Abstract
Indoleamine 2,3-dioxygenase (IDO), which is highly expressed in human glioblastoma and involved in tumor immune escape and resistance to chemotherapy, is clinically correlated with tumor progression and poor clinical outcomes, and is a promising therapeutic target for glioblastoma. IDO inhibitors are marginally efficacious as single-agents; therefore, combination with other therapies holds promise for cancer therapy. The aim of this study was to investigate the anti-tumor effects and mechanisms of the IDO inhibitor PCC0208009 in combination with temozolomide. The effects of PCC0208009 on IDO activity inhibition, and mRNA and protein expression in HeLa cells were observed. In the mouse glioma GL261 heterotopic model, the effects of PCC0208009 on l-kynurenine/tryptophan (Kyn/Trp), tumor growth, flow cytometry for T cells within tumors, and immunohistochemistry for IDO and Ki67 were examined. In the rat glioma C6 orthotopic model, animal survival, flow cytometry for T cells within tumors, and immunohistochemistry for proliferating cell nuclear antigen (PCNA) and IDO were examined. The results show that PCC0208009 is a highly effective IDO inhibitor, not only directly inhibiting IDO activity but also participating in the gene regulation of IDO expression at the transcription and translation levels. PCC0208009 significantly enhanced the anti-tumor effects of temozolomide in GL261 and C6 models, by increasing the percentages of CD3+, CD4+, and CD8+ T cells within tumors and suppressing tumor proliferation. These findings indicate that PCC0208009 can potentiate the anti-tumor efficacy of temozolomide and suggest that combination of IDO inhibitor-based immunotherapy with chemotherapy is a potential strategy for brain tumor treatment.
Keywords: glioblastoma; immunotherapy; indoleamine 2,3-dioxygenase; temozolomide
Introduction
Glioblastoma multiforme (GBM) is the most common and aggressive primary brain cancer.1 Even after standard treatment, the prognosis of GBM remains poor, may due to the potently immunosuppressive tumor environment.2–4 Indoleamine 2,3-dioxygenase (IDO, also known as IDO1), a key enzyme in the metabolism of the essential amino acid tryptophan (Trp) along the l-kynurenine (Kyn) pathway, induces immune tolerance with local tryptophan depletion and produces toxic tryptophan catabolites.5 Recent studies show that IDO is highly expressed in human glioblastoma,6,7 increases the recruitment of regulatory T cells, clinically correlates with drug resistance, tumor progression, and poor clinical outcomes,3,8,9 and suggest that IDO is a promising therapeutic target for glioblastoma.3,5 Several IDO inhibitors, such as indoximod and PF-06840003, have been entered in phase 1/2 clinical trials for brain tumor therapy.
In preclinical models, IDO inhibitors are only marginally efficacious as single-agents and can enhance the anti-tumor effects of multiple classes of chemotherapy agents.10–12 Therefore, the combination of IDO inhibitors with chemotherapy holds promise for cancer therapy.13,14 Temozolomide (TMZ) is a standard chemotherapeutic agent for malignant glioma. Although TMZ improves the prognosis for patients with glioma, its clinical efficacy is partial and limited. Many adjuvant therapies have been evaluated for use with TMZ, but no further improvement in prognosis has been reported.15
PCC0208009 (PCC) is an IDO inhibitor with a chemical structure as shown in Figure 1. It is the compound of Example 1 in the Bristol-Myers Squibb Company patent (WO2015/031295 A1). The studies in our laboratory have shown that PCC can effectively inhibit the activity of IDO with an IC50 of 4.52 nM at the cellular level. There are no previous reports on PCC. However, the efficacy and mechanisms of PCC at the cellular level and in animal models remain to be elucidated. Therefore, the aim of this study was to investigate the underlying mechanisms for the effects of PCC on IDO, and the anti-tumor functions of PCC combined with TMZ in mouse heterotopic transplantation and rat orthotopic implantation glioma models.
Figure 1.

Chemical structure of PCC0208009.
Methods
Chemicals and reagents
PCC was obtained from Shanghai Send Pharmaceutical, with purity >98.5%, the molecular formula C29H35N7O and a molecular weight of 487.6. For in vitro tests, PCC was dissolved in dimethyl sulfoxide (DMSO) and diluted with Dulbecco’s Modified Eagle Medium (DMEM) to the desired concentration. For in vivo tests, PCC was reconstituted in aqueous 1% sodium carboxymethyl cellulose (SCMC, w/v) to the desired concentrations. TMZ, produced by Merck, was diluted to the appropriate concentrations with 1% SCMC.
Phycoerythrin-Cyanine 7 (PE-Cy™7) hamster anti-mouse CD3e (Catalog No. 552774), phycoerythrin (PE) rat anti-mouse CD4 (Catalog No. 553049), fluorescein isothiocyanate (FITC) rat anti-mouse CD8a (Catalog No. 561966), FITC mouse anti-rat CD3 (Catalog No. 557354), PE-Cy™5 mouse anti-rat CD4 (Catalog No. 554839), and PE mouse anti-rat CD8a (Catalog No. 554857) were all purchased from BD Biosciences. Antibodies against rabbit anti-mouse/rat IDO (Catalog No. ab106134), rabbit anti-rat proliferating cell nuclear antigen (PCNA) (Catalog No. ab15497), and rabbit anti-mouse Ki67 (Catalog No. ab16667) were purchased from Abcam. Rabbit anti-human IDO antibody (Catalog No. #86630) was purchased from Cell Signaling Technology. Mouse anti-human β-actin antibody (Catalog No. AF0003), horseradish peroxidase (HRP)-labeled goat anti-rabbit and goat anti-mouse IgG (H+L) were purchased from Beyotime Biotechnology. A high-capacity RNA-to-cDNA Kit (Catalog No.4387406) and Power SYBR® green PCR master mixtures (Catalog No. 4367659) were purchased from Thermo Fisher. Tumor dissociation kit (Catalog No. 130-096-730) was purchased from Miltenyi Biotec.
Cell culture and animals
The mouse glioma cell line GL261 and rat glioma cell line C6 were provided by Cell Bank, Chinese Academy of Sciences. The human cervical cancer cell line HeLa was gifted by Dr Hongbo Wang at Yantai University. All cell lines were cultured in DMEM supplemented with 10% fetal bovine serum (FBS), 50 IU/mL penicillin, and 50 µg/mL streptomycin sulfate and maintained at 37°C in a humidified air atmosphere containing 5% CO2.
Five-to-six weeks old male C57BL/6J mice and male Sprague–Dawley (SD) rats (weighed 200 ± 20 g) were purchased from Beijing Vital River Laboratory Animal Technology. The animal production license number was SCXK (Jing) 2016-0011. The animals were quarantined and equilibrated to the new environment for at least 5 days and maintained in a specific pathogen-free environment with free access to sterilized food and water. The animal room was maintained on a 12 h light/dark cycle at 21°C ± 5°C and 55% ± 15% relative humidity. All of the experiments related to animals were performed in accordance with the Guidelines for the Care and Use of Experimental Animals of the Experimental Animal Research Committee in Yantai University.
Effects of PCC on the viability and proliferation of HeLa cells
HeLa cells were seeded into 96-well plates at 6 × 103 cells/well. After culture for 10–12 h, the culture medium was replaced with fresh medium with 100 ng/mL interferon gamma (IFN-γ), or PCC at 25, 50, 100, or 200 nM with or without 100 ng/mL IFN-γ, and medium containing 0.1% DMSO was used as the vehicle treatment. At 72 h after the addition of drugs, cell viability and proliferation were observed. In the viability assay, adherent cells were washed, trypsinized, and counted using a CountStar IC1000 Automated Cell Counter (Ruiyu-Biotech), and viability of the counted cells was confirmed by 0.1% trypan blue exclusion, which was indicated as a percentage of the living cells. In the proliferation test, cells were assayed using the 3-[4,5-dimethylthiazol-2yl]-2,5-diphenyl tetrazolium bromide (MTT) method. The cell proliferation rate of control cells was represented as 100%, and the relative cell proliferation rates of the other groups were calculated.
Inhibition effects of PCC on IDO activity in HeLa cells
HeLa cells were seeded in 96-well plates at 6 × 103 cells/well. After culture for 10–12 h, the medium was replaced with fresh medium only or medium containing 100 ng/mL IFN-γ. Except for the vehicle group, the other groups were all induced by IFN-γ for 24 h. Then, the medium was replaced with fresh medium only, medium containing 100 ng/mL IFN-γ, or containing 100 nM PCC. At 0.5, 1, 2, 5, 10, 24, 48, and 72 h after the addition of drugs, the cell supernatants were harvested for determination of l-kynurenine (Kyn) and tryptophan (Trp) by liquid chromatography tandem-mass spectrometry (LC-MS/MS).
Effects of PCC on IDO expression in HeLa cells
HeLa cells were seeded in 6-well plates at 2 × 105 cells/well. After culture for 10–12 h, the medium was replaced with fresh medium, 100 ng/mL IFN-γ, or 100 ng/mL IFN-γ plus PCC at 50, 100, and 200 nM, respectively. After incubation for 48 h, cells were harvested. The expression of IDO at the protein and mRNA levels was detected by Western blot and quantitative real-time polymerase chain reaction (qRT-PCR), respectively.
Western blot was performed as described previously.16 Proteins were extracted from the harvested cells using radio immunoprecipitation assay (RIPA) lysis buffer. The cell lysates containing 50 μg protein were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membrane. The membrane was blocked for 2 h at room temperature with tris-buffered saline containing 0.05% Tween-20 (TBS-T) and 5% skimmed milk, and then, incubated with rabbit anti-human monoclonal antibody against IDO (1:1000), followed by incubation with HRP-conjugated goat anti-rabbit IgG (1:2000) for 2 h. Finally, the protein levels were detected using BeyoECL Plus reagent and exposed to the film. Normalization of results was ensured by running a parallel Western blot with the β-actin antibody used as an internal control. The optical density was quantified using Image-Pro Plus 6.0 software.
In the qRT-PCR assay, the total RNA was extracted from the harvested cells. Then, reverse transcription to cDNA was performed using SuperScript® III reverse transcriptase according to the manufacturers’ instructions. qRT-PCR was performed using SYBR Green MasterMix on an ABI Prism 7500 fast system (PerkinElmer) under the following amplification parameters: 95°C for 10 min, (95°C, 15 s; 60°C, 15 s; 72°C, 30 s) ×40 cycles, 72°C for 6 min, and 2-ΔΔΔCt was used for statistical analysis of results. Relative expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The primers used in this experiment were as follows: IDO1 forward: 5′-GCCCTTCAAGTGTTTCACCAA-3′; IDO1 reverse: 5′-CCAGCCAGACAAATATATGCGA-3′; GAPDH forward: 5′-GGTCGGAGTCAACGGATTTG-3′; GAPDH reverse: 5′-ATGAGCCCCAGCCTTCTCCAT-3′.
Mouse glioma GL261 heterotopic transplantation model
Five-week-old male C57BL/6J mice were inoculated subcutaneously in the dorsal scapula with 0.2 mL of Matrigel containing 3 × 106 cells under ketamine–xylazine anesthesia. The day of tumor inoculation was designated day 1. Tumor width (W) and length (L) of xenografts were measured by Vernier caliper, and tumor volume (TV) was calculated according to the formula: V = 0.5 × L × W2.
Effects on the pharmacodynamic biomarker Kyn/Trp
When the tumor volumes reached approximately 300–400 mm3, the mice were intragastrically (i.g.) administered a single dose of PCC at 100 mg/kg. At 0, 2, 4, and 8 h after administration, plasma and tumor were collected for the detection of Trp, Kyn, and PCC, with five animals per time point. Trp, Kyn, and PCC were all measured by LC-MS/MS.
Anti-tumor effects of PCC in combination with TMZ
When the tumors reached approximately 100 mm3, the mice were randomized into four groups: Vehicle, PCC, TMZ, and PCC plus TMZ; each group contained 10 mice. PCC was i.g. administered at 100 mg/kg twice daily, TMZ was i.g. administered at 100 mg/kg once every 2 days, and the vehicle group was i.g. administered 1% SCMC twice daily. The dosing volume was 0.1 mL/10 g. During the study, the body weight of animals and tumor volumes were measured once every 3 or 5 days. At the end of the study, tumors were collected and quickly weighed. The mean tumor weight (MTW) in each group was obtained. The inhibition rate (IR in %) was calculated by the following formula: IR (%) = [(MTWvehicle − MTWtreatment)/MTWvehicle] × 100. Five tumors were randomly selected for flow cytometry analysis, and three tumors were randomly selected for immunohistochemical detection.
Flow cytometry analysis
Tumor-infiltrating lymphocytes (TILs) were harvested from tumors using tumor dissociation kit (Miltenyi Biotec) according to the instructions. Red blood cell lysis working solution was added to the cell suspensions to remove erythrocytes and incubated for 15 min, the cells were centrifuged at 300g for 10 min, and then washed and adjusted to 107 cells/mL with phosphate-buffered saline (PBS). Three-color staining of lymphocytes was performed with PE-Cy™7-CD3e, PE-CD4, and FITC-CD8a using standard staining methods. FACS analysis was performed with Accuri™ C6 Flow Cytometer running CFlow Plus software.
Immunohistochemical staining
The tumors were fixed in 4% paraformaldehyde solution, processed, and embedded in paraffin, and the tumor sections (4 µm) were processed for immunohistochemical staining for IDO and Ki67 as described previously.17 Briefly, sections were blocked with 3% normal goat serum and 0.1% Triton X-100, and incubated with antibodies against IDO (1:100) and Ki67 (1:200) overnight at 4°C; sections were then incubated with the biotinylated secondary antibody for 30 min, followed by avidin–biotin–peroxidase complex for 45 min at 37°C. Immunoreactivity signals were developed with 0.05% diaminobenzidine in Tris-HCl buffer (0.1 M, pH 7.6) containing 0.03% H2O2. Protein positive cells were stained brown in the cytoplasm. Sections were then mounted and examined under high-power microscope (200×), and each specimens was randomly selected for three vision test areas as the total area. The positive expressions for IDO and Ki67 were analyzed by the IPP software. The positive area of the protein expression was defined as follows: The integrated optical density (IOD) = the positive area × the average optical density.
Rat glioma C6 orthotopic implantation model
SD rats were anesthetized by intraperitoneal injection with 10% chloral hydrate (0.35–0.5 mL/100 g) and immobilized with a stereotactic frame for tumor implantation. A 0.6-mm-diameter bur hole was drilled at 3 mm right lateral and 1 mm anterior to the bregma. With antiseptic technique, 106 cells in 8 µL PBS were stereotactically implanted into the caudate nucleus using a Hamilton syringe at a depth of 5 mm from the dura mater. The day of tumor inoculation was designated day 1. Animals were used in the experiments on day 5.
Distribution of PCC in the rat brain
After tumor inoculation for 15 days, rats were i.g. administered a single dose of PCC at 50 mg/kg. At 0.5, 2.5, and 6.5 h after dosing, the cerebrum and cerebellum were harvested for detection of PCC content using LC-MS/MS.
Animal survival study
According to the body weight, animals were randomly divided into four groups: Vehicle, PCC, TMZ, and PCC plus TMZ. Each group contained 10 animals. PCC was i.g. administered at 50 mg/kg twice daily, TMZ was i.g. administered at 50 mg/kg once every 2 days, and the vehicle group was i.g. administered with 1% SCMC twice daily, from day 5 to day 35. The dosing volume was 0.2 mL/100 g. During the study, the body weight was measured twice every week and the survival times of rats were recorded and analyzed. Animals were kept in the study until the rats were dead or dying.
Flow cytometry and immunohistochemical staining
In this experiment, the grouping, dose, and route of administration were the same as described in the section “Animal survival study.” The differences were that the animals in this study were treated from day 5 to day 26, and each group contained 10 animals. At the end of treatment, tumors from five rats were sampled for flow cytometry analysis, and tumors from three rats were sampled for immunohistochemical detection. T lymphocytes from the tumors were prepared as described above. Three-color staining of lymphocytes was performed with FITC-CD3, PE-Cy™5-CD4, and PE-CD8a. The tumors were immunohistochemically stained for PCNA and IDO, and brains from normal rats were also immunohistochemically stained for PCNA.
Statistical analysis
The results are presented as the mean ± standard deviation and P < 0.05 was considered statistically significant. The data were analyzed by two-tailed unpaired Student’s t-tests for paired groups or one-way analysis of variance (ANOVA) for three or more groups. Survival time was calculated using the Kaplan–Meier method in each treated group and compared by the logrank test.
Results
Effect of PCC on the viability and proliferation of HeLa cells
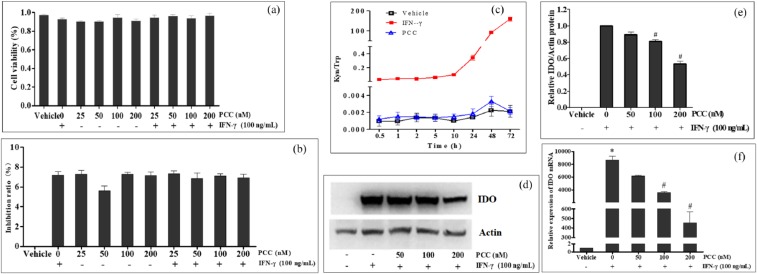
The viability and proliferation of HeLa cells were examined after cells were treated with IFN-γ and PCC alone, or PCC plus IFN-γ. The results of the viability assay are shown in Figure 2(a); the percentages of living cells in the PCC and IFN-γ alone groups or in the combination treated groups had no significant differences compared with the vehicle group (P > 0.05). The results of the proliferation test are shown in Figure 2(b); the IRs in PCC and IFN-γ alone or in combination treated groups also showed no significant differences compared with the vehicle group (P > 0.05), with IRs less than 8%. These results indicated that 25–200 nM PCC or/and 100 ng/mL IFN-γ had no obvious effects on the viability and proliferation of HeLa cells.
Figure 2.
Effects of PCC0208009 (PCC) on HeLa cells and IDO activity and expression in vitro. HeLa cells were treated with 100 ng/mL IFN-γ, or PCC at 25–200 nM with or without 100 ng/mL IFN-γ. The viability (a) and proliferation (b) of cells were observed 72 h after the addition of drugs. HeLa cells were induced by IFN-γ for 24 h and then treated with 100 ng/mL IFN-γ or 100 nM PCC. At different time points after the addition of drugs, Kyn and Trp in the supernatants were determined by liquid chromatography tandem-mass spectrometry, and Kyn/Trp was calculated (c). HeLa cells were treated with 100 ng/mL IFN-γ, or 100 ng/mL IFN-γ plus PCC at 50, 100, and 200 nM. After incubation for 48 h, the expression of IDO was detected by Western blot for the protein level (d and e) and qRT-PCR for the mRNA level (f). All experiments were repeated three times. *P < 0.05, compared with the vehicle group. #P < 0.05, compared with the IFN-γ group.
Effect of PCC on IDO activity and expression in HeLa cells
IDO is highly expressed in HeLa cells induced by IFN-γ, which is widely used for activity screening of IDO inhibitors. After 10–20 h induction with 100 ng/mL IFN-γ, IDO was observed to be highly expressed in HeLa cells by Kyn/Trp determination. The IDO inhibition effects of PCC in HeLa cells were detected after 24 h induction with IFN-γ in this study. As shown in Figure 2(c), IDO activity (Kyn/Trp) was maintained at a high level in the IFN-γ group, and was highly expressed 10 h later. In the PCC group, the activity of IDO was effectively inhibited to the level of the vehicle group, from the drug addition to at least 72 h.
HeLa cells were treated with IFN-γ or IFN-γ plus PCC for 48 h, and the expressions of IDO at protein and mRNA levels were detected. The results from Western blot analysis are shown in Figure 2(d) and (e); the expression levels of IDO protein were almost undetectable in the vehicle group, but were significantly increased by IFN-γ treatment. The IDO protein expression induced by IFN-γ was dose-dependently suppressed by PCC, which showed significant differences at 100 and 200 nM (P < 0.05). The results from qRT-PCR are shown in Figure 2(f); IDO mRNA was expressed at low levels in the vehicle group and was significantly increased by IFN-γ treatment compared with the vehicle group (P < 0.05). The IDO mRNA expression induced by IFN-γ was dose-dependently suppressed by PCC, which showed significant differences at all doses compared with the IFN-γ group (P < 0.05).
PCC on Kyn/Trp in GL261 subcutaneous mouse model
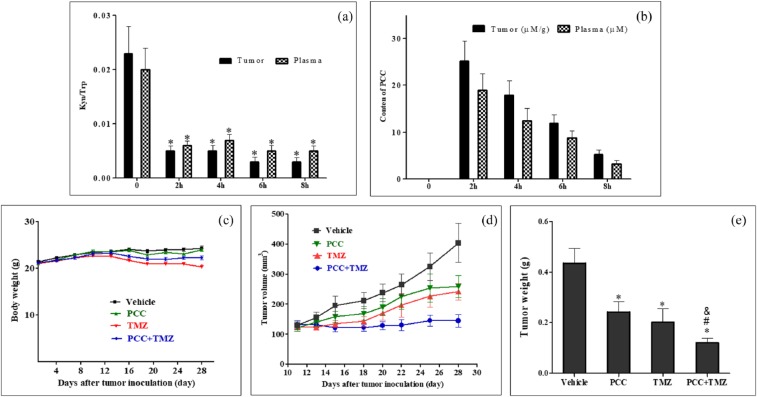
To investigate the biochemical mechanism of PCC in vivo, mouse model bearing GL261 were generated. The levels of the pharmacodynamic biomarkers Kyn/Trp and PCC in the plasma and tumors were determined at different time points after mice were i.g. administered a single dose of PCC at 100 mg/kg. As shown in Figure 3(a), PCC was highly distributed in the tumors and plasma. Compared to pre-dose (0 h), the ratios of Kyn/Trp in the tumor and plasma samples were all significantly decreased from 2 to 8 h after drug administration (P < 0.05) (Figure 3(b)).
Figure 3.
Anti-tumor effects of PCC0208009 (PCC) and TMZ on GL261 tumor-bearing mice: (a, b) Mice were i.g. administered a single dose of PCC at 100 mg/kg. At 0, 2, 4, and 8 h after dosing, plasma and the tumor were collected for determination of PCC and Kyn/Trp (n = 5). *P < 0.05, compared with the values at 0 h. (c–e) Mice were i.g. administered with Vehicle (1% SCMC, twice daily), PCC (100 mg/kg, twice daily), TMZ (100 mg/kg, once every 2 days), or PCC plus TMZ, n = 10. During the study, the body weight of animals and tumor volumes were measured once every 5 days and once every 3/4 days: (c) body weight curve, (d) tumor growth curve, and (e) mean tumor weight. *P < 0.05, compared with the vehicle group; #P < 0.05, compared with the PCC group; &P < 0.05, compared with the TMZ group.
Combinatorial treatment with PCC and TMZ on GL261 subcutaneous mouse model
To explore the anti-tumor effects and mechanisms of PCC in combination with TMZ on tumor growth in vivo, mouse GL261 subcutaneous model was prepared. Mice were treated with Vehicle, PCC, TMZ, or PCC plus TMZ. The body weights of animals and the volumes and weights of tumors were measured. Tumor samples were analyzed by flow cytometry for T cell populations and were immunohistochemically examined for IDO and Ki67 expression.
Body weights of tumor-bearing mice
As shown in Figure 3(c) and Table 1, compared with the vehicle group, no significant effects on the body weight of animals were observed in the PCC group during the study (P > 0.05). From day 12 and day 16 to the end of this study, mouse body weights in the TMZ and PCC plus TMZ groups were noted to have significant decreases compared with the vehicle group (P < 0.05). The body weight decreased by 12.56% and 12.66% in the TMZ group and PCC plus TMZ group, respectively, compared to that at the beginning of the study (P < 0.05). However, no decrease in body weight was observed between the PCC plus TMZ group and the TMZ group (P > 0.05), indicating that PCC did not increase the side effects of TMZ.
Table 1.
Effects of PCC0208009 (PCC) and TMZ on body weights and GL261 tumor weights.
| Groups | Body weight
(g) |
||||
|---|---|---|---|---|---|
| Pre-treatment (day 11) | Post-treatment (day 28) | Weight gain (%) | Tumor weight (g) | Inhibition rate (%) | |
| Vehicle | 23.06 ± 1.33 | 24.23 ± 2.32 | 5.07 | 0.432 ± 0.21 | − |
| PCC | 23.75 ± 1.25 | 23.83 ± 1.82 | 0.34 | 0.248 ± 0.15* | 42.59 |
| TMZ | 22.62 ± 1.74 | 19.78 ± 2.92& | −12.56 | 0.203 ± 0.17* | 53.01 |
| PCC + TMZ | 22.99 ± 1.37 | 20.08 ± 2.24& | −12.66 | 0.126 ± 0.06*#& | 70.83 |
TMZ: temozolomide.
Mice were i.g. administered with Vehicle (1% SCMC, twice daily), PCC (100 mg/kg, twice daily), TMZ (100 mg/kg, once every two days), or PCC plus TMZ, n=10. The percentage of body weight gain was calculated based on that at the beginning of study. Tumor weights were observed on day 28, and the inhibition rate of tumor weight was calculated. *P < 0.05, compared with the vehicle group; #P < 0.05, compared with the PCC group;
P < 0.05, compared with the TMZ group.
Tumor volume and tumor weight
The suppression of tumor growth was observed in the PCC, TMZ and PCC plus TMZ groups, and PCC combined with TMZ showed stronger suppression of tumor growth than PCC or TMZ alone (Figure 3(d)). At the end of the study, the mean tumor weights in PCC, TMZ, and combinatorial treatment groups were obviously smaller than those in the vehicle group (P < 0.05), and the tumor IRs were 42.59%, 53.01%, and 70.83%, respectively (Figure 3(e) and Table 1). No significant difference in tumor weight was observed between the PCC group and TMZ group (P > 0.05). The mean tumor weights in the PCC plus TMZ group were less than those in the PCC and TMZ groups (P < 0.05).
T cell subtypes in GL261 tumor
To understand the immunological changes in animals treated with PCC and TMZ, T cell populations within tumors were analyzed. As shown in Figure 4(a) and (b), compared with the vehicle group, the percentages of CD3+, CD4+, and CD8+ T cells in the PCC group were noted to have slight increases (P > 0.05) while there were significant decreases in the TMZ group (P < 0.05). In the PCC plus TMZ group, the percentage of CD3+ cells was significantly higher than those in the vehicle and TMZ groups (P < 0.05), and the percentages of CD4+ and CD8+ were higher than those in the vehicle group (P > 0.05) and significantly higher than those in the TMZ group (P < 0.05).
Figure 4.
Effects of PCC0208009 (PCC) on T cell subtypes and expressions of IDO and Ki67 within GL261 tumors. Mice were i.g. administered with Vehicle (1% SCMC, twice daily), PCC (100 mg/kg, twice daily), TMZ (100 mg/kg, once every 2 days), or PCC plus TMZ, n = 10. On day 28, five tumors from each group were analyzed by flow cytometry with PE-Cy™7-CD3e, PE-CD4, and FITC-CD8a. Three tumors from each group were analyzed by immunohistochemical analysis for Ki67 and IDO expression: (a) representative flow graphs, (b) results of T cell populations. (c and d) Ki67 detection and (e and f) IDO detection. *P < 0.05 compared with the vehicle group, #P < 0.05 compared with the TMZ group.
IDO and Ki67 expression in GL261 tumor
Immunohistochemical detection was carried out to observe the changes in IDO and Ki67 expression in GL261 tumors. The Ki67 protein is a cellular marker for proliferation.18 As shown in Figure 4(c) and (d), Ki67 expression was noted at a high level in the vehicle group. Considerable decreases in Ki67 expression were observed in the PCC and TMZ groups (P > 0.05), and a significant decrease was observed in the PCC plus TMZ group compared to the vehicle group (P < 0.05). There was no significant difference in Ki67 expression between the TMZ and PCC groups (P > 0.05). The expression of IDO is shown in Figure 4(e) and (f). The high expression level of IDO was observed in GL261 tumors in the vehicle group, while IDO expression in the PCC group was significantly decreased (P < 0.05).
Combinatorial treatment with PCC and TMZ on C6 orthotopic rat model
The syngeneic intracranial orthotopic model of rat glioma C6 were prepared by implanting tumor cells into the caudate nucleus of SD rats. The day of tumor inoculation was designated day 1.
Distribution of PCC in rat brain
At 15 days after tumor inoculation, the rats were i.g. administered a single dose of PCC at 50 mg/kg, and the cerebrum and cerebellum were harvested for the detection of PCC content. The PCC contents in the cerebrum and cerebellum were 122.3 and 113.7 nM/g at 0.5 h, 70.6 and 63.5 nM/g at 2.5 h, 32.8 and 34.3 nM/g at 6.5 h, respectively. These results indicated that PCC could cross the blood-brain barrier and distribute into the brain.
Animal survival and body weights
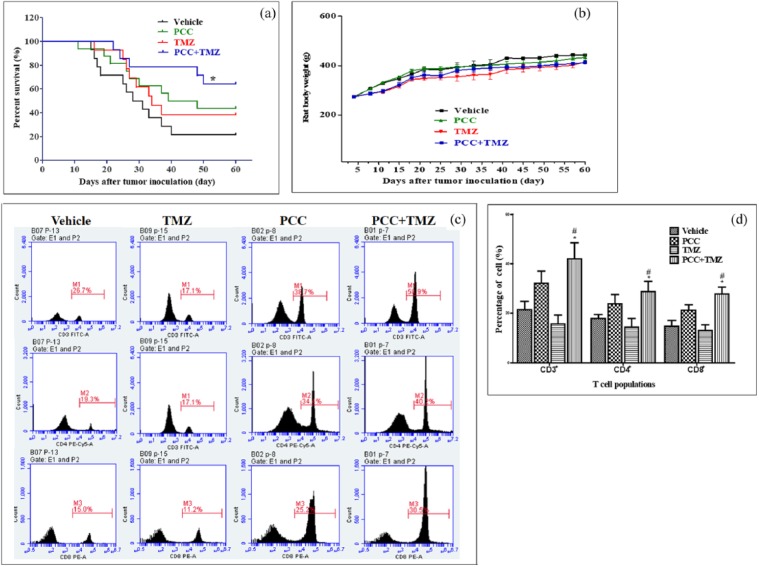
The animal survival curve is shown in Figure 5(a). No significant prolongation in animal survival was observed in the PCC or TMZ groups compared with the vehicle group (P > 0.05). Animal survival in the PCC plus TMZ group was significantly prolonged than compared with the vehicle group (P < 0.05) and compared with the PCC and TMZ groups (P < 0.05).
Figure 5.
Effects of PCC0208009 (PCC) and TMZ on survival of C6-bearing rats and T cell subtypes within tumors. Rats were i.g. administered with Vehicle (1% SCMC, twice daily), PCC (50 mg/kg, twice daily), TMZ (50 mg/kg, once every 2 days), or PCC plus TMZ, from day 5 to day 35, n = 10. The survival time and body weight of animals were recorded and analyzed. Animals were treated from day 5 to day 28 according to the above protocol, tumor from 5 rats were analyzed by flow cytometry with mouse anti-rat FITC-CD3, PE-Cy™5-CD4, and PE-CD8a: (a) animal survival curve, (b) body weights of animals, (c) representative flow graphs, and (d) results of T cell populations. *P < 0.05 compared with the vehicle group; #P < 0.05 compared with the TMZ group.
As shown in Figure 5(b), compared with the vehicle group, no significant effects on the body weight of rats were observed in the PCC group during the study (P > 0.05); from day 8 to the end of this study and from day 8 to day 21, rat body weights in the TMZ group and PCC plus TMZ group were noted to show significant decreases (P < 0.05). However, no additional decrease in body weight was observed between the PCC plus TMZ group and the TMZ group (P > 0.05), indicating that PCC did not increase the side effects of TMZ in this model.
T cell subtypes in C6 tumor
To understand the immunological mechanisms of underlying the anti-tumor effects of PCC and combinatorial treatment of PCC and TMZ, T cell populations within tumors were analyzed. As shown in Figure 5(c) and (d), compared with the vehicle group, the percentages of CD3+, CD4+, and CD8+ T cells in the PCC group were increased, ranging from 40% to 70%, and were slightly decreased in the TMZ group. In the PCC plus TMZ group, the percentages of CD3+, CD8+, and CD4+ T cells were significantly increased compared with the vehicle and TMZ groups by approximately about twofold.
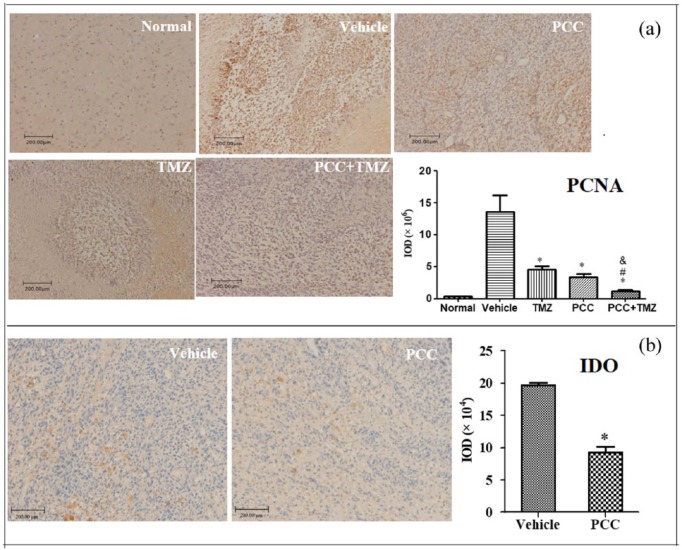
PCNA and IDO expression in C6 tumor by immunohistochemistry
The results from immunohistochemical staining are shown in Figure 6. PCNA expression is a useful prognostic and diagnostic biomarker for gliomas.19 The expression of PCNA was very low in the brain tissue and highly expressed in the tumor of the vehicle group (Figure 6(a)). Compared with the vehicle group, PCNA in the PCC, TMZ, and PCC plus TMZ groups was significantly decreased (P < 0.05). The PCNA in the PCC plus TMZ group was significantly decreased compared to the PCC and TMZ groups (P < 0.05). IDO expression in the PCC group was significantly decreased in the C6 tumor compared with that in the vehicle group (P < 0.05) (Figure 6(b)).
Figure 6.
Immunohistochemical detection for PCNA and IDO expression in C6 tumors. Rats were i.g. administered with Vehicle (1% SCMC, twice daily), PCC (50 mg/kg, twice daily), TMZ (50 mg/kg, once every 2 days), or PCC plus TMZ, from day 5 to day 35. Brain from normal rats and tumor from three rats in each group were immunohistochemically stained for PCNA or IDO. *P < 0.05 compared with the vehicle group; #P < 0.05 compared with the PCC group; &P < 0.05 compared with the TMZ group. Mice were i.g. administered with Vehicle (1% SCMC, twice daily), PCC (100 mg/kg, twice daily), TMZ (100 mg/kg, once every 2 days), or PCC plus TMZ, n = 10. The percentage of body weight gain was calculated based on that at the beginning of study. Tumor weights were observed on day 28, and the inhibition rate of tumor weight was calculated. *P < 0.05, compared with the vehicle group; #P < 0.05, compared with the PCC group; &P < 0.05, compared with the TMZ group.
Discussion
The clinical responses to most immunotherapy strategies are poor, mainly due to the immunosuppressive network within the local tumor microenvironment.20 IDO is a key immunosuppressive enzyme that modulates the anti-tumor immune response by promoting regulatory T cell generation and blocking effector T cell activation. Targeting IDO is a promising approach that may reverse the complex process of immune escape and induce a potent anti-tumor response. However, in preclinical models of cancer, single-agent therapy with IDO inhibitor showed modest anti-tumor activity. Therefore, combination IDO inhibitors incorporating other tumor therapies including chemotherapy will be required for maximal therapeutic benefit.
PCC is an effective IDO inhibitor, yet whether it has cytotoxic activity is unknown. In vitro experiments indicated that PCC had no effect on the viability and proliferation of HeLa cells, regardless of whether they were treated with IFN-γ. Other experiments also indicated that PCC up to 10 μM had no obvious direct cytotoxic activity against common tumor cell lines (data not shown). After induction with IFN-γ, IDO activity was highly increased in HeLa cells, which could be effectively inhibited to normal levels by PCC, lasted for at least 72 h, and other IDO inhibitors also inhibited IDO activity, but the maintenance time was much shorter, less than 36 h. Based on this result, we speculated that PCC affects IDO expression in HeLa cells. The results from Western blot and qRT-PCR analyses showed that PCC significantly decreased IDO expression induced by the IFN-γ at the protein and mRNA levels. In the study of mouse GL261 and rat C6 models, PCC decreased the expression of IDO protein in tumors. The above results indicated that PCC was related to the regulation of IDO expression at the transcription and translation levels. However, the mechanistic basis for this inhibition is not yet clear, and further studies are needed.
The activity of IDO can be estimated by measuring serum Kyn concentrations or Kyn/Trp.12,21 The IDO inhibitory effects of PCC in vivo were evaluated in mouse model bearing GL261. Kyn/Trp in the tumor and plasma was significantly decreased after drug administration. The trend and extent were comparable between the tumor and plasma, and Kyn/Trp could serve as a biomarker for the therapeutic activity of PCC or other IDO inhibitors.
The anti-tumor functions of PCC combined with TMZ were evaluated in the mouse GL261 and rat C6 models. PCC in combination with TMZ significantly suppressed tumor growth and prolonged animal survival and enhanced anti-tumor effects. This could be reflected by Ki67 and PCNA expression. Ki67 protein and PCNA are useful markers for cell proliferation. PCC in combination with TMZ could significantly decrease the expression of these two markers, indicating that combinatorial treatment could more effectively inhibit tumor cell proliferation.
In the study of T cell populations and immunohistochemical staining, TMZ, as a cytotoxic drug, showed decreased effects on all subtypes within tumors, indicating that TMZ also killed T cells when it killed the tumor cells; this might be a reason for drug resistance and relapse. PCC alone slightly increased the percentages of T cells within tumors but significantly increased them when in combination with TMZ. This indicated tha PCC and TMZ could more effectively increase the proportion of T cells in tumor. However, the mechanistic basis for the enhancement of anti-tumor effect was not clear, though it is speculated that TMZ could promote the release of tumor antigens by killing tumor cells, and PCC could reverse the immune tolerance of the tumor microenvironment, with these two factors interacting to enhance the anti-tumor immune responses.
In summary, PCC is a highly effective IDO inhibitor that not only directly inhibits the IDO activity but also participates in the regulation of IDO expression at the transcription and translation levels. PCC0208009 combined with TMZ enhanced the anti-tumor effects in animal models by increasing the percentages of CD3+, CD4+, and CD8+ T cells within tumor and suppressing tumor proliferation. These findings suggest that the combination of IDO inhibitor-based immunotherapy with chemotherapy is a potential strategy for brain tumor treatment.
Acknowledgments
S.S. and G.D. contributed equally to this work and should be considered co-first authors.
Footnotes
Declaration of conflicting interests: The authors declare no potential conflicts of interest with respect to the study, authorship, and/or publication of this article.
Funding: This work was supported by the National Natural Science Foundation of China (Grant No. 81473188), Shandong Provincial Natural Science Foundation (Young and Middle-Aged Scientists Research Awards Fund) (Grant No. BS2015YY012), Project of Shandong Province Higher Educational Science and Technology Program (Grant No. J15LM03), Doctoral Fund of Yantai University (Grant No. YX13B29), and Taishan Scholar Project awarded to Zimei Wu and Bo Liu.
References
- 1. Hanif F, Muzaffar K, Perveen K, et al. (2017) Glioblastoma multiforme: A review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pacific Journal of Cancer Prevention: APJCP 18: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cohen JV, Kluger HM. (2016) Systemic immunotherapy for the treatment of brain metastases. Frontiers in Oncology 6: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sordillo PP, Sordillo LA, Helson L. (2017) The kynurenine pathway: A primary resistance mechanism in patients with glioblastoma. Anticancer Research 37: 2159–2171. [DOI] [PubMed] [Google Scholar]
- 4. Huang B, Zhang H, Gu L, et al. (2017) Advances in immunotherapy for glioblastoma multiforme. Journal of Immunology Research 2017: 3597613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Selvan SR, Dowling JP, Kelly WK, et al. (2016) Indoleamine 2,3-dioxygenase (IDO): Biology and target in cancer immunotherapies. Current Cancer Drug Targets 16: 755–764. [DOI] [PubMed] [Google Scholar]
- 6. Mitsuka K, Kawataki T, Satoh E, et al. (2013) Expression of indoleamine 2,3-dioxygenase and correlation with pathological malignancy in gliomas. Neurosurgery 72: 1031–1038; discussion 1038–1039. [DOI] [PubMed] [Google Scholar]
- 7. Zhai L, Ladomersky E, Lauing KL, et al. (2017) Infiltrating T Cells increase IDO1 expression in glioblastoma and contribute to decreased patient survival. Clinical Cancer Research 23: 6650–6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wainwright DA, Balyasnikova IV, Chang AL, et al. (2012) IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clinical Cancer Research 18: 6110–6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhai L, Dey M, Lauing KL, et al. (2015) The kynurenine to tryptophan ratio as a prognostic tool for glioblastoma patients enrolling in immunotherapy. Journal of Clinical Neuroscience: Official Journal of the Neurosurgical Society of Australasia 22: 1964–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanihara M, Kawataki T, Oh-Oka K, et al. (2016) Synergistic antitumor effect with indoleamine 2,3-dioxygenase inhibition and temozolomide in a murine glioma model. Journal of Neurosurgery 124: 1594–1601. [DOI] [PubMed] [Google Scholar]
- 11. Muller AJ, DuHadaway JB, Donover PS, et al. (2005) Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nature Medicine 11: 312–319. [DOI] [PubMed] [Google Scholar]
- 12. Meng X, Du G, Ye L, et al. (2017) Combinatorial antitumor effects of indoleamine 2,3-dioxygenase inhibitor NLG919 and paclitaxel in a murine B16-F10 melanoma model. International Journal of Immunopathology and Pharmacology 30: 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Austin CJ, Rendina LM. (2015) Targeting key dioxygenases in tryptophan-kynurenine metabolism for immunomodulation and cancer chemotherapy. Drug Discovery Today 20: 609–617. [DOI] [PubMed] [Google Scholar]
- 14. Muller AJ, Prendergast GC. (2005) Marrying immunotherapy with chemotherapy: Why say IDO? Cancer Research 65: 8065–8068. [DOI] [PubMed] [Google Scholar]
- 15. Kim WJ, Newman WC, Amankulor NM. (2017) Phase I/II trial of combination of temozolomide chemotherapy and immunotherapy with fusions of dendritic and glioma cells in patients with glioblastoma. Neurosurgery 81: N11. [DOI] [PubMed] [Google Scholar]
- 16. Du G, Zhu H, Yu P, et al. (2013) SMND-309 promotes angiogenesis in human umbilical vein endothelial cells through activating erythropoietin receptor/STAT3/VEGF pathways. European Journal of Pharmacology 700: 173–180. [DOI] [PubMed] [Google Scholar]
- 17. Zhu H, Zou L, Tian J, et al. (2013) SMND-309, a novel derivative of salvianolic acid B, protects rat brains ischemia and reperfusion injury by targeting the JAK2/STAT3 pathway. European Journal of Pharmacology 714: 23–31. [DOI] [PubMed] [Google Scholar]
- 18. Wu L, Yun Z, Tagawa T, et al. (2014) Activation of CD1d-restricted natural killer T cells can inhibit cancer cell proliferation during chemotherapy by promoting the immune responses in murine mesothelioma. Cancer Immunology, Immunotherapy 63: 1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lv Q, Zhang J, Yi Y, et al. (2016) Proliferating cell nuclear antigen has an association with prognosis and risks factors of cancer patients: A systematic review. Molecular Neurobiology 53: 6209–6217. [DOI] [PubMed] [Google Scholar]
- 20. Cavallo F, De Giovanni C, Nanni P, et al. (2011) 2011: The immune hallmarks of cancer. Cancer Immunology, Immunotherapy 60: 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Jong RA, Nijman HW, Boezen HM, et al. (2011) Serum tryptophan and kynurenine concentrations as parameters for indoleamine 2,3-dioxygenase activity in patients with endometrial, ovarian, and vulvar cancer. International Journal of Gynecological Cancer 21: 1320–1327. [DOI] [PubMed] [Google Scholar]