Abstract
Parkinson’s disease (PD) is the second most common age-related neurodegenerative disease in the elderly and the patients suffer from uncontrolled movement disorders due to loss of dopaminergic (DA) neurons on substantia nigra pars compacta (SNpc). We previously reported that transplantation of human fetal midbrain-derived neural precursor cells restored the functional deficits of a 6-hydroxy dopamine (6-OHDA)-treated rodent model of PD but its low viability and ethical issues still remain to be solved. Albeit immune privilege and neural differentiation potentials suggest mesenchymal stem cells (MSCs) from various tissues including human placenta MSCs (hpMSCs) for an alternative source, our understanding of their therapeutic mechanisms is still limited. To expand our knowledge on the MSC-mediated PD treatment, we here investigated the therapeutic mechanism of hpMSCs and hpMSC-derived neural phenotype cells (hpNPCs) using a PD rat model. Whereas both hpMSCs and hpNPCs protected DA neurons in the SNpc at comparable levels, the hpNPC transplantation into 6-OHDA treated rats exhibited longer lasting recovery in motor deficits than either the saline or the hpMSC treated rats. The injected hpNPCs induced delta-like ligand (DLL)1 and neurotrophic factors, and influenced environments prone to neuroprotection. Compared with hpMSCs, co-cultured hpNPCs more efficiently protected primary neural precursor cells from midbrain against 6-OHDA as well as induced their differentiation into DA neurons. Further experiments with conditioned media from hpNPCs revealed that the secreted factors from hpNPCs modulated immune responses and neural protection. Taken together, both DLL1-mediated contact signals and paracrine factors play critical roles in hpNPC-mediated improvement. First showing here that hpMSCs and their neural derivative hpNPCs were able to restore the PD-associated deficits via dual mechanisms, neuroprotection and immunosuppression, this study expanded our knowledge of therapeutic mechanisms in PD and other age-related diseases.
Keywords: cell therapy, Parkinson's disease, hpNPCs, Neuroprotection, Immunosupression
Introduction
Parkinson’s disease (PD) is characterized by the selective loss of dopaminergic (DA) neurons in the substanta nigra pars compacta (SNpc) region, resulting in reduced striatal dopamine, and accumulations of toxic protein aggregates containing α-synuclein, called Lewy bodies. Because the dopamine controls movement and coordination, PD patients experience various motor symptoms including tremor, bradykinesia, rigidity, and postural instability as well as dementia and psychiatric disturbance. Dopamine replenishment with L-3,4- dihydroxyphenylalanine (L-DOPA), an immediate dopamine precursor, is still a routine treatment so far. However, the pharmacological therapies including L-DOPA and antioxidants have shown short-term positive effects at early stages, usually require increased drug dosage, and are unable to block further disease progress. Recently, a stem cell-based replacement therapy has been suggested as an alternative treatment for recovering the loss of DA neurons in PD: the cellular replacement of DA neurons with human stem cells has been a potentially successful for PD patients1–3.
Many lines of evidence suggest that altered modulation of inflammatory responses is deeply engaged in diverse neuronal diseases and, in particular, microglia associated neuroinflammation is a major contributor of neurodegenerative diseases and brain aging4. Microglia cells, a type of glia cells derived from myeloid precursors in bone marrow, are the main brain-resident immunological cells sensing brain injuries and aging5. Depending on the environment, microglia cells exist in three distinct morphologies serving diverse functional roles: amoeboid, ramified, or reactive/activated microglia. Activated microglia cells undergo proliferation and transformation from a ramified state into an activated, amoeboid morphology. Intensity of activation stimuli is able to determine a pool of secreted factors from the activated microglia cells: microglia cells exposed to weak stimuli secret anti-inflammatory factors and growth factors, and regulate cellular maintenance and innate immunity. However, either too aggressive or too passive stimuli induce the cells to release proinflammatory factors or cytotoxic substrates generating massively detrimental neurotoxic effects6–10. In addition, when microglia cells are chronically and excessively activated, they themselves serve as stimulators generating proinflammatory factors or reactive oxygen species (ROS) in responses to neuron injuries, and alter synaptic functions and environment, commonly referred to reactive microgliosis11. The chronically activated microglia cells are responsible for persisting inflammatory activity and chronic neuronal damages in age-related neurodegenerative disorders including Alzheimer’s disease (AD)12,13, PD14,15, and Huntington’s disease (HD)16,17.
The placenta is composed of three layers, amnion, chorion, and deciduas and is an excellent source of mesenchymal stem cells (MSCs) because it is discarded after delivery. In particular, amniotic membrane produced diverse growth factors including transforming growth factor β (TGF-β), epidermal growth factor, and hepatocyte growth factor (HGF)18, and expressed various anti-angiogenic and inflammation regulatory proteins such as angiogenin, interleukin (IL)-1 receptor antagonist, IL-10, tissue inhibitors of metalloproteinase (TIMPs)-1, -2, -3, -4, growth-related oncogene (GRO), and monocyte chemoattractant protein-1 (MCP-1)19,20. Furthermore, placenta-derived MSCs can strongly inhibit T-lymphocyte proliferation and block differentiation and maturation of monocytes into dendritic cells21,22. Due to their potentials for immune modulation as well as their low immunogenicity, placenta-derived MSCs are a very attractive cell source for allogenic cell transplantation: the hpMSC treatment was reported to inhibit bleomycin-induced lung fibrosis, post ischemic cardiac dysfunction, and bile duct ligation (BDL)-induced liver fibrosis in their animal models20–25. Particularly, the amnion-derived placenta MSCs (hpMSCs) with ectodermal lineage characters are appealing as a regenerative medicine for neurodegenerative diseases26,27. The MSCs from first trimester human placenta were reported to be able to differentiate DA neurons and to recover the motor defects in a rat model of PD, which suggests that the MSCs from placenta are able to work in the PD model28. We recently reported that transplantation of human fetal neural progenitor cells, which was obtained from 14 weeks old fetal midbrain, restored the motor dysfunctions and loss of DA neurons in the 6-OHDA treated PD rats29. In addition, we showed the hpMSC mediated recovery of memory deficits in an AD animal model, Tg2576: the intravenously infused hpMSCs were capable of improving spatial memory function, suppressing amyloid plaque formation, and inactivating microglia in the aged AD mice by a paracrine manner30. Albeit many investigations have proved therapeutic potentials of placental derived stem cells and their derivatives in neurodegenerative diseases, especially in PD, most of them have focused on their possible neurogenic effects. Despite the critical influence of microglia functions on PD pathogenesis, fewer studies have explored effects of placenta-derived stem cells on microglia associated inflammation and neuronal survival in neurodegenerative diseases as well as their therapeutic mechanism.
Here, we systematically characterized hpMSCs and then investigated the therapeutic potential and mechanism of differentiated neural phenotype cells (hpNPCs) from hpMSCs in a 6-OHDA-treated rodent model of PD. Compared with the saline and hpMSC-treated ones, the hpNPC-transplanted PD rat models demonstrated better performances in DA neuron associated motor tasks for a longer period. In addition, the injected hpNPCs were able to protect DA neurons in the substantia nigra (SN) against 6-OHDA more efficiently than even hpMSCs via suppression of microglia and astrocyte activation and production of multiple neurotrophic factors. Taken together, we anticipate that allogenic transplantation using hpNPCs derived from hpMSCs are able to restore the physiological deficits of PD through dual mechanisms, neuroprotection and immune suppression and constitute a new therapeutic strategy for neurodegenerative diseases.
Materials and Methods
See the detailed information in the supplementary materials.
Cell Culture
Human term placentas (≥37 gestational weeks) were obtained from donors with their informed consent according to the Institutional Review Board of the CHA General Hospital (Seoul, Korea). The hpMSCs were isolated from the amniotic membranes of three donors and then cultured as described previously30. hpNPCs were acquired by a following culture method: hpMSCs were seeded as a sphere form on dishes coated with 15 μg/ml of polyornithine (PLO; Sigma-Aldrich, St. Louis, MO, USA ) and 4 μg/ml of fibronectin (FN; Sigma-Aldrich) and then incubated using complete proliferation medium additionally containing 25 ng/ml of FGF4 (R&D, Minneapolis, MN, USA) and 1 μg/ml of heparin (Sigma-Aldrich) for 6 days at 37°C with 5%.
Growth Curve and Population Doubling Level
For the acquisition of hpMSCs growth curves, hpMSCs from passages 1–10 were seeded at 5×105 cells/75 T density with culture medium. hpMSCs were harvested and counted every 3 days. The number of accumulated cells was calculated with the formula for population doubling level (PDL).
Characterization of hpMSCs
AP staining: Alkaline phosphatase staining was performed with the StemTAGTM Alkaline phosphatase Staining Kit (Cell Biolabs, Inc., San Diego, CA, USA ) according to manufacturer’s recommendations.
Quantitative reverse transcription polymerase chain reaction: Total RNA was isolated from cells with TRIzol (Ambion, Thermo Fisher Scientific, Waltham, MA, USA). The cDNA was synthesized from 1 μg of total RNA using the Superscript II reverse transcriptase, as recommended in the manufacturer’s protocol. Reverse transcription polymerase chain reaction (RT-PCR) was conducted as described in the previous report29,31. Primer sequences are shown in Suppl. Table 1.
Flow cytometry analysis: The phenotype of hpMSCs was analyzed by flow cytometry using antibodies described in Suppl. Table 2. After the cells were washed, they were then analyzed by fluorescent-activated cell sorting (FACS) Calibur (BD Bioscience, San Jose, CA, USA) with the Cell Quest software.
Immunocytochemistry: Cells were fixed and permeabilized. The cells were visualized with fluorescence microscopy or confocal fluorescence microscopy (LSM 510 confocal microscope, Zeiss) as described previously29,31.
Immunoblot analysis: Whole cell lysates were normalized with the Bradford reagent (Bio-Rad, Hercules, California, USA), and 30–50 μg of the lysate was subjected to 8–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Darmstadt, Germany). Antibody information is in Suppl. Table 2. Immunoreactions were conducted using the enhanced chemiluminescence Western blotting system (Millipore).
Analysis of the Differentiation Potential of Human hpMSCs
We assessed the differentiation potential of hpMSCs during adipogenic, osteogenic and chondrogenic induction as described previously32.
Mouse Primary Central Nervous System Neural Precursor Culture, Co-cultures and Conditional Medium Treatments
Primary central nervous system (CNS) neural precursor (NP) cells were prepared and then co-cultured with none, hpMSC or hpNPCs as previously described33. Briefly, brain tissues were dissected from the anlages of the cortex, ventral parts of the midbrain (VM) of mouse embryos at embryonic day 14 (E14). Dissected tissues were dissociated into single cell suspension by pipetting. NP cells were then seeded on 10-cm culture dishes pre-coated with PLO/FN. NP cells were cultured in serum free N2 medium, 1% penicillin/streptomycin with 20 ng/ml basic fibroblast growth factor (bFGF) at 37°C and 5% CO2. Undifferentiated NP cells were co-cultured with either hpMSCs or hpNPCs. Conditioned Media (CM) from hpNPCs were collected during 3 days of differentiation without medium changes, filtered and then kept at −70°C until use.
Animals
Female adult Sprague-Dawley rats (220–250 g, 8 weeks old) were housed at room temperature (22–23°C) in standard 12 h light/dark cycles with free access to food and water. The experimental procedure was performed according to the animal care guidelines of the Institutional Animal Care and Use Committees (IACUC:170027).
6-OHDA Lesions and Transplantation
6-OHDA PD rats were generated as described previously32. 6-OHDA was injected unilaterally into right medial forebrain bundle (MFB; anterior-posterior (AP) −4.4, medial-lateral (ML) −1.2, dorsal-ventral (DV) −7.8, tooth bar set at −2.3 and AP −4.0, ML −0.8, DV −8.0, tooth bar set at 3.4 (all coordinates in millimeter relative to the bregma)) at a rate of 0.6 μl/min29. The 6-OHDA animal model was confirmed by amphetamine-induced rotation. Cells (2×1.5×105/rat) or a saline solution were transplanted into two sites in the brain (coordinates in the AP, ML and DV relative to the bregma and dura (1) 0.07, −0.30, −0.55; (2) −0.10, −0.40, −0.50; incisor bar set at zero). Cyclosporine A was used daily to suppress immune rejection.
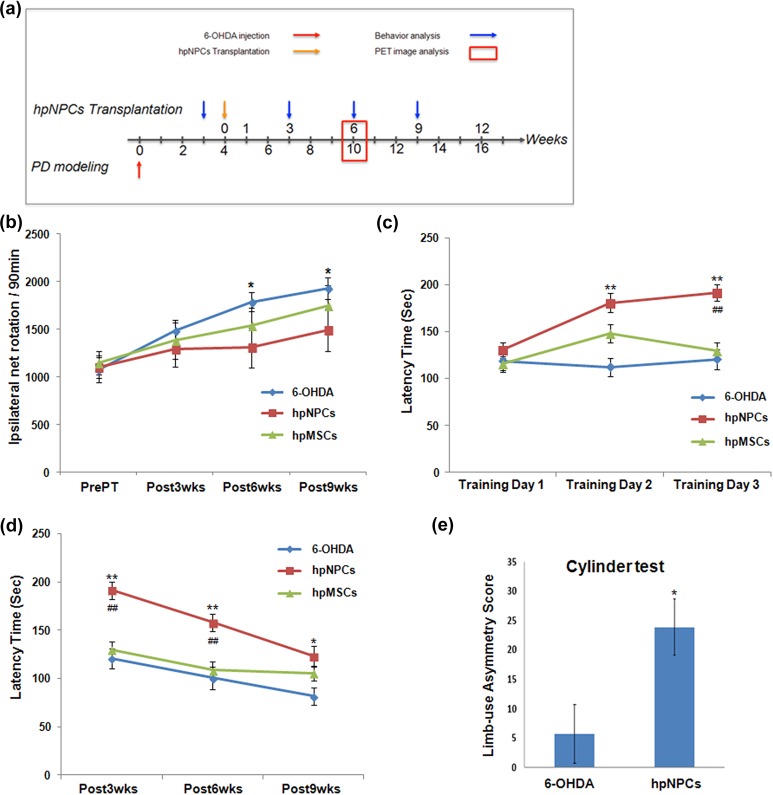
Experimental paradigm: The experimental timeline is described in Figure 2(a).
Figure 2.
Transplanted hpNPCs restored motor deficits of a rodent model of PD. (a) The experimental timeline. (b) In the rotation test, amphetamine-induced (5 mg/kg, i.p.) rotation scores per min exhibited a better performance by the hpNPC-transplanted PD rats compared with either saline or hpMSC-injected ones. (c) Rotarod tests were conducted at 3 weeks after transplantation as motor learning tasks. For the short-term motor memory task, the rotarod tests were performed during consecutive 3 days as training. In a training course, the hpNPC treatment enhanced memory of motor tasks compared with 6-OHDA (*) and hpMSC (#) treatment. (d) For the long-term motor memory task, the rotarod tests were repeated at 6 and 9 weeks after transplantation. Albeit all animals displayed declined motor activity, the hpNPC-transplanted rats showed better performance of significance compared with 6-OHDA (*) and hpMSC (#)-treated ones. (e) Asymmetry score in the cylinder task exhibited improved performance by the hpNPC-transplanted PD rats. ** and ##, P<0.01 and * and #, P<0.05 for each comparison. The data are expressed as the means ± SEM. 6-OHDA: 6-hydroxy dopamine; hpMSC: human placenta mesenchymal stem cell; hpNPC: hpMSC-derived neural phenotype cell; i.p.: intra-peritoneally; PD: Parkinson’s disease; SEM: standard error of the mean
Tissue Processing, Immunohistochemistry and Immunofluorescence
Immunohistochemistry was performed as previously described30 on free-floating cryomicrotome-cut sections (40-μm thick) that encompassed the entire brain. The primary antibody information is Suppl. Table 2. The Vectastain Elite ABC kit (Vector Laboratories., Burlingame, CA, USA) was used as a secondary antibody. Tissues were visualized with fluorescence microscopy or confocal fluorescence microscopy (LSM 510 confocal microscope, Zeiss).
Positron Emission Tomography Analysis
To measure dopaminergic depletion and the functional effects of cell transplantation, F-18 FP- CIT positron emission tomography (PET) analysis using an Inveon PET scanner (Siemens Medical Solutions, Inc., Knoxville, TN, USA)34 was performed at 6 weeks after transplantation as described previously29.
Behavioral Analyses
Animal models were divided into three groups: Sham 6-OHDA (n = 12), hpMSCs (n=8), and hpNPCs (n = 12).
Rotation test: Amphetamine-induced ipsilateral turning was measured for 90 min at 7 days before cell transplantation and at 3, 6, 9 and 12 weeks after cell transplantation.
Rotarod test: The accelerating rotarod test was conducted using an ACCELER rotarod treadmill for rat. After adaptation to a fixed speed (4 rpm) for 3 min, the rats were placed on a horizontal plastic rod that was rotating at an initial speed of 4 rpm, and the rotational velocity of the rod was linearly increased from 4 to 50 rpm within 5 min. The time that each rat was able to maintain its balance walking on the top of the rod was measured. This test was performed at 3, 6, and 9 weeks after cell transplantation.
Cylinder test: Spontaneous movement was measured by placing animals in a transparent cylinder (height, 40 cm; diameter, 20 cm). Spontaneous activity was video recorded for 5 min. A total of six patterns of movement (Left Foreleg Touch, Right Foreleg Touch, First Left of Both Foreleg Touch, First Right of Both Foreleg Touch, Both Foreleg Touch, and only Raise Upper Body) were assessed by viewing the spontaneous movement of rats. The number of forelimb steps was measured by two experimenters blind to the treatment group.
Safety Tests
Teratoma formation assay and karyotype analysis were conducted as describe previously29. Cells (5×105) were injected into the testis of (Central Lab. Animal Inc., Korea). Teratoma formation in the testis of 6-week-old BALB/c-nu Sic male mice was detected by hematoxylin and eosin staining after cell injection (5×105). The chromosomes were then visualized using G-band staining after cell were treated with 0.05 μg/ml colcemid for 1–2 h.
Statistics
The quantitative results of immunohistochemistry were analyzed using either Student’s t tests or two-way analysis of variance followed by a least significant difference post-hoc test (SPSS Version 24.0, IBM Inc., Armonk, New York, USA). A mixed model analysis of variance procedure was used to account for random effects in rats. Data are presented as the means ± standard error of the mean (SEM). A P-value of <0.05 was considered significant.
Results
Stem Cells (hpMSCs) from Human Term Placenta Preserved features of MSCs
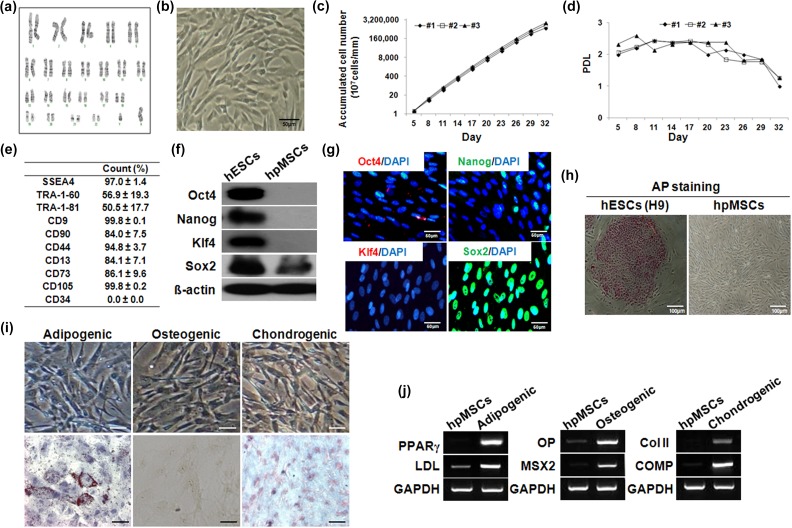
Upon karyotyping, hpMSCs isolated from placenta amniotic membranes contained a Y chromosome, confirming their human fetal origin (Figure 1(a)). As stereotypical MSCs, the hpMSCs retained a very flattened and asymmetrical spindle-shaped morphology, which is strongly reminiscent of mesenchymal cells (Figure 1(b)). The hpMSCs were isolated from the amniotic membranes of three donors and cultured as described in ‘Materials and Methods’. All of the isolated hpMSCs demonstrated that the number of accumulated cells sharply and steadily increased up to 32 days, and during a same period, their PDL was sustained without morphological change, indicating the robust proliferation of the cells (Figure 1(c) and (d)). Flow cytometry confirmed expression of MSC markers in the hpMSCs: they were positive for the MSC-related surface markers CD44 (94.8±3.7%), CD13 (84.1±7.1%), CD73 (86.1±9.6%), CD105 (99.8±0.2%), and CD90 (84.0±7.5%), but were not positive for the hematopoietic stem cell marker CD34 (0.0±0.0%) (Figure 1(e)). The hpMSCs also expressed SSEA-4 (97.0±1.4%) and markers of embryonic stem cells (ESCs), including TRA-1-60 (56.9±19.3%), CD9 (99.8±0.1%), and TRA-1-81(50.5±17.7%). The proteins Oct4, Nanog, and Klf4 were not detected and Sox2 was expressed in freshly isolated hpMSCs by immunoblotting, whereas all four factors were detected in human ESC (hESC) H9 cells, a positive control (Figure 1(f)). Consistently, immunostaining of the hpMSCs revealed that the cells were positive for only Sox2, but were devoid of the Oct4, Nanog, and Klf4 proteins (Figure 1(g)). Alkaline phosphatase enzyme activity, a marker for hESCs, was undetectable in hpMSCs, indicating that the isolated MSCs were not contaminated with hESCs (Figure 1(h)). In addition to proliferation capacity, their differentiation potentials into adipocytes, osteoblasts, and chondrocytes were tested: the hpMSCs were positively stained with Oil Red O for adipogenic properties, with von Kossa for the osteogenic properties, and with Alcian blue for the chondrogenic properties (Figure 1(i)). Expression of lineage specific markers confirmed multipotency of hpMSCs: peroxisome proliferator-activated receptor-gamma (PPARγ) and lipoprotein lipase (LDL) for adipogenic differentiation, osteopontin (OP) and MSX2 for osteogenic differentiation, and type II collagen (Col II) and Cartilage Oligomeric Matrix Protein (COMP) for chondrogenic differentiation, indicating that hpMSCs maintain key features of MSCs including proliferation and differentiation capacities (Figure 1(j)).
Figure 1.
Characterization and maintenance of hpMSCs from human term placenta. (a) Karyotyping of hpMSCs. (b) Morphology of hpMSCs. (c) Growth curves of the hpMSCs. (d) PDL of hpMSCs. (e) FACS analysis of the immunophenotypic surface profile of hpMSCs. The hpMSCs were negative for CD34 (hematopoietic and endothelial cell markers) and positive for SSEA4, TRA-1-60, and TRA-1-81 (ES markers) and for CD9 and CD44, CD13, and CD90 (MSC markers). (f, g) The protein levels of stem cell makers such as OCT4, NANOG, KLF4, and SOX2 were measured by immunoblotting and immunostaining. (h) Alkaline phosphatase staining. The scale bar indicates a length of 100 μm. (i, j) Differentiation potential of hpMSCs. The following staining methods were employed; Oil Red O staining for adipogenic differentiation, Von Kossa staining for osteogenic differentiation, and Alcian blue staining for chondrogenic differentiation. The mRNA level of PPARγ and LDL were expressed for adipogenic differentiation and OP and MSX2 were expressed for osteogenic differentiation, and Col II and COMP were expressed for chondrogenic differentiation. The scale bar indicates a length 50 μm. Col II: type II collagen; COMP: Cartilage Oligomeric Matrix Protein; FACS: fluorescent-activated cell sorting; hpMSC: human placenta mesenchymal stem cell; LDL: lipoprotein lipase; OP: osteopontin; PDL: population doubling level; PPARγ: peroxisome proliferator-activated receptor-gamma.
Motor Dysfunctions of a Rodent Model of PD were Restored by Transplantation of hpNPCs
Neural stem cells or precursor cells have been reported to have therapeutic potentials for neurodegenerative diseases including PD; the transplanted human fetal NP cells into rats with PD symptoms were able to rehabilitate their motor deficits and to inhibit loss of dopaminergic (DA) neurons29. However, neural stem cell transplantation in clinical application is impeded by limitation in securing enough number of cells without ethical concerns and immune responses against allogenic transplantation. To solve the problems, hpNPCs (hpMSC-derived neural phenotype cells) were obtained from the spherical culture condition under FGF4 containing proliferation media for 6 days: compared with hpMSCs, the cells cultured in a spherical form were more positive for neuronal markers, Nestin and Tuj1 (Suppl. Figure 1(a)). To induce neuronal differentiation rather than other cells after transplantation, the hpNPCs were then transplanted into the brain in a rodent model of PD. As described at Figure 2(a), a PD rat model was generated by injection of 6-OHDA into the right MFB of 8-week-old female adult Sprague-Dawley rats. After confirming their damage with a rotation test, the afflicted rats were applied to transplantation with saline, hpMSCs, or the hpNPCs and then subjected to the behavior assays. First, we measured amphetamine-induced rotational behavior at 1 week pre-transplantation and at 3, 6, and 9 weeks after transplantation to assess the extent of motor impairment or improvement. 6-OHDA-treated rats displayed steadily increased ipsilateral rotations over weeks, indicating that the DA neuron loss associated motor dysfunctions kept worse. Whereas the hpMSC-injected PD rats showed increased abnormal rotations to the 6-OHDA rats at comparable levels, the hpNPC-treated rats maintained a lower number of turns of significance at 6 and 9 weeks after transplantation compared with both the 6-OHDA and the hpMSC-treated ones, suggesting that the injected hpNPCs mitigated PD-associated motor deficits (Figure 2(b)). Next, the motor memory was assessed by measuring the latency to fall off an accelerating rod in a rotarod test. This test was split into short-term and long-term motor memory tests. To measure short-term memory, motor learning process was analyzed during three training days at 3 weeks after transplantation.
Although all tested animals ran similar length of time at the first training day, the hpNPC-treated rats displayed significantly improved performance following 2 days: from second day, the hpNPC-transplanted rats ran longer time compared with the 6-OHDA ones (**) and on third day, they exhibited better performance over the 6-OHD and the hpMSC-treated ones (##) (Figure 2(c)). The superior performance achieved by the hpNPC-treated rat was maintained up to 9 weeks after transplantation, although the effects diminished over weeks: the hpNPC-treated animals carried out running for longer time of significance over other two groups at 6 and 9 weeks after treatment indicating the hpNPCs were able to improve motor memory (Figure 2(d)). The cylinder test, another asymmetric behavior task, was performed to evaluate the use of the impaired forelimb while exploring and rearing in a transparent cylinder. A significant group effect on the number of impaired forelimb touches was observed: rats transplanted with the hpNPCs demonstrated significantly increased use of the impaired forepaw arm compared with the sham group (Figure 2(e)). The restored motor defects in the afflicted rats suggested that the transplanted hpNPCs have potentials to recover 6-OHDA associated neuronal damages.
The Transplanted hpNPCs Protected DA Neurons Against 6-OHDA Implicated Cell Death
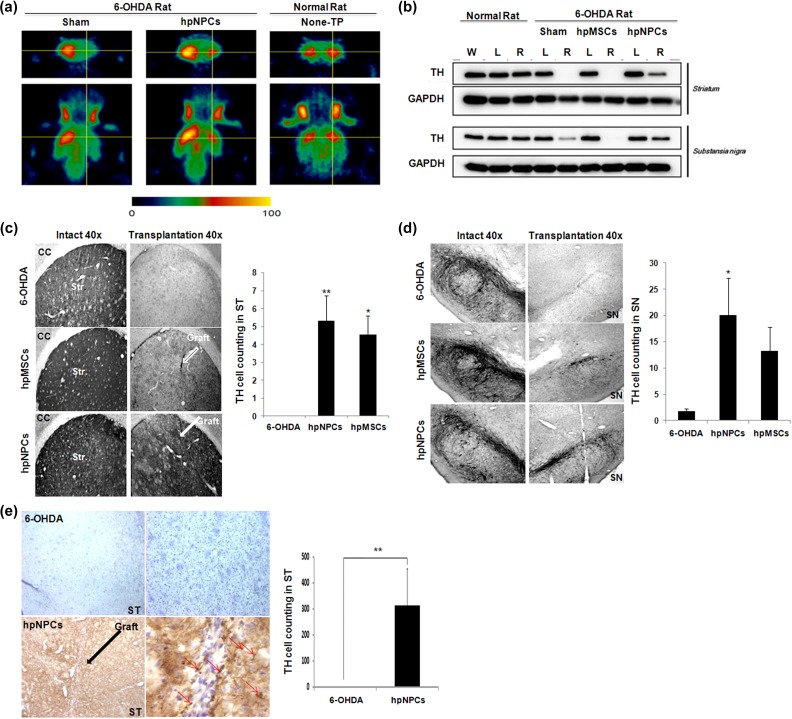
Recovery of DA neuron associated motor defects by the injected hpNPCs suggests that the transplanted hpNPCs are able to protect loss of DA neurons. The hpNPC-mediated restoration of DA was supported by PET analysis that was conducted 6 weeks after transplantation using radiolabeled ligands for dopamine transporters (DATs): while the 6-OHDA-treated rats, compared with a normal rat, exhibited reduced signals indicating loss of DA terminal functionality, the hpNPC-transplanted PD rats manifested partial retrieval of DA loss (Figure 3(a)). Next, we also examined tyrosine hydroxylase (TH) protein, a rate-limiting enzyme in DA synthesis, by immunoblotting to assess the in vivo survival of the DA neurons in the striatum and the SN after cell transplantation into the stratum of 6-OHDA-lesioned rats. Whereas, it was not or barely detected in the lesioned striatum and SN of 6-OHDA and hpMSCs treated groups respectively, TH protein was observed in the lesioned striatum and lesioned SN of the hpNPC-injected group, indicating that transplantation of hpNPCs, not hpMSCs, were able to protect TH-positive cells against 6-OHDA-treated rats (Figure 3(b)). The enhanced viability of TH-positive cells was further supported by immunohistochemical staining with TH antibody for DA neurons. TH-immunoreactivity (IR) was plentiful in the striatum of an intact site whereas the decreased of TH-IR was observed on a 6-OHDA injected site. The implantation of either hpNPCs or hpMSCs remarkably inhibited the loss of striatal TH-IR induced by 6-OHDA injection compared with the 6-OHDA rats (Figure 3(c)). In addition, significantly higher number of DA neurons on the SN of the hpNPCs transplanted rats existed compared with 6-OHDA treatment rats (Figure 3(d)). The hpMSCs injection protected TH cells but it was not significant. The significant number of DA neurons was observed in striatum even at 12 weeks after hpNPCs transplantation (p< 0.05, Figure 3(e)). Taken together, hpNPCs are able to restore 6-OHDA mediated motor dysfunction via protecting DA neurons against cell death and hpMSCs themselves have partial potency for recovering functional deficits of 6-OHDA treated rats.
Figure 3.
The hpNPCs were able to protect DA neurons in 6-OHDA treated rats. (a) PET image analysis. The 18F FP-CIT PET image with coronal and transaxial slices showed that 6-OHDA mediated dopaminergic impairment (sham) was partially recovered after transplantation of the hpNPCs. (b) The hpNPC-injected 6-OHDA-lesioned rats showed partial recovery of TH protein in both the striatum and substantia nigra at 12 weeks after cell transplantation compared with either saline or the hpMSC-treated ones. (c) TH-immunoreactivity exhibited that a rich TH fiber network in the striatum to the lesion after transplantation was lower in the 6-OHDA-lesioned side of striatum. (d) Immunohistochemical staining and quantification of TH-positive cell bodies in the substantia nigra. (e) Immunohistochemical staining and quantification of TH-positive cell bodies in the striatum. ***, P<0.001; **, P<0.01 and *, P<0.05 for each comparison 6-OHDA: 6-hydroxy dopamine; DA: dopamine; hpMSC: human placenta mesenchymal stem cell; hpNPC: hpMSC-derived neural phenotype cell; PET: positron emission tomography; ST: striatum; TH: tyrosine hydroxylase.
The Transplanted hpNPCs Survived and Differentiated into Neurons at the Grafted Sites
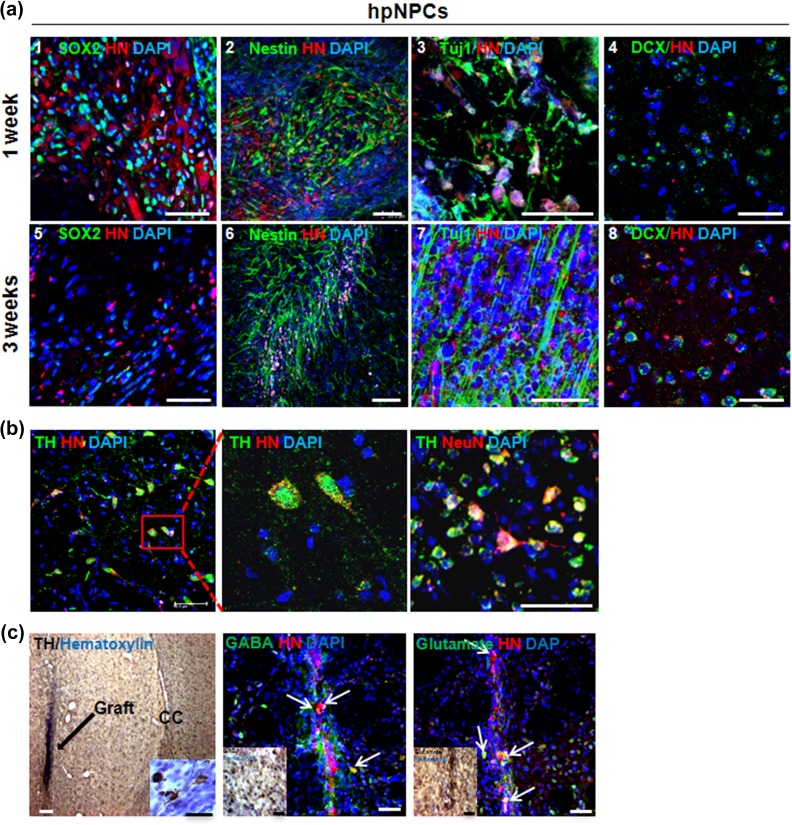
A graft site was easily identified in brain sections followed by immunohistochemical staining for human-specific human nuclei (HN) marker: the transplanted cells around the vertical needle tract stained positive for HN (Suppl. Figure 2(a)). The presence of the transplanted cells was confirmed by fluorescent in situ hybridization against human centromere 17 (CEP17) within the striatum of hpNPCs group (Suppl. Figure 2(b)). The subpopulation of HN+ cells was co-immunostained with antibodies against SOX2 (Figure 4(a) 1 and 5), Nestin (Figure 4(a) 2 and 6) and Tuj1 (Figure 4(a) 3 and 7) at 1 and 3 weeks after transplantation. At 1 and 3 weeks after transplantation, some of the transplanted cells were co- expressed with doublecortin (DCX) and dispersed in the striatum, indicating migratory potentials of the injected cells (Figure 4(a) 4 and 8). The HN and TH co-stained hpNPCs at 6 weeks post-transplantation indicated that the transplanted cells were able to differentiate into DA neurons in the striatum region at 6 weeks after transplantation (Figure 4(b)). Interestingly, the transplanted cells were able to differentiate into other types of neurons at 12 weeks after transplantation: some cells were co-expressed with gamma-aminobutyric acid (GABA) and glutamate+ cells (Figure 4(c)). To test whether hpNPCs generate teratomas, hpNPCs were transplanted into testis of severe combined immunodeficiency (SCID) mice. No teratoma formation was observed in vivo at 6 or 36 weeks after injection (Suppl. Figure 3). Taken together, the results showed that hpMSC-derived hpNPCs were able to survive and differentiate into neurons including TH-positive neurons without teratoma formation after transplantation into a rat PD model.
Figure 4.
The transplanted hpNPCs were able to survive and differentiate into diverse neurons including DA neurons in 6-OHDA-treated rats. (a) Immunostaining of the transplanted hpNPCs at the grafted sites displayed survival and neuronal differentiation of the injected hpNPCs. At 1 week (1–4) and 3 weeks (5–8) after transplantation, the brains were isolated from the hpNPC-treated rats and were stained with multiple neuronal markers with HN (scale bar: 100 μm): SOX2 (pluripotent stem cell marker, 1 and 5), Nestin (neural precursor marker, 2 and 6), Tuj-1 (neuronal marker, 3 and 7) and DCX (migration marker 4 and 8). (b) The transplanted cells were positive for both TH and NeuN in the striatum region at 6 weeks post-transplantation scale bar is 100μm (c) The transplanted hpNPCs were differentiated into GABA and glutamate-positive neurons at 12 weeks after transplantation. The scale bar indicates a length of 50 μm. 6-OHDA: 6-hydroxy dopamine; DA: dopamine; DCX: doublecortin; HN: human nucleic marker; hpMSC: human placenta mesenchymal stem cell; hpNPC: hpMSC-derived neural phenotype cell; TH: tyrosine hydroxylase.
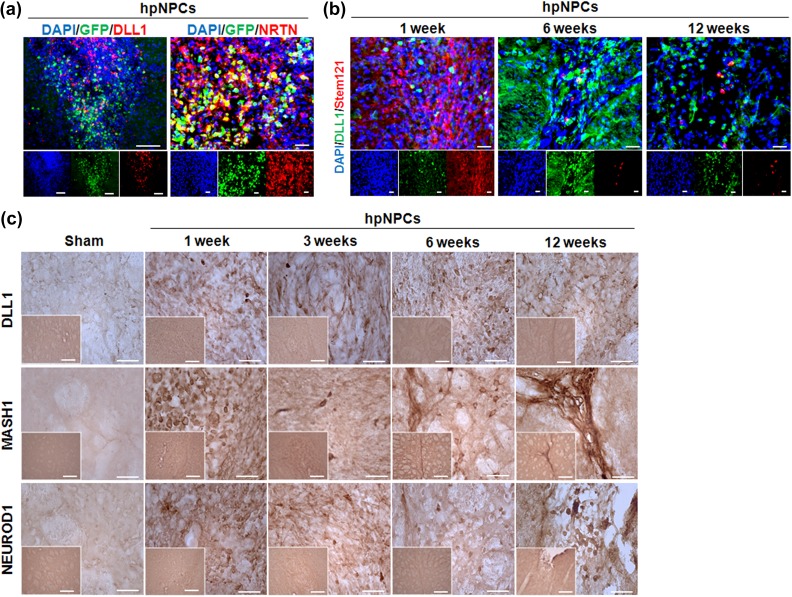
The Transplanted hpNPCs Expressed DLL1 and Neurturin, and Altered Microenvironment in the Transplanted Striatum
We next looked for putative factors contributing to hpNPC-mediated neuroprotection. Because the injected hpNPCs influenced neighboring cells at the grafted site (Figure 4) and DLL-Notch-mediated cell contact signals are critical in neuronal survival in PD, we examined activation of DLL-Notch mediated cell contact signals35,36. In addition, since neurturin (NRTN) among neurotrophic factors was reported to protect neurons in PD and regulate Notch signals for protect cardiac progenitor cells, expression of NRTN was also probed37,38. A majority of injected green fluorescent protein (GFP)-tagged hpNPCs expressed DLL1 and subpopulation of GFP-hpNPCs was overlapped with NRTN positive cells, indicating that Notch signals and the NRTN contribute the survival of hpNPCs at the graft and their migration position (Figure 5(a)). To test whether the DLL1-positive hpNPCs affected their environment by cell contact signals, the transplanted regions were co-stained with DLL-1 and a human cytosolic stem cell marker, Stem121. Although hpNPCs were barely detected at 6 weeks after injection, abundance of the DLL1 positive cells dramatically increased at 6 weeks and then declined until 12 weeks after injection, indicating that most of the DLL1-expressing cells were endogenous at 6 weeks after injection and the transplanted hpNPCs functioned through altering their environment (Figure 5(b)). Next, we examined the expression of DLL1 and its effect on neurogenesis by immunohistochemistry after cell transplantation. Compared with the sham controls, the cluster of cells in the graft site were DLL1 positive antibody at 1, 3, 6 and 12 weeks post-transplantation and Notch target MASH1 and NeuroD1 protein levels increased at 1, 3, 6 and 12 weeks post- transplantation as well. Interestingly, DLL1, MASH1, and NeuroD1 increased on the margin of the striatum in the hpNPCs transplanted PD rats at 6 weeks after injection, indicating that the transplanted hpNPCs migrated into the margin of the striatum ad 6 weeks post-transplantation and then modulated their environments favoring to neuronal differentiation (Figure 5(c)). This result suggested that the injected hpNPCs were well differentiated into neural or neuronal cells in the host rat brain. Taken together, our results suggested that the transplanted hpNPCs have potentials to enhance survival of the afflicted DA neurons by both cell contacts and paracrine factors including NRTN.
Figure 5.
The transplanted hpNPCs expressed DLL1 and influenced environments. (a) The transplanted GFP-tagged hpNPCs were positive for DLL1 and NRTN. Scale bar is 20μm. (b) Regardless of disappearance of the injected hpNPCs, DLL1 positive cells were detected around the graft sites. The scale bars represent 20μm. (c) Immunohistochemical staining of DLL1, MASH1, and NEUROD1-positive cell bodies in the striatum (scale bar: 50 and 20μm). DAPI: 4’,6-diamidino-2-phenylindole; DLL: delta-like ligand; GFP: green fluorescent protein; hpMSC: human placenta mesenchymal stem cell; hpNPC: hpMSC-derived neural phenotype cell; NRTN: neurturin.
Secreted Factors from hpNPCs Enhanced Cell Survival and Neuronal Differentiation as well as Modulated Microglial Activation in Mouse NP Cells Culture
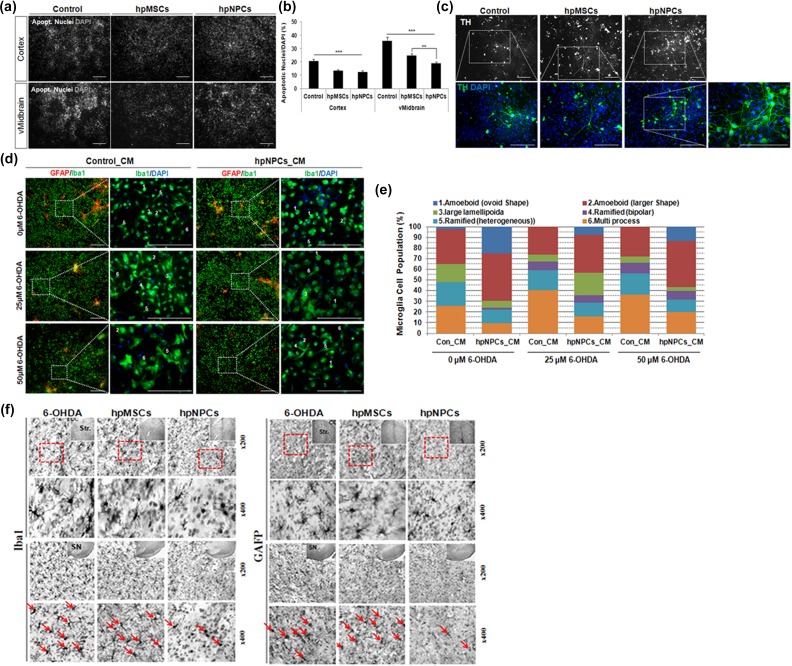
Recent studies demonstrated that diffusible factors secreted from the transplanted MSCs induced neurogenesis and neuroprotection, and modulated microglial activation, called paracrine activity39–41. To test whether the paracrine factors released from hpNPCs promote survival and neuronal differentiation of neighboring precursors, undifferentiated mouse NPCs from either cortex or ventral midbrain were co-cultured with either hpMSCs or hpNPCs under differentiation condition33 and then apoptotic nuclei containing apoptotic cells were measured at differentiation day 14. As shown Figure 6(a–b), all co-cultured NPCs from both cortex and midbrain displayed remarkable reduction in the number of apoptotic cells. In particular, the hpNPCs more efficiently suppressed cell death of NPCs from midbrain of significance compared with the hpMSCs. In addition, the hpNPCs were able to induce differentiation of NPCs into TH-positive cells more efficiently at 7 days after differentiation than the hpMSCs, indicating that secreted factors from hpNPCs may have attributes enhancing both neuroprotection and differentiation into TH-positive DA neurons (Figure 6(c)). Immune-modulatory potentials of paracrine factors from hpNPCs was assessed in the experiment where the activated microglial cells and astrocytes by 6-OHDA were treated with CM from hpNPCs for 3 days in vitro. After both microglia and astrocytes were treated with differential concentrations of 6-OHDA (0, 25, and 50 μM), activation of microglial cells and astrocytes in the presence of CM were estimated by morphological changes of microglial cells with Iba1-imunoreactivity (green) or astrocytes with glial fibrillary acidic protein (GFAP)-immunoreactivity (red). Although remarkable changes in astrocytes were not detected in any conditions, microglia showed profound morphological changes in the presence of 6-OHDA and CM: both 25 and 50 μM of 6-OHDA-activated microglia, whose activation was suppressed by CM (Figure 6(d)). For detailed assessment, microglia cells were categorized into six subtypes according to their shapes, ovoid shape amoeboid (1), larger shape amoeboid (2), large lamellipodia (3), bipolar ramified (4), heterogeneous ramified (5) and multiple extend process (6)42–44. Quantification of the microglia population revealed that the CM treatment altered the proportions of the subtypes induced by 6-OHDA. The 6-OHDA treatment increased population of activate microglias, subtype 4–6, and decreased the resting cells with residual amoeboid morphology. This population shift was reversed by hpNPC-CM: the portion of round shaped resting microglia increased in the expense of reduction in the activated microglia population (Figure 6(e)). Next, we examined whether the effect of transplanted hpNPCs was associated with inhibition of 6-OHDA-induced glial cells activation in PD rats. Parallel to CM-mediated immune suppression, the hpNPC transplantation into the 6-OHDA-treated rats mitigated activation of immune responses at 12 weeks after transplantation compared with controls and even hpMSC-treated rats: enlarged cell soma and numerous process containing ramified Iba1 positive microglias and GFAP-positive astrocytes were detected on striatum and SN regions in the 6-OHDA-treated and 6-OHDA-plus-hpMSC-treated rats, and their populations were substantially reduced by transplantation of hpNPCs (Figure 6(f)). Taken together, the hpNPCs have potentials to modulate immune responses of damaged brains with secreted factors in a paracrine manner.
Figure 6.
hpNPCs protected neural precursor cells via paracrine factor associated immune modulation. (a) Apoptotic cells were estimated by cells with apoptotic nuclei at differentiation day 14 for cell survival in mouse fetal cortical and midbrain neuronal cells co-cultured with media only, hpMSCs or hpNPCs. (Scale bar: 100μm). (b) Quantification of apoptotic nuclei/DAPI in co-culture of mouse fetal cortex- or midbrain-derived neuronal cells with media only, hpMSCs or hpNPCs. ***, p< 0.0001and **, P<0.01. (c) Shown in upper panels were determined 14 days after differentiation by immunocytochemical assay for dopaminergic neuron marker, TH (white). The boxed area in the upper panel exhibiting mature TH+ DA neurons (green) is enlarged in the lower panel (scale bar: 100 μm). (d) After treatment with 6-OHDA (0, 25, 50 μM), activated microglias and astrocytes were treated with CM from hpNPCs for 3 days in vitro and stained with Iba1 (green) or astrocytes with GFAP (red). The CM from hpNPC-treated microglial cells were less activated. The boxed area in the left panel showing diverse morphology of Iba+ microglial cells is a higher magnification in the right panel (scale bar: 100μm). (e) In the presence of CM from hpNPCs, quantification of activated microglial cells were counted by morphology of Iba+ cells (green) after treatment of 6-OHDA for 3 days. (f) Photomicrographs of Iba-1 and GFAP in the ST and SN after intracerebral injections of 6-OHDA with saline, hpMSCs or hpNPCs. The hpNPC-injected rats displayed suppression of microglial or astrocytic activation compared with saline and hpMSC-injected ones. 6-OHDA: 6-hydroxy dopamine; DAPI: 4’,6-diamidino-2-phenylindole; DA: dopamine; GFAP: glial fibrillary acidic protein; hpMSC: human placenta mesenchymal stem cell; hpNPC: hpMSC-derived neural phenotype cell; SN: substantia nigra; ST: striatum; TH: tyrosine hydroxylase.
Discussion
Among the neurodegenerative diseases, PD is one with high frequency in the elderly over 60 years and devastates lives of both the affected individuals and their kin. Chronic oxidative stresses and damages, mitochondrial dysfunction, and genetic mutations lead to loss of DA neurons in midbrain, pathological features of PD, but growing results support aging as a primary factor in PD pathogenesis45–48. Because loss of DA neurons causes decline of dopamine and uncontrolled movement of the patients, the current therapeutic treatments are administration of L-DOPA and DA agonists, and deep bran stimulation49. Although these approaches are able to mitigate PD symptoms, they are temporary in relief and not able to control a complex pathogenesis of PD. To protect loss of DA in a midbrain region in PD, the stem cell, especially MSC, based therapies have been shed light as an alternative approach and have showed promising results. However, the therapeutic mechanism of non-neural MSC-mediated functional recovery has not been clearly demonstrated.
Placenta becomes an attractive extra embryonic tissue as a fetal pluripotent stem cell source upon several reasons. First, because human placentas are usually discarded after birth, the acquisition of the stem cells from the placenta is free of ethical issues50. Secondly, placenta has two sides, a fetal consisting of amnion and chorion and a maternal side consisting of deciduas, and the diverse stem cells from both sides exhibit not only attributes of MSCs but also differentiation capacities into different lineages such as ectodermal neuronal and retinal cells, endodermal pancreatic beta cells51. Thirdly, the placental MSCs secrete large amounts of proangiogenic and antiapoptotic cytokines and have immunosuppressive properties such as suppressing T-cell proliferation52,53. In addition, compared with the MSCs from bone marrow, cord blood, and adipose tissue, the placental MSCs were recently reported to grow faster and to undergo slower senescence, implying that the placenta MSCs are available for long-term culture54. Nonetheless, characteristic analysis of placental stem cells, in particular hpMSCs, was not fully conducted. Our studies first confirmed hpMSCs as stereotypical MSCs: along with long lasting proliferation capacity, they were positive for MSC markers CD44, CD73, and CD105, as well as ESC markers SSEA4, TRA-1-60, and TRA1- 81 but negative for a hematopoietic marker CD34. The hpMSCs were able to induce appropriate genes for particular lineages in differentiation conditions (Figure 1(e)). Among MSC markers, CD90(Thy-1), which is a marker for ESCs and neural cells as well, was expressed in majority of the isolated hpMSCs. The CD90 positive population among the MSCs isolated from term placenta displayed wide spectra: almost all umbilical cord derived MSCs (UC-MSCs) (99%) are positive for CD90 but a small portion (about 26%) of Wharton’s jelly-derived MSCs (WJ-MSCs) expresses it. A large population of CD90-positive among the hpMSCs here may result from their origin, a maternal amniotic membrane, because the MSCs from maternal side of placenta were CD90 positive52–54. The high level of the CD90 in the hpMSCs is intriguing in that the CD90 is expressed in cells of the immune and nervous systems, and is engaged in modulating T-cell activation, inflammation, modulating neuronal growth and signal transmission, and tumor suppression55,56. It is worthwhile to study the role of CD90 in hpMSC mediated immune modulation. Among four stem cell factors, only Sox2 was clearly detected in the hpMSCs at protein and cellular level (Figure 1(f)). Considering that none of the MSCs, including bone marrow, adipose, cord blood, and placenta MSCs, expressed all four factors, their particular patterns of expression in MSCs are likely dependent of their origin and culture conditions54. In our study, only Sox2 was expressed at detectable levels in the all prepared hpMSCs, indicating that subpopulations of stem cells from placenta have particular regulatory signals for Sox2 expression and functions. At this moment, we do not know the mechanism but Sox2 expression in the hpMSCs may endow hpMSCs with distinct potentials for their cellular behaviors including differentiation potential. Because Sox2 is one of early neuronal lineage markers and CD90 is of late neuronal ones, the relatively high level of CD90 and SOX2 expression in hpMSCs suggests that the hpMSCs have strong capacities both for immune modulation and for differentiation into neuronal cells. Upon these two properties, hpMSCs are regarded as a good candidate of cell therapies for neurodegenerative diseases including PD.
hpNPCs are able to Recover Physiological Deficits of a Rat Model of PD via Dual Mechanisms, Neuroprotection and Immune Modulation
We recently reported that the transplantation of human fetal midbrain derived neural progenitor cells was able to restore functional deficits of 6-OHDA-treated PD rats: the damaged rat grafted with the fetal progenitor cells exhibited a significant recovery in motor asymmetry 12 weeks post-graft along with elevated level of dopamine in the area of the lesioned striatum29. Due to ethical concerns and limitations on supply in fetal stem cells or fetal NP cells, we have looked for the alternatives and in this study, presented therapeutic potentials of the hpNPCs. Compared with either the saline or hpMSC-treated rats, the hpNPC-transplanted PD rats exhibited significant recovery in motor dysfunctions (Figure 2(b–e)). Along with convalescing motor activities, the hpNPC-xenografted PD rats partially regained DA and TH-positive population in both SN and striatum (Figure 3). The hpNPC-mediated restoration may provide good opportunities to substitute the hpNPCs for fetal neural progenitor cells in many therapies. Because both hpMSCs and hpNPCs are relatively easier to secure a large amount of supply from placenta, this possibility may also help to develop novel stem cell-based therapies in diverse diseases as well as to understand underlying mechanism of cell therapies.
Abnormal innate and adaptive immune responses including microglia activation are a critical feature of PD: toxic molecules, such as α-synuclein, released from damaged neurons activate microglia and the activated microglial cells, in turns, secret proinflammatory factors and ROS, which further aggravate neuronal damages. Moreover, modified α-synuclein is recognized as an antigen and recruit effector T-cells activating microglia57. Growing results suggest that regulation of immune responses in PD is considered as a critical target in developing therapies and in animal model of PD, MSCs and NP cells protected DA neurons and suppressed activation of astrocyte and microglia through paracrine factors39,40. In this study, we showed that the hpNPC-mediated recovery in the 6-OHDA-treated rats was associated with, at least, immunosuppression and neuroprotection, where both paracrine factors and cell contact interactions at the grafted sites play key roles (Figures 5 and 6). Co-culture experiments of activated glial cells with the hpNPCs reveal that the hpNPCs produce diffusible factors enhancing survivability and neuronal differentiation, including into TH-positive neurons, of the primary NP cells from fetal cortex and midbrain (Figure 6(a–c)). Less Activated microglia cells and astrocytes treated with the CM from the hpNPCs indicate that hpNPCs are able to suppress immune responses with diffusible factors (Figure 6(d)). Interestingly, while co-cultured NP cells from cortex were protected at comparable levels by both hpMSCs and hpNPCs, the hpNPCs are more efficient in protection of NP cells from midbrain than hpMSCs. A bank of secreted factors promoting DA neuron protection and differentiation have been identified including Wnt1, Wnt5a, and sonic hedgehog (SHH), some of which were expressed in hpMSCs41,58,59. Furthermore, the hpMSCs secrete immunosuppressive TGFβ, interleukin (IL)-10, and Insulin degrading enzyme (IDE), and mitigated astrocyte/microglia activation30,31. Because hpNPCs are derived from hpMSCs, it is a reasonable expectation that hpNPCs are able to produce diffusible factors with similar functions. However, two cells display differential restoration capacities of motor defects, DA neuron loss, and long-term immune modulation, suggesting that hpMSCs and hpNPCs secrete differential as well as common diffusible molecules regulating immune responses and survival signals. Now the secreted molecules and metabolites in the CMs from both conditions are under investigation to search relevant new factors and novel mechanisms.
Along with soluble factors, a DLL1-Notch signal is able to contribute the hpMSC/hpNPC associated suppression of astrocyte/microglia activation as well as neuroprotection (Figure 5(c)): the high levels of DLL1 expressed in hpNPCs was paralleled with the increased survival of DA neurons in the SN of the PD rat model in our study. Notch was reported to repress microglia activation through Notch mediated suppression of tumor necrosis factor (TNF) release as well as the expression of MHC-II and OX40 L on mast cells, which induce the differentiation of Th260,61. In addition, the silencing of DLL1 caused a decrease in TH expression, showing that DLL-Notch signals enhance viability and differentiation of DA neurons62,63. In a rat model of PD, α-synuclein was reported to bind a Notch 1 promoter with p53 protein and repressed its expression as well as Notch mediated neurogenesis in PD35,64. Mildronate, a small molecule with charged nitrogen and oxygen atoms, protected DA neurons in 6-OHDA-treated rats via stimulation of Notch 3 signaling and suppression of GFAP and inducible nitric oxide synthase (iNOS; an inflammation marker)36. These reports indicate that Notch signals not only modulate transport of external signals but can also affect neighbors via cell contacts and secretion factors. In this study, the GFP-tagged hpNPCs in the grafted site here expressed both DLL1 and NRTN and induced DLL1 expression around the injection site for a long period (Figures 4 and 6). In particular, when AAV- NRTN (CERE-120) was injected into the striatum and SN, it prevented the emergence of motor symptoms shown in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated young adult monkeys for up to 10 months65. Induction of DLL1 and NRTN supports the idea that the hpNPCs are able to change the environment to the favorable condition for survival and neuronal differentiation via both cell contact and paracrine manners. Interestingly, CM injected into the SN and striatum (STR) into a rat model of PD relieved PD-associated functional and physiological deficits41. Protective capacity of CM was comparable with that of the transplanted NPCs, indicating the secreted molecules are critical therapeutic factors. Co-culture experiments of NP cells either with hpNPCs or hpMSCs fit into their conclusions even though the better protection and DA differentiation were observed from hpNPC co-cultured midbrain cells. In this study, the transplanted hpNPCs induced not only neurotrophic factors including NRTN but also DLL-Notch-mediated cell contact signals. These two distinct signals are beneficial in treatment: the secreted factors are able to influence larger areas and the cell contact signals improve neuroprotection and immunosuppression at graft and adjacent areas. It will be interesting to see whether cell transplantation and CM have differential therapeutic effects on PD treatment and which mechanisms/pathways redeem each other if each of them has their own drawbacks.
Hereto we characterized the human placenta MSCs, hpMSCs, and investigated the therapeutic potentials of hpMSCs and their neural phenotype cells, hpNPCs, with a PD rat model. The present study is the first report to compare efficiencies of functional recovery of PD-associated deficits between placenta MSCs and their neural derivatives and to delineate the non-neural MSC-mediated therapeutic mechanisms: immunosuppression and neuroprotection via diverse diffusible factors and cell contact signals. Here we suggest that the hpMSCs and hpNPCs are good cellular sources for cell therapies and their medical applications are plausible in wide ranging of neural diseases including PD and aging.
Supplementary Material
Acknowledgments
We are grateful to Yun-hwa Jeong and to the Clinical Statistics Center of CHA University for providing statistical analysis. Han Wool Kim, Hyun-Seob Lee, and Jun Mo Kang contributed equally to the first authorship.
Footnotes
Ethical Approval: The experimental procedure was performed according to the Institutional Review Board of the CHA General Hospital (Seoul, Korea) and animal care guidelines of the Institutional Animal Care and Use Committees (IACUC:170027).
Statement of Human and Animal Rights: The experimental procedure was performed according to the animal care guidelines of the Institutional Animal Care and Use Committees (IACUC:170027).
Statement of Informed Consent: Statement of Informed Consent is obtained from donors with their informed consent according to the Institutional Review Board of the CHA General Hospital (Seoul, Korea).
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Bio & Medical Technology Development Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (NRF-2017M3A9B4025699 and NRF-2017M3A9B4025709).
Supplemental Material: Supplementary material for this article is available online.
References
- 1. Pakkenberg B, Moller A, Gundersen HJ, Mouritzen Dam A, Pakkenberg H. The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson’s disease estimated with an unbiased stereological method. J Neurol Neurosurg Psychiatry. 1991;54:30–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McNaught KS, Belizaire R, Isacson O, Jenner P, Olanow CW. Altered proteasomal function in sporadic Parkinson’s disease. Exp Neurol. 2003;179:38–46. [DOI] [PubMed] [Google Scholar]
- 3. Murrell W, Wetzig A, Donnellan M, Feron F, Burne T, Meedeniya A, Kesby J, Bianco J, Perry C, Silburn P., Mackay-Sim A. Olfactory mucosa is a potential source for autologous stem cell therapy for Parkinson’s disease. Stem Cells. 2008;26:2183–2192. [DOI] [PubMed] [Google Scholar]
- 4. Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Santambrogio L, Belyanskaya SL, Fischer FR, Cipriani B, Brosnan CF, Ricciardi-Castagnoli P, Stern LJ, Strominger JL, Riese R. Developmental plasticity of CNS microglia. Proc Natl Acad Sci USA. 2001;98:6295–6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175:4320–4330. [DOI] [PubMed] [Google Scholar]
- 7. Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–139. [DOI] [PubMed] [Google Scholar]
- 8. Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502. [DOI] [PubMed] [Google Scholar]
- 9. Town T, Tan J, Flavell RA, Mullan M. T-cells in Alzheimer’s disease. Neuromolecular Med. 2005;7:255–264. [DOI] [PubMed] [Google Scholar]
- 10. Wilkinson B, Koenigsknecht-Talboo J, Grommes C, Lee CY, Landreth G. Fibrillar beta-amyloid- stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J Biol Chem. 2006;281(30):20842–20850. [DOI] [PubMed] [Google Scholar]
- 11. Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. [DOI] [PubMed] [Google Scholar]
- 12. Rogers J, Luber-Narod J, Styren SD, Civin WH. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer’s disease. Neurobiol Aging. 1988;9:339–349. [DOI] [PubMed] [Google Scholar]
- 13. McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett. 1987;79:195–200. [DOI] [PubMed] [Google Scholar]
- 14. Zecca L, Zucca FA, Wilms H, Sulzer D. Neuromelanin of the substantia nigra: a neuronal black hole with protective and toxic characteristics. Trends Neurosci. 2003;26:578–580. [DOI] [PubMed] [Google Scholar]
- 15. Wilms H, Rosenstiel P, Sievers J, Deuschl G, Zecca L, Lucius R. Activation of microglia by human neuromelanin is NF-kappaB dependent and involves p38 mitogen-activated protein kinase: implications for Parkinson’s disease. FASEB J. 2003;17:500–502. [DOI] [PubMed] [Google Scholar]
- 16. Singhrao SK, Neal JW, Morgan BP, Gasque P. Increased complement biosynthesis by microglia and complement activation on neurons in Huntington’s disease. Exp Neurol. 1999;159:362–376. [DOI] [PubMed] [Google Scholar]
- 17. Pavese N, Gerhard A, Tai YF, Ho AK, Turkheimer F, Barker RA, Brooks DJ, Piccini P. Microglial activation correlates with severity in Huntington disease: a clinical and PET study. Neurology. 2006;66:1638–1643. [DOI] [PubMed] [Google Scholar]
- 18. Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20:173–177. [PubMed] [Google Scholar]
- 19. Hao Y, Ma DH, Hwang DG, Kim WS, Zhang F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000;19(3):348–352. [DOI] [PubMed] [Google Scholar]
- 20. Wolbank S, Hildner F, Redl H, van Griensven M, Gabriel C, Hennerbichler S. Impact of human amniotic membrane preparation on release of angiogenic factors. J Tissue Eng Regen Med. 2009;3:651–654. [DOI] [PubMed] [Google Scholar]
- 21. Magatti M, De Munari S, Vertua E, Gibelli L, Wengler GS, Parolini O. Human amnion mesenchyme harbors cells with allogeneic T-cell suppression and stimulation capabilities. Stem Cells. 2008;26:182–192. [DOI] [PubMed] [Google Scholar]
- 22. Magatti M, De Munari S, Vertua E, Nassauto C, Albertini A, Wengler GS, Parolini O. Amniotic mesenchymal tissue cells inhibit dendritic cell differentiation of peripheral blood and amnion resident monocytes. Cell Transplant. 2009;18:899–914. [DOI] [PubMed] [Google Scholar]
- 23. Cargnoni A, Gibelli L, Tosini A, Signoroni PB, Nassuato C, Arienti D, Lombardi G, Albertini A, Wengler GS, Parolini O. Transplantation of allogeneic and xenogeneic placenta-derived cells reduces bleomycin-induced lung fibrosis. Cell Transplant. 2009;18:405–422. [DOI] [PubMed] [Google Scholar]
- 24. Cargnoni A, Di Marcello M, Campagnol M, Nassuato C, Albertini A, Parolini O. Amniotic membrane patching promotes ischemic rat heart repair. Cell Transplant. 2009;18:1147–1159. [DOI] [PubMed] [Google Scholar]
- 25. Sant’Anna LB, Cargnoni A, Ressel L, Vanosi G, Parolini O. Amniotic membrane application reduces liver fibrosis in a bile duct ligation rat model. Cell Transplant. 2011;20:441–453. [DOI] [PubMed] [Google Scholar]
- 26. Chang CJ, Yen ML, Chen YC, Chien CC, Huang HI, Bai CH, Yen BL. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells. 2006;24:2466–2477. [DOI] [PubMed] [Google Scholar]
- 27. Evangelista M, Soncini M, Parolini O. Placenta-derived stem cells: new hope for cell therapy? Cytotechnology. 2008;58:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park S, Kim E, Koh SE, Maeng S, Lee WD, Lim J, Shim I, Lee YJ. Dopaminergic differentiation of neural progenitors derived from placental mesenchymal stem cells in the brains of Parkinson’s disease model rats and alleviation of asymmetric rotational behavior. Brain Res. 2012;1466:158–166. [DOI] [PubMed] [Google Scholar]
- 29. Moon J, Schwarz SC, Lee HS, Kang JM, Lee YE, Kim B, Sung MY, Hoglinger G, Wegner F, Kim JS, Hyung-Min C, Sung WC, Kwang YC, Kwang-Soo K, Johannes S. Preclinical analysis of fetal human mesencephalic neural progenitor cell lines: characterization and safety in vitro and in vivo. Stem Cells Transl Med. 2017;6:576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim KS, Kim HS, Park JM, Kim HW, Park MK, Lee HS, Lim DS, Lee TH, Chopp M, Moon J. Long-term immunomodulatory effect of amniotic stem cells in an Alzheimer’s disease model. Neurobiol Aging. 2013;34(10):2408–2420. [DOI] [PubMed] [Google Scholar]
- 31. Kim KS, Park JM, Kong T, Kim C, Bae SH, Kim HW, Moon J. Retinal angiogenesis effects of tgf-beta1 and paracrine factors secreted from human placental stem cells in response to a pathological environment. Cell Transplant. 2016;25:1145–1157. [DOI] [PubMed] [Google Scholar]
- 32. Kim MJ, Shin KS, Jeon JH, Lee DR, Shim SH, Kim JK, Cha DH, Yoon TK, Kim GJ. Human chorionic-plate-derived mesenchymal stem cells and Wharton’s jelly-derived mesenchymal stem cells: a comparative analysis of their potential as placenta-derived stem cells. Cell Tissue Res. 2011;346:53–64. [DOI] [PubMed] [Google Scholar]
- 33. Bae EJ, Lee HS, Park CH, Lee SH. Orphan nuclear receptor Nurr1 induces neuron differentiation from embryonic cortical precursor cells via an extrinsic paracrine mechanism. FEBS Lett. 2009;583:1505–1510. [DOI] [PubMed] [Google Scholar]
- 34. Bao Q, Newport D, Chen M, Stout DB, Chatziioannou AF. Performance evaluation of the inveon dedicated PET preclinical tomograph based on the NEMA NU-4 standards. J Nucl Med. 2009;50:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Crews L, Mizuno H, Desplats P, Rockenstein E, Adame A, Patrick C, Winner B, Winkler J, Masliah E. Alpha-synuclein alters Notch-1 expression and neurogenesis in mouse embryonic stem cells and in the hippocampus of transgenic mice. J Neurosci. 2008;28:4250–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klusa VZ, Isajevs S, Svirina D, Pupure J, Beitnere U, Rumaks J, Svirskis S, Jansone B, Dzirkale Z, Muceniece R, Kalvinsh I, Vinters HV. Neuroprotective properties of mildronate, a small molecule, in a rat model of Parkinson’s disease. Int J Mol Sci. 2010;11:4465–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Herzog CD, Brown L, Kruegel BR, Wilson A, Tansey MG, Gage FH, Johnson EM, Jr, Bartus RT. Enhanced neurotrophic distribution, cell signaling and neuroprotection following substantia nigral versus striatal delivery of AAV2-NRTN (CERE-120). Neurobiol Dis. 2013;58:38–48. [DOI] [PubMed] [Google Scholar]
- 38. Ishida H, Saba R, Kokkinopoulos I, Hashimoto M, Yamaguchi O, Nowotschin S, Shiraishi M, Ruchaya P, Miller D, Harmer S, Poliandri A, Kogaki S, Sakata Y, Dunkel L, Tinker A, Hadjantonakis AK, Sawa Y, Sasaki H, Ozono K, Suzuki K, Yashiro K. GFRA2 identifies cardiac progenitors and mediates cardiomyocyte differentiation in a ret-independent signaling pathway. Cell Rep. 2016;16:1026–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kan I, Barhum Y, Melamed E, Offen D. Mesenchymal stem cells stimulate endogenous neurogenesis in the subventricular zone of adult mice. Stem Cell Rev. 2011;7:404–412. [DOI] [PubMed] [Google Scholar]
- 40. Nicaise C, Mitrecic D, Pochet R. Brain and spinal cord affected by amyotrophic lateral sclerosis induce differential growth factors expression in rat mesenchymal and neural stem cells. Neuropathol Appl Neurobiol. 2011;37:179–188. [DOI] [PubMed] [Google Scholar]
- 41. Teixeira FG, Carvalho MM, Panchalingam KM, Rodrigues AJ, Mendes-Pinheiro B, Anjo S, Manadas B, Behie LA, Sousa N, Salgado AJ. Impact of the secretome of human mesenchymal stem cells on brain structure and animal behavior in a rat model of Parkinson’s Disease. Stem Cells Transl Med. 2017;6(2):634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fujita H, Tanaka J, Toku K, Tateishi N, Suzuki Y, Matsuda S, Sakanaka M, Maeda N. Effects of GM-CSF and ordinary supplements on the ramification of microglia in culture: a morphometrical study. Glia. 1996;18:269–281. [DOI] [PubMed] [Google Scholar]
- 43. Kim YJ, Park HJ, Lee G, Bang OY, Ahn YH, Joe E, Kim HO, Lee PH. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia. 2009;57:13–23. [DOI] [PubMed] [Google Scholar]
- 44. Kim DS, Ross PJ, Zaslavsky K, Ellis J. Optimizing neuronal differentiation from induced pluripotent stem cells to model ASD. Front Cell Neurosci. 2014;8:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Levy G. The relationship of Parkinson disease with aging. Arch Neurol. 2007;64:1242–1246. [DOI] [PubMed] [Google Scholar]
- 46. Driver JA, Logroscino G, Gaziano JM, Kurth T. Incidence and remaining lifetime risk of Parkinson disease in advanced age. Neurology. 2009;72:432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reeve A, Simcox E, Turnbull D. Ageing and Parkinson’s disease: why is advancing age the biggest risk factor? Ageing Res Rev. 2014;14:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rodriguez M, Rodriguez-Sabate C, Morales I, Sanchez A, Sabate M. Parkinson’s disease as a result of aging. Aging Cell. 2015;14:293–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Foltynie T, Hariz MI. Surgical management of Parkinson’s disease. Expert Rev Neurother. 2010;10(6):903–914. [DOI] [PubMed] [Google Scholar]
- 50. Parolini O, Alviano F, Bagnara GP, Bilic G, Buhring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N, Miki T, Marongiu F, Nakajima H, Nikaido T, Portmann-Lanz CB, Sankar V, Soncini M, Stadler G, Surbek D, Takahashi TA, Redl H, Sakuragawa N. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta-derived stem cells. Stem Cells. 2008;26:300–311. [DOI] [PubMed] [Google Scholar]
- 51. Abdulrazzak H, Moschidou D, Jones G, Guillot PV. Biological characteristics of stem cells from foetal, cord blood and extraembryonic tissues. J R Soc Interface. 2010;7(Suppl. 6):S689–S706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vellasamy S, Sandrasaigaran P, Vidyadaran S, George E, Ramasamy R. Isolation and characterisation of mesenchymal stem cells derived from human placenta tissue. World J Stem Cells. 2012;4(6):53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Talwadekar MD, Kale VP, Limaye LS. Placenta-derived mesenchymal stem cells possess better immunoregulatory properties compared to their cord-derived counterparts-a paired sample study. Sci Rep. 2015;5:15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Heo JS, Choi Y, Kim HS, Kim HO. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med. 2016;37:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sundberg M, Jansson L, Ketolainen J, Pihlajamaki H, Suuronen R, Skottman H, Inzunza J, Hovatta O, Narkilahti S. CD marker expression profiles of human embryonic stem cells and their neural derivatives, determined using flow-cytometric analysis, reveal a novel CD marker for exclusion of pluripotent stem cells. Stem Cell Res. 2009;2:113–124. [DOI] [PubMed] [Google Scholar]
- 56. Moraes DA, Sibov TT, Pavon LF, Alvim PQ, Bonadio RS, Da Silva JR, Pic-Taylor A, Toledo OA, Marti LC, Azevedo RB, Oliveira DM. A reduction in CD90 (THY-1) expression results in increased differentiation of mesenchymal stromal cells. Stem Cell Res Ther. 2016;7(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mosley RL, Hutter-Saunders JA, Stone DK, Gendelman HE. Inflammation and adaptive immunity in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2:a009381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Abeliovich A, Hammond R. Midbrain dopamine neuron differentiation: factors and fates. Dev Biol. 2007;304:447–454. [DOI] [PubMed] [Google Scholar]
- 59. Schwartz CM, Tavakoli T, Jamias C, Park SS, Maudsley S, Martin B, Phillips TM, Yao PJ, Itoh K, Ma WRao MS, Arenas E, Mattson MP. Stromal factors SDF1alpha, sFRP1, and VEGFD induce dopaminergic neuron differentiation of human pluripotent stem cells. J Neurosci Res. 2012;90(7):1367–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cao Q, Lu J, Kaur C, Sivakumar V, Li F, Cheah PS, Dheen ST, Ling EA. Expression of Notch-1 receptor and its ligands Jagged-1 and Delta-1 in amoeboid microglia in postnatal rat brain and murine BV-2 cells. Glia. 2008;56:1224–1237. [DOI] [PubMed] [Google Scholar]
- 61. Nakano N, Nishiyama C, Yagita H, Koyanagi A, Akiba H, Chiba S, Ogawa H, Okumura K. Notch signaling confers antigen-presenting cell functions on mast cells. J Allergy Clin Immunol. 2009;123:74–81 e1. [DOI] [PubMed] [Google Scholar]
- 62. Christophersen NS, Gronborg M, Petersen TN, Fjord-Larsen L, Jorgensen JR, Juliusson B, Blom N, Rosenblad C, Brundin P. Midbrain expression of Delta-like 1 homologue is regulated by GDNF and is associated with dopaminergic differentiation. Exp Neurol. 2007;204:791–801. [DOI] [PubMed] [Google Scholar]
- 63. Bauer M, Szulc J, Meyer M, Jensen CH, Terki TA, Meixner A, Kinkl N, Gasser T, Aebischer P, Ueffing M. Delta-like 1 participates in the specification of ventral midbrain progenitor derived dopaminergic neurons. J Neurochem. 2008;104:1101–1115. [DOI] [PubMed] [Google Scholar]
- 64. Desplats P, Spencer B, Crews L, Pathel P, Morvinski-Friedmann D, Kosberg K, Roberts S, Patrick C, Winner B, Winkler J, Masliah E. alpha-Synuclein induces alterations in adult neurogenesis in Parkinson disease models via p53-mediated repression of Notch1. J Biol Chem. 2012;287:31691–31702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bartus RT, Herzog CD, Chu Y, Wilson A, Brown L, Siffert J, Johnson EM, Jr, Olanow CW, Mufson EJ, Kordower JH. Bioactivity of AAV2-neurturin gene therapy (CERE-120): differences between Parkinson’s disease and nonhuman primate brains. Mov Disord. 2011;26:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.