In study 2, versus placebo, TSSCPM significantly improved at 12 hours postdose in cetirizine 5-mg QAM group and numerically improved at 24 hours postdose in cetirizine 10-mg QHS group.
Conclusion
Regardless of dosing regimen, cetirizine demonstrates effective 24-hour relief of SAR symptoms, particularly on TSSCAM, which assesses overnight and early morning symptom control.
Keywords: allergic rhinitis, antihistamine, cetirizine, chlorpheniramine, dosing time, over-the-counter, seasonal allergic rhinitis, symptom severity, treatment compliance
Introduction
Allergic rhinitis affects approximately 10% to 40% of the population worldwide.1,2 Second-generation antihistamines are the most commonly used medications for the treatment of allergy symptoms.3,4 Cetirizine is an oral second-generation H1-receptor antagonist that is available as an over-the-counter medication. Published data demonstrate that cetirizine effectively relieves the symptoms of allergic rhinitis.5–8
Studies indicate that allergic rhinitis-related symptoms have circadian variations in some patients.9–15 Of the 246 subjects with hay fever surveyed in a study, approximately 75% reported that they experienced their most troublesome symptom at its maximum intensity overnight or in the morning.11 Variations in allergic rhinitis symptom severity may be related to diurnal elevations in the levels of inflammatory mediators or cortisol.9,16
The objective of this analysis was to examine the effects of different cetirizine dosing regimens over a 2-week treatment period as well as at 12- and 24-hour postdose intervals. Presented here are the data from 2 studies in which subjects with seasonal allergic rhinitis (SAR) were randomized to treatment with cetirizine, the active comparator chlorpheniramine, or placebo. Additionally, a post hoc analysis of subject-assessed morning and evening total symptom severity complex (TSSC) is described, which evaluated the effects of cetirizine on TSSC at 12- (TSSC12) and 24 hours (TSSC24) postdose in the 2 studies.
Methods
Subjects
Subjects were ≥18 years of age in study 1 and ≥12 years of age in study 2. Included subjects were males or females with a documented history of grass pollen-related (study 1) or ragweed pollen-related (study 2) allergic rhinitis. Allergic sensitivity was verified by skin testing or a radioallergosorbent test. Subjects had to have acute seasonal exacerbations of symptoms attributable to grass pollen sensitivity, with a minimum score of 8 (excluding nasal congestion) in study 1 or ragweed pollen sensitivity, with a minimum score of 10 (excluding nasal congestion) in study 2, as assessed by the investigator rating scale at both the screening visit and study initiation. Subjects with perennial allergic rhinitis were included, provided they experienced an acute seasonal exacerbation attributable to grass (study 1) or ragweed (study 2) pollen. Study 1 was conducted at 3 centers, and study 2 was conducted at 5 centers, in various types of clinics (eg, Veterans Administration hospital, medical centers, and allergy clinics) across the United States.
Key exclusion criteria were identical for both studies. Subjects were excluded if they were taking oral steroid therapy with unstable dosing or could not stop the use of nasal steroids or cromolyn for 1 week before study initiation or the use of oral antihistamines for 72 hours before study initiation. Subjects who required treatment with drugs with antihistaminic or sedating properties were undergoing immunotherapy or intended to make major alterations in the home environment were also excluded, as were women of childbearing potential. Subjects were prohibited from using any concomitant intranasal or oral therapy for the relief of rhinitis symptoms during the studies.
Study Design
Study 1 was a multicenter, randomized, double-blind, parallel-group study comparing the efficacy and safety of 3 different cetirizine dosing regimens with placebo. Study 2 was a multicenter, randomized, double-blind, parallel-group study comparing the efficacy and safety of 2 different cetirizine dosing regimens with placebo and with an active comparator, chlorpheniramine. These studies were conducted during grass (study 1) and ragweed (study 2) seasons. Institutional review board approval was obtained before the initiation of both studies. In the case of minors, the investigator had to obtain the written consent of the parent or legal guardian of the patient and assent of the minor patient.
In study 1, subjects were randomized to receive cetirizine 10-mg once daily in the morning (QAM), cetirizine 10-mg once daily at bedtime (QHS), cetirizine 5-mg twice daily (BID), or placebo for 2 weeks. In study 2, subjects were randomized to cetirizine 5-mg QAM, cetirizine 10-mg QHS, chlorpheniramine 8-mg BID, or placebo for 2 weeks. To maintain the blind, medications were administered in a dummy-double design, with all subjects taking 2 capsules of study medication in the morning and the evening in both studies. In both studies, study visits were conducted at baseline and at the end of weeks 1 and 2.
Symptom Scores and Efficacy Assessments
Nasal congestion, itchy nose, sneezing, watery nasal discharge, itchy eyes, watery eyes, and itchy mouth were assessed in study 1. The same symptoms were assessed in study 2, with the exclusion of itchy mouth. Each symptom was rated on a 4-point scale: 0 = none, 1 = mild, 2 = moderate, and 3 = severe. Subjects rated symptoms reflectively, evaluating symptoms over the previous 12 hours, once at 10 am, and once at 10 pm every day prior to dosing using a self-assessment card. In consultation with the subject, the investigator rated symptom severity at each visit using the same scale as the subject. The TSSC was the sum of the mean daily symptom severity ratings of all individual symptoms, averaged over the treatment period. For study 1, TSSC was the sum of severity ratings of 6 symptoms, excluding nasal congestion. For study 2, TSSC was the sum of severity ratings of 5 symptoms, excluding nasal congestion. The decision to exclude nasal congestion from the TSSC was made prior to initiating the studies. The global evaluation was assigned numerical values as follows: 0 = failure/no relief, 1 = fair/some relief, 2 = good/considerable relief, and 3 = excellent/complete relief. The global evaluation was determined at the end of study by the investigator (study 1) or subject (study 2). Study end points were the subject and investigator TSSC over the entire treatment period, subject and investigator severity ratings for each individual symptom over the entire treatment period, and global evaluation of the effectiveness of study drug.
A post hoc analysis of TSSC assessed by subjects in the morning (TSSCAM) and evening (TSSCPM), 12 and 24 hours after dosing, depending on when the dose was taken, was conducted. Since symptoms were scored reflectively over the previous 12 hours, TSSCAM evaluated symptoms overnight and in the early morning while TSSCPM evaluated symptoms during the day and early evening. For the post hoc analysis, the TSSCAM and TSSCPM scores were averaged separately over the treatment period.
Safety Assessments
All adverse events (AEs) that were reported by subjects or observed by the investigators were recorded. Clinical laboratory evaluations were performed within 96 hours of study drug initiation and on the last day of therapy.
Statistical Methods
In study 1, enrollment was planned for 240 subjects (80 at each site); 204 subjects entered the study. In study 2, enrollment was planned for 300 subjects (60 at each site); 314 subjects entered the study. Subjects who took study medication for <3 days and discontinued because of reasons other than lack of response or exacerbation of symptoms were not evaluated for efficacy. All subjects who took doses of study drug for ≥3 days (≥6 doses) were evaluated for efficacy. Subjects who discontinued early prior to the third day because of lack of adequate response were included in the analysis using the initial severity score or last valid ratings carried forward to the subsequent missing week. Subjects who took ≥1 dose of study medication were evaluated for safety. Both subject-rated and investigator-rated TSSC and individual symptoms were compared among treatment groups using linear models that included center, treatment, evaluation week, and AM/PM (subject analysis only) as main effects, along with first- and second-order interactions, with the initial severity score by an investigator as a covariate. The sum of squares for each effect was computed using SAS PROC GLM (Release 85.5; SAS Institute, Cary, NC) and was used to compute the required F statistics. Baseline covariates and any interaction effects were statistically significant if the F statistic associated with the effect was as large as the 10% critical point of the F distribution. The overall treatment effect was declared significant if the associated F statistic was as large as the 5% critical point. When the overall treatment effect was declared significant, pairwise comparisons of treatments were made based on the main effects model, including terms for center, treatment, evaluation week, and AM/PM (subject analysis only), with initial investigator-rated severity score as a covariate (at the 5% level of significance). This controls the familywise error rate at the 5% level under the configuration, for example, that all population means are equal.
The global assessment of therapeutic effect was compared among treatment groups using the generalized Mantel–Haenszel statistic stratified by study center. Overall treatment differences were statistically significant if the observed value of the statistic was as large as the 5% critical point of the χ2 distribution. The investigator baseline symptom ratings were used as an initial covariate for both investigator and subject ratings. The proportion of subjects reporting somnolence was compared among treatment groups using a likelihood ratio χ2 test. The post hoc analysis of subject-assessed TSSCAM and TSSCPM was performed using an analysis of covariance model, with terms for treatment and center and investigator-assessed baseline score as covariates. Each post hoc test was made at the 5% significance level.
Results
Subject Demographics and Baseline Characteristics
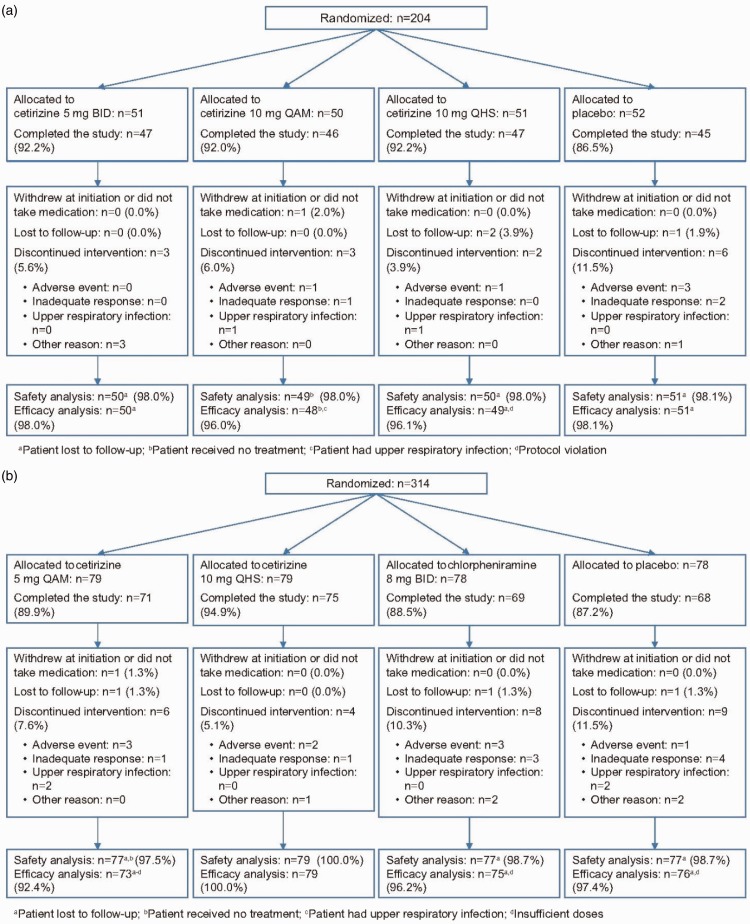
For study 1, 204 subjects were randomized. Of these subjects, 198 were included in the efficacy assessments; 3 subjects were lost to follow-up, 1 withdrew at initiation, 1 had an upper respiratory tract infection throughout the study, and 1 was in violation of the protocol. The latter 2 subjects were included in the safety analysis (n = 200). The median treatment duration was 15 days in the cetirizine 10-mg QAM (range 5, 21), cetirizine 10-mg QHS (range 4, 22), and cetirizine 5-mg BID (range 8, 20) groups; placebo was administered a median 15 days (range 5, 22). For study 2, 314 subjects were randomized. Of these subjects, 303 were included in the efficacy assessments; 3 subjects were lost to follow-up, 1 withdrew early without taking any study medication, 6 discontinued early because of AEs, and 1 discontinued because of an upper respiratory tract infection. The 6 subjects who discontinued early because of AEs and the 1 subject who discontinued because of an upper respiratory infection were included in the safety analysis (n = 310). The median treatment duration was 15 days in the cetirizine 5-mg QAM (range 3, 20), cetirizine 10-mg QHS (range 5, 21), chlorpheniramine 8-mg BID (range 2, 23), and placebo (range 6, 23) treatment groups. Patient disposition during the studies is shown in Figure 1(a) and (b).
Figure 1.
Disposition of patients in (a) study 1 and (b) study 2. BID, twice-daily; QAM, once-daily morning; QHS, once-daily bedtime.
At baseline, there were no significant differences among treatment groups except for race in study 1 (P = .02) and weight in men in study 2 (P = .02; Table 1); these differences were not considered clinically relevant. At baseline, the severity of each individual SAR symptom and TSSC were similar among the treatment groups for each study (Table 1).
Table 1.
Subject Demographics and Investigator-assessed Symptom Severity at Baseline.
| Demographicsa |
Study 1 |
Study 2 |
||||||
|---|---|---|---|---|---|---|---|---|
| Placebo |
Cetirizine |
Placebo |
Cetirizine |
Chlorphen-iramine | ||||
| 10-mg QAM | 10-mg QHS | 5-mg BID | 5-mg QAM | 10-mg QHS | 8-mg BID | |||
| (n = 51) | (n = 49) | (n = 50) | (n = 50) | (n = 77) | (n = 77) | (n = 79) | (n = 77) | |
| Age, y, mean ± SD | 40.1 ± 14.3 | 38.5 ± 10.8 | 40.5 ± 14.0 | 41.6 ± 11.8 | 35.0 ± 12.5 | 30.3 ± 13.5 | 29.9 ± 11.6 | 31.7 ± 13.8 |
| Raceb,c | ||||||||
| White, n (%) | 45 (88.2) | 49 (100.0) | 49 (98.0) | 47 (94.0) | 74 (96.1) | 71 (92.2) | 74 (93.7) | 71 (92.2) |
| Other, n (%) | 6 (11.8) | 0 | 1 (2.0) | 3 (6.0) | 3 (3.9) | 5 (6.5) | 5 (6.3) | 6 (7.8) |
| Male/female, n (%) | 28/23 (54.9/45.1) | 25/24 (51.0/49.0) | 31/19 (62.0/38.0) | 31/19 (62.0/38.0) | 64/13 (83.1/16.9) | 61/16 (79.2/20.8) | 67/12 (84.8/15.2) | 62/15 (80.5/19.5) |
| Symptoms,d mean ± SD | (n = 51) | (n = 48) | (n = 49) | (n = 50) | (n = 76) | (n = 73) | (n = 79) | (n = 75) |
| Itchy nose | 2.18 ± 0.84 | 1.96 ± 0.74 | 2.02 ± 0.75 | 1.82 ± 0.77 | 1.92 ± 0.89 | 1.77 ± 0.91 | 1.81 ± 0.85 | 1.95 ± 0.84 |
| Sneezing | 2.20 ± 0.57 | 2.13 ± 0.79 | 2.12 ± 0.83 | 1.98 ± 0.82 | 2.12 ± 0.78 | 2.01 ± 0.90 | 1.96 ± 0.81 | 2.20 ± 0.87 |
| Runny nose | 2.20 ± 0.75 | 2.29 ± 0.71 | 2.15 ± 0.74 | 2.02 ± 0.84 | 2.21 ± 0.77 | 2.23 ± 0.81 | 2.08 ± 0.83 | 2.29 ± 0.80 |
| Itchy eyes | 1.90 ± 0.90 | 1.87 ± 1.00 | 2.00 ± 0.91 | 2.08 ± 0.83 | 2.15 ± 0.77 | 1.97 ± 0.93 | 2.08 ± 0.86 | 1.91 ± 0.86 |
| Watery eyes | 1.47 ± 0.97 | 1.54 ± 1.09 | 1.53 ± 1.04 | 1.92 ± 0.92 | 1.85 ± 0.83 | 1.62 ± 1.01 | 1.91 ± 0.83 | 1.68 ± 0.84 |
| Itchy palatee | 1.12 ± 1.13 | 1.35 ± 1.21 | 1.29 ± 1.22 | 1.04 ± 0.99 | n/a | n/a | n/a | n/a |
| Nasal congestion | 2.04 ± 0.77 | 2.08 ± 0.68 | 1.94 ± 0.90 | 1.92 ± 0.63 | 2.33 ± 0.66 | 2.27 ± 0.71 | 2.39 ± 0.74 | 2.44 ± 0.72 |
| TSSCf | 11.06 ± 2.49 | 11.15 ± 2.90 | 11.10 ± 2.81 | 10.86 ± 2.54 | 10.26 ± 2.19 | 9.60 ± 1.88 | 9.84 ± 1.89 | 10.03 ± 2.16 |
Abbreviations: BID, twice-daily; n/a, not applicable; QAM, once-daily morning; QHS, once-daily bedtime; SD, standard deviation; TSSC, total symptom severity complex.
aSafety evaluable subjects.
bSignificant difference among treatment groups in study 1 (P = .02).
cMissing value for 1 subject treated with 5-mg QAM in study 2.
dEfficacy evaluable subjects.
eNot assessed in study 2.
fTSSC is the sum of individual symptoms, excluding nasal congestion.
TSSC Over 2 Weeks
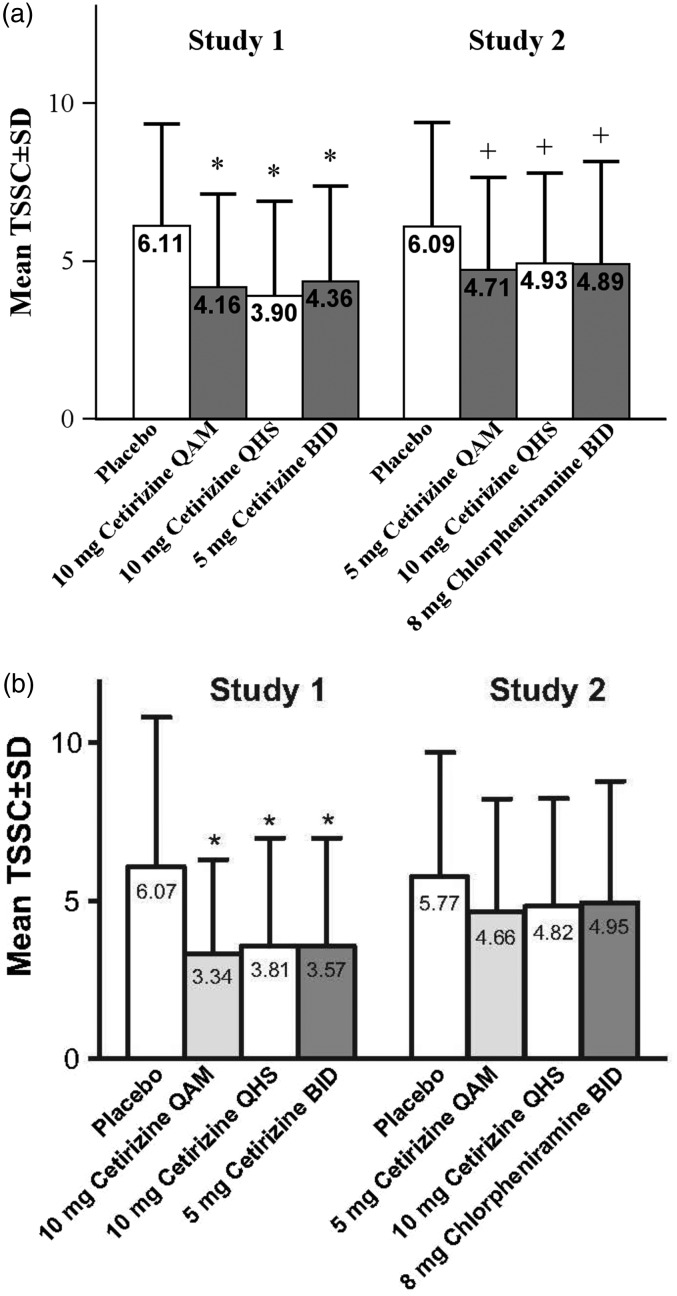
In study 1, cetirizine was significantly more efficacious at reducing TSSC versus placebo (P ≤ .003) in all 3 dosing groups when assessed by both subject (Figure 2(a)) and investigator (Figure 2(b)). There were no significant differences among the cetirizine treatment groups. In study 2, the 2 cetirizine groups and the active comparator group had a significantly lower subject-assessed TSSC versus placebo (P ≤ .04; Figure 2(a)). Investigator-assessed TSSC for the active treatment groups was numerically lower versus placebo (Figure 2(b)); however, this difference was not statistically significant.
Figure 2.
Observed mean TSSC assessed by (a) subject and (b) investigator. TSSC is the sum of 6 symptoms in study 1 and 5 symptoms in study 2; TSSC does not include nasal congestion in either study. *P ≤ .003 versus placebo. †P ≤ .04 versus placebo. BID, twice-daily; QAM, once-daily morning; QHS, once-daily bedtime; SD, standard deviation; TSSC, total symptom severity complex.
Individual Symptom Severity Ratings
In study 1, both subject- and investigator-rated severity for each of the 7 individual symptoms demonstrated that cetirizine was more efficacious in improving most individual SAR-related symptoms (Table 2). When assessed by the subject, all 3 cetirizine dosing regimens were significantly more efficacious compared with placebo (P < .05) in alleviating sneezing, runny nose, itchy nose, itchy eyes (except the 5-mg BID group), and watery eyes (except the 5-mg BID group). Similarly, when assessed by the investigator, all 3 cetirizine dosing regimens were significantly more effective than placebo (P < .05) in alleviating sneezing, runny nose, itchy nose (except the 10-mg QAM group), itchy eyes, and watery eyes (except the 5-mg BID group and the 10-mg QAM group). There were no significant differences among the 3 cetirizine groups for relieving any individual symptom.
Table 2.
Observed Mean Symptom Severity Ratings for the Entire Treatment Period as Assessed by Subject and Investigator.a
|
Study 1 |
Study 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Symptom, mean ± SD | Placebo |
Cetirizine |
Cetirizine |
Chlorphen-iramine | ||||
| 10-mgQAM | 10-mg QHS | 5-mg BID | Placebo | 5-mg QAM | 10-mg QHS | 8-mg BID | ||
| (n = 45/40)b | (n = 46/45)b | (n = 45/43)b | (n = 46/41)b | (n = 71/68)b | (n = 72/68)b | (n = 76/69)b | (n = 71/65)b | |
| Itchy nose | ||||||||
| Subject | 1.23 ± 0.70 | 0.87 ± 0.65c | 0.77 ± 0.60c | 0.81 ± 0.64c | 1.34 ± 0.78 | 0.97 ± 0.71c | 1.07 ± 0.79 | 1.07 ± 0.80c |
| Investigator | 1.07 ± 0.90 | 0.76 ± 0.81 | 0.74 ± 0.80c | 0.60 ± 0.77c | 1.28 ± 0.96 | 1.01 ± 0.95 | 1.06 ± 0.96 | 1.04 ± 0.94 |
| Sneezing | ||||||||
| Subject | 1.18 ± 0.65 | 0.71 ± 0.61c | 0.56 ± 0.54c | 0.64 ± 0.57c | 1.20 ± 0.76 | 0.82 ± 0.62c | 0.85 ± 0.62c | 0.96 ± 0.73c |
| Investigator | 1.21 ± 1.08 | 0.56 ± 0.81c | 0.43 ± 0.71c | 0.51 ± 0.74c | 1.15 ± 1.04 | 0.80 ± 0.84 | 0.90 ± 0.89 | 0.98 ± 0.93 |
| Runny nose | ||||||||
| Subject | 1.29 ± 0.68 | 0.95 ± 0.69c | 0.89 ± 0.64c | 0.81 ± 0.56c | 1.38 ± 0.76 | 1.16 ± 0.68 | 1.15 ± 0.68 | 1.16 ± 0.77 |
| Investigator | 1.27 ± 1.04 | 0.79 ± 0.87c | 0.77 ± 0.87c | 0.62 ± 0.73c | 1.25 ± 0.99 | 1.17 ± 0.94 | 1.18 ± 0.94 | 1.16 ± 1.05 |
| Itchy eyes | ||||||||
| Subject | 1.14 ± 0.72 | 0.71 ± 0.63c | 0.76 ± 0.74c | 0.94 ± 0.78 | 1.21 ± 0.80 | 0.96 ± 0.79 | 1.04 ± 0.73 | 0.92 ± 0.81 |
| Investigator | 1.18 ± 1.04 | 0.66 ± 0.84c | 0.80 ± 0.91c | 0.87 ± 0.98c | 1.20 ± 1.00 | 0.92 ± 0.97 | 0.99 ± 0.96 | 0.93 ± 0.97 |
| Watery eyes | ||||||||
| Subject | 0.82 ± 0.67 | 0.52 ± 0.56c | 0.54 ± 0.75c | 0.75 ± 0.73 | 0.96 ± 0.76 | 0.79 ± 0.74 | 0.82 ± 0.69 | 0.79 ± 0.74 |
| Investigator | 0.86 ± 0.95 | 0.37 ± 0.59c | 0.61 ± 0.85 | 0.71 ± 0.91 | 0.93 ± 0.91 | 0.75 ± 0.90 | 0.69 ± 0.82 | 0.83 ± 0.95 |
| Itchy palated | ||||||||
| Subject | 0.50 ± 0.67 | 0.29 ± 0.45 | 0.39 ± 0.55 | 0.42 ± 0.55 | n/a | n/a | n/a | n/a |
| Investigator | 0.46 ± 0.78 | 0.22 ± 0.58 | 0.43 ± 0.73 | 0.27 ± 0.57 | n/a | n/a | n/a | n/a |
| Nasal congestion | ||||||||
| Subject | 1.53 ± 0.75 | 1.41 ± 0.84 | 1.35 ± 0.60 | 1.31 ± 0.68 | 1.79 ± 0.74 | 1.67 ± 0.72 | 1.67 ± 0.75 | 1.71 ± 0.82 |
| Investigator | 1.39 ± 1.00 | 1.27 ± 0.95 | 1.14 ± 0.90 | 1.20 ± 0.91 | 1.71 ± 0.88 | 1.63 ± 0.89 | 1.62 ± 0.92 | 1.68 ± 0.97 |
Abbreviations: BID, twice-daily; n/a, not applicable; QAM, once-daily morning; QHS, once-daily bedtime; SD, standard deviation.
aStatistical analyses were conducted based on the main effects model, including terms for center, treatment, evaluation week, and AM/PM (subject analysis only), with the initial severity score assessed by investigator as a covariate.
bSubject numbers are listed as (subject/investigator) for each assessment, respectively, and varied slightly among the different symptoms because of scattered missing data. These numbers represent the population of patients that were available for evaluation.
cSignificantly different compared with placebo (P < .05).
dNot assessed in study 2.
In study 2, both cetirizine dosing regimens and the active comparator were significantly more efficacious compared with placebo (P < .05) at relieving subject-assessed sneezing and itchy nose symptom severity (except the 10-mg QHS dose for itchy nose; Table 2). There were no significant differences among the active treatment groups. Investigator-rated symptom severity was numerically lower for both cetirizine dosing regimens and the active comparator versus placebo for all individual symptoms; however, there were no significant differences between groups.
Efficacy at 12 and 24 Hours After Cetirizine Dosing
In study 1, post hoc analysis demonstrated that the subject-assessed TSSC12 and TSSC24 were statistically lower for cetirizine 10-mg QAM, 10-mg QHS, and 5-mg BID compared with placebo (P ≤ .05). In study 2, the 5-mg QAM dose produced statistically lower TSSC12 and TSSC24 compared with placebo (P ≤ .05). For the 10-mg QHS group, TSSC12 was statistically lower compared with placebo (P ≤ .05), while TSSC24 was numerically lower.
Effect of Cetirizine on Morning and Evening Symptoms Over 2 Weeks
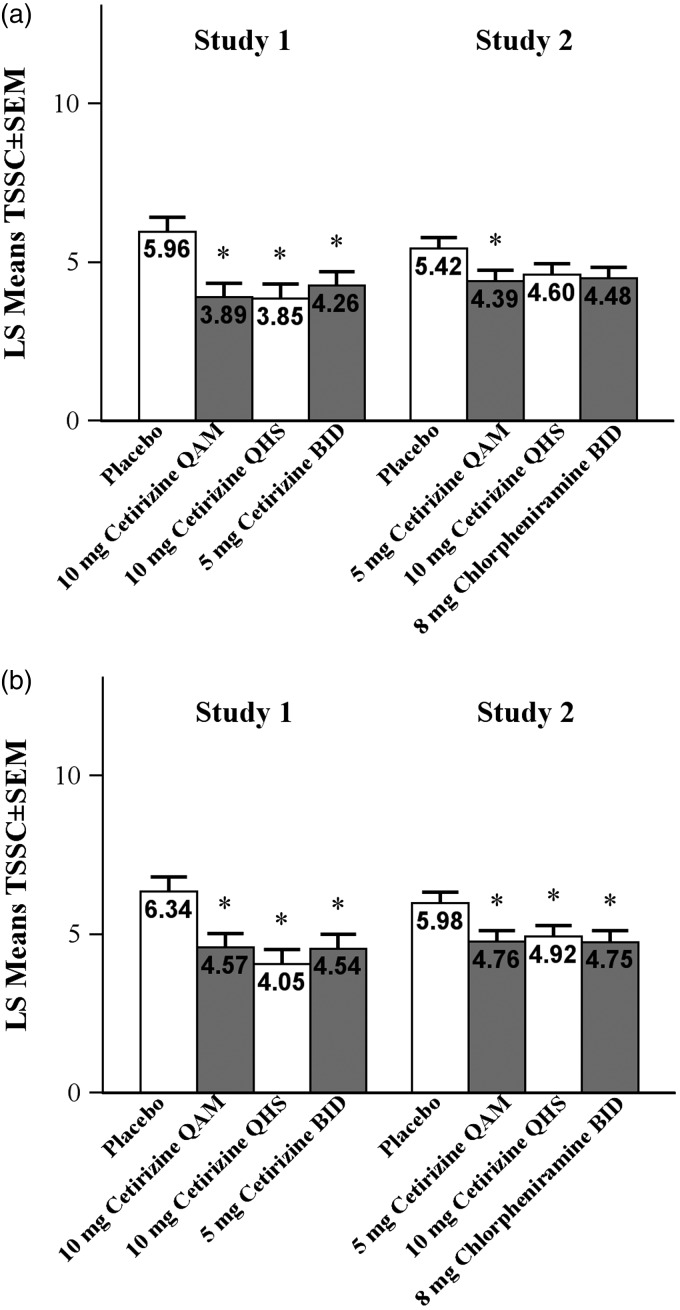
TSSCAM improvement over 2 weeks was evaluated by TSSC24 for the QAM dosing groups and by TSSC12 for the QHS dosing groups for both studies (Figure 3(a)). Subject-assessed TSSCAM reflectively evaluated symptom severity overnight and in the early morning. In both studies, post hoc analysis demonstrated that TSSCAM improvements over 2 weeks were statistically superior for all the cetirizine groups at 12 and 24 hours postdose compared with placebo (P ≤ .03). In study 1, the adjusted mean TSSCAM was lower in the cetirizine 10-mg QHS group (4.05) at 12 hours postdose compared with the cetirizine 10-mg QAM group (4.57) at 24 hours postdose; the difference was −0.52 (95% confidence interval [CI], −1.77 to 0.73). In study 2, the adjusted mean TSSCAM was higher in the cetirizine 10-mg QHS group (4.92) at 12 hours postdose compared with the cetirizine 5-mg QAM group (4.76) at 24 hours postdose; the difference was 0.16 (95% CI, −0.77 to 1.08).
Figure 3.
Mean TSSC in the (a) morning and (b) evening assessed by subject. For QAM dosing, evening scores are 12 hours postdose and morning scores are 24 hours postdose. For QHS, evening scores are 24 hours postdose and morning scores are 12 hours postdose. *P ≤ .05 versus placebo. BID, twice-daily; QAM, once-daily morning; QHS, once-daily bedtime; TSSC, total symptom severity complex.
TSSCPM over 2 weeks was evaluated by TSSC12 for the QAM dosing groups and by TSSC24 for the QHS dosing groups for both studies (Figure 3(b)). Subject-assessed TSSCPM reflectively evaluated symptom severity during the day and in the early evening. Post hoc analysis demonstrated that, compared with the placebo group, TSSCPM improvements were statistically superior in the cetirizine 10-mg QAM group at 12 hours postdose, 10-mg QHS group at 24 hours postdose, and 5-mg BID group at 12 and 24 hours in study 1 (P ≤ .006). In study 2, compared with placebo, TSSCPM improvements were statistically superior in the cetirizine 5-mg QAM group (P = .033) at 12 hours postdose and numerically lower in the cetirizine 10-mg QHS group at 24 hours postdose. In study 1, the adjusted mean TSSCPM was lower in the cetirizine10-mg QHS group (3.85) at 24 hours postdose compared with the cetirizine 10-mg QAM group (3.89) at 12 hours postdose; the difference was −0.04 (95% CI, −1.26 to 1.18). In study 2, the adjusted mean TSSCPM was higher in the cetirizine 10-mg QHS group (4.60) at 24 hours postdose compared with the cetirizine 5-mg QAM group (4.39) at 12 hours postdose; the difference was 0.22 (95% CI, −0.72 to 1.15).
Global Evaluation of Treatment
A global assessment of the therapeutic treatment effect over 14 days was recorded by investigators in study 1 and by subjects in study 2. In study 1, the proportion of investigators reporting “good” or “excellent” treatment effects was greater in the cetirizine 10-mg QAM group, 10-mg QHS group, and 5-mg BID group versus the placebo group (55.3%, 65.9%, and 56.2% vs 43.1%, respectively). Within the cetirizine groups, subjects in the 10-mg QHS group had the largest proportion of “good” or “excellent” responders and the lowest proportion of “failure” responders. The mean global rating of the therapeutic effect was higher for the cetirizine 10-mg QHS group (1.8) than the 10-mg QAM (1.5), 5-mg BID (1.6), and placebo groups (1.2). The observed differences in the distributions of ratings did not reach statistical significance (P = .070).
In study 2, the proportion of subjects who reported “considerable relief” or “complete relief” was slightly greater in the cetirizine 5-mg QAM and cetirizine 10-mg QHS groups versus the placebo group (34.3% and 29.5% vs 28.4%, respectively). The mean global ratings of the therapeutic effect were similar for the cetirizine 5-mg QAM, 10-mg QHS, and placebo groups (mean ratings of 1.1, 1.1, and 1.0, respectively). There were no statistically significant differences among treatments with respect to the distributions of the subjects’ global assessments (P = .25).
Safety
In study 1, 59% of subjects receiving placebo reported AEs; 55%, 46%, and 54% of subjects treated with cetirizine 10-mg QAM, 10-mg QHS, and 5-mg BID reported AEs (Table 3). In study 2, 55% of subjects treated with chlorpheniramine reported AEs; 51%, 43%, and 46% of subjects receiving placebo, cetirizine 5-mg QAM, and cetirizine 10-mg QHS reported AEs. The most commonly reported AEs in both studies were somnolence and headache. Differences in somnolence rates between the cetirizine and placebo groups were not statistically significant in either study. Dry mouth and pharyngitis occurred occasionally in each study and were reported more frequently in the active treatment groups compared with placebo. Most AEs were mild to moderate in severity (Table 3). No serious AEs were reported.
Table 3.
Summary of AEs in the Safety Population.
|
Study 1 |
Study 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Placebo |
Cetirizine |
Placebo |
Cetirizine |
Chlorphen-iramine | ||||
| 10-mg QAM | 10-mg QHS | 5-mg BID | 5-mg QAM | 10-mg QHS | 8-mg BID | |||
| (n = 51) | (n = 49) | (n = 50) | (n = 50) | (n = 77) | (n = 77) | (n = 79) | (n = 77) | |
| Subjects with AEs, n (%) | 30 (58.8) | 27 (55.1) | 23 (46.0) | 27 (54.0) | 39 (50.6) | 33 (42.9) | 36 (45.6) | 42 (54.5) |
| Subjects who discontinued because of AEs, n (%) | 3 (5.9) | 1 (2.0) | 1 (2.0) | 0 | 1 (1.3) | 3 (3.9) | 2 (2.5) | 3 (3.9) |
| AEs in ≥10% of subjects, n (%) | ||||||||
| Headache | 15 (29.4) | 12 (24.5) | 10 (20.0) | 9 (18.0) | 17 (22.1) | 14 (18.2) | 11 (13.9) | 14 (18.2) |
| Somnolence | 6 (11.8) | 6 (12.2) | 9 (18.0) | 13 (26.0) | 8 (10.4) | 13 (16.9) | 17 (21.5) | 18 (23.4) |
| Dry mouth | 0 | 7 (14.3) | 4 (8.0) | 1 (2.0) | 3 (3.9) | 5 (6.5) | 4 (5.1) | 11 (14.3) |
| Pharyngitis | 2 (3.9) | 5 (10.2) | 3 (6.0) | 4 (8.0) | 3 (3.9) | 5 (6.5) | 6 (7.6) | 5 (6.5) |
| Total number of AEs | 65 | 62 | 57 | 54 | 62 | 69 | 70 | 81 |
| Severity, n (%) | ||||||||
| Mild | 39 (60.0) | 35 (56.5) | 40 (70.2) | 41 (75.9) | 35 (56.4) | 46 (66.7) | 35 (50.0) | 49 (60.5) |
| Moderate | 16 (24.6) | 24 (38.7) | 13 (22.8) | 11 (20.4) | 14 (22.6) | 20 (29.0) | 27 (38.6) | 24 (29.6) |
| Severe | 8 (12.3) | 3 (4.8) | 4 (7.0) | 2 (3.7) | 12 (19.4) | 3 (4.3) | 7 (10.0) | 7 (8.6) |
| Not specified | 2 (3.1) | 0 | 0 | 0 | 1 (1.6) | 0 | 1 (1.4) | 1 (1.2) |
Abbreviations: AE, adverse event; BID, twice-daily; QAM, once-daily morning; QHS, once-daily bedtime.
Discussion
In these 2 studies, SAR symptom severity was significantly improved with cetirizine over 2 weeks of treatment as reflected by subject-assessed TSSC. Cetirizine treatment was well tolerated; fewer AEs were reported in the cetirizine groups versus the placebo groups. These data are consistent with previous studies that demonstrated the efficacy and safety of cetirizine for the relief of allergic rhinitis symptoms.5–8
A post hoc analysis demonstrated that, in study 1, cetirizine significantly improved subject-evaluated TSSC12 and TSSC24 compared with placebo, regardless of dosing time. In study 2, compared with placebo, cetirizine produced significant improvements in TSSC12 in the 10-mg QHS and 5-mg QAM dosing groups. TSSC24 was also significantly improved with cetirizine 5-mg QAM dosing and was numerically improved with 10-mg QHS dosing.
TSSCAM, assessing symptom severity overnight and in the early morning, was significantly improved, compared with placebo, in all of the cetirizine groups in both studies, regardless of the dosing schedule. TSSCPM, assessing symptom severity during the day and in the early evening, was significantly improved in all of the cetirizine dosing groups in study 1 and in the cetirizine 5-mg QAM group in study 2. In study 2, TSSCPM in the cetirizine 10-mg QHS group was numerically lower than placebo; however, the difference did not reach statistical significance.
The medical literature suggests that symptoms of SAR vary in intensity throughout the day in some individuals, with the most severe symptoms occurring overnight and in the early morning hours.9–13 Different mechanisms have been cited to explain the circadian rhythm of allergy symptoms, including fluctuations in the levels of inflammatory mediators or cortisol throughout a 24-hour period.9,16 The diurnal variations in SAR symptom severity have spurred researchers to evaluate the effects of chronotherapy or the administration of drugs in synchrony with the biological diurnal rhythms of different conditions, including rhinitis.13,15,17,18 Indeed, in a chronotherapeutic study, the evening administration of the first-generation antihistamine mequitazine was shown to be most effective for the relief of allergic rhinitis in patients with predominantly morning symptoms.19 More recently, second-generation antihistamines have not shown this same chronotherapeutic effect.17,18
In this post hoc analysis, it was shown that cetirizine improved symptom severity, particularly overnight and in the early morning, regardless of the dosing schedule. As there was a numerically greater reduction in TSSCAM symptoms when cetirizine 10-mg was administered the prior evening compared to the previous morning in study 1, we further evaluated the statistical extent of the difference in a manner previously utilized by researchers to assess the equivalence of second-generation antihistamines.20 The difference in the adjusted mean TSSCAM between the QHS and QAM groups was −0.52 (95% CI, −1.77 to 0.73). The 95% CI was then compared to an equivalence limit of 0.65. This equivalence limit was calculated according to the Food and Drug Administration’s (FDA) Guidance for Industry Non-Inferiority Clinical Trials21 and was determined by taking one half of the smallest of the 95% confidence limits of the effect of cetirizine 10-mg observed in a pooled analysis of studies. Therefore, the QAM and QHS regimens cannot be considered “equivalent” in this exercise; however, the difference between the 2 regimens was not statistically different either.
The determination of optimal administration times for some medications allows patients and prescribers to selectively reduce symptoms at the time of greatest intensity. This approach offers advantages for improved symptom control. Conversely, for some patients, this may limit dosing flexibility and treatment compliance.22,23 The efficacy end point of subject TSSC shows that cetirizine significantly improved SAR symptom severity over 2 weeks regardless of dosing schedule. Additionally, the post hoc analysis indicates a trend toward improved overnight and early morning symptom relief regardless of the timing of the cetirizine administration. Thus, the results demonstrate that patients treated with cetirizine for the reduction of SAR symptom severity have flexibility in when they take their medication. Although our analysis of these studies provides insight into the effects of alternative cetirizine dosing schedules on diurnal symptoms, these studies were not specifically designed for this purpose. Therefore, it would be ideal to conduct additional studies that evaluate the chronotherapeutic effects of cetirizine. These chronotherapeutic studies would be designed to evaluate the effect of cetirizine dosing regimens on symptom severity at multiple times of the day in patients with regulated sleep-wake patterns who experience particularly troublesome allergic rhinitis symptoms overnight and in the early morning.
A limitation of these studies was that they were conducted in 1985, before the draft FDA allergic rhinitis guidance for industry was widely disseminated.24 Thus, the study designs were different in some details compared with more recent trials. For example, these studies lacked placebo run-in periods, and the study protocol did not specify any predetermined primary end points. Additionally, although adults and children evaluated symptoms BID in this study, as is recommended in the FDA guideline,24 the 45 subjects (14.52%) who were 12 to <18 years of age in the second study may have been less able to self-rate their symptoms as accurately as adult subjects. Strengths of the studies were that they were multicenter, randomized, double-blind, placebo-controlled, and well powered, with assessments by both patients and investigators.
Conclusions
Once-daily cetirizine effectively relieves SAR symptoms over a 24-hour period, regardless of morning or evening dosing. A post hoc analysis demonstrated that, regardless of dosing regimen, cetirizine improves symptom severity, particularly overnight and in the early morning, when symptoms may be most troublesome.
Ethical Approval
This study was approved by our institutional review board.
Statement of Human and Animal Rights
This article does not contain any studies with animal subjects.
Statement of Informed Consent
In the case of minors, the investigator obtained the written consent of the subject's parent or legal guardian and the assent of the minor subject.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M. K. Patel, M.-M. Wu, and X. Tian are employees of Johnson & Johnson Consumer Inc. E. R. Urdaneta was an employee of Johnson & Johnson Consumer Inc. while authoring this publication. K. B. Franklin is a consultant to Johnson & Johnson Consumer Inc.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The clinical studies were funded by Pfizer. Support for publication and analysis of the studies was provided by Johnson & Johnson Consumer Inc., McNeil Consumer Healthcare. Editorial support was provided by Erin Scott, PhD, and Danielle Gross, PhD, from Complete Publication Solutions, LLC, which was funded by Johnson & Johnson Consumer Inc.
References
- 1.Blaiss MS. Allergic rhinoconjunctivitis: burden of disease. Allergy Asthma Proc. 2007; 28:393–397. [DOI] [PubMed] [Google Scholar]
- 2.Pawankar R, Canonica GW, Holgate ST, Lockey RF, Blaiss M. The WAO white book on allergy: Update 2013. http://www.worldallergy.org/UserFiles/file/WhiteBook2-2013-v8.pdf. Accessed November 27, 2017.
- 3.Bousquet J, Khaltaev N, Cruz AA, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008; 63(suppl 86):8–160. [DOI] [PubMed] [Google Scholar]
- 4.Navarro A, Valero A, Rosales MJ, Mullol J. . Clinical use of oral antihistamines and intranasal corticosteroids in patients with allergic rhinitis. J Investig Allergol Clin Immunol. 2011; 21:363–369. [PubMed] [Google Scholar]
- 5.Day JH, Briscoe M, Widlitz MD. Cetirizine, loratadine, or placebo in subjects with seasonal allergic rhinitis: effects after controlled ragweed pollen challenge in an environmental exposure unit. J Allergy Clin Immunol. 1998; 101:638–645. [DOI] [PubMed] [Google Scholar]
- 6.Mansmann HC, Jr, Altman RA, Berman BA, et al. Efficacy and safety of cetirizine therapy in perennial allergic rhinitis. Ann Allergy. 1992; 68(4):348–353. [PubMed] [Google Scholar]
- 7.Falliers CJ, Brandon ML, Buchman E, et al. Double-blind comparison of cetirizine and placebo in the treatment of seasonal rhinitis. Ann Allergy. 1991; 66:257–262. [PubMed] [Google Scholar]
- 8.Skoner DP, LaForce CF, Nathan RA, et al. Effect of cetirizine on symptom severity and quality of life in perennial allergic rhinitis. Allergy Asthma Proc. 2014; 35:338–345. [DOI] [PubMed] [Google Scholar]
- 9.Aoyagi M, Watanabe H, Sekine K, et al. Circadian variation in nasal reactivity in children with allergic rhinitis: correlation with the activity of eosinophils and basophilic cells. Int Arch Allergy Immunol. 1999; 120(suppl 1):95–99. [DOI] [PubMed] [Google Scholar]
- 10.Binder E, Holopainen E, Malmberg H, Salo O. Anamnestic data in allergic rhinitis. Allergy. 1982; 37:389–396. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson PA, Bogie W. Diurnal variation in the symptoms of hay fever: implications for pharmaceutical development. Curr Med Res Opin. 1973; 1:395–400. [DOI] [PubMed] [Google Scholar]
- 12.Reinberg A, Gervais P, Levi F, Smolensky M, Del Cerro L, Ugolini C. Circadian and circannual rhythms of allergic rhinitis: an epidemiologic study involving chronobiologic methods. J Allergy Clin Immunol. 1988; 81:51–62. [DOI] [PubMed] [Google Scholar]
- 13.Storms WW. Pharmacologic approaches to daytime and nighttime symptoms of allergic rhinitis. J Allergy Clin Immunol. 2004; 114:S146–S153. [DOI] [PubMed] [Google Scholar]
- 14.Reinberg A, Levi F, Guillet P, Burke JT, Nicolai A. Chronopharmacological study of antihistamines in man with special references to terfenadine. Eur J Clin Pharmacol. 1978; 14:245–252. [DOI] [PubMed] [Google Scholar]
- 15.Smolensky MH, Lemmer B, Reinberg AE. Chronobiology and chronotherapy of allergic rhinitis and bronchial asthma. Adv Drug Deliv Rev. 2007; 59:852–882. [DOI] [PubMed] [Google Scholar]
- 16.Fidan V, Alp HH, Gozeler M, Karaaslan O, Binay O, Cingi C. Variance of melatonin and cortisol rhythm in patients with allergic rhinitis. Am J Otolaryngol. 2013; 34:416–419. [DOI] [PubMed] [Google Scholar]
- 17.Haye R, Hoye K, Berg O, Frones S, Odegard T. Morning versus evening dosing of desloratadine in seasonal allergic rhinitis: a randomized controlled study [ISRCTN23032971]. Clin Mol Allergy. 2005; 3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marmouz F, Giralt J, Izquierdo I. Morning and evening efficacy evaluation of rupatadine (10 and 20 mg), compared with cetirizine 10 mg in perennial allergic rhinitis: a randomized, double-blind, placebo-controlled trial. J Asthma Allergy. 2011; 4:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reinberg A, Gervais P, Ugolini C, Del Cerro L, Bicakova-Rocher A. A multicentric chronotherapeutic study of mequitazine in allergic rhinitis. Annu Rev Chronopharmacol. 1985; 3:441–444. [Google Scholar]
- 20.Hampel F, Ratner P, Mansfield L, Meeves S, Liao Y, Georges G. Fexofenadine hydrochloride, 180 mg, exhibits equivalent efficacy to cetirizine, 10 mg, with less drowsiness in patients with moderate-to-severe seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2003; 91:354–361. [DOI] [PubMed] [Google Scholar]
- 21.Non-Inferiority Clinical Trials to Establish Effectiveness. Guidance for Industry. U.S. Department of Health and Human Services, FDA, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), November 2016.
- 22.Buetow S, Henshaw J, Bryant L, O'Sullivan D. Medication timing errors for Parkinson’s disease: perspectives held by caregivers and people with Parkinson’s in New Zealand. Parkinsons Dis. 2010. ;2010:432983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coleman CI, Limone B, Sobieraj DM, et al. Dosing frequency and medication adherence in chronic disease. J Manag Care Pharm. 2012; 18:527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guidance for Industry Allergic Rhinitis: Clinical Development Programs for Drug Products. U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER), April 2000. Clin. https://www.fda.gov/downloads/drugs/guidances/ucm071293.pdf. Accessed December 12, 2017.