Abstract
Exercise pulmonary hypertension (ePH) is an underappreciated form of exertional limitation. Despite normal resting pulmonary artery pressures, patients with ePH demonstrate early pulmonary vascular changes with reduced pulmonary arterial compliance (PAC) and vascular distensibility (α). Recent data suggest that targeted vasodilator therapy may improve hemodynamics in ePH, but it is not well-known whether such medications alter pulmonary vascular distensibility. Thus, we sought to evaluate if vasodilator therapy improved α a marker of early pulmonary vascular disease in ePH. Ten patients performed supine exercise right heart catheterization (exRHC) with bicycle ergometer to peak exercise. Patients diagnosed with ePH were treated with pulmonary vasodilators. A repeat symptom-limited exercise RHC was performed at least six months after therapy. Patients with ePH had evidence of early pulmonary vascular disease, as baseline PAC and α were reduced. After pulmonary vasodilator therapy, a number of peak exercise hemodynamics statistically improved, including a decrease of total pulmonary resistance and pulmonary vascular resistance, while cardiac output increased. Importantly, vasodilator therapy partially reversed the pathogenic decreases of α at the time of repeat exRHC. Pulmonary vascular distensibility, α, a marker of early pulmonary vascular disease, improves in ePH after therapy with pulmonary vasodilators.
Keywords: early pulmonary vascular disease, exercise hemodynamics, exercise pulmonary hypertension, pulmonary hypertension, vascular distensibility
Introduction
Exercise pulmonary hypertension (ePH) is an underappreciated form of exertional limitation. Recent work has demonstrated that the pressure-flow relationship of mean pulmonary artery pressure (mPAP) to cardiac output (CO) measured during invasive incremental exercise delineates normal from abnormal hemodynamics.1–5 ePH likely resides on a continuum between normal health and manifest pulmonary arterial hypertension (PAH), though it is unclear if ePH exists as a distinct clinical entity. Despite normal resting PAPs, ePH patients have evidence of early pulmonary vascular changes.1,4,6–10 Pulmonary artery compliance (PAC) has been identified as a marker of early pulmonary vascular disease. PAC is reduced in resting pulmonary arterial hypertension (PAH) and inversely correlates with pulmonary vascular resistance (PVR) at rest and with exercise.11 PAC is also decreased in ePH patients compared to controls.6,11
The mechanical descriptor alpha (α), a measure of pulmonary vascular distensibility, is a validated and sensitive indicator of early pulmonary vascular disease and is reduced in patients with ePH.6 The distensibility of the resistive pulmonary vessels is abnormally decreased and cannot accommodate the increased blood flows in pulmonary vascular disease, resulting in a reduced α value. Alpha is a dynamic measurement of the percentage change in diameter per mmHg increase in the distending pressure of the pulmonary vasculature. Alpha can be calculated from the right heart catheterization (RHC) measures, mPAP, total pulmonary resistance (TPR), and pulmonary artery wedge pressure (PAWP) over a range of CO measurements.6,12 Resting PAC directly correlates with exercise α in patients with ePH.6 Clinically α correlates with peak oxygen consumption (VO2) during exercise testing and was a strong predictor of cardiovascular survival independent of VO2 in the heart failure population.13 It has been shown to predict future PH in patients with connective tissue disease.14 Alpha may complement to our understanding of early pulmonary vascular disease in at-risk patients and help define abnormal pulmonary vascular response to exercise. Pulmonary vascular distensibility can be modulated by chronic phosphodiesterase-5 inhibition in patients with heart failure with reduced ejection fraction.13 It is currently unclear, however, whether α is a static measure or modifiable with pulmonary vasodilator therapies in patients with normal resting cardiopulmonary hemodynamics and a diagnosis of ePH.15
Endothelial dysfunction may contribute to the pathology of ePH as endogenous nitric oxide production during exercise is reduced, which may contribute to the abnormal pulmonary vascular response to exercise.16,17 It may be rational to target endothelial dysfunction and abnormal pulmonary vascular response to exercise with pulmonary vasodilators in an attempt to restore endothelial function. There are limited data demonstrating a beneficial response to pharmacologic intervention in ePH. Two open-label studies demonstrated improved hemodynamics after initiation of the endothelial receptor antagonist ambrisentan in ePH, despite differences in the hemodynamic definition of ePH.18,19 Other studies have shown that treatment with pulmonary vasodilators was safe and effective.20–22
We hypothesize that patients with an abnormal pulmonary vascular response to exercise would experience hemodynamic improvement after treatment with pulmonary vasodilators, and in particular α, a measure of pulmonary resistive vessel distensibility, would increase after therapy.
We retrospectively analyzed ten patients that performed symptom-limited, incremental supine exercise RHC (exRHC) to test whether treatment with pulmonary vasodilators in a mixed cohort would improve cardiopulmonary hemodynamics. Patients were diagnosed with ePH according to current accepted hemodynamic recommendations.2,4,5 Patients were treated with pulmonary vasodilators and returned for a repeat exRHC for a reassessment of the pulmonary vascular response.
Materials and methods
Exercise right heart catheterization
Before exercise testing, a PA catheter (Edwards, Irvine, CA, USA) was placed in the right internal jugular vein under ultrasound guidance. The zero reference level was mid thoracic.23 After a discussion with the patient regarding personal fitness, an incremental ramp of 10–25 W every 2–3 min was selected (Medical Positioning Incorporated, Kansas City, MO, USA) while maintaining 55–65 rpm. The study performed was a symptom-limited exRHC in the supine position. Patients were coached to pedal until they were unable to exercise any further. At the time of the repeat exRHC, once patients were symptomatic and reached a similar stage/time as the initial exRHC, they were permitted to discontinue exercise. Individuals were not instructed to exercise for a longer duration. Final hemodynamic measurements obtained were considered peak effort and are annotated as peak exercise for this study, which is consistent with the methods utilized in prior studies.3,4,6 The patient’s body position was consistent between studies and all individuals performed exercise in the supine position at zero degrees.
Exercise hemodynamics (right atrium [RA], PA, PAWP, CO, PA saturation) were recorded every 2–3 min (Xper Cardio Physiomonitoring System; Philips, Melborne, FL, USA). Thermodilution CO was analyzed after averaging the sum of triplicate measurements at rest and peak exercise, and 1–2 measurements during each exercise interval and post-exercise measurements. In order to account for respiratory variation during exercise, hemodynamic measurements were averaged over several respiratory cycles.24 Measurements were recorded at four stages: supine rest; legs up; exercise; and post-exercise. Patients remained supine for the duration of the study. Supine resting measurements were obtained for approximately 10–20 min before obtaining hemodynamic measurements. The exercise bicycle was attached to the catheterization lab table before the individual’s arrival and once the resting RHC study was complete, the bicycle was slid toward the patient for the exercise portion of the study. Minimal exertion by the patients was required to place their feet into the bicycle pedals. The legs-up measurements were obtained after 3 min of rest and the post-exercise measurements were obtained after completing exercise.
Pulmonary function testing
Spirometry to determine forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) was performed within six months of the exRHC. Diffusing capacity for carbon monoxide (DLco%) percent predicted was calculated using Neas prediction equations and corrected for hemoglobin and carboxyhemoglobin.25 DLco% was not corrected for lung volumes.
Study design
We performed an analysis of ten consecutive supine exRHC. ePH was defined as either a mPAPpeak ≥30 mmHg and TPRpeak ≥ 3.0 WU, or slope of the mPAP/CO ratio > 3.0,2,4 and a PAWPpeak < 25 mmHg during supine bicycle ergometry. At least four hemodynamic values from resting to peak exercise were required for analysis. Exclusion criteria included a resting mPAP >25 mmHg, resting PAWP > 15 mmHg, structural cardiac abnormalities, severe mitral/aortic valvular disease, or left ventricular ejection fraction by echocardiogram < 45%. The RAP, PAWP, and mPAP collected from all patients, including those patients with underlying lung disease, were measured by averaging pressures over the respiratory cycle as previously described.24 Supine position with “legs up” was the baseline value in which the exercise hemodynamic values were compared. The aim of the study was to evaluate hemodynamic response to pulmonary vasodilators, with particular attention to α and PAC.
Patient population
Patients underwent exRHC to evaluate a pulmonary vascular contribution to exertional dyspnea or exercise intolerance. The diagnosis of lung disease was confirmed by review of the patient’s chart, pulmonary function testing, and chest imaging. The diagnosis of scleroderma was made using the American College of Rheumatology classification criteria.26 None of the patients were on PAH-specific therapies at the time of the initial exRHC. The University of Pittsburgh IRB approved the study (protocol no. PRO11070366).
Selection of pulmonary vasodilators
Patients were initiated on PAH-specific therapy at the discretion of the attending PH physician in line with clinical practice patterns at the University of Pittsburgh. Medications were selected after a discussion with the patient weighing risks and benefits of treatment for ePH. The patients agreed to initiate therapy to treat symptoms related to an abnormal pulmonary vascular response to exercise.
Statistical analysis
We compared hemodynamic values before and after treatment using a paired t-test (or Wilcoxon signed rank test) for continuous variables or McNemar test for categorical variables, respectively. Categorical values are shown as numbers (n) and percent (%). Continuous variables are shown as mean ± SEM. Correlations were calculated by the Spearman method. Statistical significance was considered < 0.05. STATA 14.2 (StataCorp., College Station, TX, USA) was utilized to analyze the data.
The α model
The mathematical derivation of the α model was described by Linehan et al.12 It is determined by calculating the slope of the diameter-pressure relationship of a particular vessel. Local vascular resistance is found by determining vessel diameter and is directly proportion to the fourth power of that diameter based on Poiseuille’s equation.12,27
For each time point during rest and exercise, mPAP, PAWP, TPR, and CO were collected using the method of successive iterations and a single value for α was determined. α was then varied to find the best-fit least-square value between measured mPAP and a calculated mPAP.28 Generally accepted values of α were in the range of a 1.5–2% increase in pulmonary vessel diameter per mmHg increase in pressure.27,28 Recent studies have demonstrated values slightly lower in control groups.6,13
Results
Baseline patient characteristics
Women comprised the majority of included patients (70%). The mean age was 66 ± 8.2 years, 50% had underlying lung disease (40% chronic obstructive pulmonary disease [COPD] and 10% interstitial lung disease). Out of the four individuals with COPD, two had CT scan evidence of severe emphysema. The two remaining COPD patients had mild centrilobular emphysema by CT scan. The two patients with severe radiographic emphysema had RV/TLC ratios of 60% and 43%, indicating presence of air trapping at rest. The remaining patients’ RV/TLC ratios were ≤ 35%, indicating a lack of air trapping at rest. The patient with ILD had mild bibasilar ILD (UIP pattern) on high-resolution CT scan that had not progressed on repeat high-resolution CT scan at three years.
Thirty percent of patients had scleroderma. The mean 6-min walk distance was 306 ± 148.2 m, demonstrating a baseline reduced ability for exertion; half of the patients with a baseline functional class (FC) recorded were WHO FC 2 at the time of the initial exRHC. The normal baseline FEV1% and FVC% with a reduced DLco% reflects a mixed population of lung disease and individuals without lung disease. The patients with COPD had a lower DLco%, though 4/5 of the non-lung disease patients had a reduced DLco% < 80% (Table 1).
Table 1.
Patient characteristics at baseline.
| n | 10 |
| Age (years) | 66 (8.2) |
| Female (n (%)) | 7 (70) |
| BMI (kg/m2) | 29.6 (6.1) |
| Lung disease (n (%)) | |
| No | 5 (50) |
| COPD | 4 (40) |
| ILD | 1 (10) |
| Scleroderma (n (%)) | 3 (30) |
| 6-minute walk distance (m) | 306 ± 148.2 |
| Functional class (n (%)) | |
| 1 | 2 (25) |
| 2 | 4 (50) |
| 3 | 2 (25) |
| TRV (m/s) | 2.7 (+/− 0.5) |
| FEV1 (%) | 84 (29.9) |
| FVC (%) | 91 (15.0) |
| DLco (%) | 49 (20.9) |
| Use of medication for ePH | |
| Sildenafil | 1 |
| Tadalafil* | 7 |
| Ambrisentan / Tadalafil | 1 |
| Riociguat | 1 |
Tadalafil (40 mg = 6; 20 mg = 1).
Baseline hemodynamics and response to therapy
Resting supine legs down
Before therapy, patients had normal right- and left-sided filling pressures with a mPAP 19 ± 1.1 mmHg, PAWP 8.4 ± 1.2 mmHg, and preserved CO 5.1 ± 0.3 L/min. The population as a whole did not demonstrate borderline PAH; however, the higher mean PVR of 2.1 ± 0.3 WU and TPR of 3.8 ± 0.2 WU may indicate higher resting vascular tone. After therapy with pulmonary vasodilators, the resting supine hemodynamics demonstrate significant improvements in a number of values including cardiac flows, PA pulse pressure, stroke volume (SV), stroke volume index (SVI), and PAC. There was a trend toward a reduced TPR post therapy (Table 2). We found that the systemic vascular resistance (SVR) was reduced in the supine legs-down position post therapy. These findings were not present in either the legs-up or maximal exercise stages.
Table 2.
Resting supine legs down hemodynamics before and after treatment.
| Pre treatment | Post treatment | P value | |
|---|---|---|---|
| n | 10 | 10 | |
| Cardiopulmonary hemodynamics | |||
| PASP (mmHg) | 35.1 ± 1.5 | 31.3 ± 1.9 | 0.07 |
| PADP (mmHg) | 11.0 ± 1.1 | 11.1 ± 1.3 | >0.9 |
| mPAP (mmHg) | 19.0 ± 1.1 | 17.8 ± 1.3 | 0.47 |
| TPR (WU) | 3.8 ± 0.2 | 3.2 ± 0.2 | 0.07 |
| PAWP (mmHg) | 8.4 ± 1.2 | 9.3 ± 1.1 | 0.63 |
| CO (L/min) | 5.1 ± 0.3 | 5.6 ± 0.3 | 0.037 |
| CI (L/min/m2) | 2.8 ± 0.2 | 3.1 ± 0.1 | 0.037 |
| TPG (mmHg) | 10.6 ± 1.6 | 10.0 ± 0.6 | 0.66 |
| PA pulse pressure (mmHg) | 24 ± 1.3 | 20 ± 1.6 | 0.017 |
| PVR (WU) | 2.1 ± 0.3 | 1.8 ± 0.1 | 0.35 |
| Stroke volume (mL) | 80.0 ± 5.3 | 88.5 ± 8.4 | 0.039 |
| Stroke volume index (mL/m2) | 42.2 ± 2.2 | 47.7 ± 3.2 | 0.035 |
| RVSWI (g*m/m2) | 11.5 ± 0.8 | 12.2 ± 1.1 | 0.58 |
| PAC (mL/mmHg) | 3.3 ± 0.3 | 4.6 ± 0.5 | 0.003 |
| PA saturation (%) | 68 ± 0.8 | 69 ± 1.0 | 0.61 |
| SVR (WU) | 21.6 ± 1.2 | 18.0 ± 0.9 | 0.008 |
| Systemic hemodynamics | |||
| HR (BPM) | 66 ± 3.3 | 66 ± 3.9 | >0.9 |
| SBP (mmHg) | 149 ± 5.2 | 147 ± 5.6 | 0.82 |
| DBP (mmHg) | 87 ± 1.4 | 79 ± 3.8 | 0.06 |
| MAP (mmHg) | 107 ± 1.9 | 102 ± 3.8 | 0.22 |
| O2 saturation (%) | 99 ± 0.5 | 97 ± 0.9 | 0.13 |
Resting supine legs up
Nine of the ten patients included in the analysis had legs up hemodynamic data available (Table 3). The legs-up position increases venous return and augments cardiac preload.29 Most values increased from the supine legs-down to the supine legs-up position. We utilized the legs-up position as the baseline to remove the influence of venous return in the legs-up position when compared to peak exercise (Table 4).
Table 3.
Resting supine legs up hemodynamics before and after treatment.
| Pre treatment | Post treatment | P value | |
|---|---|---|---|
| n | 9 | 9 | |
| Cardiopulmonary hemodynamics | |||
| PASP (mmHg) | 40.9 ± 3.3 | 37.2 ± 2.6 | 0.21 |
| PADP (mmHg) | 15.7 ± 1.4 | 13.8 ± 1.1 | 0.24 |
| mPAP (mmHg) | 24.1 ± 1.7 | 21.6 ± 1.3 | 0.21 |
| TPR (WU) | 4.5 ± 0.3 | 3.6 ± 0.3 | 0.049 |
| PAWP (mmHg) | 10.9 ± 1.4 | 12.7 ± 0.9 | 0.39 |
| CO (L/min) | 5.4 ± 0.3 | 6.2 ± 0.3 | 0.06 |
| CI (L/min/m2) | 2.9 ± 0.2 | 3.3 ± 0.2 | 0.07 |
| TPG (mmHg) | 13.2 ± 2.4 | 8.8 ± 2.8 | 0.15 |
| PA pulse pressure (mmHg) | 25 ± 2.2 | 23 ± 2.5 | 0.31 |
| PVR (WU) | 2.4 ± 0.3 | 1.8 ± 0.2 | 0.11 |
| SV (mL) | 78.3 ± 6.8 | 90.0 ± 7.9 | 0.037 |
| SVI (mL/m2) | 42.3 ± 2.6 | 48.6 ± 3.0 | 0.036 |
| RVSWI (g*m/m2) | 15.4 ± 1.8 | 15.2 ± 1.3 | >0.9 |
| PAC (mL/mmHg) | 3.5 ± 0.5 | 3.6 ± 0.6 | 0.9 |
| SVR (WU) | 19.8 ± 1.5 | 16.7 ± 1.2 | 0.09 |
| Systemic hemodynamics | |||
| HR (BPM) | 70 ± 3.2 | 69 ± 3.4 | 0.67 |
| SBP (mmHg) | 152 ± 5.1 | 151 ± 6.4 | 0.89 |
| DBP (mmHg) | 87 ± 2.8 | 83 ± 3.8 | 0.22 |
| MAP (mmHg) | 109 ± 1.9 | 106 ± 4.2 | 0.41 |
| O2 saturation (%) | 98 ± 0.9 | 97 ± 1.0 | 0.47 |
Table 4.
Peak exercise hemodynamics before and after treatment.
| Pre treatment | Post treatment | P value | |
|---|---|---|---|
| n | 10 | 10 | |
| Exercise parameters | |||
| Duration of exercise (min) | 12.1 ± 1.4 | 11.5 ± 1.2 | 0.55 |
| Estimated METS | 4.8 ± 0.6 | 5.3 ± 0.7 | 0.16 |
| Systemic Hemodynamics | |||
| Maximum workload (W) | 74 ± 12.1 | 84 ± 13.5 | 0.22 |
| HR (mmHg) | 114 ± 10.0 | 109 ± 9.3 | 0.11 |
| SBP (mmHg) | 177 ± 7.6 | 186 ± 7.8 | 0.32 |
| DBP (mmHg) | 98 ± 5.7 | 111 ± 7.8 | 0.28 |
| MAP (mmHg) | 124 ± 6.0 | 136 ± 6.9 | 0.18 |
| O2 saturation (%) | 92 ± 5 | 90 ± 4.9 | 0.55 |
| O2 (LPM), n = 3 | 4 ± 2 | 5 ± 3.3 | 0.34 |
| Exercise hemodynamics | |||
| PASP (mmHg) | 67.4 ± 3.7 | 59.4 ± 3.3 | 0.009 |
| PADP (mmHg) | 25.1 ± 1.3 | 23.3 ± 2.0 | 0.37 |
| mPAP (mmHg) | 39.2 ± 1.8 | 35.3 ± 2.1 | 0.08 |
| TPR (WU) | 4.4 ± 0.2 | 3.2 ± 0.3 | 0.003 |
| Slope mPAP/CO ratio (mmHg/L/min) | 3.4 ± 0.5 | 2.8 ± 0.5 | 0.32 |
| Δ mPAP/ Δ CO (WU) | 4.3 ± 1.0 | 3.2 ± 0.6 | 0.33 |
| PAWP (mmHg) | 16.7 ± 2.0 | 19.2 ± 2.2 | 0.39 |
| CO (L/min) | 9.1 ± 0.6 | 11.3 ± 0.8 | 0.005 |
| CI (L/min/m2) | 5.0 ± 0.3 | 6.1 ± 0.4 | 0.019 |
| TPG (mmHg) | 22.5 ± 2.5 | 20.9 ± 2.3 | 0.47 |
| PVR (WU) | 2.5 ± 0.3 | 1.7 ± 0.2 | 0.026 |
| PA pulse pressure (mmHg) | 42 ± 3.4 | 36 ± 2.9 | 0.004 |
| SV (mL) | 83.9 ± 7.2 | 113.5 ± 18.4 | 0.005 |
| SVI (mL/m2) | 45.3 ± 3.1 | 60.3 ± 8.8 | 0.022 |
| RVSWI (g*m/m2) | 25.7 ± 2.3 | 29.6 ± 3.2 | 0.23 |
| PAC (mL/mmHg) | 2.1 ± 0.2 | 3.3 ± 0.5 | 0.005 |
| PA saturation (%) | 42 ± 3.6 | 42 ± 1.9 | >0.9 |
| SVR (WU) | 12.9 ± 1.7 | 11.0 ± 0.8 | 0.21 |
| Exercise PH classification | |||
| mPAP > 30 (n (%)) | 10 (100) | 7 (70) | 0.08 |
| TPR > 3 (n (%)) | 9 (90) | 8 (80) | 0.32 |
| ePH* (n (%)) | 9 (90) | 7 (70) | 0.16 |
| Slope mPAP/CO ratio > 3 (n (%)) | 6 (60) | 5 (50) | 0.56 |
| Δ mPAP/ Δ CO > 3 (n (%)) | 7 (70) | 5 (50) | 0.32 |
| ePH combination definition† (n (%)) | 9 (90) | 8 (80) | 0.32 |
mPAP > 30 mmHg and TPR > 3.0.
†Either ePH or slope of the mPAP/CO > 3 or Δ mPAP/Δ CO > 3.
Peak exercise
Ten included patients met ePH criteria and none of the population demonstrated a PAWPpeak >25 mmHg (Table 4). The majority of patients were treated with tadalafil (70%); others were treated with sildenafil (10%), ambrisentan/tadalafil combination (10%), and riociguat (10%). Participants were treated for an average of 283 ± 119.8 days after initial and follow-up exRHC. When comparing peak exercise pre- and post-therapy duration of therapy, work achieved or estimated METS performed did not increase. The majority of patients stopped exercise due to dyspnea: six for dyspnea, three for dyspnea and fatigue, and one due to general fatigue.
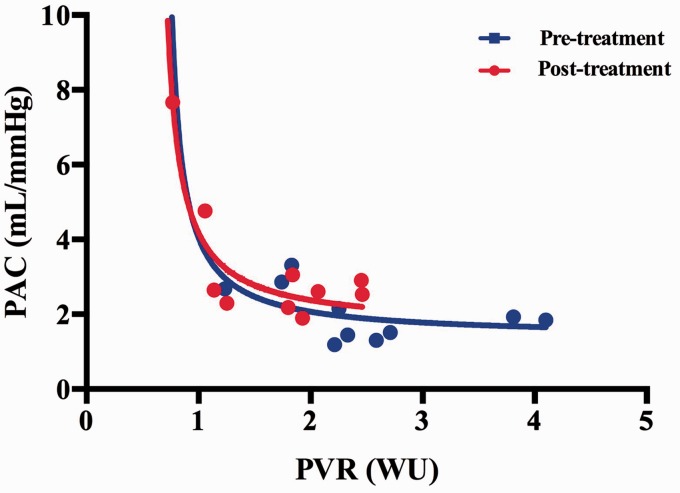
We note significant improvements in a number of post-therapy peak exercise pulmonary vascular parameters (Table 4). There were significant increases in cardiac flows with increased CO and CI post therapy after treatment with pulmonary vasodilators. Heart rate did not improve post therapy; however, SV and SVI did significantly improve. There were significant improvements in vascular resistance with decreases in TPR and PVR. Fig. 1 demonstrates the shift of the PVR to PAC graph to the left and upward after the initiation of pulmonary vasodilators. This indicates that our ePH cohort experienced a reduced PVR and increased PAC post-pulmonary vasodilator therapy. There was a trend toward a decrease in mPAP post therapy; however, this did not reach statistical significance. We demonstrate that PA pulse pressure decreased and PAC improved with therapy.
Fig. 1.
Peak pulmonary artery compliance vs. pulmonary vascular resistance pre-treatment (blue line) and post-treatment (red line). Plotted values are mean values for PAC and PVR.
While treatment with pulmonary vasodilators improved hemodynamics, we did not find a significant change in the number meeting criteria for ePH (Table 4).
Pulmonary vascular distensibility
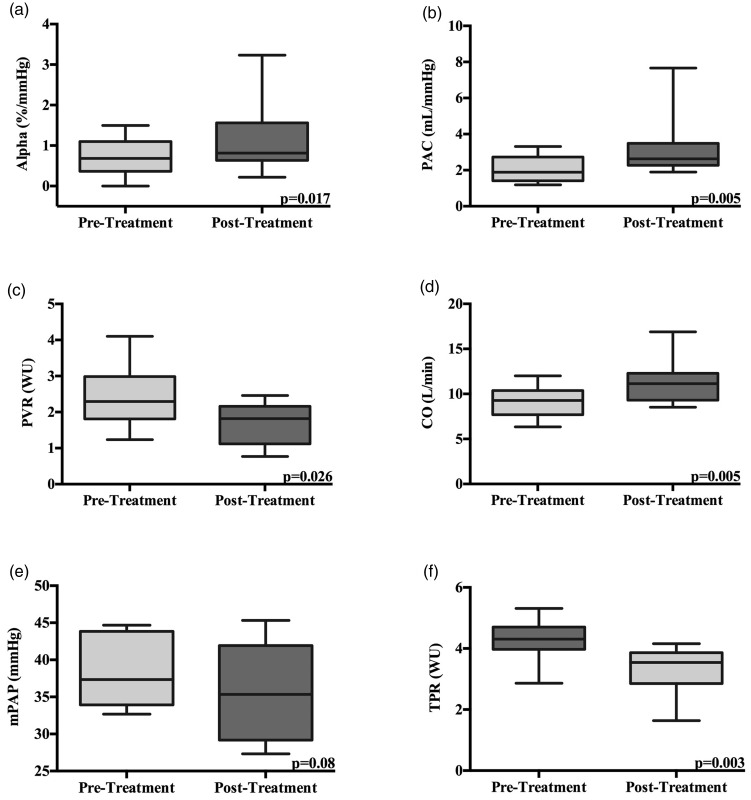
All patients in the cohort had α calculated pre- and post-vasodilator treatment. There were an average of 5.9 ± 1.1 pre-treatment and 6.2 ± 1.1 post-treatment exercise hemodynamic measurements per patient. We assessed whether treatment with a variety of pulmonary vasodilators improved abnormal pulmonary vascular distensibility. We demonstrate that α was reduced at 0.69 ± 0.15 %/mmHg before treatment and significantly improved to an average of 1.15 ± 0.27 %/mmHg after therapy (P = 0.017). This represents a 40% improvement in α (Fig. 2). Pulmonary vascular distensibility did not correlate with age, either before or after treatment.
Fig. 2.
Peak exercise hemodynamics measured before and after therapy. (a) Alpha; (b) PAC; (c) PVR; (d) cardiac output; (e) mPAP; (f) TPR. Box plots show median, IQR, and minimum and maximum values.
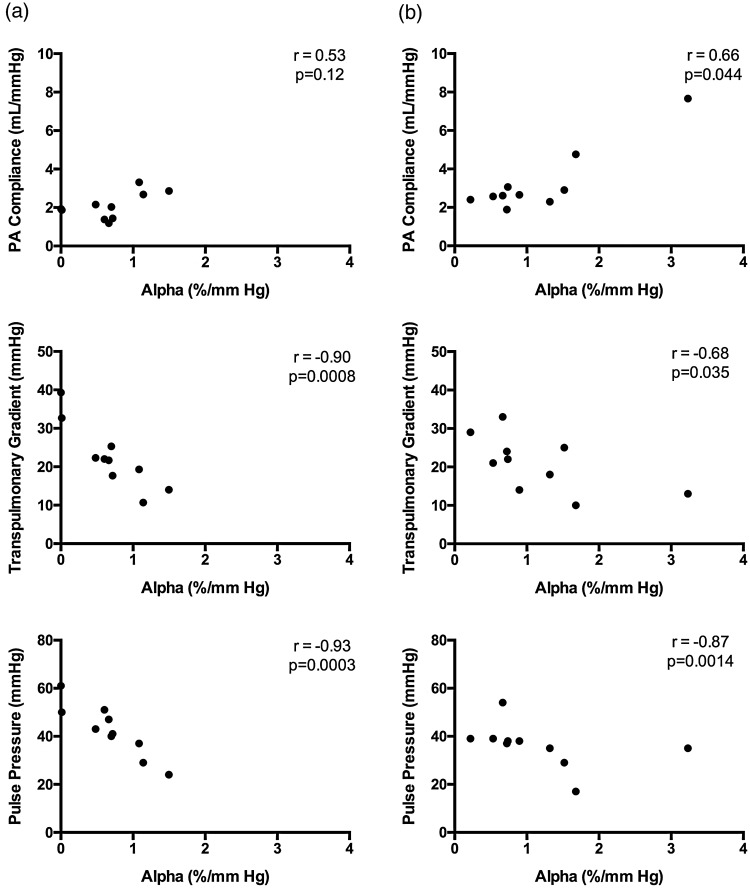
We calculated Spearman correlation for peak values before and after therapy for α (Fig. 3). We found a significant inverse correlation between α calculated from exercise values and peak PVR before therapy (r = –0.70, P = 0.025) and non-significant after therapy (r = –0.45, P = 0.19). Alpha had a non-significant correlation with PAC pre-therapy (r = 0.53, P = 0.12) and a significant post-therapy peak correlation (r = 0.66, P = 0.044) (Fig. 3). Alpha showed a strong inverse correlation with pre-therapy exercise values of PA pulse pressure (r = –0.93, P = 0.0003) and after therapy (r = –0.87, P = 0.0014), a strong inverse correlation with mPAP (r = –0.82, P = 0.003), and a non-significant inverse correlation after therapy at peak exercise (r = –0.32, P = 0.4). The transpulmonary gradient (TPG) had a strong inverse correlation with α before and after therapy at peak exercise (r = –0.90, P = 0.0008) and (r = –0.68, P = 0.035), respectively. TPR did not correlate with α at peak exercise before or after therapy.
Fig. 3.
Relationship of α with peak exercise hemodynamics. (a) Pre-therapy Spearman correlation demonstrated a non-significant correlation with PAC and strongly significant inverse correlations with TPG and PA pulse pressure. (b) Post-therapy Spearman correlation demonstrated a significant correlation with PAC and strongly significant inverse correlations with TPG and PA pulse pressure.
Baseline DLco% and pulmonary vascular disease
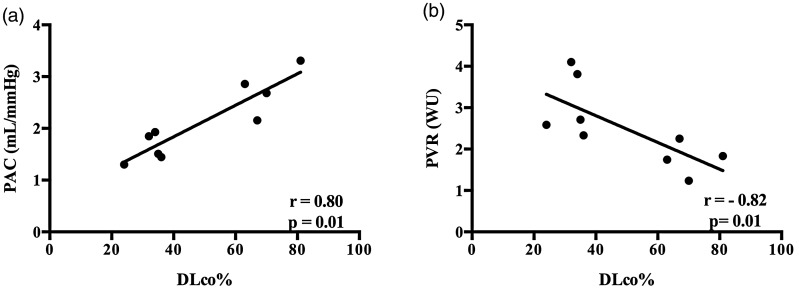
DLco% performed around the time of the exRHC demonstrates a strong correlation between maximum exercise PAC and PVR before the initiation of therapy (Fig. 4). There was not a significant correlation of DLco% with mPAP, TPR, or α at maximum exercise before therapy. Post-therapy hemodynamic values did not correlate with DLco%.
Fig. 4.
Correlation of resting DLco% with peak exercise PAC and PVR. Resting DLco% strongly correlated with (a) PAC and (b) PVR at peak exercise.
Submaximal exercise hemodynamics
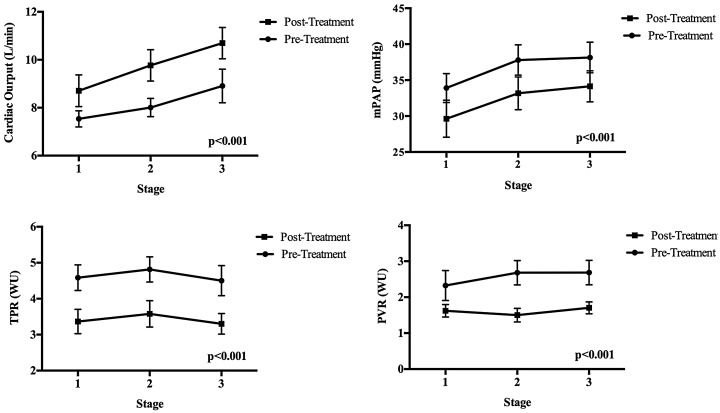
We evaluated the hemodynamic response to exercise during submaximal workloads for the first three exercise stages (Fig. 5). Each stage represents a 10–25 W increment in workload. We demonstrate that TPR, PVR, and mPAP are reduced with increased CO during post-treatment measures, indicating that at lower workloads there are improved flows, pressure, and resistance.
Fig. 5.
Hemodynamics at submaximal stages of exercise. Each stage of exercise represents approximately 10–25 W of workload representing < 4 METS effort consistent with activities of daily living. TPR, PVR, and mPAP are reduced with increased CO during post-treatment measures, indicating that at lower workloads there are improved flows, pressure, and resistance.
Follow-up
Of the ten patients treated with pulmonary vasodilators, seven remained on therapy at the time of this manuscript. One patient discontinued tadalafil due to self-reported increase in libido. Two patients were lost to follow-up, which included one patient on sildenafil and another on combination therapy. There were no deaths while on therapy.
Discussion
We demonstrate that a mixed population of patients with normal resting hemodynamics diagnosed with ePH exhibit evidence of early pulmonary vascular disease. This finding is supported by a reduced PAC at rest and a reduced α measured at peak exercise. We note significant improvements in a number of post-therapy peak exercise hemodynamic values including TPR, PVR, SV, SVI, CO, CI, pulmonary artery systolic pressure (PASP), PAC, and pulse pressure compared to pre-treatment values. To our knowledge, this is the first study to demonstrate that α a measure of resistive vessel distensibility and early pulmonary vascular disease improves with pulmonary vasodilators in ePH. In total, these findings offer insight into patients with early pulmonary vascular disease that α can be followed after the initiation of pulmonary vasodilators for improvement in vascular distensibility.
We show that PAC is decreased at rest and peak exercise and improved after treatment. The reduced PAC in our population is similar to that seen in resting PH.30 Increased PAC has been implicated as a potential contributor to the development of endothelial dysfunction and PH in humans with early pulmonary vascular disease.31 Monocrotaline rat models of PH suggest similar findings that, before the development of smooth muscle hypertrophy and endothelial cell dysfunction, there is an interruption of the internal elastic lamina layer within the pulmonary arteries.30,32 The disruption in the elastic lamina function results in a loss of PAC a precursor to distal proliferative vasculopathy.33,34 Additionally, we demonstrate that resting PAC can revert to near normal levels, suggesting that intervention in early pulmonary vascular disease may improve endothelial function and subsequent distal vasculopathy.
Our study population demonstrate a similar reduction in pre-treatment α compared to other studies with ePH or manifest PAH.6,13 This suggests a common pulmonary vascular response to exercise with abnormal vascular distensibility of the pulmonary resistive vessels as a contributor. In patients diagnosed with ePH, a reduced α occurs before the manifestation of resting PAH and may be a sensitive marker for early disease detection. Alpha is decreased in older age.13,28 Although our population is older, we did not find a correlation of age with α, making age an unlikely main contributor to reduced pulmonary vascular distensibility.
We are the first to show an improvement in α after treatment for ePH.18,19 In our population, α increased after the initiation of pulmonary vasodilators from 0.69 ± 0.15 to 1.15 ± 0.27 %/mmHg, though it did not entirely normalize. This provides insight into the disease process of ePH where loss of vascular distensibility can at least be partially reversed with pulmonary vasodilator therapy. We identified significant correlations with α and peak values of TPG and PA pulse pressure before and after therapy. This suggests that pulsatile hemodynamics strongly associate with mechanical distensibility and could reflect RV function during exercise.35
Our study evaluated the long-term effect of a variety of vasodilating medications for an average of 283 ± 119.8 days. Saggar and Segrera treated ePH patients with ambrisentan for a six-month duration.18,19 Our study produced similar post-treatment hemodynamic effects despite differences in the definition of ePH utilized, duration of therapy, and the class of pulmonary vasodilators selected.18,19 We show a statistically significant improvement in TPR at peak exercise, though mPAP demonstrates a trend toward improvement. In our population, the peak exercise mPAP decreased 3.9 mmHg after treatment. This finding is in line with post-treatment changes in mPAP from the Saggar (decreased 4.1 mmHg) and Segrera (decreased 5.2 mmHg) studies.18,19 The borderline statistical significance in mPAP is likely related to our small sample size.
Our patients were treated with a variety of pulmonary vasodilators. Based upon attending physician preference, the majority of our patients were treated with PDE5i, which differs from prior publications that primarily utilized ambrisentan monotherapy.18,19 We demonstrate that use of PDE5i and riociguat can be safe and well tolerated in a mixed population of ePH patients. Our population is too small to compare hemodynamic responses by pulmonary vasodilator class. A randomized controlled study comparing the difference in hemodynamic response to pulmonary vasodilators, singly or in combination, in ePH may be warranted.
From our data, it is clear that pulmonary vasodilators in general can improve exercise hemodynamics. We show that CO and SV increased significantly. It is presumed that the improved CO is a result of lower vascular resistance after treatment; however, the effect of pulmonary vasodilators on the myocardium cannot be entirely discounted.36 There may be direct myocardial interaction independent of the pulmonary vascular effects with use of a PDE5i.37 An improvement in right ventricular myocardial relaxation and contractility could contribute to increases in CO and SV.36
It is of interest that half of our study participants had background lung disease. Current proposed definitions of ePH are rooted in exercise hemodynamics and do not exclude the presence of background lung disease.2,4,5,38 Cardiopulmonary hemodynamics obtained during exercise in patients with obstructive lung disease can result in air trapping and potential overestimation of pressures. We performed a digital averaging of the mPAP and PAWP over several respiratory cycles in all patients to best estimate transmural mPAP and PAWP, and account for changes in intrathoracic pressure.24 Respiratory pressure swings during exercise affect mPAP and PAWP similarly; therefore, TPG and PVR should be relatively unaffected by intrathoracic pressure changes.38 Patients aged > 50 years are considered to have an abnormal peak PVR during exercise of > 1.20 WU.8 These criteria apply to all patients in our population and reflect the presence of exercise pulmonary vascular disease, and should mirror changes advocated by the ERS statement on pulmonary hemodynamics during exercise.5,39 In our population, the PVR at maximum exercise in all individuals was elevated to 2.5 WU and decreased significantly to 1.7 WU indicating improved post-therapy ePH, but residual disease.
The DLco% was reduced in our patients despite normal spirometry measures. This is likely due to the mix of patients with and without parenchymal lung disease, though 90% of the study participants had a DLco < 80%. A reduced DLco% has been associated with pulmonary vascular disease in at-risk patients, such as scleroderma or lung disease, two populations in our study.18,38,40 Scleroderma patients with a DLco% < 60% have been shown to have evidence of early pulmonary vascular disease detected by exRHC despite normal resting PAP.40 Moderate to severe COPD patients with normal resting PAP and reduced DLco% can exhibit hemodynamic findings of ePH.38 We demonstrate in a mixed population with and without parenchymal lung disease that DLco% strongly correlates with PAC and PVR at peak exercise before therapy (Fig. 4). This may reflect a pre-existing abnormality of the vascular resistance vessels of the pulmonary vascular bed at rest that is unmasked with exercise. We do not have follow-up DLco% data on individuals after therapy. Whether DLco% improves after treatment for ePH is not known. Larger studies may help determine the predictive role of DLco% in ePH.
All of our patients were treated with pulmonary vasodilators and there were no differences in oxygen use before and after therapy at maximal exercise. Two of the four participants diagnosed with COPD had increased resting O2 use, which is a known effect with pulmonary vasodilators in obstructive lung disease.41 Exercise testing may be a reasonable modality to identify patients with an abnormal pulmonary vascular response to exercise that may respond favorably to pulmonary vasodilators.
Despite the hemodynamic improvements at peak exercise after treatment, we did not demonstrate an improvement in exercise capacity. Our methodology likely affected this outcome. Although individuals performed a symptom-limited study, once a similar duration of exercise time or stage was achieved, participants were permitted to discontinue exercise. Individuals were not encouraged to exercise further. Therefore, we would not expect a major difference in exercise capacity before and after therapy. Prior ePH treatment trials did not report exercise bicycle duration or workload at the time of repeat exRHC, so a comparison cannot be performed.18,19 Saggar and Segrera reported improved exercise capacity with the submaximal measure of 6MWD after therapeutic intervention in ePH patients.18,19 We did not have repeat 6MWD data in all participants after therapy to make such a comparison.
In order to evaluate the hemodynamic response to submaximal workloads, we plotted TPR, CO, and mPAP during the first three stages of exercise before and after therapy (Fig. 5). We demonstrate that at workloads less than peak exercise, levels closer to activities of daily living have lower mPAP, PVR, TPR, and higher CO at each stage of exercise. Thus, at similar levels of effort, individuals experienced improved hemodynamics after treatment. This may imply that less work was required to achieve similar hemodynamics and may translate to less perceived effort at submaximal levels.
Some participants may have performed a submaximal exercise study. We did not perform simultaneous cardiopulmonary exercise data to evaluate effort. We had patients complete a maximal effort study limited by symptoms. The PA (mixed venous) saturation at peak exercise was 42% before and after therapy in our participants, which is higher than expected for a maximum exercise study. A decrease in PA saturation during exercise reflects increased metabolic demands and oxygen extraction. Previous ePH studies demonstrate a range of 33 ± 8% to 51.2 ± 8% at peak exercise in the upright and supine positions, respectively.18,39 Supine PA saturations may vary according to body position over different cardiac outputs and may be 10% higher in the supine position compared to an upright position.42 PA saturations measured at maximum exercise in normal participants can decrease to approximately 24%, while patients with resting PAH have mixed venous saturations decrease to 38% at peak exercise in the supine position.43–45 It is reasonable that the elevated PA saturation in ePH may be due to oxygen extraction abnormalities,39 a relatively low oxygen peak oxygen consumption, or impaired cardiac output during exercise. Faria-Urbina et al. demonstrated that ePH patients had reduced maximum exercise systemic oxygen extraction that unmasked a pre-existing skeletal muscle abnormality after treatment with ambrisentan.39 Further investigation into the mechanisms of skeletal muscle dysfunction and oxygen extraction in ePH may be warranted.
Some of our patients may have performed a submaximal study. The proposed definition of ePH is independent of workload. Once mPAP > 30 mmHg with CO < 10 L/min and TPR > 3.0 WU, ePH can be diagnosed, even at submaximal workloads.4,5 An abnormal pulmonary vascular response to exercise diagnosed as ePH can be present in individuals at risk for PAH (scleroderma, congenital heart disease), left heart disease, lung disease, or a combination of disease states.5 Therefore reaching maximal exercise may not be necessary in patients once the definition of ePH is met.4,5
Almost all dyspneic patients, with few exceptions, experience symptoms when upright and mobile. In the resting upright position, PAP and CO are lower.46 In the upright position, PVR is elevated compared to supine due to the de-recruitment of the pulmonary vasculature from lower cardiac output due to reduced venous return. Once exercise is initiated, upright and supine PVR values mirror one another and should decrease in normal individuals.47 In spite of the supine position, the abnormal pulmonary pressure flow relationship should remain abnormal in our population.
Prior studies have demonstrated that patients with a mPAP in the range of 21–24 mmHg are at risk for ePH.9,48–50 Our population contained a mix of lung disease and scleroderma patients, two populations at risk for developing abnormal hemodynamics. This demonstrates that at-risk populations with normal resting mPAP may have an abnormal pulmonary vascular response to exercise, therefore confrontational exercise testing may be diagnostically helpful.9,48
It is unclear whether treatment of ePH delays progression to resting PAH. None of our patients advanced to resting PAH after an average follow-up duration of 283 days, though this study was not designed to detect progression to resting PAH. It is unclear if changes in the hemodynamics post-vasodilator therapy are significant or improve morbidity or mortality.2,4,8 It is reasonable, however, that early restoration of endothelial dysfunction with pulmonary vasodilators could be critical to preventing or slowing vascular disease.
To date, no data have demonstrated a decreased survival in ePH patients with a reduced α value. Heart failure patients with a low α of < 0.7 %/mmHg had a reduced survival related to cardiovascular death.13 Although mechanistic differences exist between heart failure and ePH, overlap related to nitric oxide signaling and prostacyclin production may contribute to abnormal pulmonary vascular function and distensibility.51–53 A long-term study of the natural history of ePH would help determine if reduced α predicts increased morbidity and mortality and whether altering α with pharmacologic intervention is beneficial.
The limitations of our study should be noted. These include a small clinical population without a control group; yet our population size is in line with previously published studies,6,18–22 thus adding to the emerging literature of this patient population. Patients exercised to symptom limitation. Gas exchange measurements were not available. Our study was not designed to detect a difference in exercise capacity. Patients were followed clinically for side effects; however, they were not systematically assessed. Two patients were lost to follow-up after the repeat RHC. No patients died after the initiation of therapy and all ten patients had a repeat RHC performed.
Further limitations include the open-label strategy utilized. Although it was evident that all patients were in a treatment arm, the main focus of this study was hemodynamic. It is unlikely that bias could influence strictly obtained hemodynamic measurements. Although there was variability in the duration of time between initiation of therapy and repeat RHC, this variation reflects current clinical practice and demonstrates that no patients developed resting PH after therapy was initiated.
In conclusion, patients diagnosed with ePH demonstrate improved peak exercise hemodynamics after treatment with a variety of pulmonary vasodilators. We are the first to show that the measure of vascular distensibilty was partially reversed in patients with ePH with pulmonary vasodilators. It is likely that α can be used to follow changes in the vascular bed in response to therapy. Larger studies are needed to better understand the natural history of ePH and the pulmonary vascular hemodynamic changes that occur after therapy.
Conflict of interest
SYC has served as a consultant for Actelion (Significant), Gilead, Pfizer, and Vivus (Modest). MGR has served as a consultant for Actelion and Gilead. The authors declare no other conflicts of interest.
Funding
This work was supported by NIH grants R01 HL124021, HL 122596, HL 138437, and UH2 TR002073 (SYC).
References
- 1.Naeije R, Vanderpool R, Dhakal BP, et al. Exercise-induced pulmonary hypertension: physiological basis and methodological concerns. Am J Respir Crit Care Med 2013; 187: 576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis GD, Bossone E, Naeije R, et al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation 2013; 128: 1470–1479. [DOI] [PubMed] [Google Scholar]
- 3.Godinas L, Lau EM, Chemla D, et al. Diagnostic concordance of different criteria for exercise pulmonary hypertension in subjects with normal resting pulmonary artery pressure. Eur Respir J 2016; 48: 254–257. [DOI] [PubMed] [Google Scholar]
- 4.Herve P, Lau EM, Sitbon O, et al. Criteria for diagnosis of exercise pulmonary hypertension. Eur Respir J 2015; 46: 728–737. [DOI] [PubMed] [Google Scholar]
- 5.Kovacs G, Herve P, Barbera JA, et al. An official European Respiratory Society statement: pulmonary haemodynamics during exercise. Eur Respir J 2017; 50: 1700578. [DOI] [PubMed] [Google Scholar]
- 6.Lau EM, Chemla D, Godinas L, et al. Loss of vascular distensibility during exercise is an early hemodynamic marker of pulmonary vascular disease. Chest 2016; 149: 353–361. [DOI] [PubMed] [Google Scholar]
- 7.Naeije R, Vonk Noordegraaf A, Kovacs G. Exercise-induced pulmonary hypertension: at last!. Eur Respir J 2015; 46: 583–586. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira RK, Agarwal M, Tracy JA, et al. Age-related upper limits of normal for maximum upright exercise pulmonary haemodynamics. Eur Respir J 2016; 47: 1179–1188. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira RKF, Faria-Urbina M, Maron BA, et al. Functional impact of exercise pulmonary hypertension in patients with borderline resting pulmonary arterial pressure. Pulm Circ 2017; 7: 654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliveira RK, Waxman AB, Agarwal M, et al. Pulmonary haemodynamics during recovery from maximum incremental cycling exercise. Eur Respir J 2016; 48: 158–167. [DOI] [PubMed] [Google Scholar]
- 11.Jain P, Rao S, Macdonald P, et al. Diagnostic performance of pulmonary capacitance at rest and during exercise in idiopathic pulmonary arterial hypertension. Heart Lung Circ 2017. doi: 10.1016/j.hlc. 2017.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Linehan JH, Haworth ST, Nelin LD, et al. A simple distensible vessel model for interpreting pulmonary vascular pressure-flow curves. J Appl Physiol (1985) 1992; 73: 987–994. [DOI] [PubMed] [Google Scholar]
- 13.Malhotra R, Dhakal BP, Eisman AS, et al. Pulmonary vascular distensibility predicts pulmonary hypertension severity, exercise capacity, and survival in heart failure. Circ Heart Fail 2016; 9: e003011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kusunose K, Yamada H, Hotchi J, et al. Prediction of future overt pulmonary hypertension by 6-min walk stress echocardiography in patients with connective tissue disease. J Am Coll Cardiol 2015; 66: 376–384. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal R, Gomberg-Maitland M. Physiologic markers of exercise as a potential screening tool for the detection of pulmonary hypertension: “alpha” few steps forward. Chest 2016; 149: 295–297. [DOI] [PubMed] [Google Scholar]
- 16.Oldham WM, Janocha A, Pappagianopoulos P, et al. Nitric oxide metabolite flux during exercise in pulmonary arterial hypertension. Eur Respir J 2011; 38: 2308. [Google Scholar]
- 17.Oldham WM, Lewis GD, Janocha AJ, et al. Nitric oxide pathway metabolite flux in exercise-induced pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 185: A6737. [Google Scholar]
- 18.Saggar R, Khanna D, Shapiro S, et al. Brief report: effect of ambrisentan treatment on exercise-induced pulmonary hypertension in systemic sclerosis: a prospective single-center, open-label pilot study. Arthritis Rheum 2012; 64: 4072–4077. [DOI] [PubMed] [Google Scholar]
- 19.Segrera SA, Lawler L, Opotowsky AR, et al. Open label study of ambrisentan in patients with exercise pulmonary hypertension. Pulm Circ 2017; 7: 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park MH, Ramani GV, Kop WJ, et al. Exercise-uncovered pulmonary arterial hypertension and pharmacologic therapy: Clinical benefits. J Heart Lung Transplant 2010; 29: 228–229. [DOI] [PubMed] [Google Scholar]
- 21.Kovacs G, Maier R, Aberer E, et al. Pulmonary arterial hypertension therapy may be safe and effective in patients with systemic sclerosis and borderline pulmonary artery pressure. Arthritis Rheum 2012; 64: 1257–1262. [DOI] [PubMed] [Google Scholar]
- 22.Yagi S, Akaike M, Iwase T, et al. Bosentan ameliorated exercise-induced pulmonary arterial hypertension complicated with systemic sclerosis. Intern Med 2010; 49: 2309–2312. [DOI] [PubMed] [Google Scholar]
- 23.Kovacs G, Avian A, Olschewski A, et al. Zero reference level for right heart catheterisation. Eur Respir J 2013; 42: 1586–1594. [DOI] [PubMed] [Google Scholar]
- 24.Boerrigter BG, Waxman AB, Westerhof N, et al. Measuring central pulmonary pressures during exercise in COPD: how to cope with respiratory effects. Eur Respir J 2014; 43: 1316–1325. [DOI] [PubMed] [Google Scholar]
- 25.Neas LM, Schwartz J. The determinants of pulmonary diffusing capacity in a national sample of U.S. adults. Am J Respir Crit Care Med 1996; 153: 656–664. [DOI] [PubMed] [Google Scholar]
- 26.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum 1980; 23: 581–590. [DOI] [PubMed] [Google Scholar]
- 27.Lalande S, Yerly P, Faoro V, et al. Pulmonary vascular distensibility predicts aerobic capacity in healthy individuals. J Physiol 2012; 590: 4279–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reeves JT, Linehan JH, Stenmark KR. Distensibility of the normal human lung circulation during exercise. Am J Physiol Lung Cell Mol Physiol 2005; 288: L419–425. [DOI] [PubMed] [Google Scholar]
- 29.Jabot J, Teboul JL, Richard C, et al. Passive leg raising for predicting fluid responsiveness: importance of the postural change. Intensive Care Med 2009; 35: 85–90. [DOI] [PubMed] [Google Scholar]
- 30.Thenappan T, Prins KW, Pritzker MR, et al. The critical role of pulmonary arterial compliance in pulmonary hypertension. Ann Am Thorac Soc 2016; 13: 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan W, Madhavan K, Hunter KS, et al. Vascular stiffening in pulmonary hypertension: cause or consequence? (2013 Grover Conference series). Pulm Circ 2014; 4: 560–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Todorovich-Hunter L, Dodo H, Ye C, et al. Increased pulmonary artery elastolytic activity in adult rats with monocrotaline-induced progressive hypertensive pulmonary vascular disease compared with infant rats with nonprogressive disease. Am Rev Respir Dis 1992; 146: 213–223. [DOI] [PubMed] [Google Scholar]
- 33.Dodson RB, Morgan MR, Galambos C, et al. Chronic intrauterine pulmonary hypertension increases main pulmonary artery stiffness and adventitial remodeling in fetal sheep. Am J Physiol Lung Cell Mol Physiol 2014; 307: L822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le VP, Stoka KV, Yanagisawa H, et al. Fibulin-5 null mice with decreased arterial compliance maintain normal systolic left ventricular function, but not diastolic function during maturation. Physiol Rep 2014; 2: e00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blyth KG, Syyed R, Chalmers J, et al. Pulmonary arterial pulse pressure and mortality in pulmonary arterial hypertension. Respir Med 2007; 101: 2495–2501. [DOI] [PubMed] [Google Scholar]
- 36.Gomez-Arroyo J, Sandoval J, Simon MA, et al. Treatment for pulmonary arterial hypertension-associated right ventricular dysfunction. Ann Am Thorac Soc 2014; 11: 1101–1115. [DOI] [PubMed] [Google Scholar]
- 37.Hutchings DC, Anderson SG, Caldwell JL, et al. Phosphodiesterase-5 inhibitors and the heart: compound cardioprotection? Heart 2018. doi: 10.1136/heartjnl-2017-312865. [DOI] [PMC free article] [PubMed]
- 38.Hilde JM, Skjorten I, Hansteen V, et al. Haemodynamic responses to exercise in patients with COPD. Eur Respir J 2013; 41: 1031–1041. [DOI] [PubMed] [Google Scholar]
- 39.Faria-Urbina M, Oliveira RKF, Segrera SA, et al. Impaired systemic oxygen extraction in treated exercise pulmonary hypertension: a new engine in an old car? Pulm Circ 2018; 8: 2045893218755325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steen V, Chou M, Shanmugam V, et al. Exercise-induced pulmonary arterial hypertension in patients with systemic sclerosis. Chest 2008; 134: 146–151. [DOI] [PubMed] [Google Scholar]
- 41.Blanco I, Santos S, Gea J, et al. Sildenafil to improve respiratory rehabilitation outcomes in COPD: a controlled trial. Eur Respir J 2013; 42: 982–992. [DOI] [PubMed] [Google Scholar]
- 42.Harms MP, van Lieshout JJ, Jenstrup M, et al. Postural effects on cardiac output and mixed venous oxygen saturation in humans. Exp Physiol 2003; 88: 611–616. [DOI] [PubMed] [Google Scholar]
- 43.Hasler ED, Muller-Mottet S, Furian M, et al. Pressure-flow during exercise catheterization predicts survival in pulmonary hypertension. Chest 2016; 150: 57–67. [DOI] [PubMed] [Google Scholar]
- 44.Sun XG, Hansen JE, Stringer WW, et al. Carbon dioxide pressure-concentration relationship in arterial and mixed venous blood during exercise. J Appl Physiol (1985) 2001; 90: 1798–1810. [DOI] [PubMed] [Google Scholar]
- 45.Epstein SE, Beiser GD, Stampfer M, et al. Characterization of the circulatory response to maximal upright exercise in normal subjects and patients with heart disease. Circulation 1967; 35: 1049–1062. [DOI] [PubMed] [Google Scholar]
- 46.Kovacs G, Berghold A, Scheidl S, et al. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J 2009; 34: 888–894. [DOI] [PubMed] [Google Scholar]
- 47.Naeije R, Chesler N. Pulmonary circulation at exercise. Compr Physiol 2012; 2: 711–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lau EM, Godinas L, Sitbon O, et al. Resting pulmonary artery pressure of 21-24 mmHg predicts abnormal exercise haemodynamics. Eur Respir J 2016; 47: 1436–1444. [DOI] [PubMed] [Google Scholar]
- 49.Bae S, Saggar R, Bolster MB, et al. Baseline characteristics and follow-up in patients with normal haemodynamics versus borderline mean pulmonary arterial pressure in systemic sclerosis: results from the PHAROS registry. Ann Rheum Dis 2012; 71: 1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Visovatti SH, Distler O, Coghlan JG, et al. Borderline pulmonary arterial pressure in systemic sclerosis patients: a post-hoc analysis of the DETECT study. Arthritis Res Ther 2014; 16: 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shao Z, Wang Z, Shrestha K, et al. Pulmonary hypertension associated with advanced systolic heart failure: dysregulated arginine metabolism and importance of compensatory dimethylarginine dimethylaminohydrolase-1. J Am Coll Cardiol 2012; 59: 1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galie N, Manes A, Branzi A. Prostanoids for pulmonary arterial hypertension. Am J Respir Med 2003; 2: 123–137. [DOI] [PubMed] [Google Scholar]
- 53.Moraes DL, Colucci WS, Givertz MM. Secondary pulmonary hypertension in chronic heart failure: the role of the endothelium in pathophysiology and management. Circulation 2000; 102: 1718–1723. [DOI] [PubMed] [Google Scholar]