Abstract
Energy healing, or healing with intent, is a complementary and alternative medicine therapy reported to be beneficial with a wide variety of conditions. We are developing a delivery technology for a method previously tested in mouse models with solid tumors (the Bengston method) independent of the presence of a healer. The goal of this study was to assess whether stored or recorded energy has an impact on breast cancer cells in vitro, using energy-charged cotton and electromagnetic recording of healers practicing the method. Expression of genes involved in cancer and inflammation pathways was measured by quantitative reverse transcription polymerase chain reaction (qRT-PCR). Treatment of cells using energy-charged cotton resulted in statistically significant changes <1.5-fold. In cells exposed to an electromagnetic recording, 37 genes of 167 tested showed a >1.5-fold change when compared to the control, and 68 genes showing statistically significant fold changes. Two genes, ATP citrate lyase (ACLY) and interleukin 1β (IL-1β), were consistently downregulated at 4 and 24 hours of exposure to the recording, respectively, in 3 independent experiments. Both ACLY and IL-1β were also downregulated in cells exposed to a hands-on delivery of the method, suggesting these 2 genes as potential markers of the healing method.
Keywords: energy medicine, audio, complementary and alternative medicine, breast cancer
Introduction
Virtually all recorded societies report that certain individuals appear to have the ability to heal. Oftentimes this healing has been associated with spiritual disciplines of one sort or another, and the healers themselves have sometimes been accorded a special status within the culture. Healers have utilized various methods of practice, including laying on of hands, prayer, and induced altered states of consciousness, to name a few. The father of Western medicine, Hippocrates, referred to this healing as “the force which flows from many people’s hands.”1
Historically, the source and mechanism of the healing evaded systematic research even as the number of clinical cases greatly increased. The foundation of modern Western healing research can be traced to the pioneering work of the late biologist Bernard Grad at McGill University. In carefully controlled experiments, Grad found that selected healers could influence the germination of plant seeds, the growth rate of plants, and the curing of seeds that had been shocked by saline solution. In addition, he was able to measure the ability of healers to reduce goiter and stimulate wound healing in mice.2–4
Since Grad’s initial work, there have been innumerable preclinical studies of healing, sometimes categorized by the target of the intended healing. Benor, for example, discusses healing action on enzymes, cells in the laboratory, fungi/yeasts, bacteria, plants, single-cell organisms, and animals that have been subjected to controlled study.5
The proliferation of healing studies has continued to rise in recent years. At present, there are several peer-reviewed journals devoted exclusively to the burgeoning field of complementary and alternative medicine (CAM), publishing both preclinical and controlled clinical studies of healing of a wide variety of conditions. In addition, there is an increasing number of peer-reviewed journals which are not focused exclusively on CAM but that are open to publishing controlled studies in these areas.
Presently, there are a wide variety of healing methods in active practice, the most well known probably being Reiki. There is virtually nothing known about how the various methods of healing converge or diverge in terms of healing efficacy. Nor are there data which can distinguish whether there are differing mechanisms of action utilized by the different healing methods. Anecdotally, Reiki, for example, is thought to be efficacious for pain and anxiety, but not for conditions such as cancer. The method utilized in the present study seems to be somewhat unique in that it has a history of apparent efficaciousness with some cancers as well as a variety of other disorders.
The Bengston Energy Healing Method
The term “energy healing” is somewhat presumptuous in that no “energy” has been isolated in any healing modality. The use of the term “energy” in the Bengston method is for practical reasons, in order to conform with common parlance. The method came about 45 years ago, when Bengston began to observe a New York-based healer treating a wide variety of conditions in a clinical setting and to investigate the possibility of developing specific healing techniques which might become reproducible by others.
After years of exploration, a method of very rapid imaging was developed that seemed to be efficacious in others reproducing the patterns produced by the original healer. Detailed descriptions of these rapid imaging techniques have been described elsewhere.6,7 Among the more interesting observations with the New York-based healer as well as those who practiced the rapid imaging healing technique was clinical success in malignant growths and lack of success with benign growths. Equally interesting, the healing could apparently be transferred and “stored” in materials and applied later without the healer present. These patterns were similar to those found experimentally by Grad, with cotton, water, and crystals apparently being especially receptive to healing storage.
In the mid-to-late 1970s, Bengston began looking to follow Grad’s experimental approach to healing and moved into systematic experimental work with animals. With David Krinsley and Marvin Wasserman at Queens College of the City of New York, Bengston started to investigate the effects of the healing techniques on biological models widely used in conventional research. The syngeneic model of C3H/HeJ mice engrafted with mammary carcinoma cell line H2712 was used, which normally results in 100% fatality of recipient mice within 27 days.
These in vivo experiments involved inexperienced and skeptical faculty and student volunteers trained for 6 weeks in the Bengston method placing their hands around mice cages for approximately 1 hour per day while practicing the rapid imaging techniques. Instead of the predicted fatality within 27 days, they found reliable full lifespan cures of the mice.8–11 Engrafted mice developed tumors within 14 days, as expected. Around day 21, tumors in treated mice started to ulcerate, and between day 28 and 35, tumors resorbed until full remission.
Because of the unconventional nature of the research, Bengston insisted on independent replication by disinterested researchers in independent labs. As of this writing, more than a dozen healing experiments using the Bengston Energy Healing method on this cancerous mouse model have been conducted in 6 independent labs.6,7
Subsequent to the determination that the healing effect was reliable, secondary correlates involving the effect of distance, dose, and whether healing can be stored were introduced. In brief, healing does not seem to diminish with distance; there is a minimum dose necessary to produce the healing effect; and treated water and cotton seem to be able to reproduce cancer cures without further human intervention (personal communication: William Bengston, PhD, 2015).
Studies of the biological effects of the technique on human healers and healees have been conducted to investigate its effects on brain activity by electroencephalogram (EEG)12 and functional magnetic resonance imaging.13 Those studies indicate reliable physiological changes in both healer and healee. Paired recordings of the healer and subject done both with the subject at a distance and in proximity to the healer showed harmonic frequency coupling across the spectra, followed by between-individual EEG frequency entrainment effects, and then by instantaneous EEG phase locking. These results suggest the presence of a connection between the healer and healee and may thus provide a mechanism for phase coupling.12 This connection seems to be stimulated by the needs of the healee more than the conscious intention of the healer.13
Additional physical correlates to healing using the Bengston method have included anomalous magnetic micropulsations14 in the surrounding space of healing events which may indicate a reduction in entropy when healing occurs. If this is the case, then perhaps it is “information” rather than “energy” that is being transmitted in the healing process. And, if it is indeed information, that would be consistent with the apparent ability to store healing in materials.
The present study investigates whether “stored” and “recorded” healing intention can induce transcriptional changes in vitro. Success in this line of research would be a preliminary step to the development of a scalable delivery system for healing.
Material and Methods
Cell Culture
Human breast carcinoma cells MDA-MB-231 (ATCC) were cultured in dubelcco's modified eagle medium (DMEM) medium (Gibco, Thermo Fisher, Waltham, Massachusetts) supplemented with 10% fetal bovine serum (FBS; Gibco) and maintained at 37°C in a humidified atmosphere with 5% CO2. The murine mammary tumor cells 4T1 (ATCC) were cultured in Roswell Park Memorial Institute Medium (RPMI) (Gibco) supplemented with 10% FBS in the same conditions and were used for the hands-on treatment.
Exposure to Energized Cotton
Sterilized 4 oz cotton rolls were acquired from a local pharmacy and treated for 30 minutes by healers trained in the Bengston method.6 100 000 cells (MDA-MB-231) were seeded in 6-well plates. After 18 hours, a 4 × 6 in piece of treated cotton was placed on top of the 6-well plates for 24 hours. Cells were collected in TRIzol and processed for qRT-PCR analysis (see below). For the control, cells were not exposed to cotton.
Recording of Energy Healing Activity
Three people practiced the Bengston healing method on cotton inside a solid steel, double-walled, electromagnetically shielded chamber (Series 81 Solid Cell; ETS-Lindgren, Cedar Park, Texas) for 5 minutes. As the treatment of cotton took place, magnetic and electromagnetic signals were recorded using 4 types of sensors: (1) 11 three-axis magneto-resistive sensors (Honeywell model HMC2003, sensitivity DC-1 KHz), (2) 2 antennas recording electromagnetic fields above 10 KHz, (3) a geomagnetometer (model IDR-321, sensitivity 8 DC-500 Hz; Integrity Design & Research, Essex Junction, Vermont), and (4) 2 custom-fabricated “Caduceus” coils, designed to cancel out transverse electromagnetic waves. Each of these 38 analog signals was digitized by a 24-bit analog-to-digital converter at 44.1 KHz (model Motu 24ai; Motu, Inc, Cambridge, Massachusetts) and custom PC software converted and saved the incoming sensor signals into the .wav audio format.
Exposure to Energy Recordings
1 × 106 cells (MDA-MB-231) in three T25 flasks were placed in an incubator at 37°C for 5 minutes to 72 hours. Two passive speakers (PCB4 K; Pyle Home, Brooklyn, New York) were placed inside the incubator and connected in phase with 18AWG speaker wires (Southwire, Carrollton, Georgia) to an amplifier (PTAU45, Pyle), itself plugged into the speaker connector of a computer from which the recording was played repeatedly using Windows Media Player. The volume of the computer and the player was at 50% setting. The volume of the amplifier was set at 50%. Speakers were placed facing down on the top shelf and cells were placed on the lower shelf, directly underneath the speakers. In control settings, the same protocol was followed except that no recording was played, using the ambient background noise as control conditions.
Hands-On Treatment of Cancer Cells
750 000 cells (MDA-MB-231 and 4T1) were plated in T25 flasks and placed in a plastic chamber in the incubator for 18 hours before initiation of treatment. For the treatment, 3 healers practiced the Bengston method: 1 healer held the chamber, while the 2 other performed the treatment remotely. During the whole time, the 3 healers were on a conference video call. Treatments took place in sessions of 1 hour. For the 2-hour treatment, cells were treated twice 1 hour with a 30-minute break. For the 4-hour treatment, cells were treated in the same fashion on 2 consecutive days. Between treatment, cells were placed back in the incubator. At the end of treatment, cells were collected in TRIzol (Thermo Fisher, Waltham, Massachusets) and samples were processed as detailed below.
Gene Expression Analysis by qRT-PCR
After exposure to treatment (treated cotton, recording, or hands-on), cells were collected in TRIzol and total messenger RNA was extracted following the manufacturer’s instructions. Complementary DNA (cDNA) was synthetized using RT2 First Strand Kit (Qiagen, Germantown, Maryland) from 250 ng of RNA and expression of genes was analyzed using RT2 Profiler PCR Arrays (Cancer Pathway Finder; cancer inflammation and immunity vrosstalk) and RT2 pPCR Primer Assay (Qiagen) according to the manufacturer’s instructions. One microliter of cDNA was used in the PCR reactions. Results were analyzed using the ΔΔCt method.
Statistical Analyses
Student t test was used to determine P values between control group (nontreated/nonexposed) and experimental group (treated/exposed). Value of P less than 0.05 were considered significant. For PCR, Ct values were analyzed using the Data Resources Center (http://Qiagen.com) and fold changes (2−ΔΔCT) were determined as the normalized gene expression (2−ΔCT) in the experimental sample divided the normalized gene expression (2−ΔCT) in the control sample. P values were calculated based on a Student t test of the replicate 2−ΔCT values for each gene in the control group and treatment groups.
Results
Effect of Energized Charged Cotton on Cell Growth and Cancer-Related Gene Expression
Results from previous studies suggest that the Bengston method seems to exhibit anticancer activity. Cotton appears to be able to store the energy derived from this method and deliver these anticancer properties. Building on these observations and the previous work by Grad, we investigated whether charged cotton could induce measurable changes in cancer cells in vitro using MDA-MB-231 carcinoma cells. Because the mechanism of the therapeutic effect of the Bengston method is unknown, we initially screened for markers using a qRT-PCR assay comprising 84 genes involved in cell growth and cancer. The list of genes is available in supplemental data S1. Cells were exposed to cotton treated by the Bengston method for 24 hours. The control consisted of cells not exposed to cotton.
Significant change in expression was found in 6 genes: CASP9, E2F4, HMOX1, IGFBP3, MCM2, and PPP1R15A (Table 1), suggesting the viability of the storage of healing information. While statistically significant, these changes in expression were all <2, suggesting a modest effect of cotton on transcriptional activity in MDA-MB-231 cells. The next step focused on the evaluation of a recording of the healing process.
Table 1.
Genes for which expression was significantly affected by exposure to energized cotton.a
| Gene Description | Gene Symbol | Energized Cotton | |
|---|---|---|---|
| Fold Change | P Value | ||
| Caspase 9, apoptosis-related cysteine peptidase | CASP9 | –1.241 | .017 |
| E2F transcription factor 4, p107/p130-binding | E2F4 | 1.123 | .023 |
| Heme oxygenase (decycling) 1 | HMOX1 | –1.310 | .034 |
| Insulin-like growth factor binding protein 3 | IGFBP3 | 1.181 | .016 |
| Minichromosome maintenance complex component 2 | MCM2 | 1.320 | .020 |
| Protein phosphatase 1, regulatory (inhibitor) subunit 15A | PPP1R15A | –1.435 | .008 |
aFold changes were obtained with the 2−ΔΔCT method, where the control was cells not exposed to cotton. The table indicates the gene name, a brief description, the fold change of expression when compared to the control, and the P value from triplicates.
Recordings of Healing Energy
In an attempt to capture the energy transferred when healers practice the Bengston method, a custom-made apparatus was designed at the Institute of Noetic Sciences. In brief, it allowed the recording of energy fields using multiple sensors while healers practiced the Bengston method to charge substances such as cotton. To isolate from surrounding contaminants, the system was set inside a Faraday chamber. Recorded data were converted into audio files and spectral and temporal analyses were performed to identify potential variations between a control situation (empty chamber or chamber containing a neutral material) and an active situation (healers practicing the Bengston method inside the chamber). Some interesting short-term temporal effects were observed in some cases versus controls, and these may be the subject of further study. However, here we focus on the more consistently observed spectral changes. Recording R18, where 3 healers were engaged in treating pieces of cotton, showed the most consistent spectral differences versus the control.
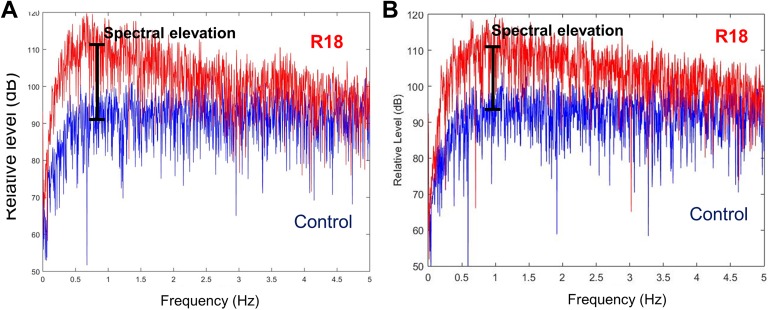
Spectral analyses covered the frequency range from below 0.1 Hz to about 20 KHz. For recording R18, the only region where significant differences were observed was at frequencies below 20 Hz, and in particular below 5 Hz. For all 11 3-axis magnetometers used to record the activity, 1 data channel (y-axis) indicated significantly elevated spectral content in the frequency range 0.25 Hz to about 3 Hz, as compared to control recordings. These elevations in spectral levels between R18 and control results were typically 6 dB or more, and in some cases 20 dB or more. Figure 1 illustrates the spectral differences in the very low frequency range for 2 independent magnetometers.
Figure 1.
Illustration of differences observed at low frequencies in R18 recording compared to control recording. Energy from 3 healers simultaneously charging cotton inside a Faraday chamber (R18, red) was recorded on 38 channels and data were converted into an audio format as .wav. The spectra from these recordings were compared to a control situation (control, blue, Faraday chamber containing a crystal). The graphs represent the spectra of R18 and control from sensor A (A) and sensor B (B). The vertical bar indicates a spectral elevation of approximately 20 dB for sensor A and 15 dB for sensor B in the frequency range between 0.5 and 1 Hz. Only in recording R18 was this elevation observed for all 11 magnetometers used in the recording apparatus.
Recording R18 Induces Gene Expression Changes in Breast Cancer Cells
Because R18 displayed highly unusual spectral content, we then investigated whether this recording could produce biological activity. Of note, the spectral elevation is located in the range of very low frequencies, thus inaudible to humans. For this reason, the control used in this series of experiment was environmental background noise. We exposed MDA-MB-231 cells to R18 and looked for gene expression changes by qRT-PCR. For the time of the exposure, cells were placed in an incubator containing 2 speakers plugged to a sound system described in Material and Methods. The control consisted of cells placed for the same amount of time in the incubator while the whole system was on but no recording was playing. Thus, any potential interference from the speakers or the amplifier was present in both the control and experimental conditions.
Initially, cells were exposed to R18 for 5 minutes, 30 minutes, 4 hours, 24 hours, 48 hours, or 72 hours. Expression of 84 genes involved in cancer pathways was screened by qRT-PCR, using the same assay as for cells treated with cotton, as described above. Previous studies suggested that there may be an active immune response induced by hands-on healing; thus, we also screened 84 genes involved in the crosstalk between immunity and cancer. Of over 167 genes tested, exposure to R18 induced statistically significant changes (P < 0.05) in the expression of 68 genes when compared to the control, for at least 1 time point tested. For 37 of these genes, the change was >1.5-fold. This interesting result suggests that the recording may exert an activity on cancer cells, though there was no clear pathway emerging from the genes affected.
There were 17 genes selected for further analysis in 2 new independent experiments based on the amplitude of the fold change, the significance of the change, and the number of time points where the changes were observed. Among them, the gene ACLY coding for ATP citrate lyase (ACLY), was consistently and significantly decreased at 4 hours of exposure to R18 in the 3 independent experiments (Table 2). ATP citrate lyase is an enzyme involved in lipid metabolism that plays a role in cancer growth. Expression of ACLY tends to decrease as early as after 5 minutes of exposure to R18 and increase after 24 hours (Table 2). In addition, IL-1β, coding for Interleukin 1 β, an important immune mediator, showed a trend to decrease at 4 hours and was consistently and significantly decreased at 24 hours of exposure to R18 (Table 2). These results identify ACLY and IL-1β as possible markers of recording R18 activity. The cells exposed to R18 did not show any phenotypical change as it relates to growth rate, apoptosis, or morphology, compared to the control samples.
Table 2.
Fold change of expression of ACLY and IL-1β in breast cancer cells exposed to R18.a
| A | ACLY | |||||
|---|---|---|---|---|---|---|
| Exp 1 | Exp 2 | Exp 3 | ||||
| Fold Change | P Value | Fold Change | P Value | Fold Change | P Value | |
| 5 minutes | –1.66 | .0004 | ||||
| 30 minutes | –1.86 | .00003 | –1.17 | .2876 | ||
| 4 hours | –1.352 | .011 | –2.11 | .00007 | –1.64 | .0009 |
| 24 hours | 1.3849 | .003 | –1.11 | .6005 | ||
| IL1β | ||||||
| Exp 1 | Exp 2 | Exp 3 | ||||
| B | Fold Change | P Value | Fold Change | P Value | Fold Change | P Value |
| 4 hours | –1.32 | .015 | –1.2 | .49088 | –1.27 | .0505 |
| 24 hours | –1.73 | .00157 | –1.61 | .0032 | ||
aMDA-MB-231 cells were exposed to R18 for 5 minutes, 30 minutes, 4 hours, and 24 hours. Expression of ACLY was analyzed by qRT-PCR using RT2 Primer assay and associated products (Qiagen). The tables indicate the fold changes and associated P values (triplicates) when comparing cells exposed to R18 to control cells in 3 independent experiments at the specified time points tested. Values of P < 0 .05 are indicated in red and fold changes >|1.5| are indicated in blue. ACLY indicates ATP citrate lyase; IL-1β, interleukin 1β.
Transcriptional Changes of ACLY and IL-1β in Cells Exposed to Hands-On Healing
The identification of reproducible changes induced by R18 led us to assess the expression of these genes in cells treated directly using the hands-on Bengston method. We reproduced conditions in which R18 was recorded, that is, 3 healers practicing the Bengston method. MDA-MB-231 cells were exposed for 1, 2, or 4 hours to the healers. To accommodate the healers’ proceedings and the time outside the incubator, the exposure was performed in sessions of 1 hour. Samples exposed for 2 hours resulted from 2 sessions of 1 hour with 30 minutes rest in between, and the 4-hour exposure consisted in the same procedure, repeated 2 days in a row. Results are presented in Table 3. Interestingly, ACLY was decreased to the same extent as with R18 at 1 and 2 hours of exposure. IL-1β however did not show significant expression change (data not shown).
Table 3.
Fold change of expression of ACLY and IL-1β in cells treated with hands-on healing.a
| ACLY | IL1β | |||
|---|---|---|---|---|
| Fold Change | P Value | Fold Change | P Value | |
| 1 hour | –1.33 | .004 | –4.07 | 3.00E –06 |
| 2 hours | –1.31 | .004 | –2.24 | .00032 |
| 4 hours | –1.03 | .903 | 1.39 | .10371 |
aCells were treated with hands-on Bengston method as described in the Material and Methods section for 1, 2, or 4 hours in triplicate. The tables indicate fold changes of expression of ACLY in MDA-MB-231 cells and IL-1β in 4T1 cells. P < 0.05 are indicated in red and fold changes >|1.5| are indicated in blue. ACLY indicates ATP citrate lyase; IL-1β, interleukin 1β.
As the next step of these investigations will be to test the anticancer activity of R18 in vivo, we also tested the expression of ACLY and IL-1β in 4T1 cells (murine breast cancer cells) that can be used as a syngeneic model in cancer in Balb/c mice. In these cells, IL-1β was decreased 2 to 4 fold, suggesting a greater effect of the healers themselves compared to R18. Expression of ACLY was not affected in this cell line (data not shown). The effect of hands-on healing seemed to decrease with exposure time for both genes in both cell lines, as there was no significant change at 4 hours of treatment.
Discussion
This study represents the first steps of the development of an alternative delivery method of the Bengston healing method in order to be able to benefit from the therapeutic effect independent of the healers. The goal was to identify a vehicle for the healing that could be studied in laboratory settings and produce consistent changes in a living model compared to a control situation. We started these experimentations in vitro using cancer cells in culture to screen by qRT-PCR changes occurring at the transcriptional level.
The first vehicle that was investigated was cotton, as it has been clinically used to transfer the “energy” and reproduce healing properties previously. However, we did not identify strong transcriptional changes in breast cancer cells treated with cotton compared to control cells.
We then assessed another method of storage by recording magnetic and electromagnetic signals originated from healers practicing the Bengston method. This was done in collaboration with the Institute of Noetic Sciences which engineered the sensors and a conversion module to produce recording in an audio format, all inside a Faraday chamber. The recording of 3 healers charging cotton induced singular signals in low frequencies that were not observed in control conditions, such as an empty chamber or the chamber containing untreated cotton. Previous studies have shown that low-frequency signals, or infrasound, can increase sensitivity to chemotherapy and increase cell permeability.15 This recording (R18) was selected for further investigations.
For the purpose of screening, we used 2 qRT-PCR assays that include each 84 genes involved in cancer pathway and cross talk between cancer and immunity, respectively, for a total of 167 genes tested. When cells were exposed to R18 at various time points, 68 genes showed a significant fold change (P < 0.05), of which 37 were >1.5. Most of these genes belonged to the cancer pathway assay. This observation was somewhat expected, as we used cancer cells and not immune cells. Among those selected for confirmation in independent experiments, 2 genes presented a significant and consistent pattern: ACLY and IL-1β.
ATP citrate lyase is an enzyme that catalyzes the conversion of citrate to cytosolic acetyl-CoA, important for lipid metabolism. This enzyme is frequently found dysregulated in cancer cells as they have an important need to produce membranes and increased lipid-based posttranslational modification of proteins. Inhibition of ACLY by small interfering RNA or chemical inhibitor reduced tumor growth of lung adenocarcinoma in nude mice.16 ATP citrate lyase is overexpressed in breast cancer cell lines compared to normal cells17 and its inhibition was recently shown to suppress growth of breast cancer cells,18 making it an attractive target for the treatment of breast cancer. The decrease in ACLY observed in this study may indicate an anticancer effect of the recording.
Interleukin 1β is an important mediator in immunity that stimulates inflammatory cytokines and amplifying inflammation. It is normally produced by monocytes, but it was shown to be upregulated in a number of solid tumors, including breast cancer.19 Interleukin 1β was shown to increase invasiveness and growth of breast cancer cells in vitro and in vivo.20 Interleukin 1β produced by monocytes of patients with renal cell carcinoma was associated with invasion and tumor growth.21 The decrease in IL-1β expression observed when cells were exposed to the recording was modest in this study. However, it was consistent across 3 independent experiments.
It is important to note that we did not demonstrate the healing properties of recording R18. The transcriptional changes indicate that R18 may have a biological effect on cancer cells. The therapeutic impact of the recording remains to be elucidated. Further studies will build on this work to investigate the use of ACLY and interleukin-1β as marker of the biological effect of the recording, a key step to proceed to the evaluation of its activity. Finally, we investigated whether the expression of these 2 genes was affected when cells were treated directly by hands-on Bengston method.
Expression of both ACLY and IL-1β were affected in human and mouse breast cancer cell line, respectively. This does not confirm the 2 genes as markers of the Bengston method but strengthen the previous findings and support the need to continue the explorations.
Interestingly, the transcriptional changes associated with the hands-on method occur earlier (1-2 hours) than with the recording (4-24 hours). For IL-1β, the amplitude of the change was much greater when using the hands-on method than the recording. These 2 observations combined suggest that some “information” may be lost in the process of recording and delivering the energy, compared to when it is delivered directly by healers.
While suggesting a potential therapeutic effect of the recording, this study emphasizes the need for a more complex model including immune cells to study the effect of the recording on tumor growth. To continue the development of a reverse-engineered energy-based therapeutic, we are planning on exposing mice engrafted with breast tumor cells to the recordings and measuring tumor development. Another area of interest is the effect of this recording on wound healing. In vitro (wound assay) and in vivo (eg, incision model) studies should be conducted to possibly identify a therapeutic indication and/or understand the mechanism of action.
Experiments related herein show that genomic changes can be induced in cultured transformed cells directly by contact with a healer and by the exposure to a recording of this energy. The genes demonstrating energy-induced changes under both conditions (ACLY, IL-1β) have been shown to play a role in cancer and/or the body’s response to cancer.
There is little doubt but that some biological response has been stimulated by both the healer and the recording of healing energy. The consequences of that genomics effect, and the careful evaluation of the several components to induce them, require further study. Multiple questions need to be addressed, such as exposure time of subjects to energy recordings, the composition of the recording itself, what equipment can best capture the healer’s input, and so on. Efforts to find answers to these questions are underway.
In summary, the following suggestive conclusions may be drawn from our results:
Reproducible biologic changes have been induced by healing energy, whether by direct hands-on healing or using a recording of healing activity.
Healing intention can be captured and released, thereby potentially allowing the phenomenon to be more widely disseminated.
Hands-on delivery of the healing intention is stronger than with the recording used in this study, suggesting the possibility that the recording did not fully capture the healing potential.
Supplemental Material
Suppementa_data for Transcriptional Changes in Cancer Cells Induced by Exposure to a Healing Method by Sarah Beseme, William Bengston, Dean Radin, Michael Turner, and John McMichael in Dose-Response
Acknowledgments
The authors thank David Dominik for both his support and suggestions for the hands-on in vitro studies. The authors thank Tanja Wright for her technical assistance in the qRT-PCR arrays.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded through donations from The Emerald Gate Charitable Trust.
Supplemental Material: Supplementary material for this article is available online.
Reference
- 1. Schiegl H. Healing Magnetism. Freiburg, Germany: Hermann Verlag KG; 1983. [Google Scholar]
- 2. Grad B, Cadoret R, Paul G. An unorthodox method of treatment on wound healing in mice. Int J Parapsychol. 1961;3:5–24. [Google Scholar]
- 3. Grad B. A telekinetic effect on plant growth. Int J Parapsychol. 1964;6:473–498. [Google Scholar]
- 4. Grad B. A telekinetic effect on plant growth II experiments involving treatment of saline in stoppered bottles. Int J Parapsychol. 1963;5:114–133. [Google Scholar]
- 5. Benor D. Healing Research: Holistic Medicine and Spirituality. Research in Healing Vol 1. Munich, Germany: Helix Editions; 1992. [Google Scholar]
- 6. Bengston WF. Commentary: a method used to train skeptical volunteers to heal in an experimental setting. J Altern Complement Med. 2007;13(3):329–331. doi:10.1089/acm.2007.6403. [DOI] [PubMed] [Google Scholar]
- 7. Bengston WF. Speaker. Hands on Healing: A Training Course in the Energy Cure. Louisville, CO: Sounds True Publishers; 2010. [Google Scholar]
- 8. Bengston WF, Krinsley D. The effect of the “laying on of hands” on transplanted breast cancer in mice. J Sci Explor. 2000;14(3):353–364. [Google Scholar]
- 9. Bengston WF. Some implications of the reported effects of Johrei on the viability and proliferation of cultured cancer cells in vitro. J Altern Complement Med. 2012;18(3):201 doi:10.1089/acm.2012.0021. [DOI] [PubMed] [Google Scholar]
- 10. Bengston W, Moga M. Resonance, placebo effects and type II errors: some implications from healing research for experimental methods. J Altern Complement Med. 2007;13(3):317–327. doi:10.1089/acm.2007.6300. [DOI] [PubMed] [Google Scholar]
- 11. Bengston WF. Spirituality, connection, and healing with intent: reflections on cancer experiments on laboratory mice In: Miller L, ed. The Oxford Handbook of Psychology and Spirituality. New York, NY: Oxford University Press; 2012. [Google Scholar]
- 12. Hendricks L, Bengston WF, Gunkelman J. The healing connection: EEG harmonics, entrainment, and Schumann’s resonances. J Sci Explor. 2010;24(4):655–666. [Google Scholar]
- 13. Bengston W. Some reflections on consciousness, intention, and healing: Dunne B, Jahn R. eds. Consciousness and the Life Force. Princeton, NJ: ICRL Press; 2017. [Google Scholar]
- 14. Moga M, Bengston WF. Anomalous DC magnetic field activity during a bioenergy healing experiment. J Sci Explor. 2010;24(3):397–410. [Google Scholar]
- 15. Rachlin K, Moore DH, Yount G. Infrasound sensitizes human glioblastoma cells to cisplatin-induced apoptosis. Integr Cancer Ther. 2013;12(6):517–527. doi:10.1177/1534735412465641. [DOI] [PubMed] [Google Scholar]
- 16. Hatzivassiliou G, Zhao F, Bauer DE, et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer cell. 2005;8(4):311–321. doi:10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 17. Yancy HF, Mason JA, Peters S, et al. Metastatic progression and gene expression between breast cancer cell lines from African American and Caucasian women. J Carcinog. 2007;6:8 doi:10.1186/1477-3163-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang D, Yin L, Wei J, Yang Z, Jiang G. ATP citrate lyase is increased in human breast cancer, depletion of which promotes apoptosis. Tumour Biol. 2017;39(4):1–10. doi:10.1177/1010428317698338. [DOI] [PubMed] [Google Scholar]
- 19. Lewis AM, Varghese S, Xu H, Alexander HR. Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. J Transl Med. 2006;4:48 doi:10.1186/1479-5876-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oh K, Lee OY, Park Y, Seo MW, Lee DS. IL-1β induces IL-6 production and increases invasiveness and estrogen-independent growth in a TG2-dependent manner in human breast cancer cells. BMC Cancer. 2016;16(1):724 doi:10.1186/s12885-016-2746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chittezhath M, Dhillon MK, Lim JY, et al. Molecular profiling reveals a tumor-promoting phenotype of monocytes and macrophages in human cancer progression. Immunity. 2014;41(5):815–829. doi:10.1016/j.immuni.2014.09.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppementa_data for Transcriptional Changes in Cancer Cells Induced by Exposure to a Healing Method by Sarah Beseme, William Bengston, Dean Radin, Michael Turner, and John McMichael in Dose-Response