Abstract
Acute kidney injury (AKI), characterized by a sharp drop in glomerular filtration, continues to be a significant health burden because it is associated with high initial mortality, morbidity, and substantial health-care costs. There is a strong connection between AKI and mechanisms of senescence activation. After ischemic or nephrotoxic insults, a wide range of pathophysiological events occur. Renal tubular cell injury is characterized by cell membrane damage, cytoskeleton disruption, and DNA degradation, leading to tubular cell death by necrosis and apoptosis. The senescence mechanism involves interstitial fibrosis, tubular atrophy, and capillary rarefaction, all of which impede the morphological and functional recovery of the kidneys, suggesting a strong link between AKI and the progression of chronic kidney disease. During abnormal kidney repair, tubular epithelial cells can assume a senescence-like phenotype. Cellular senescence can occur as a result of cell cycle arrest due to increased expression of cyclin kinase inhibitors (mainly p21), downregulation of Klotho expression, and telomere shortening. In AKI, cellular senescence is aggravated by other factors including oxidative stress and autophagy. Given this scenario, the main question is whether AKI can be repaired and how to avoid the senescence process. Stem cells might constitute a new therapeutic approach. Mesenchymal stem cells (MSCs) can ameliorate kidney injury through angiogenesis, immunomodulation, and fibrosis pathway blockade, as well as through antiapoptotic and promitotic processes. Young umbilical cord–derived MSCs are better at increasing Klotho levels, and thus protecting tissues from senescence, than are adipose-derived MSCs. Umbilical cord–derived MSCs improve glomerular filtration and tubular function to a greater degree than do those obtained from adult tissue. Although senescence-related proteins and microRNA are upregulated in AKI, they can be downregulated by treatment with umbilical cord–derived MSCs. In summary, stem cells derived from young tissues, such as umbilical cord–derived MSCs, could slow the post-AKI senescence process.
Keywords: acute kidney injury, cell cycle arrest, Klotho, telomeres, oxidative stress, mesenchymal stromal cells
Introduction
Acute kidney injury (AKI), previously known as acute renal failure, is a syndrome characterized by a sharp drop in the glomerular filtration rate with consequent deterioration of renal function, ultimately leading to the need for dialysis in a great portion of cases. The concept of AKI has undergone significant reexamination in recent years. Traditionally, emphasis has been placed on a pronounced acute reduction in renal function, manifested by azotemia accompanied by oliguria or anuria, with consequent fluid overload and electrolyte abnormalities. However, recent evidence suggests that even relatively mild injury and impairment of renal function, manifested by small changes in serum creatinine or decreased urine output, are predictors of serious clinical consequences. Many patients with AKI have a mixed etiology, often consisting of the coexistence of sepsis, ischemia–reperfusion injury (IRI), and the use of nephrotoxic medications. AKI, a condition that is becoming increasingly prevalent, represents a significant health burden: It affects approximately 25% of hospitalized patients, especially critically ill patients in intensive care units, and is associated with high mortality and morbidity, as well as having a substantial impact on health-care costs1,2. To date, no single therapy has been shown to improve the outcome of AKI.
Unless a fatal outcome occurs, renal function impairment after AKI has been considered to be a reversible process. In the past, the resolution of AKI and the recovery of renal function have generally been considered to be efficient processes, the traditional view being that they would have no impact on long-term renal function in surviving patients. However, recent studies suggest that there is a strong correlation between AKI and the progression to chronic kidney disease (CKD)3,4. Although the mechanisms involved in CKD progression after AKI are still largely unknown, there is growing evidence that the reduced regenerative capacity is linked to a process of senescence activation. In this review, we will focus on the understanding of AKI as a condition of renal senescence and the emerging stem cell therapies that might play a role as treatment strategies.
AKI as a Condition for Accelerated Kidney Aging
After an ischemic or nephrotoxic AKI insult, a wide range of pathophysiological events occur, particularly in the proximal tubule cells, the kidney cells that are most vulnerable to hypoxia and nephrotoxins5. Cell injury, in this setting, is characterized by cell membrane damage, cytoskeleton disruption, and DNA degradation, leading to tubular cell death by necrosis and apoptosis6. Complete kidney recovery has been observed in the majority of surviving patients, mainly in cases of mild kidney injury. However, in severe injuries or in previously damaged or aged kidneys, abnormal tubular regeneration can occur, defining a maladaptive response of the kidney to AKI4,7. The maladaptive response is characterized by interstitial fibrosis, tubular atrophy, and capillary rarefaction, which impede the complete morphological and functional recovery of the kidneys, thus indicating a strong link between AKI and the progression to CKD. In fact, seminal studies have shown an intriguing association between the development of fibrosis and cell cycle arrest of proximal tubular epithelial cells after acute injury8. This fibrogenic process is likely mediated by upregulated production of profibrotic factors, such as transforming growth factor-β (TGF-β) and connective tissue growth factor, characterized by activation and proliferation of fibroblasts and perivascular pericytes, which in turn induce extracellular matrix production and tubulointerstitial inflammation, with chronic activation of macrophages7,9,10. During the process of abnormal kidney repair, tubular epithelial cells can assume a senescence-like phenotype8.
Cellular senescence may occur as result of cell cycle arrest due to increased expression of cyclin kinase inhibitors, downregulation of Klotho expression, and telomere shortening. Cells can also be induced to senescence in response to elevated oxidative stress11. In this review, we will discuss the mechanisms potentially involved in post-AKI renal senescence.
AKI-induced Cell Cycle Arrest as a Condition of Renal Senescence
Under normal conditions, kidney cells have a low turnover, remaining in the G0 phase, a quiescent state. Ischemic or toxic insults trigger a cascade of cellular events leading to subsequent tubular epithelial cell death by necrosis and apoptosis. Remnant quiescent surviving renal tubular cells enter the cell cycle, proliferating and dedifferentiating into new tubular cells, a process that is important for repopulating the tubules12. Shortly after AKI, when the acute stress drives the damaged renal tubular cells to enter the cell cycle, there is rapid, massive induction of cyclin-dependent kinase inhibitor (p21Waf1/Cip1), a protein recognized as a cell cycle inhibitor, blocking the cell cycle at the G1/S phase7,13. The induction of this early antiproliferative response due to cell cycle arrest after an acute kidney insult, an apparently paradoxical phenomenon, provides more time for DNA damage repair, avoiding uncontrolled progression toward cell death or malignant transformation. In addition, p21Waf1/Cip1 activation modulates apoptosis and necrosis in the kidney14, helping mitigate injury. Hypoxia and other stresses can also activate the ataxia telangiectasia mutated/ataxia telangiectasia (ATM ATR) signaling pathway15, which can block cell cycle progression through p21Waf1/Cip1 or by inducing the protein kinases, Checkpoint kinase 1 (Chk1) and Checkpoint kinase 2 (Chk2), which can promote cell cycle arrest at the G2/M checkpoint16–18.
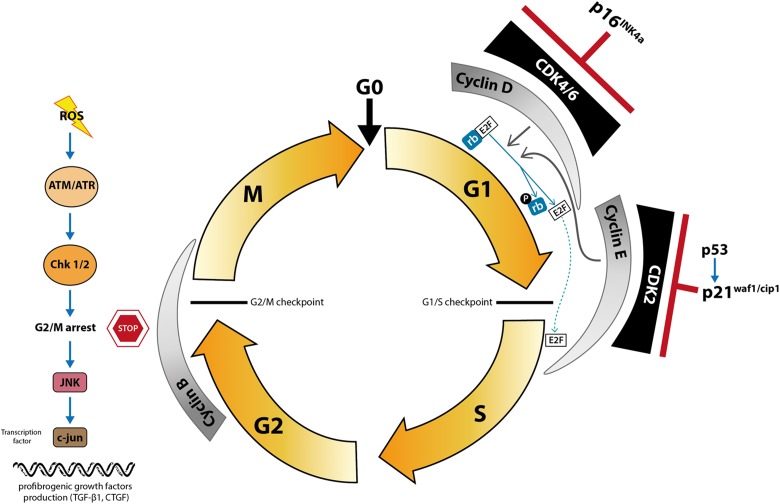
The cell cycle is highly regulated by different classes of proteins (Fig. 1). Cyclins and cyclin-dependent kinases (CDKs) are two important classes of proteins that, uncoupled, have a low level of kinase activity. However, when they bind to each other to form heterocomplexes, the kinase subunit is activated, inducing progression through the cell cycle. The third class of proteins consists of CDK inhibitors, which act by inhibiting the CDK complex, consequently inhibiting the cell cycle. In addition, the retinoblastoma protein (pRb) represents an important substrate for CDK function during the cell cycle, at the G1 checkpoint, providing a negative control of the cell cycle. The pRb, a tumor suppressor protein, prevents excessive cell growth by inhibiting cell cycle progression. When the pRb is inactivated by phosphorylation, the E2F transcription factor is released, allowing cell cycle progression.
Fig. 1.
Mechanism of cell cycle arrest induced fibrosis. In addition to the quiescent state (G0), the cell cycle includes 4 tightly controlled phases: G1, S (DNA synthesis), G2, and M (mitosis). Cyclin D and cyclin E are cell cycle regulatory proteins synthesized in the G1 phase (in its early and late portions, respectively) but degraded when the cells enter the S phase. Cyclin B is required for progression from G2 to M, and an increased cyclin B/cyclin D ratio might represent accumulation of cells in G2/M, which can occur in maladaptative repair. The p16INK4a and p21Waf1/Cip1 proteins bind to cyclin-dependent kinase–cyclin heterodimers, causing cell cycle arrest in the G0 to G1 phase, inhibiting cell proliferation. Hypoxia and reactive oxygen species may activate protein kinases Chk1 and Chk2 through ataxia telangiectasia mutated/ataxia telangiectasia and Rad3-related protein kinase signaling pathway, promoting cell cycle arrest in G2/M checkpoint. An excess of G2/M-arrested cells activates the Jun N-terminal kinase pathway, increasing levels of the transcription factor c-jun, which upregulates profibrotic cytokine production.
There are two important families of CDK inhibitors:—the Cip/Kip and inhibitor of cyclin-dependent kinase 4 (INK4) families. Members of both families inhibit the cell cycle, maintaining cells in the G1 phase.
The most well-known member of the Cip/Kip family is the previously mentioned p21Waf1/Cip1, a 21-kDa protein that binds to CDK2, inhibiting the CDK2–cyclin E complex, thus promoting cell cycle arrest13,19. In addition to participating in cell cycle progression, CDK2 is required in order to trigger pathways of necrosis and apoptosis14. In the kidney, nuclear p21 is located in the proximal and distal tubules. The p21 gene is induced after DNA damage, leading to activation of p53 and induction of p21Waf1/Cip114. After the initial insult, there is a rapid upregulation of p21Waf1/Cip1 in the kidney, although not of other Cip/Kip family members such as p27 and p5720. The upregulated expression of p21Waf1/Cip1 observed in different models of AKI20,21 prevents DNA-damaged cells from entering the cell cycle by directly inhibiting CDK2 activity22, thus avoiding cell death by necrosis or apoptosis21. In fact, p21 knockout mice induced to AKI show increased susceptibility to ischemia and nephrotoxins, characterized by more severe renal function impairment, morphologic changes, and overall mortality21,23. Once activated, p21Waf1/Cip1 can promote protection against a subsequent renal insult24. p21Waf1/Cip1 proteins have a wide spectrum of activities, depending on the cell type and the circumstances of their induction14. In addition to the beneficial effects on tubular cells, p21Waf1/Cip1 might play a role in enhancing the progression to CKD by inducing TGF-β production, ultimately leading to fibrosis25, as reported for other cell cycle inhibitory factors. In the renal ablation model, a lack of p21Waf1/Cip1 diminishes cell cycle arrest, avoiding long-term renal dysfunction and interstitial fibrosis26.
The INK4 family consists of 2 proteins: p16INK4a, a cyclin kinase inhibitor, and p19ARF, a p53 stabilizer. p16INK4a binds to the CDK4/6 kinase subunit of cyclin D, causing cell cycle arrest in the G0 to G1 phase, thus reducing the proliferation of tubular epithelial cells. In AKI models, the absence of p16INK4a promotes regenerative cell proliferation and better outcomes after kidney injury27,28.
Cellular senescence is characterized by permanent growth arrest, accompanied by characteristic morphological remodeling and metabolic changes, with a pro-inflammatory secretome phenotype, referred to as the senescence-associated secretory phenotype or senescence-messaging secretome, as described by van Deursen18. Senescent cells stain positive for senescence-associated activity of β-galactosidase (β-gal).
The role of the cell cycle inhibitory proteins p21Waf1/Cip1 and p16INK4a-Rb as potent early cell cycle arrest mediators is considered a crucial mechanism of cellular senescence. In cultured cells undergoing senescence, p21Waf1/Cip1 protein has been shown to be overexpressed29. On the other hand, decreased expression of p16INK4a and p19 results in decreased senescence with extended cell life span. Studies involving human kidney biopsy samples have shown that p16INK4a expression is low to undetectable in young individuals, whereas it is markedly increased in adult and elderly individuals30. Because the levels of INK4a proteins increase with age, they are recognized as biomarkers of aging31. Therefore, p16INK4a, like β-gal, is considered a biomarker of cellular senescence.
Both aging and AKI can upregulate the expression of cell cycle inhibitory proteins, such as p21Waf1/Cip1, p53, and p16INK4a, blocking the cell cycle and thus promoting cell cycle arrest32. However, the exact mechanisms involved in AKI, which is apparently a transient and reversible process, with possible late changes, are still largely unknown. Senescence, rather than representing a static end point, seems to be a dynamic process. It has long been known that, although p16INK4a expression leads to cell cycle arrest within 24 h after induction, sustained p16INK4a expression (for ≥6 d) is required in order to induce senescence in human cells33. In our previous studies using the IRI model, we have shown overexpression of p16INK4a, p21Waf1/Cip1, and TGF-β within 2 d after the ischemic insult, as well as that the expression of β-gal and p16INK4a remains high in ischemic animals, even at 7 d after of the insult34. Therefore, it is likely that AKI, through cell stress and DNA damage, triggers cell cycle arrest, leading to a sustained process of senescence. Post-AKI senescence could, therefore, be a consequence of maladaptative repair35,36.
Yang et al. demonstrated that distinct types and severity of kidney injury can behave differently regarding cell cycle arrest8. When moderate, reversible IRI is induced, mice present abrupt renal dysfunction, although kidney function returns to normal levels in 7 d. That model of AKI results in transient cell cycle changes, with increased numbers of G2/M phase cells only from day 1 to day 5. The authors showed that mice thus induced to AKI do not present kidney interstitial fibrosis in the long term8. Kidney injuries that are more severe such as severe IRI and nephrotoxic insult (acute aristolochic acid toxic nephropathy) feature abrupt renal dysfunction and delayed recovery. In those models of AKI, G2/M-arrested cells are prominent in the long term, persisting in large numbers up to day 42, and leading to significant interstitial fibrosis in multiple organs8.
An excess of G2/M-arrested tubular cells represents the maladaptative response to AKI because such cells can activate mitogen-activated protein kinase pathways, such as the Jun N-terminal kinase (JNK) pathway, leading to increased levels of the transcription factor c-jun, which ultimately upregulates profibrotic cytokine production8, as illustrated in Fig. 1. The evidence that interstitial fibrosis is not merely a consequence of kidney dysfunction that is more severe comes from models of unilateral kidney injury. When unilateral IRI or unilateral ureteral obstruction is employed, there is no kidney dysfunction as defined by the serum creatinine level. However, the affected kidneys develop persistent proximal tubule G2/M populations, which remain large until at least 1 mo after the insult. Even in the absence of initial renal dysfunction, such kidneys develop pronounced interstitial fibrosis over time8. In addition, inhibition of the ATM gene reduces G2/M arrest and inhibits JNK, which reduces the influence of downstream pathways activated by G2/M-arrested cells, thus decreasing profibrogenic factor production. Inhibition of p53, which can permit cell cycle progression, might also diminish renal fibrosis after AKI. Consistently, induction of G2/M arrest with drugs (such as a CDK1 inhibitor or paclitaxel, a microtubule stabilizing agent) increases profibrogenic gene expression, and that condition can reverse as the drugs wash out8. In mice null for proteins that promote the G2/M transition, recovery after AKI is delayed and there is significant interstitial fibrosis37.
Deficiency of the Klotho Gene as a Mechanism of AKI-induced Premature Senescence
In 1997, Kuro-o et al. identified an aging-suppressor gene designated Klotho. The authors demonstrated that disruption of this gene in mice, which leads to reduced Klotho protein expression, induces diverse aging-associated features such as a shortened life span, growth retardation, skin atrophy, hearing loss, reduced cognitive function, decreased bone mineral density, and premature arteriosclerosis38. In contrast, overexpression of the Klotho gene in transgenic mice has been associated with a longer life span39. The Klotho gene is also involved in human aging, having been associated with longevity40. Serum protein levels of Klotho decrease with age, and low levels are considered early predictors of atherosclerosis41. Low levels of circulating Klotho protein have also been identified in humans and animals with CKD42,43.
The Klotho gene is predominantly expressed in the kidney, specifically in the kidney tubules (distal and proximal convoluted tubules)38,44,45. Cell lines derived from the inner medullary collecting duct also express the Klotho gene. In addition, the kidney represents an important target of the Klotho protein, which has a broad range of renal effects, including regulation of renal 1,25-(OH)2-vitamin D3 production, as well as phosphate, calcium, and potassium homeostasis (Fig. 2).
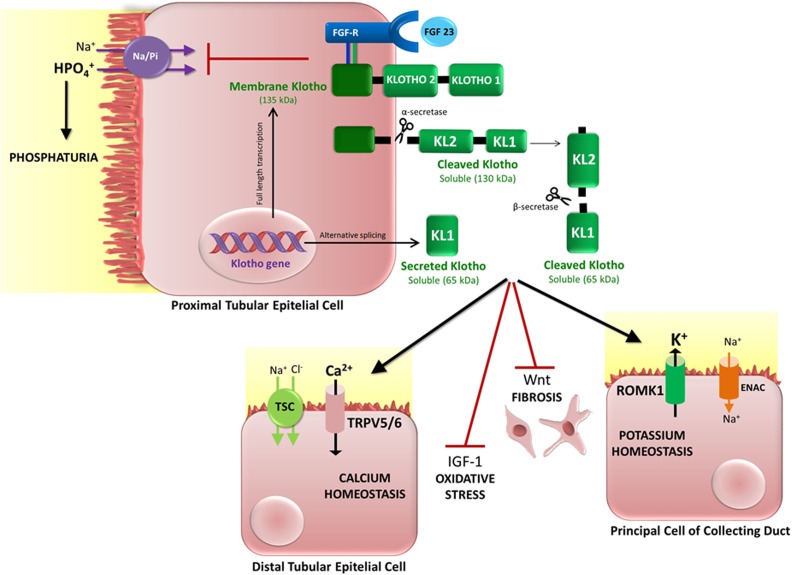
Fig. 2.
Main functions of the Klotho protein. Membrane Klotho is a 135-kDa transmembrane protein that interacts with the fibroblast growth factor receptor (FGF-R) as a co-receptor for FGF-23, regulating renal phosphate excretion and metabolism. Once cleaved by α-secretases, membrane Klotho becomes a soluble, 130-kDa peptide that is released from the cell surface, subsequently entering the bloodstream and the urine. At these sites, 130-kDa Klotho can undergo further cleavage by β-secretases, producing 65-kDa soluble peptides. Alternative splicing of the Klotho gene also creates a 65-kDa soluble Klotho, known as “secreted Klotho,” which is released into the bloodstream and urine with no need for enzymatic cleavages. All forms of soluble Klotho develop paracrine and systemic functions: In the distal nephron, soluble Klotho activates the transient receptor potential cation channel subfamily V (TRPV) calcium channels TRPV5 and TRPV6, increasing calcium reabsorption, and the renal outer medullary 1 potassium channel, increasing potassium secretion. In addition, Klotho inhibits Wnt signaling activity, thus contributing to the blocking of fibrogenic mechanisms. Klotho protein also regulates the oxidative stress through insulin growth factor 1 (IGF-1) signaling. Inhibition of IGF-1 permits forkhead box O3 action, elevating levels of the antioxidant enzymes manganese superoxide dismutase and catalase.
Klotho is a transmembrane protein, expressed on the cell surface, that interacts with the fibroblast growth factor (FGF) receptor as a co-receptor for FGF-23 (Fig. 2). Alternative cleavages of transmembrane Klotho generate cleaved (130 kDa) Klotho, which is released in blood and urine, and secreted (65-kDa) Klotho, which can have multiple functions46 (Fig. 2). Phosphate homeostasis is regulated by the interaction of Klotho protein with FGF-23, which induces renal phosphate excretion (phosphaturia). Phosphate homeostasis likely plays an important role in the aging process, an assumption supported by reports that control of hyperphosphatemia in Klotho-deficient (kl/+) and FGF-23-deficient mice can prevent premature aging syndrome47.
Several other mechanisms have been shown to play a role in Klotho depletion-induced cell senescence. Knockdown of endogenous Klotho promotes augmentation of senescence in the cultured cells. Administration of exogenous Klotho significantly decreases senescence in the endothelial cells48. Klotho deficiency also enhances cell senescence by increasing oxidative stress49. The biological activity of Wnt in Klotho knockout mice may contribute to increasing senescence in progenitor cells as described by Liu et al50.
Klotho is an antiapoptotic protein, as demonstrated in human umbilical vein endothelial cells51. In the kidney, Klotho deficiency significantly increases apoptosis49. In contrast, enhancement of Klotho expression by genetic manipulation decreases the number of apoptotic cells, as well as improving renal function and morphology, after acute and chronic kidney damage49,52.
In a mouse model of IRI-induced AKI, Hu et al. found reduced Klotho expression in the kidneys as well as in urine and blood, although Klotho expression was restored upon recovery53. Reductions in kidney and plasma levels of Klotho occur earlier than does the production of neutrophil gelatinase-associated lipocalin (NGAL), a known biomarker of kidney injury53. Patients with AKI show drastic reductions in urinary Klotho levels. To determine whether Klotho plays a pathogenic role, the authors induced ischemic AKI in mice with different levels of endogenous Klotho expression, ranging from heterozygous Klotho-haploinsufficient mice to wild-type mice and transgenic mice overexpressing Klotho53. In comparison with what was observed in wild-type mice, the levels of Klotho protein during AKI were found to be lower in haploinsufficient mice and higher in transgenic mice. The authors also found that the haploinsufficient mice showed functional and histological alterations that were more extensive than those seen in the wild-type mice, whereas those changes were milder in the transgenic mice, implying that Klotho is renoprotective. Among the rats with AKI, those receiving recombinant Klotho showed higher Klotho protein expression, less kidney damage, and lower NGAL levels than did the vehicle-treated control group53. Therefore, Klotho deficiency likely renders the kidney more susceptible to injury, accelerates renal fibrogenesis, retards renal tissue regeneration, and, in some cases, promotes chronic progression. Klotho supplementation can provide benefits, preventing or slowing the progression to CKD and renal senescence.
Telomere Shortening
Telomeres are noncoding nucleotide TTAGGG sequences repeated at the end of the chromosomes, acting as defensive caps that protect genetic material from damage and consequently from cell death triggered by DNA repair pathways. Telomerase is the enzyme responsible for maintaining telomeres. Most human cells do not express telomerase, the exceptions being highly proliferative cells such as germ cells, skin cells, and bone marrow54. At each cell division, some base pairs are lost and telomeres shorten progressively55,56. Therefore, telomere length declines with age, characterizing replicative senescence. Activation of DNA repair pathways triggered by telomere shortening can enhance p21Waf1/Cip1 and p16INK4a to halt proliferation and minimize replicative stress53,57,58. Telomere attrition causes chromosome instability, cellular senescence, and apoptosis59.
In addition to replicative senescence, cells can become senescent after a noxious stimulus, and this stress-induced senescence can lead to telomere shortening59. In short-term evaluations, telomere shortening is not a feature of ischemic AKI in the first 2 d after the insult despite the presence of greater oxidative stress34. However, renal IRI can lead to significant telomere shortening at 30 d after AKI induction, that shortening being more pronounced in telomerase-deficient mice60. Telomere attrition and telomerase deficiency have been shown to aggravate the course of kidney injury60. In aging kidneys, the increased susceptibility to injury and lack of sufficient repair might involve telomere shortening and other mechanisms of increased senescence. Although telomerase-deficient mice can present the same degree of AKI severity as do their wild-type littermates after IRI, the recovery of renal function is significantly delayed in the former60,61.
Other Mechanisms
Oxidative injury can be a mechanism involved in stress-induced cell senescence. In the kidney, mitochondrial electron transport chain and reduced nicotinamide–adenine dinucleotide–ubiquinone oxidases are the main sources of reactive oxygen species (ROS)62. The mitochondria constitute a target of ROS effects, because mitochondrial dysfunction and loss of mitochondrial membrane could be consequences of oxidative stress, resulting in altered mitochondrial permeability and release of cytochrome c, thus inducing cell death63. Highly reactive oxygen molecules can cause significant modification of lipids, DNA, and proteins64. In addition, oxidative stress can activate injury mitogen-activated protein kinase pathways as well as p53 and p21Waf1/Cip165, leading to higher cell apoptosis rates, activation of inflammation, and the development of stress-induced senescence. Autophagy pathways become activated66, resulting in increased cell expression of β-gal67. In mitochondrial dysfunction, respiratory chain disruption results in superoxide overproduction65,68. In that context, the mitochondrial antioxidant manganese superoxide dismutase (MnSOD) is particularly important for promoting cytoprotection69. In ischemic AKI, the antioxidant enzyme heme oxygenase-1 is stimulated, which increases MnSOD levels. In models of AKI, the disease is more severe and mortality is higher in mice lacking heme oxygenase-1 than in wild-type mice70. However, AKI can also make heme oxygenase-1 unable to stimulate MnSOD34, thus impairing ROS scavenging. Aging mesangial cells exhibit downregulation of the mitochondrial antioxidants MnSOD and thioredoxin reductase 2, accompanied by upregulation of the microRNA (miR)-335 and miR-34a, together with high levels of ROS. Overexpression of miR-335 and miR-34a can induce oxidative-stress related premature senescence of young mesangial cells, whereas antisense miR-335 and miR-34a inhibit senescence of old mesangial cells71. Antioxidant compounds, such as N-acetylcysteine, can delay stress-induced senescence72,73.
Another pathway that could be involved in renal senescence after AKI is inflammation. Inflammation is a multifactorial response that is needed in order to eradicate harmful pathogens and mediate tissue repair after injury. However, excess unresolved inflammation can promote fibrosis, tissue damage, and early senescence74. The release of cytokines and neutrophil/macrophage recruitment to the site of injury are considered hallmark features of the early inflammatory response, which is followed by adaptive immunity cell recruitment in later stages. Recent studies have suggested that T cells also participate in early inflammatory responses in AKI75. The recruitment of immune effector cells is facilitated by the upregulation of adhesion molecules in various cell types within the kidney76. It has been suggested that the immune signature of inflammation during IRI has many similarities with that of inflammation occurring in response to a microbial pathogen77. Cellular damage and its associated molecular products are thought to be key triggers of inflammation after acute tissue injury. Necrotic cells present damage-associated molecular patterns in the extracellular spaces, which subsequently activate pattern recognition receptors, such as Toll-like receptors and pyrin domain-containing 3 inflammasome, which are expressed in epithelial cells, endothelial cells, dendritic cells, macrophages, and lymphocytes78,79. Activated renal parenchyma cells and dendritic cells also secrete chemokines, and changes in the expression of pro-inflammatory and anti-inflammatory mediators by resident and recruited cell populations are important determinants of the injury and repair phases80. A balance between pro-inflammatory and anti-inflammatory factors is extremely important for tissue repair. However, AKI often results in an abnormal repair process resulting from sustained secretion of profibrotic cytokines and leading to post-AKI fibrosis and kidney senescence78. Virtually, all immune cells have been implicated in AKI, some—such as neutrophils, monocytes/macrophages, dendritic cells, natural killer T cells, natural killer cells, and B cells—being thought to be deleterious, whereas others—such as Tregs—are likely protective81. M1 macrophages contribute to inflammation and tissue injury in the injury phase, whereas M2 macrophages exert anti-inflammatory effects in postischemic kidneys and facilitate renal tubular regeneration during the recovery phase. In addition, increased numbers of activated and effector memory T cells have been found in the postischemic kidneys as late as 6 wk after IRI, suggesting that T cells are also involved in long-term structural changes in postischemic kidneys82. Numerous studies have suggested that a robust inflammatory process engaging innate and adaptive immune responses causes initial renal injury and mediates long-term structural changes including interstitial fibrosis or repair. It is known that acute injury results in microvascular damage and vessel loss in the kidney and that such changes are typically persistent. Various studies of biopsies of renal transplants have suggested that ischemia imposes early sustained loss of peritubular capillaries in the transplanted graft. The loss of peritubular capillaries might represent a single, common pathway toward progressive damage and senescence83.
Autophagy is a cell process triggered by lysosome-mediated degradation of damaged organelles and plays a protective role after cell stress situations. This process can be affected by aging, autophagy having been shown to be impaired in aged murine macrophages84. Removal of damaged organelles and protein aggregates occurs when those substrates are circumscribed into double-membrane vesicles called autophagosomes, which subsequently fuse with lysosomes62. Formation of an autophagosome requires the coordinated action of several protein complexes such as the so-called autophagy-related proteins and phosphatidylinositide-3 kinase. Successful autophagosome formation can be marked by increased expression of the protein light chain 3-II85. ROS can trigger mitochondrial autophagy86, although extremely severe insults can lead to lysosomal permeabilization, impairing autophagic mechanisms62, and clearance of damaged organelles indirectly inhibits excessive ROS production, protecting injured tissues62. The p62 protein recognizes cellular waste and initiates an autophagy response in cells87. There is evidence of p62-dependent Kelch-like ECH-associated protein 1 (Keap1) degradation that enhances nuclear factor (erythroid-derived 2)-Like 2 (Nrf2) to exert antioxidant effects88. Although other molecules from apoptotic pathways, such as those in the B-cell lymphoma-2 family, hypoxia-inducible factor (HIF), and p53, can induce autophagy89, the mechanisms involved in triggering this process in AKI are still unknown85. The mammalian target of rapamycin (mTOR) pathways can also modulate autophagy, the mTOR complex 1 (mTORC1) pathway inducing autophagy and the mTORC2 pathway acting as a negative regulator of autophagy in response to starvation. However, in the setting of AKI, the mTORC2 pathway can induce autophagy by activating serine/threonine kinase signaling, thus promoting cell survival as well as protecting against tubular cell apoptosis and AKI.
Autophagy is induced in response to kidney injury. In septic AKI, autophagy increases in the early stages, although the decline that follows is associated with proximal tubular dysfunction90. Inhibition of the autophagy pathway aggravates necrosis, apoptosis, and kidney injury in short-term evaluations after ischemia reperfusion91. In addition, autophagy can reduce the level of mature TGF-β1, which would theoretically suppress kidney fibrosis92. However, in long-term evaluations, a lack of autophagy can promote better kidney healing after ischemic injury91. Dramatically injured cells typically do not survive after noxious stimulus; if they do, maladaptative repair, a pro-inflammatory state, and the senescent phenotype usually follow. Healthy healing occurs when potentially viable cells proliferate and contribute to tubular repair. Thus, autophagy can protect cells from death in an acute scenario, although it might subsequently allow more fibrosis and senescence. In fact, the enzyme β-gal, which is expressed in autophagy activation, is a hallmark of cell senescence.
AKI as a Condition for Progression to CKD
Clinical data show that patients with AKI, even after complete recovery, progress to a decline in renal function that is more pronounced than that occurring in individuals without a history of AKI93. Patients who survive AKI are 9 times more susceptible to developing CKD and 3 times more likely to develop end-stage renal disease (ESRD) than are those with no history AKI or of requiring dialysis during hospitalization94,95. A single AKI episode increases the risk of ESRD by 28 times and doubles the mortality risk96. It is estimated that nearly one fourth of the increase in ESRD prevalence between 1988 and 2002 was attributable to AKI97.
CKD is an independent predictor of AKI development in intensive care patients98, and acute illness will frequently accelerate progression of preexisting CKD. It is possible that, if the acute injury develops in CKD patients with minimal renal reserve, in whom repair mechanisms are already disabled, the progression to ESRD will occur more rapidly, even if the adaptive repair occurs after AKI. Otherwise, maladaptive repair can also trigger fibrosis and capillary rarefaction, thus rapidly worsening the preexisting CKD7.
Although fibrosis is not necessarily progressive, fibrotic tissue can reduce renal mass and functional reserve and can even can induce systemic hypertension99. Therefore, the additional loss of renal mass caused by unsuccessful repair of AKI can trigger hemodynamically mediated processes that will damage the nephrons, thus hastening the progression to CKD100. Shear stress in the remaining nephrons can result in further loss of glomeruli, and marked proteinuria demonstrates significant podocyte injury. Activation of the mTORC2 pathway and consequent activation of serine/threonine kinase 2 can protect podocytes from apoptosis and foot process effacement, promoting podocyte survival and perhaps further slowing the progression to CKD101. Other well-established pathways of progression in CKD, such as the renin–angiotensin–aldosterone axis, probably play an important role in determining post-AKI progression to CKD. In fact, some studies have demonstrated that inhibition of renin–angiotensin–aldosterone can confer protection against CKD progression in the setting of AKI102–104.
In AKI, the behavior of HIF constitutes an important mechanism. It can be protective, given that HIF activation before the induction of AKI has been shown to reduce the degree of kidney injury in experimental models105. However, when chronic kidney hypoxia is already present, as it is in CKD, activation of HIF can actually exacerbate tissue injury106. Therefore, preexisting CKD can contribute to the exacerbation of fibrosis in some AKI scenarios.
Klotho deficiency activates the expression of Wnt, activating the tubulointerstitial renal fibrosis process107. Activation of Wnt/β-catenin signaling due to Klotho deficiency can arrest cells at the G2/M phase of the cell cycle, inducing the production of TGF-β and connective tissue growth factor108.
It is known that aging individuals and CKD patients are more susceptible to CKD progression after AKI. Individuals in either situation are in a state of Klotho deficiency. The decrease in Klotho expression is dependent on the severity of the insult (duration of ischemia) and on the time since the insult109, demonstrated that, in their ≥30-min bilateral IRI model (Bi-IRI group) and unilateral nephrectomy plus contralateral IRI model (Npx-IRI group), Klotho expression normalized by day 10 after ischemia–reperfusion injury, decreased again around day 14, and declined even further by day 28. Although renal Klotho (protein and messenger RNA [mRNA]) expression was reduced in both models, renal Klotho protein expression at 20 wk after IRI was slightly higher in the Bi-IRI group mice. Kidney histology showed glomerular collapse, tubular dilation, tubulointerstitial infiltration, and fibrosis. The levels of fibrotic markers (α-smooth muscle actin, connective tissue growth factor, and collagen I) showed a robust increase in the Npx-IRI group and a less severe but still significant increase in the Bi-IRI group. Therefore, at 20 wk, there was a difference in severity between the Npx-IRI and Bi-IRI models, although all of the animals had CKD with predominant fibrotic components. The authors also applied the Bi-IRI model in kl/+ mice and mice of the transgenic mouse line Tg-Kl (in which there is ubiquitous overexpression of mouse Klotho, with Klotho levels approximately 150% of normal). Notably, they found that, at 20 wk, renal Klotho protein levels in the Tg-Kl mice were comparable to those seen in the wild-type mice not subjected to AKI, although they were much lower in the kl/+ mice than in the wild-type mice. The authors also reported that, in comparison with what they observed in the wild-type mice, renal α-smooth muscle actin, connective tissue growth factor, and collagen I were lower in the Tg-Kl mice and higher (consistent with renal fibrosis) in the kl/+ mice. Therefore, kl/+ mice have a higher risk of developing fibrosis (similar to that of aging individuals and patients with CKD). In a previous study, our group also demonstrated that, by day 49 after IRI, senescence was more apparent in the rats submitted to IRI alone than in those submitted to IRI and treated with human umbilical cord–derived mesenchymal stem cells (MSCs), Klotho expression being significantly lower in the former group34.
Cell Therapy Perspectives for the Treatment of Premature Renal Senescence
There is a great body of evidence showing that the pathophysiological mechanisms triggered after AKI-induced cell damage are associated with activation of the senescence machinery. There is yet no effective treatment for AKI, with supportive therapy being the only option.
In this context, adult stem cells—derived from the bone marrow, from other sources, or even from the kidneys—can play a role in this healing process, creating prospects for new therapeutic approaches such as cell therapy. The potential regenerative properties of stem cells have opened opportunities for investigation of the potential beneficial effects of the administration of stem cells in improving the renal outcomes of AKI in animal models110,111.
The bone marrow was the first tissue to be explored as a source of stem cells. Although studies have shown that bone marrow–derived stem cells can engraft into the kidney and participate in normal tubular epithelial cell turnover and repair after AKI, the main mechanism involves paracrine effects rather than cell transdifferentiation111. It has become quite clear that the expected potential ability of stem cells to transdifferentiate into renal cells and replace damaged cells is a rare phenomenon.
Besides hematopoietic stem cells, the bone marrow contains MSCs that have been shown to provide a wide range of beneficial effects when administered in different experimental models of kidney diseases and in some clinical trials112,113. Adipose-derived MSCs (ADMSCs) have also become a very attractive source of MSCs, with regenerative capacity similar to bone marrow–derived MSCs. ADMSCs have additional advantages considering that their harvesting is minimally invasive, there are no ethical issues regarding their use, and there are fewer safety concerns. Furthermore, some authors have suggested that ADMSCs have more robust anti-inflammatory and immunomodulatory effects than do bone marrow–derived MSCs114,115.
It is well known that MSCs possess the ability to secrete a variety of soluble factors in a paracrine fashion116. Experimental studies have shown that MSCs can ameliorate kidney injury through different mechanisms, such as anti-inflammatory, antiapoptotic, and other immunomodulatory effects, besides enhancing angiogenesis. Alternatively, horizontal cell-to-cell communication may occur through the release of extracellular microvesicles derived from stem cells, which can mediate the transfer of proteins, mRNA, miR, and other molecules117, with potential application for therapeutic intervention.
A number of studies have shown that MSCs are efficient in recovering renal function in experimental models of AKI34,118–125. The significant findings of small animal studies served as the basis for preclinical and clinical trials. A phase I clinical trial was designed to determine whether the administration of allogeneic MSCs is safe in patients who are at a high risk of developing AKI after undergoing on-pump cardiac surgery112. Preliminary data from that trial show that kidney function was preserved for up to 16 mo and that none of the patients required dialysis. Another phase I study involving three patients who developed AKI after cisplatin treatment for a solid tumor demonstrated that intravenous injection of autologous MSCs was safe and improved renal function (NCT01275612). Nevertheless, additional clinical studies are needed in order to show the real benefit of stem cells for post-AKI recovery of renal function.
Another key aspect of regeneration in which stem cell therapy could play a role is avoiding senescence. As discussed above, AKI likely induces cell aging, possibly through mechanisms closely related to cell cycle arrest and Klotho downregulation. In this setting, it is of note that umbilical cord–derived MSCs are more effective in reducing the expression of cell cycle inhibitors than do bone marrow–derived MSCs or ADMSCs126. In experimental IRI, our group has recently reported that treatment with umbilical cord–derived MSCs, besides ameliorating kidney function, downregulates the upregulated expression of senescence markers (β-gal, p21Waf1/Cip1, and p16INK4a) and miRs (miR-29a and miR-34a)34. In comparison with adult ADMSCs, young (postnatal) cells appear to be more effective in the treatment of AKI34. Our study also showed that Wharton’s jelly-derived MSCs have a superior capacity to increase Klotho protein levels and therefore to protect the tissue against senescence when compared with ADMSCs. In mechanistic terms, this study showed that Wharton’s jelly-derived MSCs protect renal tissues against senescence resulting from oxidative stress, as well as increasing the renal expression of Klotho protein and MnSOD (Table 1).
Table 1.
Cell Therapy and Senescence Markers in Kidney Tissue.
| Model | Recipients | Cell Treatment | Senescence Markers | Klotho | Ref. |
|---|---|---|---|---|---|
| AKI-IRI | Adult rats | WJ-hMSCs | ↓ p16 ↓ p21 ↓ TGF-β ↓ β-galactosidase ↓ miR-29a ↓ miR-34a | ↑ | Rodrigues et al.34 |
| Aging | Old mice | Old BMCs | ↑ Collagen IV ↑ PAI-1 ↓ PDGF-B | ↓ | Yang et al.127 |
| Aging | Old mice | Young BMCs | ↓ Collagen IV ↓ PAI-1 ↑ PDGF-B | ↑ | Yang et al.127 |
Abbreviations: AKI, acute kidney injury; IRI, ischemia–reperfusion injury; WJ-hMSCS, Wharton’s jelly-derived human mesenchymal stem cells; BMCs, bone marrow–derived cells; PAI-1, plasminogen activator inhibitor-1; PDGF-B, platelet-derived growth factor subunit B; microRNA, miR.
There is also some evidence that young cells can restore youthful features in aged tissues, possibly due to some regenerative factors that are not present in older cells. In parabiosis studies involving heterochronic couples (aged and young animals that share a common circulation), the proliferative and regenerative capacity of aged tissues has been shown to improve when they are put in contact with young tissues128. These youth-associated factors can be RNAs, and some miRs that are responsible for the inhibition of antioxidants and consequently heightening of oxidative stress can promote the cell senescence phenotype, because the anti-miR effects can direct older cells back toward the young phenotype71. In addition, when aged mice are exposed to a lethal dose of radiation and then treated with bone marrow–derived cells, age-matched cells, or cells from young donors, differences can be observed among the groups. Recipients of cells from young donors are more successful in maintaining a younger phenotype, whereas recipients of age-matched cells show higher renal expression of senescence-related proteins (such as β-gal, plasminogen activator inhibitor-1, platelet-derived growth factor subunit B, fibroblast-specific protein-1, p21Waf1/Cip1, and p16INK4a), elevated deposition of collagen IV in the mesangium, and low Klotho expression127, as shown in Table 2.
Table 2.
Expression of Markers of Aging by Stem Cell Source.
In fact, the younger the cells are, the more youth-related factors are present. A comparison among cells harvested from the umbilical cord, from neonates at fourth day after birth, from their mothers, and from adult volunteers showed that levels of soluble Klotho are markedly more elevated in the cells harvested from the umbilical cord. Furthermore, we found that umbilical cord–derived MSCs presented more Klotho expression than did ADMSCs (see Table 2).
Hematopoietic cells derived from umbilical cord blood present longer telomeres than do bone marrow–derived cells, and studies of hematopoietic stem cell transplantation have shown that telomeres are longer in recipients of umbilical cord cells than in recipients of cells from other sources54. That would lead us to think that longer telomeres could be a benefit of using younger cells in cytotherapy. However, comparable telomere lengths have been demonstrated in MSCs from different sources such as dental papilla tissue, umbilical cord matrix, and adipose tissue130.
Telomerase activity presents a noncanonical enhancing activity of antioxidant enzymes such as SODs and catalases, conferring protection against oxidative stress in a telomere-length independent function131. Nevertheless, telomerase activity does not seem to differ between cells from young tissues and those from older tissues130. Therefore, it seems that, despite the fact that shorter telomeres and lower telomerase activity can confer a poorer prognosis in AKI, the mechanism by which young stem cells are better in repairing AKI does not seem to include telomere biology.
Some important studies have demonstrated that kl/+ mice show impaired progenitor cell function in various tissues including renal tissue. In the literature, there is convincing evidence that raising Klotho protein levels can ameliorate AKI and perhaps CKD53. Nevertheless, all of the studies involving Klotho have been conducted at the experimental level, and it is quite possible that upregulation of Klotho protein expression by exogenous Klotho can become a new therapeutic tool in kidney disease.
In this regard, stem cells/progenitor cells for kidney regeneration are more likely to target a cascade of mechanisms, whereas the use of one pharmacological agent targets only a limited number of pathogenic pathways. Therefore, perhaps a combination of the two could lead a better therapeutic approach. Further experimental and clinical studies, as well as phase I, II, and III trials, are needed. The use of younger MSCs, such as umbilical cord–derived MSCs and kidney progenitor cells (isolated from amniotic fluid or preterm neonate urine), could be an interesting alternative to the use of other tissue-specific renal progenitor cells132,133 and could also play a role in kidney regeneration.
Conclusion
In conclusion, the post-AKI progression of renal function includes mechanisms of senescence activation. There is as yet no single therapy that has been shown to alter the outcome of AKI. Stem cell therapy might play a role as a treatment strategy and provide improved outcomes in the coming years.
Acknowledgments
The authors are grateful to Dr. Camilla Fanelli, PhD, for her excellent technical assistance with the figures.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: Lucia Andrade is the recipient of a grant from the Brazilian Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, National Council for Scientific and Technological Development; Grant No. 302835/2009-1) and coordinator of a project grant from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo Research Foundation; Grant No. 2010/19012-0). Irene L. Noronha is the recipient of a grant from CNPq (Grant No. 461785/2014-5) and a grant from Brazilian Ministry of Health (CIPETRO).
References
- 1. Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. [DOI] [PubMed] [Google Scholar]
- 2. Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121(11):4210–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol. 2012;2(2):1303–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11(5):264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Molitoris BA. Therapeutic translation in acute kidney injury: the epithelial/endothelial axis. J Clin Invest. 2014;124(6):2355–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7(4):189–200. [DOI] [PubMed] [Google Scholar]
- 7. Basile DP, Bonventre JV, Mehta R, Nangaku M, Unwin R, Rosner MH, Kellum JA, Ronco C, ADQI XIII Work Group. Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol. 2016;27(3):687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16(5):535–543, 1p following 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duffield JS. Macrophages and immunologic inflammation of the kidney. Semin Nephrol. 2010;30(3):234–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ko GJ, Boo CS, Jo SK, Cho WY, Kim HK. Macrophages contribute to the development of renal fibrosis following ischaemia/reperfusion-induced acute kidney injury. Nephrol Dial Transplant. 2008;23(3):842–852. [DOI] [PubMed] [Google Scholar]
- 11. Vlassara H, Torreggiani M, Post JB, Zheng F, Uribarri J, Striker GE. Role of oxidants/inflammation in declining renal function in chronic kidney disease and normal aging. Kidney Int Suppl. 2009;76(114):S3–S11. [DOI] [PubMed] [Google Scholar]
- 12. Humphreys BD, Czerniak S, DiRocco DP, Hasnain W, Cheema R, Bonventre JV. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci U S A. 2011;108(22):9226–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 CDK-interacting protein cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75(4):805–816. [DOI] [PubMed] [Google Scholar]
- 14. Price PM, Safirstein RL, Megyesi J. The cell cycle and acute kidney injury. Kidney Int. 2009;76(6):604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bencokova Z, Kaufmann MR, Pires IM, Lecane PS, Giaccia AJ, Hammond EM. ATM activation and signaling under hypoxic conditions. Mol Cell Biol. 2009;29(2):526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goodarzi AA, Block WD, Lees-Miller SP. The role of ATM and ATR in DNA damage-induced cell cycle control. Prog Cell Cycle Res. 2003;5:393–411. [PubMed] [Google Scholar]
- 17. Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15(17):2177–2196. [DOI] [PubMed] [Google Scholar]
- 18. van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509(7501):439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Price PM, Safirstein RL, Megyesi J. Protection of renal cells from cisplatin toxicity by cell cycle inhibitors. Am J Physiol Renal Physiol. 2004;286(2):F378–F384. [DOI] [PubMed] [Google Scholar]
- 20. Megyesi J, Udvarhelyi N, Safirstein RL, Price PM. The p53-independent activation of transcription of p21 waf1/cip1/sdi1 after acute renal failure. Am J Physiol. 1996;271(6 Pt 2):F1211–F1216. [DOI] [PubMed] [Google Scholar]
- 21. Megyesi J, Safirstein RL, Price PM. Induction of p21waf1/cip1/sdi1 in kidney tubule cells affects the course of cisplatin-induced acute renal failure. J Clin Invest. 1998;101(4):777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu F, Megyesi J, Safirstein RL, Price PM. Identification of the functional domain of p21(waf1/cip1) that protects cells from cisplatin cytotoxicity. Am J Physiol Renal Physiol. 2005;289(3): F514–F520. [DOI] [PubMed] [Google Scholar]
- 23. Megyesi J, Andrade L, Vieira JM, Safirstein RL, Price PM. Positive effect of the induction of p21waf1/cip1 on the course of ischemic acute renal failure. Kidney Int. 2001;60(6):2164–2172. [DOI] [PubMed] [Google Scholar]
- 24. Iwakura T, Fujigaki Y, Fujikura T, Ohashi N, Kato A, Yasuda H. Acquired resistance to rechallenge injury after acute kidney injury in rats is associated with cell cycle arrest in proximal tubule cells. Am J Physiol Renal Physiol. 2016;310(9):F872–F884. [DOI] [PubMed] [Google Scholar]
- 25. Megyesi J, Tarcsafalvi A, Li S, Hodeify R, Hti Lar Seng NS, Portilla D, Price PM. Increased expression of p21waf1/cip1 in kidney proximal tubules mediates fibrosis. Am J Physiol Renal Physiol. 2015;308(2):F122–F30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Megyesi J, Price PM, Tamayo E, Safirstein RL. The lack of a functional p21(Waf1/Cip1) gene ameliorates progression to chronic renal failure. Proc Natl Acad Sci U S A. 1999;96(19):10830–10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee DH, Wolstein JM, Pudasaini B, Plotkin M. Ink4a deletion results in improved kidney regeneration and decreased capillary rarefaction after ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2012;302(1):F183–F191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Braun H, Schmidt BM, Raiss M, Baisantry A, Mircea-Constantin D, Wang S, Gross ML, Serrano M, Schmitt R, Melk A. Cellular senescence limits regenerative capacity and allograft survival. J Am Soc Nephrol. 2012;23(9):1467–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Noda A, Ning Y, Venable SF, Pereira-Smith OM, Smith JR. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994;211(1):90–98. [DOI] [PubMed] [Google Scholar]
- 30. Melk A, Schmidt BM, Takeuchi O, Sawitzki B, Rayner DC, Halloran PF. Expression of p16ink4a and other cell cycle regulator and senescence associated genes in aging human kidney. Kidney Int. 2004;65(2):510–520. [DOI] [PubMed] [Google Scholar]
- 31. Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/arf expression is a biomarker of aging. J Clin Invest. 2004;114(9):1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chkhotua AB, Abendroth D, Froeba G, Schelzig H. Up-regulation of cell cycle regulatory genes after renal ischemia/reperfusion: differential expression of p16(INK4A), p21(Waf1/Cip1) and p27(kip1) cyclin-dependent kinase inhibitor genes depending on reperfusion time. Transpl Int. 2006;19(1):72–77. [DOI] [PubMed] [Google Scholar]
- 33. Dai CY, Enders GH. P16 ink4a can initiate an autonomous senescence program. Oncogene. 2000;19(13):1613–1622. [DOI] [PubMed] [Google Scholar]
- 34. Rodrigues CE, Capcha JM, de Bragança AC, Sanches TR, Gouveia PQ, de Oliveira PA, Malheiros DM, Volpini RA, Santinho MA, Santana BA, Calado RD, Noronha IL, Andrade L. Human umbilical cord-derived mesenchymal stromal cells protect against premature renal senescence resulting from oxidative stress in rats with acute kidney injury. Stem Cell Res Ther. 2017;8(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O’Sullivan ED, Hughes J, Ferenbach DA. Renal aging: causes and consequences. J Am Soc Nephrol. 2017;28(2):407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Canaud G, Bonventre JV. Cell cycle arrest and the evolution of chronic kidney disease from acute kidney injury. Nephrol Dial Transplant. 2015;30(4):575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zahedi K, Revelo MP, Barone S, Wang Z, Tehrani K, Citron DP, Bissler JJ, Rabb H, Soleimani M. Stathmin-deficient mice develop fibrosis and show delayed recovery from ischemic-reperfusion injury. Am J Physiol Renal Physiol. 2006;290(6):F1559–F1567. [DOI] [PubMed] [Google Scholar]
- 38. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse Klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. [DOI] [PubMed] [Google Scholar]
- 39. Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309(5742):1829–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arking DE, Krebsova A, Macek M, Sr, Macek M, Jr, Arking A, Mian IS, Fried L, Hamosh A, Dey S, McIntosh I, Dietz HC. Association of human aging with a functional variant of Klotho. Proc Natl Acad Sci U S A. 2002;99(2):856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Keles N, Caliskan M, Dogan B, Keles NN, Kalcik M, Aksu F, Kostek O, Aung SM, Isbilen B, Oguz A. Low serum level of Klotho is an early predictor of atherosclerosis. Tohoku J Exp Med. 2015;237(1):17–23. [DOI] [PubMed] [Google Scholar]
- 42. Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22(1):124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barker SL, Pastor J, Carranza D, Quiñones H, Griffith C, Goetz R, Mohammadi M, Ye J, Zhang J, Hu MC, Kuro-o M, Moe OW, Sidhu SS. The demonstration of αKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant. 2015;30(2):223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24(9):3438–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lim K, Groen A, Molostvov G, Lu T, Lilley KS, Snead D, James S, Wilkinson IB, Ting S, Hsiao LL, Hiemstra TF, Zehnder D. α-Klotho expression in human tissues. J Clin Endocrinol Metab. 2015;100(10):E1308–E1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hu MC, Kuro-o M, Moe OW. Renal and extrarenal actions of Klotho. Semin Nephrol. 2013;33(2):118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kuro-o M. Klotho. Pflugers Arch. 2010;459(2):333–343. [DOI] [PubMed] [Google Scholar]
- 48. Maekawa Y, Ohishi M, Ikushima M, Yamamoto K, Yasuda O, Oguro R, Yamamoto-Hanasaki H, Tatara Y, Takeya Y, Rakugi H. Klotho protein diminishes endothelial apoptosis and senescence via a mitogen-activated kinase pathway. Geriatr Gerontol Int. 2011;11(4):510–516. [DOI] [PubMed] [Google Scholar]
- 49. Haruna Y, Kashihara N, Satoh M, Tomita N, Namikoshi T, Sasaki T, Fujimori T, Xie P, Kanwar YS. Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc Natl Acad Sci U S A. 2007;104(7):2331–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317(5839):803–806. [DOI] [PubMed] [Google Scholar]
- 51. Ikushima M, Rakugi H, Ishikawa K, Maekawa Y, Yamamoto K, Ohta J, Chihara Y, Kida I, Ogihara T. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun. 2006;339(3):827–832. [DOI] [PubMed] [Google Scholar]
- 52. Sugiura H, Yoshida T, Mitobe M, Yoshida S, Shiohira S, Nitta K, Tsuchiya K. Klotho reduces apoptosis in experimental ischaemic acute kidney injury via hsp-70. Nephrol Dial Transplant. 2010;25(1):60–68. [DOI] [PubMed] [Google Scholar]
- 53. Hu MC, Shi M, Zhang J, Quiñones H, Kuro-o M, Moe OW. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78(12):1240–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gadalla SM, Savage SA. Telomere biology in hematopoiesis and stem cell transplantation. Blood Rev. 2011;25(6):261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schmitt R, Cantley LG. The impact of aging on kidney repair. Am J Physiol Renal Physiol. 2008;294(6):F1265–F1272. [DOI] [PubMed] [Google Scholar]
- 56. Hochegger K, Koppelstaetter C, Tagwerker A, Huber JM, Heininger D, Mayer G, Rosenkranz AR. P21 and mTERT are novel markers for determining different ischemic time periods in renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2007;292(2):F762–F768. [DOI] [PubMed] [Google Scholar]
- 57. Suzui N, Yoshimi N, Kawabata K, Mori H. The telomerase activities in several organs and strains of rats with ageing. Lab Anim. 1999;33(2):149–154. [DOI] [PubMed] [Google Scholar]
- 58. Joosten SA, van Ham V, Nolan CE, Borrias MC, Jardine AG, Shiels PG, van Kooten C, Paul LC. Telomere shortening and cellular senescence in a model of chronic renal allograft rejection. Am J Pathol. 2003;162(4):1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wills LP, Schnellmann RG. Telomeres and telomerase in renal health. J Am Soc Nephrol. 2011;22(1):39–41. [DOI] [PubMed] [Google Scholar]
- 60. Westhoff JH, Schildhorn C, Jacobi C, Hömme M, Hartner A, Braun H, Kryzer C, Wang C, von Zglinicki T, Kränzlin B, Gretz N, Melk A. Telomere shortening reduces regenerative capacity after acute kidney injury. J Am Soc Nephrol. 2010;21(2):327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cheng H, Fan X, Lawson WE, Paueksakon P, Harris RC. Telomerase deficiency delays renal recovery in mice after ischemia-reperfusion injury by impairing autophagy. Kidney Int. 2015;88(1):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sureshbabu A, Ryter SW, Choi ME. Oxidative stress and autophagy: crucial modulators of kidney injury. Redox Biol. 2015;4:208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. [DOI] [PubMed] [Google Scholar]
- 64. Davies KJ. Protein damage and degradation by oxygen radicals. I. General aspects. J Biol Chem. 1987;262(20):9895–9901. [PubMed] [Google Scholar]
- 65. Hosohata K. Role of oxidative stress in drug-induced kidney injury. Int J Mol Sci. 2016;17(11):1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Scherz-Shouval R, Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007;17(9):422–427. [DOI] [PubMed] [Google Scholar]
- 67. Young AR, Narita M, Ferreira M, Kirschner K, Sadaie M, Darot JF, Tavaré S, Arakawa S, Shimizu S, Watt FM. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009;23(7):798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kruidering M, Van de Water B, de Heer E, Mulder GJ, Nagelkerke JF. Cisplatin-induced nephrotoxicity in porcine proximal tubular cells: mitochondrial dysfunction by inhibition of complexes i to iv of the respiratory chain. J Pharmacol Exp Ther. 1997;280(2):638–649. [PubMed] [Google Scholar]
- 69. Davis CA, Nick HS, Agarwal A. Manganese superoxide dismutase attenuates cisplatin-induced renal injury: importance of superoxide. J Am Soc Nephrol. 2001;12(12):2683–2690. [DOI] [PubMed] [Google Scholar]
- 70. Tracz MJ, Juncos JP, Croatt AJ, Ackerman AW, Grande JP, Knutson KL, Kane GC, Terzic A, Griffin MD, Nath KA. Deficiency of heme oxygenase-1 impairs renal hemodynamics and exaggerates systemic inflammatory responses to renal ischemia. Kidney Int. 2007;72(9):1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bai XY, Ma Y, Ding R, Fu B, Shi S, Chen XM. Mir-335 and mir-34a promote renal senescence by suppressing mitochondrial antioxidative enzymes. J Am Soc Nephrol. 2011;22(7):1252–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chen Q, Fischer A, Reagan JD, Yan LJ, Ames BN. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc Natl Acad Sci U S A. 1995;92(10):4337–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Small DM, Bennett NC, Roy S, Gabrielli BG, Johnson DW, Gobe GC. Oxidative stress and cell senescence combine to cause maximal renal tubular epithelial cell dysfunction and loss in an in vitro model of kidney disease. Nephron Exp Nephrol. 2012;122(3–4):123–130. [DOI] [PubMed] [Google Scholar]
- 74. Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140(6):771–776. [DOI] [PubMed] [Google Scholar]
- 75. Linfert D, Chowdhry T, Rabb H. Lymphocytes and ischemia-reperfusion injury. Transplant Rev (Orlando). 2009;23(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ratliff BB, Rabadi MM, Vasko R, Yasuda K, Goligorsky MS. Messengers without borders: mediators of systemic inflammatory response in AKI. J Am Soc Nephrol. 2013;24(4):529–536. [DOI] [PubMed] [Google Scholar]
- 77. Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Anders HJ, Schaefer L. Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J Am Soc Nephrol. 2014;25(7):1387–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kim HJ, Lee DW, Ravichandran K, O’Keys D, Akcay A, Nguyen Q, He Z, Jani A, Ljubanovic D, Edelstein CL. Nlrp3 inflammasome knockout mice are protected against ischemic but not cisplatin-induced acute kidney injury. J Pharmacol Exp Ther. 2013;346(3):465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kurts C, Panzer U, Anders HJ, Rees AJ. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13(10):738–753. [DOI] [PubMed] [Google Scholar]
- 81. Rabb H, Griffin MD, McKay DB, Swaminathan S, Pickkers P, Rosner MH, Kellum JA, Ronco C; Acute Dialysis Quality Initiative Consensus XIII Work Group. Inflammation in AKI: current understanding, key questions, and knowledge gaps. J Am Soc Nephrol. 2016;27(2):371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ascon M, Ascon DB, Liu M, Cheadle C, Sarkar C, Racusen L, Hassoun HT, Rabb H. Renal ischemia-reperfusion leads to long term infiltration of activated and effector-memory t lymphocytes. Kidney Int. 2009;75(5):526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens. 2004;13(1):1–7. [DOI] [PubMed] [Google Scholar]
- 84. Stranks AJ, Hansen AL, Panse I, Mortensen M, Ferguson DJ, Puleston DJ, Shenderov K, Watson AS, Veldhoen M, Phadwal K, Cerundolo V, Simon AK. Autophagy controls acquisition of aging features in macrophages. J Innate Immun. 2015;7(4):375–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kaushal GP, Shah SV. Autophagy in acute kidney injury. Kidney Int. 2016;89(4):779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang Y, Nartiss Y, Steipe B, McQuibban GA, Kim PK. ROS-induced mitochondrial depolarization initiates park2/Parkin-dependent mitochondrial degradation by autophagy. Autophagy. 2012;8(10):1462–1476. [DOI] [PubMed] [Google Scholar]
- 87. Rusten TE, Stenmark H. P62, an autophagy hero or culprit? Nat Cell Biol. 2010;12(3):207–209. [DOI] [PubMed] [Google Scholar]
- 88. Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor nrf2 through inactivation of keap1. Nat Cell Biol. 2010;12(3):213–223. [DOI] [PubMed] [Google Scholar]
- 89. Livingston MJ, Dong Z. Autophagy in acute kidney injury. Semin Nephrol. 2014;34(1):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hsiao HW, Tsai KL, Wang LF, Chen YH, Chiang PC, Chuang SM, Hsu C. The decline of autophagy contributes to proximal tubular dysfunction during sepsis. Shock. 2012;37(3):289–296. [DOI] [PubMed] [Google Scholar]
- 91. Baisantry A, Bhayana S, Rong S, Ermeling E, Wrede C, Hegermann J, Pennekamp P, Sörensen-Zender I, Haller H, Melk A, Schmitt R. Autophagy induces prosenescent changes in proximal tubular s3 segments. J Am Soc Nephrol. 2016;27(6):1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ding Y, Kim S, Lee SY, Koo JK, Wang Z, Choi ME. Autophagy regulates TGF-β expression and suppresses kidney fibrosis induced by unilateral ureteral obstruction. J Am Soc Nephrol. 2014;25(12):2835–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sawhney S, Marks A, Fluck N, Levin A, McLernon D, Prescott G, Black C. Post-discharge kidney function is associated with subsequent ten-year renal progression risk among survivors of acute kidney injury. Kidney Int. 2017;92(2):440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG; University of Toronto Acute Kidney Injury Research Group. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302(11):1179–1185. [DOI] [PubMed] [Google Scholar]
- 95. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Hsu CY. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76(8):893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hsu CY, Vittinghoff E, Lin F, Shlipak MG. The incidence of end-stage renal disease is increasing faster than the prevalence of chronic renal insufficiency. Ann Intern Med. 2004;141(2):95–101. [DOI] [PubMed] [Google Scholar]
- 98. Malhotra R, Kashani KB, Macedo E, Kim J, Bouchard J, Wynn S, Li G, Ohno-Machado L, Mehta R. A risk prediction score for acute kidney injury in the intensive care unit. Nephrol Dial Transplant. 2017;32(5):814–822. [DOI] [PubMed] [Google Scholar]
- 99. Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med. 2002;346(12):913–923. [DOI] [PubMed] [Google Scholar]
- 100. Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol. 2015;26(8):1765–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Canaud G, Bienaimé F, Viau A, Treins C, Baron W, Nguyen C, Burtin M, Berissi S, Giannakakis K, Muda AO, Zschiedrich S, Huber TB, Friedlander G, Legendre C, Pontoglio M, Pende M, Terzi F. Akt2 is essential to maintain podocyte viability and function during chronic kidney disease. Nat Med. 2013;19(10):1288–1296. [DOI] [PubMed] [Google Scholar]
- 102. Barrera-Chimal J, Prince S, Fadel F, El Moghrabi S, Warnock DG, Kolkhof P, Jaisser F. Sulfenic acid modification of endothelin b receptor is responsible for the benefit of a nonsteroidal mineralocorticoid receptor antagonist in renal ischemia. J Am Soc Nephrol. 2016;27(2):398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mejía-Vilet JM, Ramírez V, Cruz C, Uribe N, Gamba G, Bobadilla NA. Renal ischemia-reperfusion injury is prevented by the mineralocorticoid receptor blocker spironolactone. Am J Physiol Renal Physiol. 2007;293(1): F78–F86. [DOI] [PubMed] [Google Scholar]
- 104. Sánchez-Pozos K, Barrera-Chimal J, Garzón-Muvdi J, Pérez-Villalva R, Rodríguez-Romo R, Cruz C, Gamba G, Bobadilla NA. Recovery from ischemic acute kidney injury by spironolactone administration. Nephrol Dial Transplant. 2012;27(8):3160–3169. [DOI] [PubMed] [Google Scholar]
- 105. Matsumoto M, Makino Y, Tanaka T, Tanaka H, Ishizaka N, Noiri E, Fujita T, Nangaku M. Induction of renoprotective gene expression by cobalt ameliorates ischemic injury of the kidney in rats. J Am Soc Nephrol. 2003;14(7):1825–1832. [DOI] [PubMed] [Google Scholar]
- 106. Haase VH. Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol. 2006;291(2):F271–F281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Surendran K, Schiavi S, Hruska KA. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol. 2005;16(8):2373–2384. [DOI] [PubMed] [Google Scholar]
- 108. Satoh M, Nagasu H, Morita Y, Yamaguchi TP, Kanwar YS, Kashihara N. Klotho protects against mouse renal fibrosis by inhibiting Wnt signaling. Am J Physiol Renal Physiol. 2012;303(12):F1641–F1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Shi M, Flores B, Gillings N, Bian A, Cho HJ, Yan S, Liu Y, Levine B, Moe OW, Hu MC. α-Klotho mitigates progression of AKI to CKD through activation of autophagy. J Am Soc Nephrol. 2016;27(8):2331–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Dellepiane S, Medica D, Quercia AD, Cantaluppi V. The exciting “Bench to bedside” Journey of cell therapies for acute kidney injury and renal transplantation. J Nephrol. 2017;30(3):319–336. [DOI] [PubMed] [Google Scholar]
- 111. Bianchi F, Sala E, Donadei C, Capelli I, La Manna G. Potential advantages of acute kidney injury management by mesenchymal stem cells. World J Stem Cells. 2014;6(5):644–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Westenfelder C, Togel FE. Protective actions of administered mesenchymal stem cells in acute kidney injury: relevance to clinical trials. Kidney Int Suppl. 2011;1(3):103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Perico N, Casiraghi F, Introna M, Gotti E, Todeschini M, Cavinato RA, Capelli C, Rambaldi A, Cassis P, Rizzo P, Cortinovis M, Marasà M, Golay J, Noris M, Remuzzi G. Autologous mesenchymal stromal cells and kidney transplantation: a pilot study of safety and clinical feasibility. Clin J Am Soc Nephrol. 2011;6(2):412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chen YT, Sun CK, Lin YC, Chang LT, Chen YL, Tsai TH, Chung SY, Chua S, Kao YH, Yen CH, Shao PL, Chang KC, Leu S, Yip HK. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med. 2011;9:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Melief SM, Zwaginga JJ, Fibbe WE, Roelofs H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med. 2013;2(6):455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–1084. [DOI] [PubMed] [Google Scholar]
- 117. Collino F, Pomatto M, Bruno S, Lindoso RS, Tapparo M, Sicheng W, Quesenberry P, Camussi G. Exosome and microvesicle-enriched fractions isolated from mesenchymal stem cells by gradient separation showed different molecular signatures and functions on renal tubular epithelial cells. Stem Cell Rev. 2017;13(2):226–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005;68(4):1613–1617. [DOI] [PubMed] [Google Scholar]
- 119. Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289(1):F31–F42. [DOI] [PubMed] [Google Scholar]
- 120. Tögel F, Cohen A, Zhang P, Yang Y, Hu Z, Westenfelder C. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev. 2009;18(3):475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N, Alison M, Remuzzi G. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15(7):1794–1804. [DOI] [PubMed] [Google Scholar]
- 122. Morigi M, Introna M, Imberti B, Corna D, Abbate M, Rota C, Rottoli D, Benigni A, Perico N, Zoja C, Rambaldi A, Remuzzi A, Remuzzi G. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26(8):2075–2082. [DOI] [PubMed] [Google Scholar]
- 123. Cóndor JM, Rodrigues CE, Sousa Moreira R, Canale D, Volpini RA, Shimizu MH, Camara NO, Noronha IeL, Andrade L. Treatment with human Wharton’s jelly-derived mesenchymal stem cells attenuates sepsis-induced kidney injury, liver injury, and endothelial dysfunction. Stem Cells Transl Med. 2016;5(8):1048–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20(5):1053–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Cao H, Qian H, Xu W, Zhu W, Zhang X, Chen Y, Wang M, Yan Y, Xie Y. Mesenchymal stem cells derived from human umbilical cord ameliorate ischemia/reperfusion-induced acute renal failure in rats. Biotechnol Lett. 2010;32(5):725–732. [DOI] [PubMed] [Google Scholar]
- 126. Jin HJ, Bae YK, Kim M, Kwon SJ, Jeon HB, Choi SJ, Kim SW, Yang YS, Oh W, Chang JW. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14(9):17986–18001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Yang HC, Rossini M, Ma LJ, Zuo Y, Ma J, Fogo AB. Cells derived from young bone marrow alleviate renal aging. J Am Soc Nephrol. 2011;22(11):2028–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–764. [DOI] [PubMed] [Google Scholar]
- 129. Ohata Y, Arahori H, Namba N, Kitaoka T, Hirai H, Wada K, Nakayama M, Michigami T, Imura A, Nabeshima Y, Yamazaki Y, Ozono K. Circulating levels of soluble alpha-Klotho are markedly elevated in human umbilical cord blood. J Clin Endocrinol Metab. 2011;96(6):E943–E947. [DOI] [PubMed] [Google Scholar]
- 130. Jeon BG, Kumar BM, Kang EJ, Ock SA, Lee SL, Kwack DO, Byun JH, Park BW, Rho GJ. Characterization and comparison of telomere length, telomerase and reverse transcriptase activity and gene expression in human mesenchymal stem cells and cancer cells of various origins. Cell Tissue Res. 2011;345(1):149–161. [DOI] [PubMed] [Google Scholar]
- 131. Trachana V, Petrakis S, Fotiadis Z, Siska EK, Balis V, Gonos ES, Kaloyianni M, Koliakos G. Human mesenchymal stem cells with enhanced telomerase activity acquire resistance against oxidative stress-induced genomic damage. Cytotherapy. 2017;19(7):808–820. [DOI] [PubMed] [Google Scholar]
- 132. Roubelakis MG, Pappa KI, Bitsika V, Zagoura D, Vlahou A, Papadaki HA, Antsaklis A, Anagnou NP. Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: comparison to bone marrow mesenchymal stem cells. Stem Cells Dev. 2007;16(6):931–952. [DOI] [PubMed] [Google Scholar]
- 133. Rangel EB, Gomes SA, Dulce RA, Premer C, Rodrigues CO, Kanashiro-Takeuchi RM, Oskouei B, Carvalho DA, Ruiz P, Reiser J, Hare JM. C-kit(+) cells isolated from developing kidneys are a novel population of stem cells with regenerative potential. Stem Cells. 2013;31(8):1644–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]