Abstract
Islet transplantation has been reported to restore normoglycemia and the overall metabolic control in type 1 diabetes mellitus (DM). In the most experienced centers, islet transplantation clinical outcome is similar to that of the whole pancreas transplantation. Long-term islet transplantation function remains a very interesting matter worth discussing. A progressive islet function decrease was reported, probably due to islet exhaustion. In 5 islet-transplanted patients with at least 3-yr follow-up and still insulin independent, their glycemic control was characterized by a blinded retrospective continuous glucose monitoring system (CGMS). Islet transplantation restored glycemic control and glucose variability. Data were compared with patients in the waiting list. All the parameters of glycemic variability tested had improved significantly in patients who had islet transplantation compared with those patients who were on the waiting list. In conclusion, islet transplantation is able to maintain a proper glucose control and normalize glycemic variability in selected patients. A blinded retrospective CGMS is a useful method to characterize glucose homeostasis deeply in vivo in islet-transplanted patients.
Keywords: islet transplantation, continuous glucose monitoring, glycemic variability
Introduction
Islet transplantation has been reported to restore normoglycemia and the overall metabolic control in type 1 diabetes mellitus (DM). Following transplantation, the patients showed a significant reduction in HbA1c along with hypoglycemic episodes, mean glucose profiles, and glycemic variability1–2. The metabolic effects are not limited to the glucose homeostasis. Even in the case of partial graft function, islet recipients normalized the alterations of protein and lipid metabolism.3 The overall metabolic improvement induced significant consequences upon the chronic complications. Islet graft function was described to delay the onset or to reduce the progression of chronic complications of retinopathy, neuropathy, nephropathy, microangiopathy, and macroangiopathy4–6. The effects on complications might be mediated by metabolic control or by a direct C-peptide7 action and, in the case of macrovascular complications, also by improved glycemic variability8.
Available data on glycemic control and on variability are limited to the first 2 yr after transplantation2,9–11. Indeed, a progressive impairment of glycemic control in long-term follow-up was reported. In 5 patients who remained insulin independent for at least 3 yr after islet transplantation, the effects of islet transplantation on glycemic variability were assessed by using different glycemic variability indexes calculated with data taken from a blinded, continuous glucose monitoring system (CGMS).
Materials and Methods
Islet Production
The Islet Transplant Program at Niguarda Hospital started in 2009, and the activities were communicated to the Ethical Committee of Niguarda Hospital. Niguarda Hospital is certified by the Italian National Centre of Transplantation for islet production and distribution. Pancreata were obtained from multiorgan cadaveric donors utilizing cold perfusion (Celsior, Genzyme, Nederland). Exclusion and inclusion criteria were applied based on the Italian guidelines12. Islets were isolated by using the automated method described by Ricordi et al.13 Pancreata were digested by a cold enzymatic blend solution (collagenase NB1 or collagenase Good Manufacturing Practice grade, SERVA, Heidelberg, Germany, or Liberase MTF, Roche, Indianapolis, IN, USA) reconstituted in 25 mM Hank’s Balanced Salt Solution (Euroclone, Pero, Italy) and supplemented with neutral protease (protease NB, SERVA, Heidelberg, Germany) or thermolysin (Roche, Indianapolis, IN, USA). Enzyme activity was assessed as previously described14. Subsequently, islets were purified with discontinuous gradient: polysucrose solutions at decreasing density 1.132, 1.108, 1.096, 1.060, and 1.037 g/L (Mediatech-Cellgro, Manassas, VA, USA). Islets were cultured at 24 °C and 5% CO2 in Miami Media 1 (Mediatech-Cellgro) supplemented with ciprofloxacin (Fresenius Kabi, Verona, Italy) for 24 to 48 h before transplantation. In vitro islet viability characterization was assessed before transplant with propidium iodide (Sigma-Aldrich, Milan, Italy) and calcein AM (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA). A preparation was considered suitable for the transplant if the number of islets was >5,000 islet equivalents (IEQ)/kg/injection, tissue volume <10 mL, and 85% cell viability. Purity was expressed as percentage of islet versus the total tissue volume. Patients were scheduled to receive up to 2 islet infusions with a target islet mass of 10,000 IEQ/kg body weight.
Islet Transplantation
Islet preparation was suspended in Ringer Lactate (Fresenius Kabi) supplemented with bicarbonate (1 mEq/mL, S.A.L.F., Bergamo, Italy) and human serum albumin 20% (Kedrion, Lucca, Italy), loaded in a 50 mL syringe, packaged in double wrap, and transported to the radiology department.
Islets were slowly infused into the portal vein through a percutaneous transhepatic injection. Immunosuppression included induction with polyclonal ALG (Thymoglobulin, Sanofi, Italy, 1 mg/kg/day for 5 d in the case of the first transplant) or Basiliximab 20 mg on days 0 and 4 (in the case of a second islet transplant). In the first transplant of islets-alone recipients, 3 mg of everolimus was added to the induction treatment 6 to 12 h before the islet transplantation and 12 to 18 h after the procedure. Maintenance immunosuppression treatment consisted of 0.1 mg/kg slow-release tacrolimus (Advagraf, Astellas, Italy) o.i.d. starting on day 1 posttransplant (BTL 10 to 15 ng/mL up to 1 mo, 6 to 10 thereout) and 1 g of mycophenolate mofetil b.i.d. starting on day 5 through day 15 and 500 mg b.i.d. thereout. Patients were also treated with 5 μg of exenatide b.i.d from day 1 for 1 mo after transplantation. Ten patients were transplanted with this protocol, and their clinical outcome was published in 201615. The current clinical outcome is still the same with 5 insulin-independent patients, 4 patients with a partial graft function (one of them was retransplanted and now is insulin independent too), and one transplant failure.
Patients
Inclusion criteria were C-peptide-negative patients with type 1 diabetes, aged between 18 and 65 yr, a minimum disease duration of 5 yr, and brittle diabetes. Brittle diabetes was defined by the occurrence of frequent acute complications (ketoacidosis or severe hypoglycemic episodes requiring third-part assistance or unawareness despite well-conducted intensive insulin therapy). The main exclusion criteria were as follows: history of cancer, active infections, glomerular filtration rate less than 45 mL/min/1.73 m2 (grade 3b CKD), and liver or coagulation abnormalities. Each patient gave written-informed consent according to the requirement of the National Transplant Agency and the Niguarda Hospital Ethical Committee. Measures of hypoglycemia unawareness (Clarke score) were calculated from questionnaires, event diaries, and self-monitoring of blood glucose records, respectively, as previously described16. The clinical characteristics of the transplanted patients and of the patients in the waiting list are described in Table 1.
Table 1.
Patient Clinical Characteristics.
| Patient | Age (Gender) | Weight (BMI) | Years of Diabetes | HbA1c % (mmol) | Clark Score | Experimental Group |
|---|---|---|---|---|---|---|
| #1 | 48 (F) | 43 (17) | 35 | 72 | 4 | Transplanted |
| #2 | 39 (F) | 56 (17) | 27 | 65 | 6 | Transplanted |
| #3 | 37 (F) | 58 (19) | 26 | 84 | 2 | Transplanted |
| #4 | 53 (M) | 69 (23) | 12 | 79 | 3 | Transplanted |
| #5 | 50 (F) | 72 (27) | 34 | 71 | 4 | Transplanted |
| #6 | 50 (F) | 57 (21) | 29 | 83 | 3 | In waiting list |
| #7 | 49 (M) | 80 (26) | 17 | 61 | 6 | In waiting list |
| #8 | 48 (M) | 71 (22) | 38 | 62 | 5 | In waiting list |
| #9 | 38 (M) | 58 (20) | 32 | 70 | 4 | In waiting list |
| #10 | 40 (F) | 60 (22) | 33 | 78 | 5 | In waiting list |
Note: Body mass index (BMI) expressed as kilogram of body weight/centimeter2 height was measured at the time of the transplant.
According to the glycemic levels, insulin administration was progressively reduced after the transplantation, and it was started again in the presence of persistent fasting glycemic levels higher than 140 or 2-h postprandial glucose values higher than 180 mg/dL or HbA1c > 52 mmol. At the last follow-up (7 yr in patient #1, 6 in patient #2, 4 in patient #3, and 3 in patients #4 and #5), a blinded retrospective continuous glucose monitoring (IPRO2, Medtronic, Minneapolis, MN, USA) was applied for a complete assessment of glycemic control as our standard of care in patients affected by type 1 DM. Five patients in the waiting list for islet transplantation were selected as control group. They were matched with the transplanted patients’ age, body mass index, and diabetes duration. Briefly, a sensor for glucose was applied in the subcutaneous space, and it was removed 4 to 6 d later. The sensor was able to measure the glucose concentration in the interstitial space that is in equilibrium with the plasma glucose concentration.
Statistical Analyses
Different parameters are available for the analyses of glucose variability17–21. The following variability and risk indexes were analyzed.
Variability indexes
Coefficient of variation is calculated as standard deviation (SD) divided by the mean of the glucose values. This is one of the most common parameters used for glycemic variability.
The mean amplitude of glucose excursion (MAGE) is calculated by using the formula as the mean height of excursions (greater than 1SD). This parameter is usually calculated from discontinuous glucose measurements by glucometer. In our article, it is calculated from continuous glucose monitoring data using a different specific algorithm22. Therefore, the values obtained by the 2 different methods cannot be compared. MAGE in its report represents the mean of the daily glucose excursions that exceeds the upper SD measured over the 24-h period. This is an integration of within-day glycemic excursions that correlates with oxidative stress in DM21.
The mean of daily differences is a measure of the day-to-day variation of the glucose pattern. It is defined as the mean of the absolute differences between glucose values on day 2 and the corresponding values on day 1 at the same time22.
CONGA-4 is the continuous overall net glycemic action, and it is a measure of the overall intraday variation of glucose recordings. The CONGA-4 is defined as the SD of the differences between the current observation and the observation of the previous 4 h. Higher CONGA values therefore indicate greater glycemic variation22.
Risk indexes
The J index is calculated with mean and SD of blood glucose measurements in mg/dL: J = 0.001 × (mean + SD)2. The J index calculated on total measurements, no weighting for minor or major variations, is an indicator of between days’ variability when compared with the SD of more than 2 consecutive days23.
The low blood glucose index (LBGI) and high blood glucose index (HBGI) formulae are implemented by converting glucose values into risk scores. If the glucose risk score is below 0, then the risk is labeled as LBGI (normal range 0.0 to 6.9), and if it is above 0, then it is labeled as HBGI (normal range 0.0 to 7.7)24. The LBGI and HBGI were developed by Kovatchev, as a logarithmic transformation of glycemic data. The LBGI and HBGI represent the frequency and extent of low and high glycemic values, respectively. They measure the variability relative to a specific length period of time. Their predictive value has been extensively demonstrated. In addition, their risk degree is very easy to interpret.
The blood glucose risk index (BGRI), mean, and SD are calculated by using the HBGI and LBGI as BGRI = HBGI + LBGI25.
The average daily risk range (ADRR) is calculated by using the HBGI and LBGI as ADRR = max of HBGI + max of LBGI26.
Data from CGM were used to define time in range of glycemic values of each patient. The time was defined in range referring to the ADA criteria that means between 70 (fasting) and 180 mg/dL (post prandial)27.
Data are expressed as mean ± SD. Descriptive statistics have been used to summarize patient’s characteristics. This includes mean and SD. Summary statistics have been reported with maximum 1 decimals as appropriate. Continuous variables have been compared by using the Wilcoxon test. Comparisons of categorical variables have been performed by means of the χ2 test. Statistical tests are based on a 2-sided significance level of 0.05. The SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA) has been used to perform statistical analyses.
The following variables have been created:
Time in range = percentage of time with sensor glucose between 70 and 180 mg/dL.
Glycemic variability indexes have been calculated as described above.
Results
The characteristics of the transplanted islet preparations and the clinical outcome are reported in Table 2. All the patients with one exception (patient #4) received a high number of transplanted islets (more than 10,000 IEQ/kg of recipient).
Table 2.
Transplantation Details.
| Patient | IEQ (IEQ/kg b.w.) | Time from Islet Transplantation (month) | Last C-peptide (ng/mL) | Last HbA1c (mmol/%) |
|---|---|---|---|---|
| #1 | 398,900 (9,277) 300,000 (6,977) | 84 | 1.7 | 41/5.9 |
| #2 | 315,000 (5,625) 484,160 (8,646) | 72 | 2.0 | 33/5.2 |
| #3 | 629,500 (10,853) | 50 | 2.3 | 50/6.7 |
| #4 | 369,200 (5,350) | 38 | 3.9 | 39/5.7 |
| #5 | 447,600 (6,216) 605,800 (8,413) | 36 | 2.9 | 41/5.9 |
Note: Islet equivalent/kilogram body weight (IEQ/kg b.w.) is presented for each infusion. When patients received 2 islet preparations in an infusion, respectively, IEQ, IEQ/kg b.w., and purity are expressed as total IEQ and total IEQ/kg b.w.
Five patients who were still insulin independent at that time were included in the study. The follow-up from the last islet infusion at the time of CGM analysis was 84 mo for patient #1, 72 mo for patient #2, 50 mo for patient #3, 38 mo for patient #4, and 36 mo for patient #5. The C-peptide values available at the last follow-up are reported in Table 1. In patient #3, 850 mg of metformin b.i.d was introduced due to a slight increase in HbA1c.
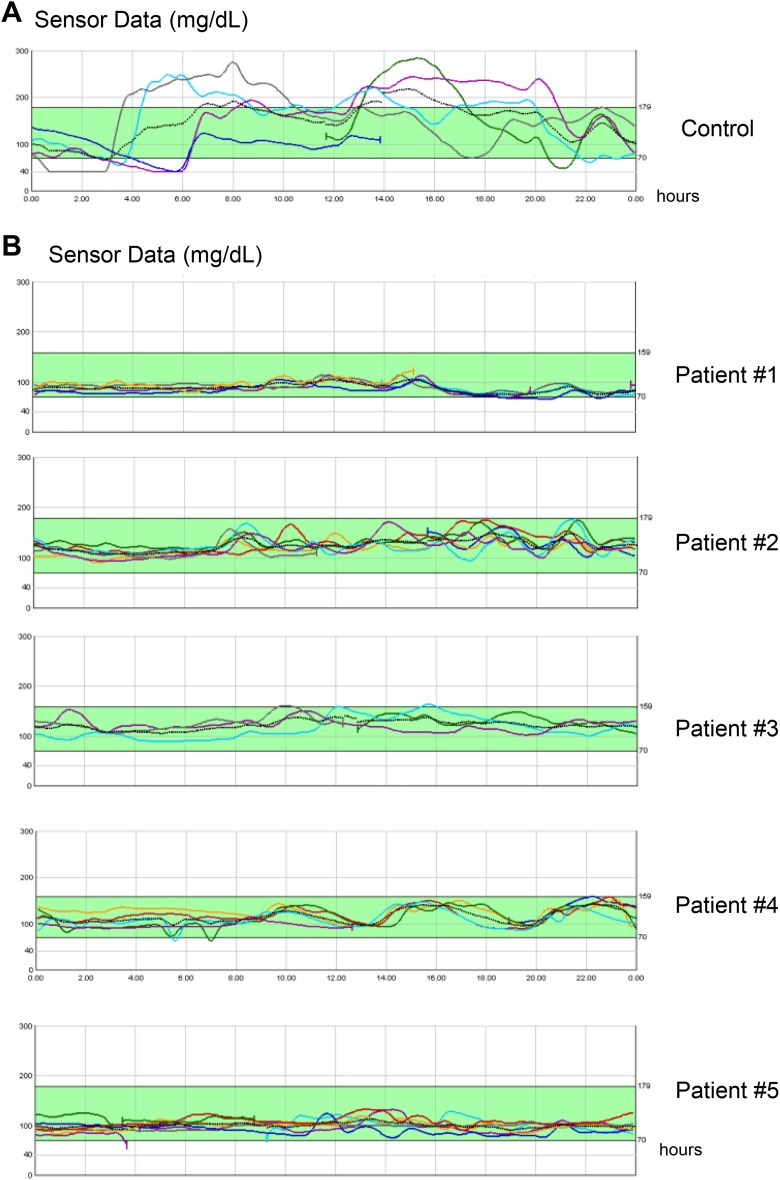
A blinded retrospective CGMS was applied to the patients. CGM data were recorded for 5, 4, 7, 7, and 5 d in transplanted patient from #1 to #5, respectively, and for 5, 5, 5, 7, and 6 d in control patients from #1 to #10, respectively. In the transplanted patients, the glucose profiles appeared well controlled and mainly within the 70- to 180-mg/dL range (Fig. 1, Table 3). A series of glycemic variability parameters were calculated on the CGM data (Table 3). After islet transplantation, all the parameters of glycemic variability significantly improved compared to the values in patients in the waiting list for islet transplantation. There was no significant correlation between the different parameters of islet variability and the length of follow-up after islet transplantation, HbA1c, or C-peptide values (data not shown). Indeed, among all the parameters for glycemic variability, BGRI showed the best direct correlation with the length of follow-up (P = 0.188) and the indirect correlation with C-peptide values (0.068), although both were not significant. Time in the range of glycemic values was significantly improved and near to be 100% in transplanted patients.
Fig. 1.
Glucose profiles achieved by retrospective continuous glucose monitoring systems. Each panel represents the glucose profiles of single patients. The glucose profiles of consecutive days (represented by different lines) of single patients are overlapped by one another. In panel A, a representative glucose profile of a patient in the waiting list for a transplant (control group) is shown. In panel B, glucose profiles of the 5 transplanted patients are reported.
Table 3.
Parameters of Glycemic Variability from the Retrospective Continuous Glucose Monitoring System.
| Indexes | Control Patients | Transplanted Patients | P Value |
|---|---|---|---|
| HbA1c (mmol, %) | 71 ± 10 (8.7 ± 1.0) | 41 ± 6 (5.9 ± 0.6) | 0.004* |
| Clarke score | 5 ± 1 | 0 | 0.001* |
| Sensor glucose mean (mg/dL) | 212 ± 47 | 109 ± 16 | 0.012* |
| Coefficient variation (%) | 37 ± 7 | 13 ± 2 | 0.012* |
| Standard deviation (mg/dL) | 77 ± 11 | 15 ± 4 | 0.012* |
| AUC (min mg/dL) | 1,058 ± 235 | 545 ± 79 | 0.012* |
| MAGE (mg/dL) | 1.2 ± 0.2 | 0.3 ± 0.1 | 0.012* |
| CONGA-4 | 94 ± 10 | 19 ± 8 | 0.012* |
| MODD (mg/dL) | 66 ± 40 | 11 ± 4 | 0.037* |
| HBGI | 19.8 ± 7.6 | 0.7 ± 0.5 | 0.012* |
| LBGI | 5.9 ± 4.1 | 1.2 ± 1.1 | 0.022* |
| BGRI | 18 ± 7 | 1 ± 1 | 0.012* |
| ADRR | 71 ± 9 | 10 ± 4 | 0.012* |
| J index | 86 ± 31 | 16 ± 5 | 0.012* |
| Time in range (%) | 31 ± 15 | 99 ± 2 | 0.008* |
Note: Glucose values are expressed in mg/dL. Data are expressed as mean ± standard deviation. Abbreviations: AUC, the area under the curve of glycemic values; MAGE, the mean amplitude of glucose excursion; CONGA-4, the continuous overall net glycemic action of the previous 4 h; MODD, the mean of daily differences; HBGI, the high blood glucose index; LBGI, the low blood glucose index; ADRR, the average daily risk range; BGRI, the blood glucose risk index.
*Indicates P value ≤ 0.05.
Discussion
In the most experienced centers, the overall success rate of islet transplantation is at present similar to the whole pancreas transplantation. However, in the long-term follow-up pancreas was reported to be more efficient than islets in taking recipients off insulin28. Islet transplantation has been reported to progressively lose its capability to maintain insulin independence, probably as a consequence of a progressive islet loss. Islet graft in our 5 patients with a follow-up longer than 3 yr provided a proper glucose control with no need of exogenous insulin administration. All the analyzed parameters (fasting C-peptide, glycemic values, and HbA1c values) confirmed a good glycemic homeostasis, even if HbA1c of patient #3 remained a bit higher than expected. Patient #3 was placed under metformin treatment due to the mild increase in HbA1c values. As previously reported, a possible progressive graft exhaustion cannot be excluded28. The use of a blinded CGMS offered an additional way to further characterize the quality of glucose control achieved by islet graft function2,9–11. In our patients, the glucose values were always within the 70- to 180-mg/dL range (nearly up to 100%) all the time in all 5 patients. In addition, the analyses of CGM data allowed the analyses of glycemic variability. The role of glycemic variability as an independent risk factor for diabetic micro and macrovascular complications, able to predict severe hypoglycemia in type 1 DM, was supported by different studies29. In our study, different parameters of glycemic variability were calculated by using data taken from the continuous glucose monitoring data. All the parameters of glycemic variability were significantly improved compared to the parameters of patients in the islet transplantation waiting list, thus confirming the good quality of glycemic control in all the transplanted patients. All these parameters taken altogether allowed to have a complete characterization of glycemic variability. These varying parameters have specific inherent properties depending upon the purpose for which they were designed. Therefore, there is not a gold standard for measuring glycemic variability30. Some parameters appeared to be indices of glycemic variability in term of acute, short-term, intraday, or interday fluctuation in blood glucose. Other indices assess the quality of glycemic control giving penalty points for glucose values that are abnormally low or high (risk indexes)30. Altogether they offer the opportunity to have a complete overview on glycemic variability.
The clinical values of all these parameters in islet-transplanted patients need further investigation. The limits of our study are that this is a single-center experience performed on a small sample. The statistical comparison can be misleading due to the sample size and heterogeneity of the 2 groups. Furthermore, no baseline characteristics were available for the patient.
However, this remains the first study on glycemic variability of long-term insulin-independent patients after islet transplantation. We suggest that one or more can be used to monitor islet graft function during follow-up and detect early signs of graft dysfunction. BGRI appeared to be among the others, the only parameter that appeared to be directly correlated with the length of follow-up and inversely with C-peptide values, although these correlations were not statistically significant. Indeed, our study is not a longitudinal one, and therefore, additional studies have to be designed for the identification of parameters for glycemic variability predictive of the graft fate. Alternatively, glycemic variability should be analyzed in patients with early signs of impaired islet graft function.
In conclusion, we believe that the CGMS might be proposed as a useful tool for islet graft function monitoring. In the 5 patients studied, transplanted islet graft function was able to maintain a good glucose control and to normalize glycemic variability even after a follow-up over a period of time longer than 3 yr.
Footnotes
Author Contributions: Federico Bertuzzi participated in research design and in the writing of the article; Barbara Antonioli, Marta Cecilia Tosca, Danila Fava, Patrizia Dorighet, and Marta Galuzzi participated in the performance of the research and data collection; Mario Marazzi, Luciano De Carlis, Andrea Lauterio, Matteo Bonomo, Antonio Gaetano Rampoldi, and Andrea De Gasperi participated in the project and in the article revision.
Ethical Approval: The activities were communicated to the Ethical Committee of Niguarda Hospital. Niguarda Hospital is certified by the Italian National Centre of Transplantation for islet production and distribution were communicated to the Ethical Committee of Niguarda Hospital. Niguarda Hospital is certified by the Italian National Centre of Transplantation for islet production and distribution.
Statement of Human and Animal Rights: Our clinical trial policies and management systems were designed to respect the human rights of trial participants according to Helsinky Declaration.
Statement of Informed Consent: An informed consent was signed by patients voluntarily before entering in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is funded by Fondazione Italiana Diabete Onlus Grant, Horizon 2020 EU project 645991—DRIVE, and Associazione Pazienti Diabetici della Provincia di Milano.
References
- 1. Shapiro AM, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol. 2017;13(5):268–277. [DOI] [PubMed] [Google Scholar]
- 2. Kessler L, Passemard R, Oberholzer J, Benhamou PY, Bucher P, Toso C, Meyer P, Penfornis A, Badet L, Wolf P, et al. Reduction of blood glucose variability in type 1 diabetic patients treated by pancreatic islet transplantation: interest of continuous glucose monitoring. Diabetes Care. 2002;25(12):2256–2262. [DOI] [PubMed] [Google Scholar]
- 3. Luzi L, Hering BJ, Socci C, Raptis G, Battezzati A, Terruzzi I, Falqui L, Brandhorst H, Brandhorst D, Regalia E, et al. Metabolic effects of successful intraportal islet transplantation in insulin-dependent diabetes mellitus. J Clin Invest. 1996;97(11):2611–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fiorina P, Shapiro AM, Ricordi C, Secchi A. The clinical impact of islet transplantation. Am J Transplant. 2008;8(10):1990–1997. [DOI] [PubMed] [Google Scholar]
- 5. Lee TC, Barshes NR, O’Mahony CA, Nguyen L, Brunicardi FC, Ricordi C, Alejandro R, Schock AP, Mote A, Goss JA. The effect of pancreatic islet transplantation on progression of diabetic retinopathy and neuropathy. Transplant Proc. 2005;37(5):2263–2265. [DOI] [PubMed] [Google Scholar]
- 6. Thompson DM, Meloche M, Ao Z, Paty B, Keown P, Shapiro RJ, Ho S, Worsley D, Fung M, Meneilly G, et al. Reduced progression of diabetic microvascular complications with islet cell transplantation compared with intensive medical therapy. Transplantation. 2011;91(3):373–378 [DOI] [PubMed] [Google Scholar]
- 7. Fiorina P, Gremizzi C, Maffi P, Caldara R, Tavano D, Monti L, Socci C, Folli F, Fazio F, Astorri E, et al. Islet transplantation is associated with an improvement of cardiovascular function in type 1 diabetic kidney transplant patients. Diabetes Care. 2005;28(6):1358–1365. [DOI] [PubMed] [Google Scholar]
- 8. Hirsch IB. Glycemic variability and diabetes complications: does it matter? of course it does! Diabetes Care. 2015;38(8):1610–1614. [DOI] [PubMed] [Google Scholar]
- 9. Gorn L, Faradji RN, Messinger S, Monroy K, Baidal DA, Froud T, Mastrototaro J, Ricordi C, Alejandro R. Impact of islet transplantation on glycemic control as evidenced by a continuous glucose monitoring system. J Diabetes Sci Technol. 2008;2(2):221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holmes-Walker DJ, Gunton JE, Payk M, Donath S, Hawthorne WJ, Loudovaris T, Anderson P, Ward GM, Kay TW, O’Connell PJ. Islet transplantation provides superior glycemic control with less hypoglycemia compared to continuous subcutaneous insulin infusion or multiple daily insulin injections. Transplantation. 2017;101(6):1268–1275. [DOI] [PubMed] [Google Scholar]
- 11. Rickels MR, Peleckis AJ, Markmann E, Dalton-Bakes C, Kong SM, Teff KL, Naji A. Long-term improvement in glucose control and counterregulation by islet transplantation for type 1 diabetes. J Clin Endocrinol Metab. 2016;101(11):4421–4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Italian Standard for the Cure of Diabetes Mellitus 2016. 126–136. (Accessed date June 2016) http://www.standarditaliani.it.
- 13. Ricordi C, Goldstein JS, Balamurugan AN, Szot GL, Kin T, Liu C, Czarniecki CW, Barbaro B, Bridges ND, Cano J, et al. National institutes of health-sponsored clinical islet transplantation consortium phase 3 trial: manufacture of a complex cellular product at eight processing facilities. Diabetes. 2016;65(11):3418–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salamone M, Seidita G, Cuttitta A, Rigogliuso S, Mazzola S, Bertuzzi F, Ghersi G. A new method to value efficiency of enzyme blends for pancreatic tissue digestion. Transplant Proc. 2010;42(6):2043–2048. [DOI] [PubMed] [Google Scholar]
- 15. Bertuzzi F, Marazzi M, De Carlis LG, Rampoldi AG, Bonomo M, Antonioli B, Tosca MC, Galuzzi M, De Gasperi A, Colussi G. Sustained islet allograft function after peritransplant treatment using exenatide with and without everolimus. Transplantation. 2016;100(11):e117–e118. [DOI] [PubMed] [Google Scholar]
- 16. Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM: a prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995;18(4):517–522. [DOI] [PubMed] [Google Scholar]
- 17. Rodbard D. Interpretation of continuous glucose monitoring data: glycemic variability and quality of glycemic control. Diabetes Technol Ther. 2009;11(1 Suppl);S55–S67. [DOI] [PubMed] [Google Scholar]
- 18. Saisho Y, Tanaka C, Tanaka K, Roberts R, Abe T, Tanaka M, Meguro S, Irie J, Kawai T, Itoh H. Relationships among different glycemic variability indices obtained by continuous glucose monitoring. Prim Care Diabetes. 2015;9(4):290–296. [DOI] [PubMed] [Google Scholar]
- 19. Cameron FJ, Donath SM, Baghurst PA. Measuring glycaemic variation. Curr Diabetes Rev. 2010;6(1):17–26. [DOI] [PubMed] [Google Scholar]
- 20. Rodbard D. Glycemic variability: challenges in interpretation. Diabetes Technol Ther. 2015;17(6):370–372 [DOI] [PubMed] [Google Scholar]
- 21. Johnson EL. Glycemic variability in type 2 diabetes mellitus: oxidative stress and macrovascular complications. Adv Exp Med Biol. 2012;771:139–154. [DOI] [PubMed] [Google Scholar]
- 22. Zaccardi F, Stefano PD, Busetto E, Federici MO, Manto A, Infusino F, Lanza GA, Pitocco D, Ghirlanda G. Group of signs: a new method to evaluate glycemic variability. J Diabetes Sci Technol. 2008;2(6):1061–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wójcicki JM. “J”-index. A new proposition of the assessment of current glucose control in diabetic patients. Horm Metab Res. 1995;27(1):41–42. [DOI] [PubMed] [Google Scholar]
- 24. McDonnell CM, Donath SM, Vidmar SI, Werther GA, Cameron FJ. A novel approach to continuous glucose analysis utilizing glycemic variation. Diabetes Technol Ther. 2005;7(2):253–263. [DOI] [PubMed] [Google Scholar]
- 25. McCall AL, Cox DJ, Crean J, Gloster M, Kovatchev BP. A novel analytical method for assessing glucose variability: using CGMS in type 1 diabetes mellitus. Diabetes Technol Ther. 2006;8(6):644–653. [DOI] [PubMed] [Google Scholar]
- 26. Kovatchev BP, Otto E, Cox D, Gonder-Frederick L, Clarke W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care. 2006;29(11):2433–2438. [DOI] [PubMed] [Google Scholar]
- 27. American Diabetes Association. Glycemic targets. Diabetes Care. 2017;40(1 Suppl):S48–S56. [DOI] [PubMed] [Google Scholar]
- 28. Niclauss N, Morel P, Berney T. Has the gap between pancreas and islet transplantation closed? Transplantation. 2014;98(6):593–599. [DOI] [PubMed] [Google Scholar]
- 29. Gorst C, Kwok CS, Aslam S, Buchan I, Kontopantelis E, Myint PK, Heatlie G, Loke Y, Rutter MK, Mamas MA. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care. 2015;38(12):2354–2369. [DOI] [PubMed] [Google Scholar]
- 30. Whitelaw BC, Choudhary P, Hopkins D. Evaluating rate of change as an index of glycemic variability, using continuous glucose monitoring data. Diabetes Technol Ther. 2011;13(6):631–636. [DOI] [PubMed] [Google Scholar]