Abstract
The aspect of treatment of autistic behaviour was investigated using valproic acid rat model of pregnant female rats. Two main groups (10 male rats/group) were treated for 6 days and then divided into six subgroups. The first group of normal rats was divided into three subgroups: (A) – control group, (B) – treated with camel milk (CAM; 2 mL/p.o) and (C) – treated with leptin (1000 µg/kg i.p) twice daily. The second group of autistic rats was randomly distributed into four subgroups as follows: (D) – positive control (autistics rats), (E) – treated with CAM, (F) – treated with a moderate dose of leptin and (G) – treated with a higher dose of leptin. Autistic behaviours of male offspring were checked by grooming and elevated pulz maze tests. Valproic acid (VPA)-induced autistic rats showed severe changes in oxidative stress markers, neurotransmitters and inflammatory cytokines, besides genotoxic manifestation of expression of tumour necrosis factor (TNF)-α, Bax and caspase-3. Leptin or CAM alone showed no signs of toxicity. CAM showed pronounced improvement in control rats than control itself. Leptin or CAM treatment of autistic animals showed a significant improvement of all measured parameters and genetic expression values. The improvement was pronounced in animals treated with CAM. These results suggest that CAM is a potential therapeutic candidate for autism via regulation of inflammatory and apoptotic pathways. Leptin plays an essential role in alleviation of autistic behaviour through antioxidant effects.
Keywords: autism, camel milk, genotoxicity, leptin, valproic acid
Introduction
Autism is a severe and pervasive neurodevelopmental disorder. It is associated with heterogeneous behaviour at earlier onset of children prior to 3 years of age.1 Autism is characterized by impairment of both verbal and non-verbal communication, social interactions, imagination, repetitive behaviour, restricted behaviour/interest, intellectual disability and attention deficit hyperactivity disorder.2 The global prevalence of autism is 0.62%, while recent epidemiological studies predict it to be 1.4% in the United States.3 Boys are more vulnerable to autism than girls with a ratio of 3.5 or 4.0 boys to 1 girl.4 There is a long debate and much more studies try to answer the question on the underlying causes, risk factors of autism and the nature of the significant increase in its prevalence. Although the etiology of autistic behaviour is quite elusive, it is considered as a multifactorial disorder which is influenced by several changes involving genetic, environmental and immunological factors as well as oxidative stress. Earlier studies report that children with autistic disorders show an elevation of plasma level of malondialdehyde (MDA). Oxidative stress will happen if the capacity of cellular antioxidants is lower than the free radical generation. The oxidative stress results in lipid peroxidation (LPO), enzyme inactivation, break of DNA strand, covalent binding to protein, nucleic acid and other damaging effects.5 In addition, glutathione (GSH) serves not only as an antioxidant, but also as a key factor for neural surviving at the early critical stage.6 Due to limited antioxidant capacity of the brain and the high requirement of energy, it is extremely vulnerable to oxidative stress. Therefore, the neurons are the first cells affected by imbalance between the released ROS and antioxidants, as a result of oxidative stress.7 There is a great interest for using natural products and antioxidants in treatment of autistic behaviours.8,9 Leptin (Ob) is an adipokine, first described 15 years ago, and secreted by adipose tissue. It plays an essential role in energy homeostasis and metabolic, neuroendocrine and reproductive functions.10 It is involved in the regulation of immune functions, cognition as well as bone metabolism.11 This adipokine is positively secreted directly proportional to the amount of white adipose tissue, therefore the plasma level of leptin is indicated for the body energy stores and state of caloric intake.12 Previous studies report that early leptin deficiency may lead to autistic behaviour and syndrome of attention deficit hyperactivity disorder (ADHD) and postnatal leptin therapy may improve the autistic behaviour.13 Camel milk (CAM) showed a unique chemical composition and possess therapeutic activities in treatment of diabetes, and autoimmune disorders such Crohn’s disease, multiple sclerosis and autism.14–17 In addition, CAM induces protective activity against hepatotoxicants and diverse carcinogens.18 On the other hand, CAM ameliorates the inflammatory responses and oxidative stress via downregulation of mitogen-activated protein kinase (MAPK) signalling pathways.19 This study aims to investigate the potential therapeutic effects of leptin and CAM against valproic acid (VPA)-induced oxidative stress and genotoxicity in autistic rats.
Materials and methods
Chemical and kits
VPA and leptin were purchased from Sigma-Aldrich (St Louis, MO, USA). CAM was purchased from the local market. Glutathione peroxidase (GPx, Code: RS505) and superoxide dismutase (SOD, Code: SD125) kits were purchased from Randox (Antrim, UK). Malondialdehyde (MDA, Code: MDA-586) kit was obtained from Oxis Research TM Co. (Foster City, CA, USA). Interleukin-1β (IL-1β, Code: IL01b02), Interleukin-6 (IL-6, Code: IL06b02) and tumour necrosis factor-alpha (TNF-α, Code: TNFa021) kits were purchased from Orgenium (Helsinki, Finland). Serotonin (Code: IB89540) and dopamine (Code: IB89538) kits were obtained from Immuno Biological Laboratories (IBL; Minneapolis, MN, USA). All other chemicals were of the highest analytical grade available.
Animals
A total of 84 adult (150–160 g) Sprague–Dawley rats comprising 70 females aged 14–16 weeks and 14 males aged 16–18 weeks were purchased and housed in a room free from any source of chemical contamination, artificially illuminated and thermally controlled in the animal house facility, Pharmacology and Chemistry Research Centre, Misr University for Science & Technology Park, 6th October City, Egypt. All animals were maintained on a standard lab diet (20% proteins), obtained from Tana Company for Oils and Soap (Tanta City, Egypt). After 1 week of acclimatization, 70 female rats were randomly distributed into 14 groups (5 rats/group). Each female was distinguished with a permanent marker, and vaginal smear was collected and investigated using optical microscope.20 After an earlier smear collection, 14 males were randomly distributed and introduced into 10 cages in the ratio of male:female at 1:5. Overnight mating between the males and females was allowed and confirmed with protein coagulates in two samples of vaginal smear (6.30 am in the morning and 6.30 pm in the evening). Females could be re-coupled if they were not pregnant after initial mating. Four days after the first observation, examination of progress of pregnancy was carried out. The day when the protein coagulates were observed was considered as the first day of gestational period.21 All the procedures described below were carried out with approval and performed in accordance with the guidelines of the ethics committee of the Faculty of Dentistry, Cairo University, Cairo, Egypt. The animals were cared for in accordance with the Guide for the Care and Use of Laboratory Animals.22
VPA-rat model of autism
On day 12.5 of the gestational period, 70 pregnant rats were divided into two major groups; the first group was treated with a single intraperitoneal injection of sodium salt of VPA (Na VPA) 600 mg/kg according to a previously described method.23 Thereafter, male offspring only were referred with autistic rats. The second group was assigned as control dams and received intraperitoneal injection with plain saline, the rat pups thus born were represented as control rats. On the 15th postnatal day, the autistic and control rats were divided into seven groups (10 rats/group), and nursed without their mothers and fed with a standard diet and water ad libitum. Animals that showed negative results of behaviour tests were excluded from this study. Autistic behaviours of male offspring were checked by preliminary behaviour tests, including grooming test and elevated pulz maze test.19,20 The following experiment was conducted on the male offspring. On the 60th day control dams (n = 30) were randomly distributed into three groups, while VPA-induced male offspring (n = 40) were randomly divided into four groups.
Experimental design
Animals within different treatment groups (10 rats/group) were treated for 6 days and divided into two main groups. The first group (n = 30), was divided into three subgroups (A), (B) and (C) and included the control group (A), the group treated with CAM (2 mL/ rat p.o) as (B)24 and the group treated with leptin (1000 µg /kg i.p) twice daily at 09:00 am and 04:00 pm as (C).25 The second group, autistic rats, was randomly distributed into four subgroups as follows: positive control group (autistics rats) without any treatment as (D), group from autistic rats and treated with CAM (2 mL/ rat p.o as (E), autistic group and treated with leptin (500 µg/kg i.p) twice daily at 09:00 am and 04:00 pm as (F) and autistic group and treated with leptin (1000 µg/kg i.p) twice daily at 09:00 am and 04:00 pm as (G).
At the end of the treatment period, the animals were fasted for about 12 h but with free access to water ad libitum. Blood samples were collected from the retro-orbital venous plexus from each animal under ether anaesthesia. Blood samples were left to clot and the sera were separated using cooling centrifugation at 3000 r/min for 15 min and stored at −20°C until analysis. The sera were used for the determination of IL-6, IL-1β and TNFα according to the instructions of the analytical kits.
After the collection of blood samples, all animals were sacrificed by cervical dislocation and samples of livers and brains were weighed (approximately 0.05–0.1 g) and homogenized in phosphate buffer (pH 7.4) to give 20% (w/v) homogenate. This homogenate was centrifuged at 1700 r/min at 4°C for 10 min and the supernatant was stored at −70°C until analysis. This supernatant from the liver sample was used for the assessment of catalase, while brain sample had been used for determination of GPx, MDA, SOD, dopamine and serotonin according to the instructions on the kits.
Gene expression
RNA extraction
Immediately after the animals’ scarification, samples of brain tissues were taken, frozen into liquid nitrogen and stored at −80°C prior to RNA extraction. RNA was extracted from 100 μg of brain tissue by the standard TRIzol® Reagent (InvitrogenTM, Carlsbad, CA, USA) extraction method and recovered in 100 μL of diethylpyrocarbonate (DEPC)-treated water. In order to remove any possible genomic DNA contamination, the total RNA samples were pre-treated using DNA-free TM DNase and removal reagents kit (Promega, Co) following the manufacturer’s protocol. The quality and integrity of the purified RNA was checked through agarose gel electrophoresis (1%) based on the integrity of 18S and 28S rRNA bands. RNA quantity was ascertained spectrophotometrically (Jenway 6505, UK) as described by Sambrook and Russel with an A260/A280 ratio between 1.7 and 1.9.26 The purified RNA samples were preserved at −80°С until use.
Reverse transcription
The complete Poly(A)+ RNA isolated from the brain samples was reverse transcribed into cDNA in a total volume of 20 μL using the High Capacity RNA to cDNA Kit PreMix Kit (iNtRON Biotechnology, Korea). The reaction tubes containing RT preparations were flash cooled in an ice chamber at −20°C until being used for cDNA amplification through semi-quantitative polymerase chain reaction (PCR).27
Semi-quantitative PCR
Genetic expression of caspase-3, TNF and Bax were studied in the brain samples using semi-quantitative PCR. Oligonucleotide PCR primer pairs were developed for caspase-3 (5-’AAATTCAAGGGACGGGTCAT-3’/5’-ATTGACACAATACACGGGATCTGT-3’), TNF (5’-CCACCACGCTCTTCTGTCTAC-3’/ 5’-ACCACCAGTTGGTTGTCTTTG-3’) and Bax (5’-AGGATGATTGCTGATGTGGATAC-3’/ 5’-CACAAAGATGGTCACTGTCTGC-3’) genes based on the published primer sequences. The specificity of the amplification products was confirmed by size estimation on a 1.2% (w/v) agarose gel ‘GAPDH’ amplification was used as the housekeeping gene in semi-quantitative PCR analysis. In a final volume of 20 μL, 1 μL (0.05 μg) of cDNA was amplified using 2 μL of dNTPs (2.5 mM each), 2 μL of 10 × PCR buffer, 0.5 μL (5 u/μL) Taq TM DNA polymerase (Segma, Co), 1 μL (10 pmoles) forward primer, 1 μL (10 pmoles) reverse primer and 12.5 μL sterilized distilled water. Thermal cycling parameters were the following: initial denaturation at 94°C for 5 min, 30 cycles of amplification (94°C for 60 s for DNA denaturation, annealing at 55–58°C for 30 s (see Table 1), and extension at 72°C for 1 min) and a final extension at 72°C for 7 min.
Table 1.
Sequences of primers used for amplification.
| Gene | Forward primer | Reverse primer | Anneal. Temp. | PCR product size | Ref. |
|---|---|---|---|---|---|
| Caspase-3 | 5-’AAATTCAAGGGACGGGTCAT-3’ | 5’-ATTGACACAATACACGGGATCTGT-3’ | 55 | 225 | Liu et al. (2002)28 |
| Bax | 5’-AGGATGATTGCTGATGTGGATAC-3’ | 5’-CACAAAGATGGTCACTGTCTGC-3’ | 56 | 300 | Liu et al. (2002)28 |
| TNF-α | 5’-CCACCACGCTCTTCTGTCTAC-3’ | 5’-ACCACCAGTTGGTTGTCTTTG-3’ | 58 | 256 | Liu et al. (2002)28 |
| GAPDH | 5′- CAAGGTCATCCATGACAACTTTG -3′ | 5′- GTCCACCACCCTGTTGCTGTAG -3′ | 58 | 496 | Wiame et al. (2000)29 |
PCR: polymerase chain reaction; TNF: tumour necrosis factor; GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
Agarose gel electrophoresis
A gel was prepared with 2% agarose containing 0.1% ethidium bromide. The DNA was visualized and photographed via ultraviolet (UV) transilluminator.
Semi-quantitative determination of PCR products
GAPDH was used as an internal control and using its specific primer (5′- CAAGGTCATCCATGACAACTTTG-3′/5′ GTCCACCACCCTGTTGCTGTAG -3′). The ethidium bromide-stained gel bands were scanned and the signal intensities were quantified by the computerized Gel-Pro (Version 3.1 for Windows 7). According to the following amplification procedure, relative expression of each gene was calculated following the formula:
The ratio between the levels of the target gene amplification product and the GAPDH (internal control) was calculated to normalize for initial variation in sample concentration as a control for reaction efficiency.30
Statistical analysis
All data were statistically analysed with the General Linear Model Procedure of the Statistical Analysis System (SAS 1982). The significance of the differences among treatment groups was determined with the Waller–Duncan k-ratio. All statements of significance were based on a probability of P ⩽ 0.05.
Results
The effect of different treatments on serum inflammatory cytokines of animals treated with VPA-induced autism are presented in Table 2, serum TNF-α, IL-1β and IL-6 were significantly increased in the group treated with VPA, while animals treated with leptin or CAM were more or less like a control. On the other hand, treatment with leptin or CAM in autistic rats showed a significant reduction in the level of the inflammatory cytokines. Autistic animals treated with a higher dose of leptin showed significant improvement in serum level of inflammatory cytokines in comparison to other groups. The results of MDA in the brain tissues (Table 3) showed a significant increase in the animals treated with VPA. Treatment with leptin or CAM showed a significant reduction in the MDA level in comparison to the control group. Autistic animals treated with leptin or CAM succeeded to decrease the MDA level and the treatment with CAM was more effective than treatment with leptin either in lower or higher dose. The enzymatic activities of SOD, GPx and catalase (Table 3) showed a significant decrease in the group treated with VPA. Treatment with leptin or CAM in autistic rats showed a significant increase in the antioxidant enzyme activities. The pronounced improvement was in treatment with higher dose of leptin. The results of this work indicated that animals treated with VPA showed severe changes in serum levels of neurohormones which is indicated by the significant increase of dopamine and decrease of serotonin. Treatment with leptin or CAM alone showed a significant increase of neuronal level of dopamine, with a significant reduction of serotonin level in the brain homogenate. On the other hand, leptin or CAM succeeded to improve the level of dopamine and serotonin in animals treated with VPA. Treatment with higher dose of leptin was the most effective than other treatments (Table 4).
Table 2.
Effect of leptin and camel milk on serum level of inflammatory cytokines of rats treated with valproic acid-induced autism.
| Treatments | TNF-Α (pg/mL) | IL-1β (pg/mL) | IL-6 (pg/mL) |
|---|---|---|---|
| Control | 18.025 ± 1.24A | 14.4 ± 4.6a | 4.9 ± 0.48a |
| VPA | 101.6 ± 6.3b | 116.05 ± 7.8b | 79.2 ± 5.3b |
| LEPT | 26.675 ± 0.99c,a | 28.62 ± 1.09a,c | 9.25 ± 0.41a,d |
| CAM | 35.225 ± 1.9c | 37.72 ± 2.1c | 16.12 ± 1.35a |
| VPA + LEPT (I) | 73.675 ± 3.5d | 82.25 ± 4.2d | 44.2 ± 2.6c |
| VPA + LEPT (II) | 49.125 ± 2.3e | 54 ± 2.4e | 17.8 ± 0.97d |
| VPA + CM | 50.575 ± 3.10e | 52.9 ± 2.9e | 31.975 ± 2.6e |
TNF: tumour necrosis factor; VPA: valproic acid; LEPT: leptin; CAM: camel milk.
Within each column, means superscript with different letters are significantly different (P < 0.05).
Table 3.
Effect of leptin and camel milk on brain lipid peroxidation, SOD, GPx and catalase in brain of rats treated with valproic acid-induced autism.
| Treatments | MDA (nM/mg protein) | SOD (U/mg protein) | GPx (U/mg protein) | Catalase (U/mg tissue) |
|---|---|---|---|---|
| Control | 33.45 ± 0.43a | 293.33 ± 14.85b | 30.06 ± 0.71a | 7.97 ± 0.18a |
| VPA | 84.77 ± 5.27b | 55.20 ± 4.21a | 5.65 ± 0.34b | 1.5 ± 0.09b |
| LEPT | 21.56 ± 1.25c | 217.29 ± 16.9c | 19.41 ± 1.36c | 5.15 ± 0.36c,f |
| CAM | 26.47 ± 1.25a | 231.01 ± 8.29c | 23.74 ± 0.91d | 6.3 ± 0.24d |
| VPA + LEPT (I) | 43.89 ± 1.75a | 101.63 ± 2.8a,d | 10.46 ± 0.41e | 2.77 ± 0.11e |
| VPA + LEPT (II) | 21.83 ± 1.42c | 164.5 ± 15.9d | 16.77 ± 1.09c, f | 4.45 ± 0.29f |
| VPA + CAM | 36.93 ± 1.76a | 148.4 ± 6.7d, e | 15.26 ± 0.73f | 4.05 ± 0.19f |
MDA: malondialdehyde; SOD: superoxide dismutase; GPx: glutathione peroxidase; VPA: valproic acid; LEPT: leptin; CAM: camel milk.
Within each column, means superscript with different letters are significantly different (P ⩽ 0.05).
Table 4.
Effect of leptin and camel milk on dopamine and sertonin (5HT) level in brain of rats treated with valproic acid-induced autism.
| Treatments | Dopamine (ng/g tissue) | 5HT (ng/g tissue) |
|---|---|---|
| Control | 6.97 ± 0.28a | 38.67 ± 1.31a |
| VPA | 28.5 ± 1.82b | 9.025 ± 0.64b |
| LEPT | 9.85 ± 0.46a | 25.17 ± 1.02c |
| CAM | 14.15 ± 0.45c | 25.15 ± 1c |
| VPA + LEPT (I) | 19.6 ± 1d | 14.15 ± 0.35d |
| VPA + LEPT (II) | 10.55 ± 0.31a,c | 27.95 ± 0.99c |
| VPA + CM | 20.25 ± 0.76d | 17.8 ± 0.57e |
VPA: valproic acid; LEPT: leptin; CAM: camel milk.
Within each column, means superscript with different letters are significantly different (P ⩽ 0.05).
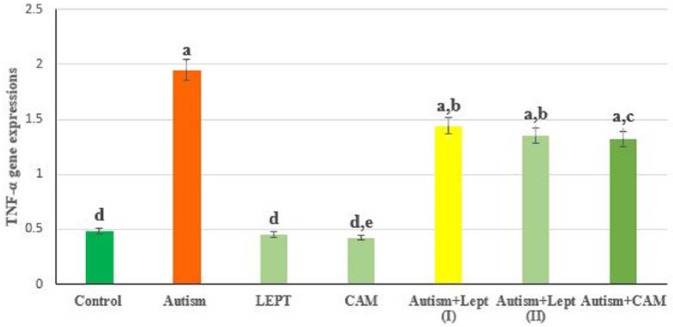
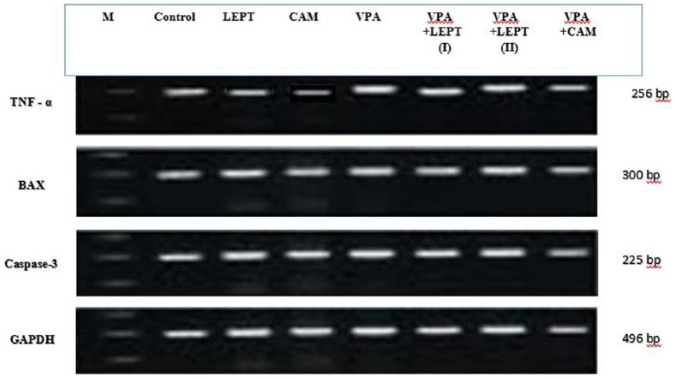
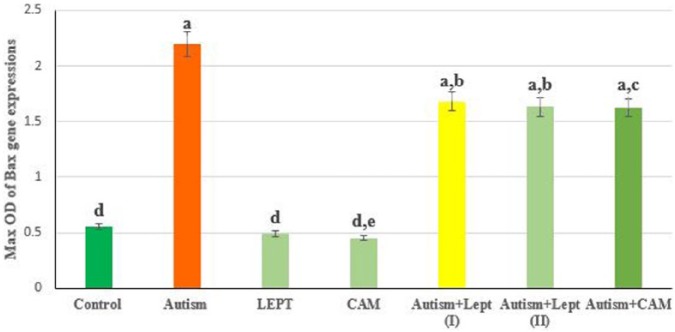
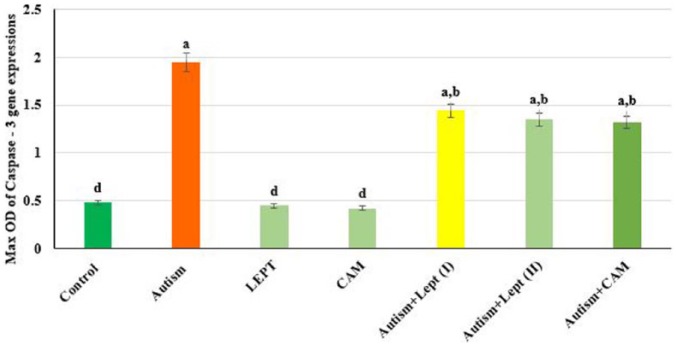
The data presented in Figures 1 and 4 are the optical density of TNF-α, Bax and caspase 3/GAPDH expression in the brain of the controls and treated animals. These results indicate that TNF-α expression was significantly increased in animals treated with VPA in comparison to the control group. However, those treated with VPA and then treated with leptin or CAM showed a significant decrease in TNF-α expression. Moreover, treatment with leptin or CAM alone did not induce any significant changes on the expression of TNF-α. In addition, the ratio between Bax/GAPDH indicated an over expression in Bax compared to the ratio between control Bax/GAPDH (Figures 2 and 4), which increased in the animals treated with VPA (2.2) compared to the control group (0.55). Animals treated with leptin and CAM did not induce any significant changes on the expression of Bax. Treatment with leptin or CAM after VPA treatment succeeded to reduce the expression of Bax. Meanwhile, the ratio of caspase-3/GAPDH was increased in VPA-treated animals (1.95) compared to control animals with caspase-3/GAPDH ratio (0.48). Treatment with leptin succeeded to reduce the ratio of expression of mRNA Caspase-3 from 1.95 in the VPA-treated group to 1.44 in the group treated with VPA plus leptin. While the treatment with a higher dose of leptin resulted in advanced improvement in the expression ratio of mRNA Bax to reach 1.35 compared to the VPA-treated group. A higher reduction in the ratio of caspase-3/GAPDH ratio was observed in the animals treated with CAM after VPA treatment (Figures 3 and 4).
Figure 1.
The ratio between TNF-α/GAPDH in brain treated with valproic acid-induced autism alone or in combination with leptin or camel milk.
Values represent mean ± SE for each group. Column superscripts with different letter are significantly different (P ⩽ 0.05).
Figure 4.
Effects of different dose of leptin (LEPT LII) or camel milk (CAM) on transcript product of brain genes (TNF-α, Bax and caspase - 3) in rats treated with valproic acid-induced autism.
Agarose gel electrophoresis of TNF-α, Bax and caspase − 3 and GAPDH RT-PCR products of different groups are presented:
Group I: Control, Group II: VPA, Group III: LEPT, Group IV: CAM, Group V: VPA + LEPT (I), Group VI: VPA + LEPT + LEPT (II), and Group VII: VPA + CAM.
Figure 2.
The max OD ratio between Bax/GAPDH in brain of rats treated with valproic acid-induced autism alone or in combination with leptin or camel milk.
Values represent mean ± SE for each group. Column superscripts with different letter are significantly different (P ⩽ 0.05).
Figure 3.
The max OD ratio between Caspase − 3/GAPDH in brain of rats treated with valproic acid-induced autism alone or in combination with leptin or camel milk.
Values represent mean ± SE for each group. Column superscripts with different letter are significantly different (P ⩽ 0.05).
Discussion
Nowadays, the interest for using natural products has increased to treat and prevent neurobehavioural disorders in humans. Accordingly, different types of natural compounds have been re-investigated and recognized as a valuable source for novel therapy in treatment of different diseases. Earlier studies reported that leptin and CAM play important roles as free radical scavenger and powerful antioxidant besides their distinguished role in the innate immune response.31,32 This study aimed to investigate the potential therapeutic effect of leptin or CAM in treatment of autism, by monitoring their effects against oxidative stress, genotoxicity and cell death in VAP-rat model of autism. The selected doses of VPA, leptin and CAM were literature based, respectively.23–25 The results of this study showed that VPA treatment on day 12.5 of the gestational period in pregnant rats induced autistic behaviour in their offspring. These results were in agreement with the previous study that reported prenatal exposure to VPA was associated with biochemical alterations and cognition impairment.33 The results of this study showed that VPA treatment induces severe changes in inflammatory cytokines: TNF-α, IL-1β, IL-6. It has been well documented that autistic individuals are more vulnerable to neuroinflammation accompanied with altered inflammatory response. The alteration of inflammatory response may be due to over activation of the monocytic (increased IL-1RA) and Th-1-like (increased IFN-gamma) in the inflammatory response system (IRS).34 In addition, animals that are pre-exposed to VPA showed ultrastructural changes in the microglial and nerve cells in different regions of the brain accompanied with increase in the permeability of the blood–brain barrier.35,36
In this study, animals pre-exposed to VPA suffer from oxidative stress, which is indicated by significant increment of lipid peroxidation (MDA) and significant reduction of enzymatic activities of SOD, GPx and catalase enzymes. There results were in agreement with earlier studies that postulated that VPA induces ROS generation and apoptosis.37 Zhang et al.38 reported that treatment with VPA induced teratogenesis in whole embryo culture model via increase in the ratio of oxidized to reduced GSH and total GSH content more than the antioxidative capacity of embryonic cells. In line with the current results, VPA treatment showed a significant increase of NO in brain tissues such as cortex, hippocampus and cerebellum indicated by oxidative stress accompanied with depletion of antioxidant enzymes and glutathione.39 The observations of this study are consistent with previous studies that depict that the uncompensated level of oxidative stress interferes with the early stage of brain development and induced autistics behaviour as in the prenatal exposure to VPA.40–42 The results of this study showed that the level of dopamine in autistic rats was significantly increased. These results were in agreement with earlier work accomplished by Narita et al.,43 which was indicated by repetitive behaviour. On the contrary, autistic animals showed markedly reduced serotonin levels in comparison to the control group.44 These observations are consistent with previous findings that report that the capacity of serotonin synthesis in the brain is reduced in autistic children, which is indicated by significant promotion of aggressive behaviour.45 These results may be attributed to a disturbance in the balance between branched and aromatic amino acids.46 On the other hand, the results of this study showed that prenatal exposure of VPA induced higher expression of TNF-α, Bax and caspase 3 pro-apoptotic proteins. These results may be due to the inhibitory effects of the repair mechanism of DNA double-strand breaks and histone deacetylase of valproate. Treatment with VPA demonstrated cytotoxic activity via induction of apoptosis and autophagy in cancer cells.47 The outcomes of this study were in agreement with previous work that demonstrated the cytotoxic activity of VPA accompanied with induction of cell death and apoptosis via upregulation of caspase 3, and pro-apoptotic regulator; Bax in ovarian cell.48 In this study, overexpression of inflammatory cytokines TNF-α is indicated by stereotypic behaviour in autistic children.49 The results of this study showed that leptin induced antioxidative stress activities which is indicated by a significant reduction of MDA and increment of SOD, GPx and catalase activities. These results are in agreement with earlier study that reveal leptin treatment induced the antioxidative defence mechanism via enhancement of the activity of certain antioxidant enzymes.50 Earlier study accomplished by Yamagishi et al.,51 suggested that leptin induced ROS generation by increasing fatty acid oxidation through activation of protein kinase A (PKA). The results of this study showed that leptin induced neuromodulatory effects against valproate-induced disturbance of neurotransmitters; dopamine and serotonin. It has been well documented that leptin plays an essential role in neurodevelopment, thus neonatal leptin deficiency resulted in reduction of adult brain volumes that accompanied with advanced adult locomotor activity.13 On the other hand, leptin treatment with autistic rats showed significant improvement of genetic expression of TNF-α, caspase 3 and BAX. The results of this study are in agreement with earlier work that report that leptin reduced apoptosis via downregulation of P53 pathway and reduced P53 half-life.52 These results may be mediated via MAPK and PI3K pathways and significant reduction of BCL2/BAX ratio.53 CAM is a rich source of different types of proteins, thus it exerts tremendous biological activities including antioxidant, anti-cancer, immunological and anti-tumour effects.54 The results of this work showed that CAM induced significant improvement of antioxidant capacity of brain homogenate at VPA-induced autistic behaviour. It is well known that CAM contains antioxidant nutrients such as magnesium and zinc. Thus, the antioxidant effects may be due to the effect of magnesium to reduce the oxidative stress and promote the vitamin E and C absorption, while zinc increases the total glutathione (GSH) and SOD.55 However, the data of this study demonstrate that CAM shows neuromodulatory effects on dopamine and serotonin. The results are in the same line as previous studies that show CAM induced GABA biogenic activity.56 It is well known that GABA acts as a negative regulator of dopamine, thus CAM reduces the level of dopamine and serotonin. CAM showed anti-inflammatory and anti-apoptotic effects indicated by significant reduction of expression of TNF-α, BAX and caspase 3. These results are in agreement with earlier studies that implicate CAM showing significant reduction of inflammatory cytokines and apoptotic markers.57 These effects may be due to the alleviation of oxidative stress that might induce cellular apoptosis and caspase-8 activation.58 These results suggest that CAM is a potential therapeutic candidate of autism via regulation of inflammatory and apoptotic pathways with leptin playing an essential role in alleviation of autistic behaviour through antioxidant activities.
Acknowledgments
The authors express extreme gratefulness and thanks to Dr Mohamed Salah, PCRC, MUST, for his kind supoprt.
Footnotes
Ethical approval and consent to participate: All the procedures described were carried out with approval and performed in accordance with the guidelines of the ethics committee of Faculty of Dentistry, Cairo University, Cairo, Egypt, and in accordance with the Guide for the Care and Use of Laboratory Animals.22
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: Self-funding.
ORCID iD: Mohamed Hamzawy  https://orcid.org/0000-0001-9018-025X
https://orcid.org/0000-0001-9018-025X
References
- 1. Christensen DL. (2016) Prevalence and characteristics of autism spectrum disorder among children aged 8 years – Autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveillance Summaries 65: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pérez-Samartín A. (2016) Acupuntura, láser y De Qi. Revista Internacional de Acupuntura 10: 16–19. [Google Scholar]
- 3. Carpenter LA, Boan AD, Wahlquist AE, et al. (2016) Screening and direct assessment methodology to determine the prevalence of autism spectrum disorders. Annals of Epidemiology 26: 395–400. [DOI] [PubMed] [Google Scholar]
- 4. Brentani H, Paula CSD, Bordini D, et al. (2013) Autism spectrum disorders: An overview on diagnosis and treatment. Revista Brasileira de Psiquiatria 35: S62–S72. [DOI] [PubMed] [Google Scholar]
- 5. Yui K, Kawasaki Y, Yamada H, et al. (2016) Oxidative stress and nitric oxide in autism spectrum disorder and other neuropsychiatric disorders. CNS & Neurological Disorders Drug Targets 15: 587–596. [DOI] [PubMed] [Google Scholar]
- 6. Perry SW, Norman JP, Litzburg A, et al. (2004) Antioxidants are required during the early critical period, but not later, for neuronal survival. Journal of Neuroscience Research 78: 485–492. [DOI] [PubMed] [Google Scholar]
- 7. Thorsen MB, Bilenberg N, Benedikz E, et al. (2016) Oxidative stress – A promising candidate in explaining the neurobiology of autism spectrum disorders. European Psychiatry 33: S182. [Google Scholar]
- 8. Bittker S. (2016) Antioxidant sulfur compounds: Potential therapies for autism? Journal of Autism 3: 1–5. [Google Scholar]
- 9. Carocho M, Ferreira IC. (2013) A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food and Chemical Toxicology 51: 15–25. [DOI] [PubMed] [Google Scholar]
- 10. Fernandes B, Dash S, Jacka F, et al. (2016) Leptin in bipolar disorder: A systematic review and meta-analysis. European Psychiatry 35: 1–7. [DOI] [PubMed] [Google Scholar]
- 11. Procaccini C, La Rocca C, Carbone F, et al. (2016) Leptin as immune mediator: Interaction between neuroendocrine and immune system. Developmental & Comparative Immunology 66: 120–129. [DOI] [PubMed] [Google Scholar]
- 12. Polyzos SA, Kountouras J, Mantzoros CS. (2015) Leptin in nonalcoholic fatty liver disease: A narrative review. Metabolism 64: 60–78. [DOI] [PubMed] [Google Scholar]
- 13. Meyer LR, Zhu V, Miller A, et al. (2014) Growth restriction, leptin, and the programming of adult behavior in mice. Behavioural Brain Research 275: 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agrawal RP, Sharma P, Gafoorunissa SJ. (2012) Effect of camel milk on glucose metabolism in adults with normal glucose tolerance and type 2 diabetes in Raica community: A crossover study. Acta Bio Medica Atenei Parmensis 82: 181–186. [PubMed] [Google Scholar]
- 15. Abdulrahman AO, Ismael MA, Al-Hosaini K, et al. (2016) Differential effects of camel Milk on insulin receptor signaling – Toward Understanding the insulin-like properties of camel milk. Frontiers in Endocrinology 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Al-Ayadhi LY, Elamin NE. (2013) Camel milk as a potential therapy as an antioxidant in autism spectrum disorder (ASD). Evidence-Based Complementary and Alternative Medicine 2013: 602834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mansour AA, Nassan MA, Saleh OM, et al. (2017) Protective effect of camel milk as anti-diabetic supplement: Biochemical, molecular and immunohistochemical study. African Journal of Traditional, Complementary, and Alternative Medicines 14: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Korashy HM, Maayah ZH, Abd-Allah AR, et al. (2012) Camel milk triggers apoptotic signaling pathways in human hepatoma HepG2 and breast cancer MCF7 cell lines through transcriptional mechanism. BioMed Research International 2012: 593195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu W-W, Kong G-Q, Ma M-M, et al. (2016) Short communication: Camel milk ameliorates inflammatory responses and oxidative stress and downregulates mitogen-activated protein kinase signaling pathways in lipopolysaccharide-induced acute respiratory distress syndrome in rats. Journal of Dairy Science 99: 53–56. [DOI] [PubMed] [Google Scholar]
- 20. Marić A, Kačarević ŽP, Čekić N, et al. (2015) Effects of between generations changes in nutrition type on vaginal smear and serum lipids in Sprague–Dawley rats. The Journal of Maternal-Fetal & Neonatal Medicine 29: 1491–1497. [DOI] [PubMed] [Google Scholar]
- 21. Ochiogu I, Uchendu C, Ihedioha J. (2008) A new and simple method of confirmatory detection of mating in albino rats (Rattus norvegicus). Animal Research International 3: 527–530. [Google Scholar]
- 22. Council NR. (2010) Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press. [Google Scholar]
- 23. Favre MR, Barkat TR, LaMendola D, et al. (2013) General developmental health in the VPA-rat model of autism. Frontiers in Behavioral Neuroscience 7: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Al-Asmari AK, Abbasmanthiri R, Al-Elewi AM, et al. (2014) Camel milk beneficial effects on treating Gentamicin induced alterations in rats. Journal of Toxicology 2014: 917608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jalali A, Morgan DA, Sivitz WI, et al. (2001) Does leptin cause functional peripheral sympatholysis? American Journal of Hypertension 14: 615–618. [DOI] [PubMed] [Google Scholar]
- 26. Sambrook JF, Russell DW. (2001) Molecular Cloning: A Laboratory Manual. New York: Cold Spring Harbor Laboratory. [Google Scholar]
- 27. Abdel-Aziem SH, Hassan AM, El-Denshary ES, et al. (2014) Ameliorative effects of thyme and calendula extracts alone or in combination against aflatoxins-induced oxidative stress and genotoxicity in rat liver. Cytotechnology 66: 457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu W, Wang G, Yakovlev AG. Identification and functional analysis of the rat caspase-3 gene promoter. Journal of Biological Chemistry. 2002; 277: 8273–8. [DOI] [PubMed] [Google Scholar]
- 29. Wiame I., Remy S., Swennen R., Sagi L., 2000. Irreversible heat inactivation of DNaseI without RNA degradation. Biotechniques 29: 252–256. [DOI] [PubMed] [Google Scholar]
- 30. Raben N, Nichols RC, Martiniuk F, et al. (1996) A model of mRNA splicing in adult lysosomal storage disease (glycogenosis type II). Human Molecular Genetics 5: 995–1000. [DOI] [PubMed] [Google Scholar]
- 31. Şerbetçi K, Uysal O, Erkasap N, et al. (2012) Anti-apoptotic and antioxidant effect of leptin on CCl4-induced acute liver injury in rats. Molecular Biology Reports 39: 1173–1180. [DOI] [PubMed] [Google Scholar]
- 32. Ebaid H, Abdel-Salam B, Hassan I, et al. (2015) Camel milk peptide improves wound healing in diabetic rats by orchestrating the redox status and immune response. Lipids in Health and Disease 14: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mabunga DFN, Gonzales ELT, Kim J-W, et al. (2015) Exploring the validity of valproic acid animal model of autism. Experimental Neurobiology 24: 285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Croonenberghs J, Bosmans E, Deboutte D, et al. (2002) Activation of the inflammatory response system in autism. Neuropsychobiology 45: 1–6. [DOI] [PubMed] [Google Scholar]
- 35. Lucchina L, Depino AM. (2014) Altered peripheral and central inflammatory responses in a mouse model of autism. Autism Research 7: 273–289. [DOI] [PubMed] [Google Scholar]
- 36. Schneider T, Przewłocki R. (2005) Behavioral alterations in rats prenatally exposed to valproic acid: Animal model of autism. Neuropsychopharmacology 30: 80–89. [DOI] [PubMed] [Google Scholar]
- 37. Tung EW, Winn LM. (2011) Valproic acid increases formation of reactive oxygen species and induces apoptosis in postimplantation embryos: A role for oxidative stress in valproic acid-induced neural tube defects. Molecular Pharmacology 80: 979–987. [DOI] [PubMed] [Google Scholar]
- 38. Zhang B, Wang X, Nazarali A. (2010) Ascorbic acid reverses valproic acid-induced inhibition of hoxa2 and maintains glutathione homeostasis in mouse embryos in culture. Cellular and Molecular Neurobiology 30: 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hishida R, Nau H. (1998) VPA-induced neural tube defects in mice. I. altered metabolism of sulfur amino acids and glutathione. Teratogenesis, Carcinogenesis, and Mutagenesis 18: 49–61. [PubMed] [Google Scholar]
- 40. Sandhya T, Sowjanya J, Veeresh B. (2012) Bacopa monniera (L.) Wettst ameliorates behavioral alterations and oxidative markers in sodium valproate induced autism in rats. Neurochemical Research 37: 1121–1131. [DOI] [PubMed] [Google Scholar]
- 41. Chauhan A, Gu F, Essa MM, et al. (2011) Brain region-specific deficit in mitochondrial electron transport chain complexes in children with autism. Journal of Neurochemistry 117: 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang Y, Yang C, Yuan G, et al. (2015) Sulindac attenuates valproic acid-induced oxidative stress levels in primary cultured cortical neurons and ameliorates repetitive/stereotypic-like movement disorders in Wistar rats prenatally exposed to valproic acid. International Journal of Molecular Medicine 35: 263–270. [DOI] [PubMed] [Google Scholar]
- 43. Narita N, Kato M, Tazoe M, et al. (2002) Increased monoamine concentration in the brain and blood of fetal thalidomide-and valproic acid–exposed rat: Putative animal models for autism. Pediatric Research 52: 576–579. [DOI] [PubMed] [Google Scholar]
- 44. Coutinho AM, Sousa I, Martins M, et al. (2007) Evidence for epistasis between SLC6A4 and ITGB3 in autism etiology and in the determination of platelet serotonin levels. Human Genetics 121: 243–256. [DOI] [PubMed] [Google Scholar]
- 45. Seo D, Patrick CJ, Kennealy PJ. (2008) Role of serotonin and dopamine system interactions in the neurobiology of impulsive aggression and its comorbidity with other clinical disorders. Aggression and Violent Behavior 13: 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maciejak P, Szyndler J, Kołosowska K, et al. (2014) Valproate disturbs the balance between branched and aromatic amino acids in rats. Neurotoxicity Research 25: 358–368. [DOI] [PubMed] [Google Scholar]
- 47. Liu M, Zhao Y, Zhang X. (2015) Knockdown of glutamate cysteine ligase catalytic subunit by siRNA causes the gold nanoparticles-induced cytotoxicity in lung cancer cells. PLoS ONE 10: e0118870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kwiecińska P, Taubøll E, Gregoraszczuk EŁ. (2012) Comparison of the effects of valproic acid and levetiracetam on apoptosis in the human ovarian cancer cell line OVCAR-3. Pharmacological Reports 64: 603–614. [DOI] [PubMed] [Google Scholar]
- 49. Patel AS, Zalcman SS. (2014) Interleukin-2 treatment induces an acquired behavioral response pattern (repetitive stereotyped movements) mediated by dopamine D1 and D2 receptors. International Neuropsychiatric Disease Journal 2: 175–185. [Google Scholar]
- 50. Fontoura P, Mello MD, Gallo-Sá P, et al. (2017) Leptin improves sperm cryopreservation via antioxidant defense. Journal of Reproduction & Infertility 18: 172. [PMC free article] [PubMed] [Google Scholar]
- 51. Yamagishi S-I, Edelstein D, Du X-L, et al. (2001) Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. Journal of Biological Chemistry 276: 25096–25100. [DOI] [PubMed] [Google Scholar]
- 52. Toro AR, Pérez-Pérez A, Gutiérrez IC, et al. (2015) Mechanisms involved in p53 downregulation by leptin in trophoblastic cells. Placenta 36: 1266–1275. [DOI] [PubMed] [Google Scholar]
- 53. Pérez-Pérez A, Toro AR, Vilarino-Garcia T, et al. (2016) Leptin reduces apoptosis triggered by high temperature in human placental villous explants: The role of the p53 pathway. Placenta 42: 106–113. [DOI] [PubMed] [Google Scholar]
- 54. Sharma C, Singh C. (2014) Therapeutic value of camel milk – A review. Advanced Journal of Pharmacie and Life Science Research 2: 7–13. [Google Scholar]
- 55. Al-Ayadhi LY, Elamin NE. (2015) Camel milk as a potential therapy as an antioxidant in autism spectrum disorder (ASD). In: Croft C. (ed.) Prenatal and Childhood Nutrition: Evaluating the Neurocognitive Connections. Oakville, ON, Canada: Apple Academic Press, pp. 345–361. [Google Scholar]
- 56. Limon A, Gallegos-Perez J-L, Reyes-Ruiz JM, et al. (2014) The endogenous GABA bioactivity of camel, bovine, goat and human milks. Food Chemistry 145: 481–487. [DOI] [PubMed] [Google Scholar]
- 57. Darwish HA, Raboh NRA, Mahdy A. (2012) Camel’s milk alleviates alcohol-induced liver injury in rats. Food and Chemical Toxicology 50: 1377–1383. [DOI] [PubMed] [Google Scholar]
- 58. Kruidenier L, Kuiper I, Lamers CB, et al. (2003) Intestinal oxidative damage in inflammatory bowel disease: Semi-quantification, localization, and association with mucosal antioxidants. The Journal of Pathology 201: 28–36. [DOI] [PubMed] [Google Scholar]