Abstract
Administration of mesenchymal stromal cells (MSCs) is a promising strategy to treat cardiovascular disease (CVD). As progenitor cells may be negatively affected by both age and comorbidity, characterization of MSC function is important to guide decisions regarding use of allogeneic or autologous cells. Definitive answers on which factors affect MSC function can also aid in selecting which MSC donors would yield the most therapeutically efficacious MSCs. Here we provide a narrative review of MSC function in CVD based on a systematic search. A total of 41 studies examining CVD-related MSC (dys)function were identified. These data show that MSC characteristics and regenerative potential are often affected by CVD. However, studies presented conflicting results, and directed assessment of MSC parameters relevant to regenerative medicine applications was lacking in many studies. The predictive ability of in vitro assays for in vivo efficacy was rarely assessed. There was no correlation between quality of study reporting and study findings. Age mismatch was also not associated with study findings or effect size. Future research should focus on assays that assess regenerative potential in MSCs and parameters that relate to clinical success.
Keywords: mesenchymal stromal cells, mesenchymal stem cells, cardiovascular disease, cellular therapy, regenerative medicine
Introduction
Cardiovascular disease (CVD) is one of the leading causes of mortality in the Western world1. Genetic makeup, exposure to risk factors, and lifestyle factors all play a role in the pathogenesis of CVD. Aging is a main driver of CVD progression2, inducing nonreversible changes in the vasculature and the heart. These changes range from remodeling of the vessel wall to tissue damage at places of turbulent blood flow and predispose previously healthy individuals to the development of CVD3,4. Aging also affects the regenerative potential of the cardiovascular system. Aged individuals have fewer circulating endothelial progenitor cells (EPCs), which has been implicated in the development and accumulation of vascular damage5,6. Direct effects of aging on tissue repair capabilities of resident tissue cells have also been shown; for instance, aged endothelial cells are less effective at migration, more prone to become senescent, and they have an altered secretion profile that contributes to the development of hypertension and atherosclerosis7–9. In turn, CVD also affects the progenitor cell compartment and regenerative potential of tissue cells. In many CVDs, an age-independent decrease in EPCs has been reported10–12. Decreased migratory potential and increased senescence are also present in endothelial cells of CVD patients.
Eventually, end organ damage develops through a final common pathway of chronic tissue hypoxia, impaired or disturbed angiogenesis, inflammation, and eventually fibrosis.
Though progress has been made, therapies for CVD with the potential to reverse disease-induced damage are still lacking. Cellular therapy approaches that aim to promote angiogenesis and enhance organ function in CVD are currently being investigated. Preclinical and clinical research, while promising at first, demonstrated no clear benefits of autologous bone marrow mononuclear cells (BM-MNCs) in various CVDs13,14. Interestingly, advanced age is one of the strongest predictors of a lack of clinical response to autologous BM-MNC therapy15. EPCs are still being evaluated clinically, but technical difficulties in acquiring and expanding cells to the numbers required for cellular therapy hamper efforts16. Mesenchymal stromal cells (MSCs), first discovered as nonhematopoietic BM precursor cells, gained interest given their regenerative properties in a wide variety of disease contexts and the relative ease of procurement and expansion. In preclinical and clinical studies, MSCs were shown to promote angiogenesis, reduce fibrosis, have immunomodulatory properties, and ultimately restore tissue function. MSCs exert their regenerative effects through close cross talk with local tissue cells and immune effector cells by both direct cell–cell contact and release of paracrine factors17. Transdifferentiation remains controversial and is unlikely to account for the observed regenerative effects18.
During the past decades, MSCs gradually transitioned from the bench to the bedside in CVD; phase III clinical trials examining potential application of MSCs as a therapeutic agent in CVD are ongoing. For instance, MSCs have been shown to be effective at improving left ventricle ejection fraction, reduction in scar size, and neoangiogenesis when administered to patients with ischemic cardiomyopathy19. In patients with critical limb ischemia (CLI), MSCs were shown to improve perfusion and wound healing20,21. As systemic anti-inflammatory effects are thought to contribute to clinical effects of MSCs in CVD22, novel applications also include diseases that have both ischemic and autoinflammatory features such as systemic sclerosis (SSc)23.
Generally, MSCs for clinical applications are isolated from BM, adipose tissue (AT), or the umbilical cord, but MSC-like cells that fulfill the International Society for Cellular Therapy’s (ISCT) minimal criteria for MSCs24 have also been identified in other tissues including organ tissue.
Variability in therapeutic efficacy between donors prompts careful consideration of the cell source to maximize clinical benefits. Currently, in MSC-based therapy, both autologous and allogeneic cells are clinically used. Investigating regenerative potential of MSCs prior to clinical administration is vital to aid identifying donors whose MSCs will yield the largest clinical effect. Donor characteristics such as gender and age have been reported to affect MSC efficacy. MSCs derived from older donors proliferate slower, have reduced differentiation capacity, and display more features of cellular senescence when compared with cells derived from younger individuals25,26.
Several studies suggest that autologous MSCs may also be functionally affected by disease. In unselected BM-derived cells, CVD dysfunction has been shown12,27, and dysfunction of MSCs has also been reported in CVD. If MSC dysfunction occurs in certain disease states, then this could reduce clinical efficacy of autologous MSC treatment and render administration of allogeneic cells more favorable. However, where MSCs were once considered completely immunoprivileged, studies have shown that immune responses directed against MSC may develop under certain circumstances28,29. For instance, senescence induced by prolonged culturing leads to loss of immunosuppressive ability. Additionally, donor characteristics such as gender and age might affect MSC efficacy, further complicating the search for a suitable cell source. Accordingly, in assessing CVD-related MSC dysfunction, age and features of cellular senescence are important factors to consider.
Here we review the currently available literature on CVD-mediated dysfunction in MSCs to assess the presence and extent of CVD-mediated dysfunction in MSCs harvested from clinically relevant sources. Additionally, the role of aging in CVD-mediated dysfunction will be explored, using the available evidence. We will discuss the implications of our findings for clinical MSC-based cell therapy.
Materials and Methods
Search Strategy
To identify studies that examined the effect of CVD on MSC (dys)function, a search was conducted in Medline/PubMed and Embase. The search strategy contained the following components: (1) MSCs and (2) disease and/or dysfunction. The search was performed on December 18, 2016. Studies were selected by 2 reviewers. The references of included studies and the studies citing them were hand searched.
We included primary studies that examined cells obtained from individuals with CVD that (partially) fulfill the minimal criteria for multipotent MSCs set by the International Society for Cell and Gene Therapy (ISCT)30. The MSCs had to be obtained from clinically relevant sites, that is, BM, AT, and umbilical cord tissue or cord blood (UCB).
We excluded studies that examined MSCs derived from animal sources. We also excluded nonprimary studies (reviews were used to snowball and enhance the keyword search).
A total of 45 studies were identified. Four studies were excluded. Two studies were excluded because they examined the effect of patient serum on healthy MSCs and the other 2 studies were excluded because the full text was not available.
Study Characteristics and Data Extraction
The following study characteristics were recorded: subject characteristics such as age and gender, MSC source, type of disease, N, passage number, growth medium, protein source (fetal bovine serum [FBS] vs. platelet lysate or alternatives), whether the cells had been freshly used or postthaw, details of the various assessments conducted, and their results. Data from reported assays were extracted to enable quantitative summaries. Raw data were gathered from the paper text or derived from digitally traced figures, using Plot Digitizer Version 2.6.8.
For analyses, “MSC dysfunction” was defined as “a difference between healthy and CVD MSCs” in one of the prespecified categories that focused on general MSC characteristics and/or regeneration: flowcytometric (FACS) marker expression of the ISCT minimal set, trilineage differentiation, proliferation, cytokine secretion, immunomodulation, angiogenesis/tissue repair, and senescence. In studies that did not report dysfunction in one of these categories but did find alterations or impairment in another area, an additional category (“any other dysfunction”) was created and scored.
Assessment of Risk of Bias
No validated “risk of bias” instrument exists for in vitro studies that utilize cells. For this purpose, findings were coded as 0 (no dysfunction) or 1 (dysfunction present). Reporting was similarly coded as 0 (not reported) and 1 (reported).
We assessed the correlation between the reporting of the study characteristics listed above and positive study results with Fisher’s exact test. The correlation between continuous variables (age of CVD or control participants, delta age) and reporting was assessed with logistical regression.
Studies containing in vivo animal experiments were assessed using the Hooijmans risk of bias tool31. Funnel plots were constructed using ratio of means (meanCVD/meancontrol). The inverse of the square root N was used to estimate the standard error32.
Other Statistical Analyses
Quantitative analyses are reported as ratio of means (meanCVD/meancontrol). As reliable estimates of variance were not available for a large portion of the included studies, no meta-analysis was performed. Distribution of study results was, however, reported by weighted mean on the square root of the sample size (N). To assess the correlation between delta age or passage number and effect ratio, data were analyzed by using mixed effects models using restricted maximum likelihood estimation.
All statistical analyses were conducted using IBM SPSS Statistics Version 24.0 or in “R” software (Version 2.15.3). A P value of <0.05 was considered statistically significant.
Results
The scope and quality of the studies widely differed. Some studies only examined the ISCT MSC minimal criteria—the presence of extracellular MSC markers and the absence of hematopoietic markers, and sometimes fewer than all 3 lineages of differentiation. Others conducted a thorough assessment of multiple MSC parameters, including senescence, immunomodulation, and tissue repair capabilities, sometimes including animal models as well. Table 1 lists the characteristics of the studies. A summary of the effect of disease on MSC function is provided in Table 2 for in vitro studies, and Table 3 details studies with an in vivo component. Below, we will discuss the studies conducted per CVD entity.
Table 1.
Study Characteristics.
| Disease | First Author | N (CVD) | N (Control) | Type of Control | Gender Balance CVD (Male/FEMALE) | Gender Balance Control (Male/Female) | Average Age CVD (Range If Provided) | Average Age Control (Range If Provided) | Source/Location |
|---|---|---|---|---|---|---|---|---|---|
| CVD | Neef et al. (2012)34 | 51 | 0 | — | 35/16 | — | 86.6 | — | Sternal BM |
| CVD | Brunt et al. (2012)35 | 22 | 0 | — | Young:7/2, aged: 11/2 | — | Young: 56.1 (47–63), aged: 73.3 (64–85) | — | Not mentioned |
| CVD | Mancini et al. (2015)33 | 50 | 0 | — | CABG: 28/13. Valve replacement: 4/5 | — | CABG: 63.4, valve: 59.7 | — | AT |
| CAD | Friis et al. (2011)39 | 15 | 16 | Young healthy | 14/1 | 5/11 | 63.64 (53–79) | 26.3 (21–38) | Iliac crest BM |
| CAD | Follin et al. (2013)36 | 7 | 7 | Healthy | 7/0 | 2/5 | 65.7 (58–76) | 41.5 (28–57) | Abdominal AT, lipoaspirate |
| CAD | Efimenko et al. (2014)40 | 64 | 31 | Without CVD that underwent surgery (appendicitis, hernia), trauma, or hip and knee replacement | 55/9 | 7/24 | (43–77) | (2–82) | sc AT |
| CAD + DM | Liu et al. (2013)38 | CAD: 10, CAD + DM2: 10 | 0 | — | — | — | CAD DM 54.40; CAD 55.8 | — | Sternal BM |
| CAD + DM | Dzhoyashvili et al. (2014)37 | 32 CAD, 28 CAD+ DM2 | 19 | No CVD that underwent general surgery or replacement of femoral or knee joints | CAD: 29/3, CAD + DM2: 17/11 | 5/14 | CAD: 62.3; CAD + DM2: 61.0 | 56.7 | sc AT harvested during surgery |
| CAD + DM2 | Phadnis et al. (2009)76 | 95 | 0 | — | — | — | (15–80) | — | Sternal BM |
| CAD | Behfar et al. (2010)42 | 12 | 0 | — | 11-Jan | — | 64.1 | — | Sternal BM |
| CAD | Grauss et al. (2007)41 | 4 | 0 | — | — | — | — | Iliac crest BM | |
| CLI | Brewster et al. (2016)45 | 8 | 4 | Young, healthy | CLI: 2/2, CLI+ DM: 2/2 | 3/1 | CLI: 72 (46–85), CLI + DM: 75.5 (67–85) | 27.5 (22–34) | Healthy: iliac crest BM. CLI: BM from amputated tibiae |
| CLI | Gremmels et al. (2014)44 | 12 | 12 | Non-CLI/PAD elective orthopedic interventions | 8/4 | 4/8 | 67.5 (29–81) | 50 (20–81) | Iliac crest BM |
| CLI | Smadja et al. (2012)43 | 11 | 4 | With peripheral thrombocytopenia, no CLI/PAD | — | — | Median age 65 | “Same age” | Iliac crest BM |
| CLI | Altaner et al. (2013)46 | 41 (Responders: 27, nonresponders: 14) | 0 | — | 35/6 | — | 66 | — | Iliac crest BM |
| HF | Dmitrieva et al. (2015)49 | 16 | 10 | Healthy | — | — | 54.4 | 42.7 | Iliac crest or sternum (not specified for whom which location was chosen) |
| HF | Golpanian et al. (2015)77 | 49 | 0 | — | <60: 21/2, >60: 23/3 | — | <60:51.95; >60: 68.86 | — | Iliac crest BM |
| HF | Minulina et al. (2014)48 | 42 (14 isolated HF, 16 obesity, 12 DM) | 13 | HSCT donors | HF: 13/1; HF + obesity: 13/3, HF + DM: 8/4 | 23.10% | HF:55.8 HF + obesity:52, HF + DM: 59.2 | 39.7 | BM iliac crest or sternum |
| DM1 | De Lima et al. (2016)51 | 21 | 10 | HSCT donors | 15/6 | 5/5 | DM1: median 16 (13–31). | 34 (19–48) | Iliac crest BM |
| DM1 | Davies et al. (2016)53 | early DM1: 10, late DM1: 12 | 19 | Healthy | Early: 9/1. Late: 7/5. | 13/6. | Median age: early: 22 (18–35), late: 42 (31–62) | 37 (21–70) | Iliac crest BM |
| DM1 | Yaochite et al. (2016)52 | 5 | 5 | HSCT donors | 5/0 | 5/0 | 23.2 | 33.1 | Iliac crest BM |
| DM2 | Trinh et al. (2016)55 | 3 | 3 | Nondiabetics who underwent cardiovascular surgery | 3/0 | 3/0 | 59 (not given per group) | 59 (not given per group) | AT |
| DM2 | Gu et al. (2012)56 | 5 | 5 | Patients undergoing breast reconstruction | 1/4 | 2/3 | 62.0 (48–74) | 55.2 (range 38–65 yr) | AT: control: abdominal flap, DM: abdominal lipoaspirate |
| DM2 + CLI | Acosta et al. (2013)62 | — | — | DM2 without CLI | — | — | — | — | AT—biopsy specimens |
| DM2 | Krawiec et al. (2016)61 | 4 DM2, 4 elderly | 7 | Healthy | 0/8 | 0/7 | 42 (39–45) | Young: 34 (26–40). Elderly: 65 (61–68) | Abdominal AT |
| DM2 | Barbagallo et al. (2016)59 | — | — | Cells obtained from supplier | — | — | — | — | AT (supplier) |
| DM2, age | Krawiec et al. (2015)60 | 4 DM2, 4 elderly | 4 | Healthy | 0/8 | 0/4 (7 Males included for testing gender effect) | — | “Held constant” | Abdominal AT |
| DM2 | Cramer et al. (2010)57 | 9 | 34 | Nondiabetic | — | — | 43 (32–64) (not provided separately) | 43 (32–64) | AT—gross specimen |
| DM2 + diabetic foot that required surgical treatment | Koci et al. (2014)58 | 18 | 14 | Nondiabetics undergoing hip or knee replacement surgery | 12.4/5.6a | 7/7 | 58 (30–84) | 61 (29–78) | AT from debridement sites or amputation |
| DM2 | Cheng et al. (2016)54 | 4 | 4 (pooled) | Nondiabetic, abdominoplasty | — | 0/4 | 59 (48–71) | — | Sections of subcutaneous AT |
| Gestational DM2 | Kim et al. (2015)64 | 4 | 3 | Mothers without DMG | 4 | 3 | — | — | Umbilical cord |
| CKD | Reinders et al. (2013)65 | 10 | 10 | Live kidney donors or undergoing orthopedic surgery | 7/3 | 5/5 | 66 | Median 64 | BM |
| CKD | Roemeling—van Rhijn et al. (2012)66 | 16 | 16 | Live kidney donors | CKD 10/6 | 6/10 | 54.5 (25–73) | 56.2 (26–72) | AT harvested from abdominal incision |
| CKD | Yamanaka et al. (2014)67 | 9 | 6 | Non-CKD | 7/3 | 4/2 | 56.2 (50–63) | 52.6 (37–64) | Subcutaneous or mesenteric AT |
| SSc | Hegner et al. (2016)74 | 6 | 6 | HSCT donors | 2/4 | Matched | Median: 50 (38–74) | Matched | Iliac crest BM |
| SSc | Cipriani et al. (2007)41 | 7 | 15 | Healthy | 1/7 | 1/14 | 42 (18–44) | 41 (22–46) | Iliac crest BM |
| SSc | Guiducci et al. (2011)69 | 5 | 5 | Healthy without AID | 0/5 | 0/5 | 42 (18–44) | 39 (20–44) | BM |
| SSc | Larghero et al. (2008)72 | 12 | 13 | 9 HSCT donors 4 Spinal surgery patients | 8/4 | — | 46.8 (33–65) | — | BM: SSc: 8 sternum aspirate, 4 iliac crest. Healthy: 9 from filters used during BM processing for allogeneic transplant. 4: spinal surgery patients. |
| SSc | Cipriani et al (2013)70 | 10 | 10 | HSCT donor | 1/9 | 1/9 | 35.8 (22–46) | 35 (23–45) | Iliac crest BM |
| SSc | Cipriani et al. (2013)71 | 10 | 10 | Purchased from supplier | 1/9 | “Matched” | 35.8 (22–46) | Matched | Iliac crest BM |
| SSc | Vanneaux et al. (2013)73 | 9 | 9 | HSCT donor | — | — | 41 (range: 25–60) | — | BM (SSc: iliac crest, healthy: BM filters) |
| SSc | Cipriani et al. (2014)101 | 10 lcSSc, 10 dcSSc | 10 | HSCT donor | 2/18 | 0/10 | 33.1 (21–46) | Matched | BM |
Abbreviations: CVD, cardiovascular disease; AID, autoimmune disease; CAD, coronary artery disease; HF, heart failure; CLI, critical limb ischemia; PAD, peripheral artery disease; DM, diabetes mellitus; DMG, gestational diabetes mellitus; CKD, chronic kidney failure; SSc, systemic sclerosis; lcSSc, limited cutaneous SSc; dcSSC, diffuse cutaneous SSc; BM, bone marrow; AT, adipose tissue; sc, subcutaneous; UCB: umbilical cord blood; CABG: coronary artery bypass graft; HSCT, hematopoietic stem cell transplant.
Table 2.
In Vitro Assessment of MSCs.
| Disease | First Author | FACS Markers | Differentiation | Proliferation | Cytokine Production | Immunomodulation | Tissue Repair/Angiogenesis | Cellular Aging | Other Dysfunction |
|---|---|---|---|---|---|---|---|---|---|
| CVD | Neef et al. (2012)34 | Present | A, O, C,: Confirmed | Higher CFUs associated with DM2, steroid treatment, COPD, impaired renal function, high euroSCORE (a measure for comorbidity), and impaired left ventricle function and a high number of MNC in the bone marrow | — | — | — | — | — |
| CVD | Brunt et al. (2012)35 | Present | M: impaired | Impaired in aged patients, also lower CFUs | — | — | — | — | WNT/β-catenin signaling reduced in age |
| CVD | Mancini et al. (2015)33 | Present | Met ISCT criteria | — | — | Suppression CD4+ proliferation: impaired in aged pt | — | — | — |
| CAD | Friis et al. (2011)39 | NS | E: N.S. | NS | — | — | Tubule formation MSCs: NS | — | — |
| CAD | Follin et al. (2013)36 | NS | E: NS | Impaired | — | — | tubule formation MSCs: NS | — | — |
| CAD | Efimenko et al. (2014)40 | NS | A, O: NS | — | Older: fewer amounts of VEGF, HGF, and ANG—similar trend in healthy patients, no direct comparisons | — | Tubule formation assay EA.hy926 cells: impaired in older patients. Possible decrease in CAD | shorter telomeres; attenuated telomerase activity | — |
| CAD + DM | Liu et al. (2013)38 | Present | — | Impaired | — | — | — | — | — |
| CAD + DM | Dzhoyashvili et al. (2014)37 | NS | A. O.: NS | PD NS, proliferation activity impaired | CAD: more VEGF, HGF and PAI-1. CAD + T2DM more PlGF, HGF, and PAI-1 | — | Tubule formation EA.hy926 cells: impaired | shorter telomeres, no difference in telomerase activity | |
| CAD + DM2 | Phadnis et al. (2009)76 | Present | P: successful | 57% could not be cultured. Aged MSCs (>30) early proliferative senescence | — | — | — | — | |
| CAD | Behfar et al. (2010)42 | Present | — | — | — | — | — | — | DM less effective in vivo |
| CAD | Grauss et al. (2007)41 | Present | NS | — | — | — | — | — | |
| CLI | Brewster et al. (2016)45 | NS | A, O: NS | NS | bFGF, HGF, MCP-1, and VEGF: NS | — | 3D in vitro invasion assay: decreased EC ingrowth in FBS, increased ingrowth of EC in PL. EC proliferation: NS. EC chemotaxis: NS | — | |
| CLI | Gremmels et al. (2014)44 | NS | A, O: NS C: impaired in older donors | NS | — | — | Scratch wound, tubule formation, proliferation all in HMECS: NS. Migration to PDGF-BB: NS | β-galactosidase and gh2ax no significant differences when age was taken into account | |
| CLI | Smadja et al. (2012)43 | NS | — | — | — | — | — | — | |
| CLI | Altaner et al. (2013)46 | NS | Responders: higher intensity of CD44 and CD90. Other markers: NS | Age-dependent increased population doubling time | IL-4, IL-6, MIP1b higher in responders. Twenty-seven other factors including VEGF, bFGF, and MCP: NS | — | — | — | Altered protein expression |
| HF | Dmitrieva et al. (2015)49 | NS | A. O: confirmed | Impaired. CFU NS | — | — | — | — | Altered gene expression |
| HF | Minullina et al. (2014)48 | NS | NS | – | — | — | — | β-galactosidase staining did not correlate with specific differentially expressed genes | Altered gene expression |
| DM1 | De Lima et al. (2016)51 | NS | A, O, C: NS | — | — | — | Migration to FBS: increased | — | Altered gene expression |
| DM1 | Davies et al. (2016)53 | NS | A, O: NS | NS, CFU NS | IL-6, CXCL1, CXCL6, and PGE2: NSHGF lower in early DM1 pts compared with late | CD3+ T cell proliferation: NS. IDO activity: NS | Scratch wound MSCs: NS | — | Altered gene expression |
| DM1 | Yaochite et al. (2016)52 | NS | A: NS | — | — | PBMC proliferation: NS | — | ||
| DM2 | Trinh et al. (2016)55 | NS | A, O: NS | NS | — | — | — | — | EGR-1 pathway upregulated |
| DM2 | Gu et al. (2012)56 | NS | — | NS | VEGF: decreased | — | HUVEC proliferation and tubule formation: NS | — | — |
| DM2 + CLI | Acosta et al. (2013)62 | — | — | — | — | — | — | — | Decreased fibrinolytic activity |
| DM2 | Krawiec et al. (2016)61 | — | — | NS | — | — | — | — | Increased thrombogenesis |
| DM2 | Barbagallo et al. (2016)59 | Not mentioned (cells bought from Lonza) | A: impaired | — | More IL-1b in undifferentiated DM2 cells. IL-6 and TNF-α higher in healthy cells | — | — | — | — |
| DM2, age | Krawiec et al. (2015)60 | — | SMC: impaired | — | — | — | Scratch wound SMCs: not compared, both induced closure. Elderly donors no DM2, less wound closure. Gender or BMI no influence | — | — |
| DM2 | Cramer et al. (2010)57 | — | A: higher, O, C: impaired | NS | — | — | — | β-galactosidase staining increased. Increased with higher glucose concentrations. More apoptosis; including more transcription of apoptotic pathway genes | Differentially expressed genes |
| DM2 + diabetic foot that required debridement/amputation | Koci et al. (2014)58 | Differences in CD105 expression between populations, both healthy and DM2 | A, C: similar, O: impaired | — | — | — | — | — | — |
| DM2 | Cheng et al. (2016)54 | NS | N: DM2 MSCs expressed more nestin and MAP2 than healthy cells | Proliferative activity impaired | — | — | — | — | — |
| Gestational DM2 | Kim et al. (2015)64 | NS | A, O: differentiation markers not induced | Impaired | — | — | — | Increased β-galactosidase staining, also increased in senescence associated genes | — |
| CKD | Reinders et al. (2013)65 | NS | A, O, C: NS | NS | MCP-1 and IL-6: NS | Autologous PBMC proliferation: NS. No differences. | — | — | Differentially expressed miRNAs, increase in oct-4 |
| CKD | Roemeling-van Rhijn et al. (2012)66 | NS | A, O: NS | NS, CFU similar | — | NS. Uremic circumstances: PBMCs did not proliferate in 10% dialysis serum but did proliferate in pre—emptive kidney transplant pt serum. Uremia/dialysis had no effect on MSC immunomodulation. (MLR activated PBMCs [irradiated allogeneic cells; PHA as control] → thymidine incorporation and anti-CD3–CD28 bead stimulation of PBMCs: also thymidine incorporation.) | - | 7AAD staining: NS | – |
| CKD | Yamanaka et al. (2014)67 | NS | A, O: NS | NS | — | — | — | β-galactosidase and β-heterochromatic foci: NS | Decreased PCAF expression and secretion |
| SSc | Hegner et al. (2016)74 | NS | A, O, C: NS | Impaired | — | — | Migration to TGF-β, MSCs: increased in SSc | — | Higher sm22a and calponin expression. Altered response to TGF-β and other cytokines |
| SSc | Cipriani et al. (2007)41 | — | A, O: NS endothelial cells less CXCR4 expression in VEGFR2 positive cells | NS, CFU NS | — | — | Chemoinvasion VEGF, SDF: impaired. Tubule formation MSCs: impaired | Telomerase activity: reduced | — |
| SSc | Guiducci et al. (2011)69 | NS | A, O: NS | NS, CFU: NS | SDF-1, VEGF: increased. TGF-β1: NS | — | Tubule formation MVECs: much better than healthy CM | — | Upregulation of TGF-β pathway in response to stimuli |
| SSc | Larghero et al. (2008)72 | NS | A, O: NS | CFU lower | — | PBMC proliferation + irradiated MSCs: anti-CD3 stimulated or MLR (normal MSCs). NS | — | — | – |
| SSc | Cipriani et al (2013)70 | NS | A, O, C: NS | Impaired | IL-6: increased. TGF-β: NS | PBMC proliferation: NS. Induction of Treg differentiation in CD4+ T + MSC coculture: better induction in both healthy and SSc T cells. Induced Tregs: less immunosuppressive but restored upon addition of both healthy and SSc MSCs | — | β-galactosidase staining: increased. p53: NS; p21: increased | — |
| SSc | Cipriani et al. (2013)71 | NS | NS | — | — | — | 3D tubule formation MSCs/MVECS: NS Migration to PDGF-BB: NS | — | Increased expression of contractile genes |
| SSc | Vanneaux et al. (2013)73 | NS | — | — | MMP2, 9; TGF-β1: NS | — | — | — | Increased expression of TGF-β signaling axis |
| SSc | Cipriani et al. (2014)101 | — | — | — | — | — | — | — | Decreased Cav-1 expression, which contributes to fibrosis |
Abbreviations: COPD, chronic obstructive pulmonary disease; NS, no significant difference; MSCs, mesenchymal stromal cells; TNF-α, tumor necrosis factor α. Disease column: CVD, cardiovascular disease; AID, autoimmune disease; CAD, coronary artery disease; HF, heart failure; CLI, critical limb ischemia; PAD peripheral artery disease; DM, diabetes mellitus; DMG, gestational diabetes mellitus; CKD, chronic kidney failure; SSc, systemic sclerosis. Differentiation column: A, adipogenic; O, osteogenic; C, chondrogenic; E, endothelial cell; P, pancreatic lineage; SMC, smooth muscle cell; CFU, colony forming units; PBMC, peripheral blood mononuclear cells; MLR, mixed lymphocyte reaction; CM, conditioned medium; FBS, fetal bovine serum; PL, platelet lysate.
Table 3.
In Vivo Assessment of Mesenchymal Stromal Cells (MSCs).
| CVD | First Author | Animal/Strain | Age (Wk) | Sex | Ncell | Nveh | Healthy Controls | Model | # Cells | Route | Timing | Follow-up | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAD + DM | Liu et al. (2013)38 | Rat—Sprague-Dawley | — | M | CAD + DM: 11; CAD: 9 | 14 | No | Suture ligation LAD | 2 × 106 | 1× subepicardial | Immediately | 4 wk | Both groups compared with control: LV function increased at 4 wk; apoptosis decreased—but DM group performed less |
| CAD | Behfar et al. (2010)42 | Mouse—nude | 8–12 | — | Naive: 10 treated: 14 | 10 | No | LAD suture ligation | 6 × 105 | 5× epicardial | 1-mo post-infarction | 20 mo | Exp 1 (no vehicle control, no n mentioned): 2/11 CAD MSCS improved ejection fraction. Exp 2: Cardiopoiesis group: Increased survival. Recovery of LV function. Less scar formation Native MSCs: better survival and LV function than control. |
| CAD | Grauss et al. (2007)41 | Mouse NOD/scid | 8–10 | M | 12 | 14–10 sham | No | LAD ligation | 2 × 105 | 5× injection in infarcted area | 15-min postinfarction | 15 d | LV better in MSC group. Blood vessel density increased in scar area and border zone. Less wall thinning |
| CLI | Gremmels et al. (2014)44 | Mouse—NMRI FoxN1 nu/nu | 8–10 | M | 60 (3/donor) | 21 | Yes | Femoral artery ligation | 1 × 105 | 5× im adductor muscle | 24-h post-ligation | 14 d | MSC-treated animals had increased blood flow recovery. NS Increased capillary density |
| CLI | Smadja et al. (2012)43 | Mouse nu/nu | 7 | M | 36 (18 control, 18 CLI) | 18 | Yes | Femoral artery ligation | 1 × 105 | iv in the eye | 5-h post-ligation | 14 d | Perfusion increased in MSC-treated animals. NS |
| DM1 | Yaochite et al. (2016)52 | Mouse—C57BL/6 | 10 | M | 18 (9 per group) | 6 | Yes | Streptozotocin ip for 5 d | 1 × 106 | 1× Intrasplenic | 20 d after last STZ dose | 30 d | Blood glucose levels, glucose tolerance, reduction in inflammation: NS (better than control group). No increase in Treg frequency, NS |
| DM2 | Trinh et al. (2016)55 | Mouse—C57BL/6 | 10 | F | DM2: 30, H: 30, DM2: mock 5, DM2 EGR-1 siRNA: 6 | 5 | Yes | Skin flap (3 × 2 cm) | 5 × 105 | 4× sc | Immediately | 7 d | Increased necrosis (digital pictures), less prominent neovascularization (histology). Knockdown of EGR1 rescued this |
| DM2 | Gu et al. (2012)56 | Mouse BALB/c Slc nu | 6 | — | DM2: 5, healthy: 15 | 15 | Yes | Skin flap (1.25 × 2.5 cm)—silicone sheet inserted to separate flap from bed. Sutured in place | 1 × 105 cells | 1× sc in the center of the flap | Immediately | 7 d | Surviving area measured using Visitrak, ratio of surviving area to total area: NS |
| DM2 | Krawiec et al. (2016)61 | Rat—Lewis | 8 | — | DM2: 7, healthy: 8, elderly: 7 | N/A, no sham | Yes | Aorta interposition (with MSC-seeded polyester urethane urea construct) | 3 × 106 | N/A | N/A | 8 wk | Patency: healthy: 100%, elderly: 71%, DM2: 28%. Patent grafts had neotissue and breakdown of the scaffold. Occluded grafts had a thrombus and no remodeling |
| CKD | Yamanaka et al. (2014)67 | Mouse—nude | 10 | — | 8 | Positive control: 8, negative control: 8 | Yes | Directed in vivo angiogenesis silicone cylinders with Trevigen’s basement membrane extract ± MSCs | 1 × 106 | sc | N/A | 9-d postimplantation | Decreased blood vessel growth in reactors (better than negative control) |
Note. Table only contains preclinical studies.
aNumber of patients is not mentioned in 2 studies; CAD, coronary artery disease; DM, diabetes mellitus; CLI, critical limb ischemia; CKD, chronic kidney disease. LAD, left anterior descending artery; im, intramuscularly; iv, intravenously; ip, intraperitoneally.
Effect of Disease on MSC Characteristics and Regenerative Potential
General CVD
Three studies examined MSCs procured from patients undergoing elective cardiovascular surgery; no control group was present. Since the findings are presented in light of a correlation with specific patient factors, and not as a difference between individuals with cardiac disease and healthy controls, we discuss these studies separately.
Mancini et al. determined CVD risk/atherosclerosis status based on the planned procedure; 41 patients who underwent coronary bypass grafting (CABG) were considered to have atherosclerosis, whereas the 9 patients scheduled for a valve replacement were “nonatherosclerotic.” Mancini et al. subsequently related CVD risk to the ability of AT-MSCs to suppress anti-CD3/CD28-bead activated CD4+ T cell proliferation. Diabetes mellitus type 2 (DM2) and atherosclerosis were independently associated with a reduction in the immune suppressive capacity of AT-MSCs. ISCT minimal criteria were also determined but not compared between groups33.
Neef et al. found in their study examining BM-MSCs derived from 51 patients that colony forming unit (CFU) numbers were increased in DM2, steroid treatment, chronic obstructive pulmonary disease (COPD), impaired renal function, high euroSCORE (a measure for comorbidity), impaired left ventricle function, and a high number of MNCs in the BM. However, in multivariate analysis, only a high MNC number in the BM and steroid treatment were predictive of a high CFU. There was no correlation between MNC numbers and age, myocardial infarction (MI) or cardiovascular risk status, so confounding by MNC number was not present. ISCT minimal criteria were not found to be altered34.
Brunt et al. performed gene expression analysis in BM-MSCS from 22 patients who underwent undefined cardiovascular surgery. The array contained 84 genes that are linked to the WNT/β-catenin signaling pathway that is involved in embryonic development and tissue repair. They found that 34 differentially expressed genes were associated with cardiovascular risk factors35.
Coronary artery disease
Coronary artery disease (CAD) MSCs did not differ from healthy MSCs with regard to ISCT minimal criteria. The majority of the studies did not include healthy controls (5/8).
Three studies showed a detrimental effect of CAD on proliferation; however, one study did not find any differences36–39.
Two studies did not find differences between diseased and healthy MSCs of BM or AT origin in a tubule forming assay36,39, whereas 2 others did find impairments. For instance, Efimenko et al. found impairment of tubulogenesis in AT-MSC from CAD patients, possibly due to decreased secretion of proangiogenic factors and increased production of antiangiogenic factors. Efimenko et al. also found a shorter telomere length and less telomerase activity in MSCs from CAD patients in comparison with healthy controls40.
Dzhoyashvili et al. also found impaired tubulogenesis in CAD AT-MSCs; both conditioned medium from MSCs derived from patients with CAD only or CAD + DM2 performed worse than healthy MSCs. There was no difference between CAD only and CAD + DM2 MSCs. Vascular endothelial growth factor (VEGF) expression secretion was increased in CAD and CAD + DM2 MSCs as compared with healthy MSCs37. Dzhoyashvili et al. then show that impairment of tubulogenesis is caused by an increase in anti-angiogenic factors rather than a decrease in pro-angiogenic factors—expression of the anti-angiogenic cytokine thrombospondin-1 was higher in CAD patients as well. Although they were not able to confirm higher thrombospondin-1 secretion in the culture medium, thrombospondin-1 mRNA levels negatively correlated with the results of the tubulogenesis assay. Both expression and secretion of PAI-1 were elevated in CAD and CAD + DM2 MSCs. Inhibition of PAI-1 partially restored angiogenesis37.
Three studies evaluated the efficacy of MSC administration in an animal model of myocardial ischemia, but these did not include healthy controls. The studies demonstrate that CAD MSCs do display regenerative features in vivo; no conclusions regarding dysfunction can be drawn41,42. Liu et al. found that DM2 in addition to CAD confers less increase in ejection fraction, myocardial contractile ability, and more myocardial apoptosis than CAD only or vehicle control. The authors speculate that this is due to a decrease in VEGF and B-cell lymphoma 2 (Bcl-2) secretion, present in only the CAD and DM2 group, as determined by significantly lower Bcl-2 and VEGF levels in the myocardium posttransplantation38.
Critical limb ischemia
CLI does not appear to affect the angiogenic potential of MSCs, not even MSCs harvested from the BM of an amputated leg.
Smadja et al. first examined the angiogenic effect of CLI BM-MSCs versus control MSCs in a hind limb ischemia model. There was no difference between CLI and control with regard to limb perfusion43.
Gremmels et al. extensively examined MSCs derived from iliac crest BM. They report no differences between CLI MSCs and healthy MSCs with regard to gene expression, in vitro tubulogenesis, scratch wound closure, and migration toward platelet-derived growth factor (PDGF)-BB. ISCT minimal criteria were mostly similar, though chondrogenic differentiation was impaired in CLI donors. Senescence, measured as β-galactosidase, was increased in CLI patients. However, these differences were age dependent; after correction for age, no significant difference was found between healthy controls and CLI patients. Senescence was not correlated with angiogenic potential. Most notably, the in vivo angiogenic potential of MSCs-CLI MSCs was similar to healthy MSCs44. Brewster et al. examined MSCs derived from 8 amputated legs versus iliac crest BM from healthy volunteers. Interestingly, even though the legs had not been perfused for some time, the resulting MSCs functioned just as well as healthy MSCs with regard to proliferation, cytokine production and endothelial cell proliferation, migration, and invasion. The only parameter that differed was MSC invasion, which was lower in the CLI group that had been cultured in FBS—there were no differences between MSCs cultured in platelet lysate. There were no differences between MSCs from CLI patients with DM2 and MSCs from CLI patients without DM245.
Altaner et al. assayed MSCs acquired from patients who participated in a clinical trial that examined the effect of autologous BM-MNC administration in CLI. While no controls were included, Altaner et al. created groups based on clinical response to BM-MNC administration, enabling correlations between the clinical efficacy of BM-MNCs and the in vitro assays of BM-MSCs. Various in vitro differences were identified; responders had increased expression of the cell surface markers CD44 and CD90. Responders also secreted more interleukin (IL)-4, IL-6, and macrophage inflammatory protein-1b (CCL4). Protein profiling of MSC lysates showed that responders expressed more Zinc finger protein (SNAI1), and nonresponders expressed more E-cadherin and pancreatic and duodenal homeobox-1 (PDX-1)46. The influence of MSC frequency in the administered BM-MNC product on therapeutic efficacy was not assessed. Given the low frequency of MSCs in unfractionated BM47, the consequences of these findings for MSC-based therapy are uncertain.
Heart failure
Two studies examined BM-MSC function in patients with ischemic heart failure as compared with healthy controls. In both studies, gene expression patterns, assayed with real-time polymerase chain reaction arrays containing either 85 or 84 prespecified transcripts, were found to reflect adaptation of cardiac tissue to ischemia. Minullina et al. used the heart failure samples to assess the relation of differentially expressed genes and patient-specific factors due to significant differences between the healthy and disease group (gender balance, age). Body mass index was the only factor that could be linked to gene expression; nonobese individuals had an increase in gene expression associated with extracellular matrix (ECM) homeostasis such as Collagen Type I Alpha 2 Chain (COL1A2) and connective tissue growth factor (CTGF) and several matrix metalloproteinases (MMPs) in nonobese individuals. Transcript levels in obese individuals were similar to healthy controls. Interestingly, β-galactosidase activity, a measure for cellular senescence, did not correlate with differential gene expression in heart failure, although the authors do not report whether healthy controls were also examined48.
In addition to gene expression analyses, Dmitreva and colleagues conducted functional assays. Differentiation was unaffected. They found a lower population doubling time at p3 in the HF group, but no differences in CFU. This difference might be caused by a higher number of a-SMA+ CD105+ cells in the heart failure samples—possibly reflecting fibroblast contamination49.
Two clinical trials comparing autologous versus allogeneic MSCs as treatment in heart failure have been conducted. While these were not geared toward assessing regenerative potential, some conclusions can be drawn. The POSEIDON study does not clearly show benefits of one over the other; both groups display favorable structural cardiac changes. However, left ventricular function was only significantly improved in the allogeneic group, whereas functional outcome measures only significantly improved in the autologous group as compared with the allogeneic group28.
The POSEIDON-DCM study in nonischemic dilated cardiomyopathy demonstrated that patients treated with allogeneic MSCs had a greater improvement in clinical parameters. Differences in circulating immune cells were also found; in the allogeneic MSC group, patients had less terminally differentiated effector memory T cells and more memory B cells with a suppressed phenotype. T cell activation (measured as CD3+ CD25+ T cells) did not differ. Importantly, patients who had received allogeneic MSCs had an increase in EPC colony forming units, whereas patients who had received autologous MSCs had not50.
Diabetes mellitus type 1
Three studies evaluated diabetes mellitus type 1 (DM1) BM-MSCs versus healthy controls. No difference between DM1 cells and healthy controls was found with regard to differentiation and proliferation51–53. Immunomodulation as measured with peripheral MNC or selected T cell proliferation was similar in 2 studies52,53. In a mouse model for diabetes, Yaochite et al. found that intrasplenically injected DM1 MSCs resulted in similar glucose control as treatment with healthy MSCs, showing that DM1 MSCs and healthy MSCs have similar therapeutic efficacy in DM152.
Gene expression profiling was conducted in 2 studies (microarrays), and differentially expressed genes were found in DM1 MSCs51,53. De Lima et al. most notably found differences in pathways involved in migration, so migratory capacity was assessed—DM1 MSCs performed better than healthy MSCs. Functional pathway analysis revealed upregulation of the sympathetic nervous system. Davies et al. found increased transcription of pathways involving growth, development and response to stress and wounding in late DM1. There were no differences in the scratch wound assay (MSCs only)51. Davies et al. also found that baseline secretion of cytokines (IL-6, CXCL1, CXCL6, and PGE2) was similar to healthy control levels. Hemocompatibility was also similar, even though late DM1 cells express more CD55 (complement receptor) on the surface of the cells. Late DM1 even had less clot formation, possibly correlating with the lower expression of CD59 as compared with healthy cells53.
Diabetes mellitus type 2
Nine studies examined the (dys)function of AT-derived MSCs (AT-MSCs) in patients with diabetes mellitus type 2 compared with nondiabetic controls. Results are mixed—some studies point toward disease-related dysfunction, whereas others find no differences at all. Proliferation of AT-MSCs was reported in several studies to be similar or even better than healthy controls, but another study found slower proliferation54–57. Similar mixed results were found in differentiation assays: osteogenic and chondrogenic differentiation was unaffected in one study55 but impaired in 2 other studies57,58, and adipogenic differentiation was reported to be increased in one study58 but completely absent in another59. The 2 studies that looked into gene expression found that DM2 AT-MSCs express genes implicated in the pathogenesis of DM2, demonstrating that AT-MSCs may be affected55,57. Tubulogenesis was unaffected56. Scratch wound healing (smooth muscle cells) was more efficient with DM2 CM60.
In vivo angiogenesis was evaluated by 2 studies, using the murine ischemic skin flap model. Trinh et al. observed a bigger necrotic surface area, less neoangiogenesis, and more CD45+ cell infiltrate in animals treated with DM2 AT-MSCs, but DM2 cells still performed better than vehicle control. Trinh et al. found EGF-1 to be involved in DM2-mediated MSC dysfunction, a pathway also affected in the DM2 disease phenotype. Inhibiting EGR-1 expression increased skin survival in the ischemic skin flap model55. Gu et al., however, did not see differences in the ischemic skin flap model with regard to necrosis56. This could be due to the heterogeneity of the protocols—different mouse species were used as well as different cell doses and injection protocols. Expression of cytokine mRNAs and secretion of cytokine proteins were evaluated in 4 studies. VEGF mRNA expression was higher in DM2 AT-MSCs in one study but lower in another study58. Gu et al. who determined elevated VEGF mRNA in DM2 AT-MSC subsequently showed that VEGF protein levels were lower in CM, but still performance in the ischemic skin flap model was similar56. Reports are similarly mixed about IL-6; some studies find higher baseline IL-6 levels (both protein and mRNA), others find lower IL-6 levels as compared with controls. It is also unclear whether transforming growth factor beta-β and bFGF secretion are affected.
Two studies point toward a possible thrombogenic effect of diabetic AT-MSCs. Krawiec et al. evaluated healthy, DM2 and elderly AT-MSCs in a murine aorta interposition model. Seeding of the constructs was successful and no differences were seen between groups. However, DM2-MSC-seeded grafts were thrombogenic—almost all grafts had to be explanted within 1-wk postimplantation—28% patency at 8 wk. Subsequent assays showed that DM2 AT-MSCs produced less fibrinolytic factors as compared with healthy MSCs61. Acosta et al. studied the secretion of fibrinolytic mediators by MSCs from DM2 patients after observing 2 instances of distal microvascular thrombosis in a clinical trial that examined intra-arterial administration of AT-MSCs for CLI. DM2 MSCs indeed displayed impaired fibrinolytic properties and secreted more plasminogen activator inhibitor type 1 and less tissue plasminogen activator than healthy MSCs, regardless of type blood serum62.
In DM2, one study reported higher senescence in DM2 AT-MSCs as compared with healthy controls. Senescence even increased with higher glucose content of the medium and could be rescued with insulin supplementation to the culture medium57.
The above studies in DM2 all examined AT-MSCs. In a review of 14 clinical studies in which autologous BM-MSCs were administered to 132 DM2 patients, van de Vyver describes that the results of MSC therapy are variable and that some patients do not respond at all63. This underscores the need for comparative studies in DM2 and healthy BM-MSCs.
Gestational diabetes mellitus
Kim et al. found that UCB-MSCs from mothers with diabetes mellitus gravidarum (DMG) proliferate slower than UCB-MSCs derived from healthy mothers. Osteogenic and adipose differentiation were negatively affected. Kim et al. also observed signs of mitochondrial dysfunction at both the gene expression and protein level in DMG UCB-MSCs. Increased β-galactosidase activity was seen in DMG MSCs64.
Chronic kidney disease
BM-MSCs from chronic kidney failure (CKD) patients were assayed extensively by Reinders et al. from FACS markers and differentiation to miRNA profiles and PBMC proliferation, but no differences were found between healthy MSCs and CKD MSCs65. Similarly, for AT-MSC, no differences in these parameters found between healthy controls and CKD MSCs. Roemeling-van Rhijn et al. assayed healthy and CKD AT-MSCs under both normal and uremic conditions with regard to suitability for cell therapy: aside from general MSC parameters, long-term genetic stability and apoptosis were taken into account. They also determined MSC-mediated suppression of PBMC proliferation. No differences between healthy and CKD MSCs were found66. Similarly, Yamanaka et al. did not find any differences between CKD and controls in ISCT minimal criteria, proliferation, and senescence. However, they do report a defect in the in vivo angiogenic potential of AT-MSCs. They also found a reduced gene and protein expression of PCAF, a regulator of HIF-α, which might underlie this angiogenesis defect67.
Systemic sclerosis
SSc is not traditionally considered a CVD, however, given the disease’s profound vascular manifestations as well as the presence of fibrosis, which is ubiquitous in CVD, research conducted in SSc MSCs might provide valuable insights—SSc can be considered a model disease in this sense68. Even though in SSc loss of angiogenic potential is a key feature, SSc MSCs retain their angiogenic and immunomodulatory properties, as shown in 2 studies, though 1 study reports impaired tubulogenesis69,70. In a coculture assay, Cipriani et al. furthermore showed that healthy endothelium downregulates SSc MSC transcription of contractile genes that are associated with a profibrotic phenotype71. Some studies report disease-specific profibrotic features in SSc MSCs, for example, higher expression of contractile genes upon TGF-β signaling, and general increased response to TGF-β in terms of receptor upregulation and increased downstream signaling72–74. Whether a priori TGF-β receptor upregulation is present is contested by contrasting findings. Interestingly, even healthy MSCs display some response to profibrotic stimuli, though they also downregulate their TGF receptors, whereas SSc MSCs upregulate these. SSc MSCs generally display a more senescent phenotype than healthy controls, as demonstrated by 2 studies with age-matched controls70,75.
Aging and MSC Dysfunction
Aging in MSC dysfunction can be considered as an additive effect “on top of” potential CVD-mediated dysfunction or as an independent factor influencing regenerative capacity. The latter can only be detected in studies that included healthy MSCs.
In studies lacking healthy controls, generally a degree of age-dependent dysfunction was observed in post hoc analyses. Proliferation was impaired in aged patients in 2 studies, but one study found no such impairment34,35,76. Extracellular markers were not affected. With regard to regenerative capacity, Mancini et al. found that age impaired immunomodulation in AT-MSCs from CVD patients33.
Brunt et al. furthermore reported impairment of myogenic differentiation in older donors. Increasing age was also associated with less activity of the WNT/β-catenin signaling pathway in MSCs. However, though the authors assert that the WNT/β-catenin alterations are mainly age dependent, the differentially expressed genes in aged patients are all but one similar to differentially expressed genes in individuals with high cardiovascular risk score as compared with people with low cardiovascular risk score35.
In studies with controls, conflicting results were seen in the small number of studies that separately analyzed the contribution of biological or cellular aging to possible dysfunction. MSCs derived from elderly CAD patients had less angiogenic potential than MSCs derived from elderly healthy patients. This was shown to be due to an age-dependent decrease in VEGF production in combination with a CAD-mediated increase in anti-angiogenic factors40. On the other hand, in a study in CLI, all differences between healthy and diseased individuals were found to be due to advanced age in the CLI group44. Additionally, aged MCs from DM2 patients had a strong prothrombogenic effect whereas age-matched healthy MSCs did not61.
The relationship between MSC donor age and treatment efficacy in heart failure was assessed by Golpanian et al. in a retrospective analysis of pooled data from 2 clinical trials. Donor age did not affect the outcomes in MSC recipients. However, in pooling, the data autologous/allogeneic recipients were mixed, and no separate analysis comparing donor age and allogeneic/autologous cells was conducted77.
Not all studies were fully age-matched, possibly introducing confounders. Therefore, we collated all the study results and tested for an association between age-mismatched groups (age matched: average ages within 10 yr) and MSC dysfunction using the χ2 test. We found that there was no association between age matching and proliferation, cytokine secretion, FACS marker expression, immunomodulation, altered differentiation, angiogenesis, senescence, or any in vitro dysfunction in general.
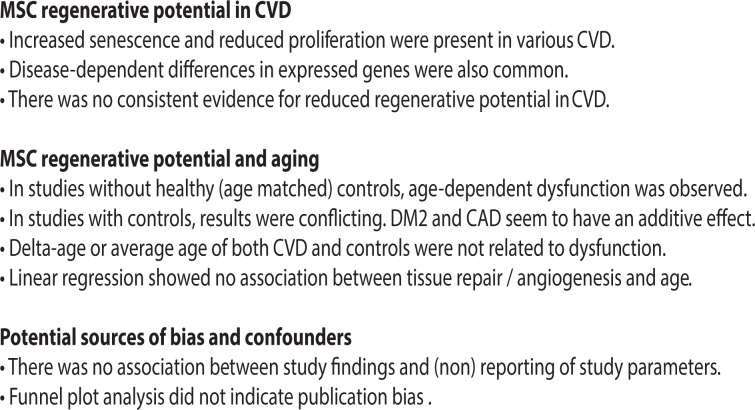
Linear regression analysis did not reveal a significant association between delta age and tissue repair/angiogenesis ratio of means (Fig. 1C). Logistic regression did not reveal significant associations between MSC dysfunction and the difference in average ages (delta age; CVD control) between groups (P = 0.583). There was also no association between MSC dysfunction and average age of CVD patients (P = 0.232), nor age of healthy controls (P = 0.597).
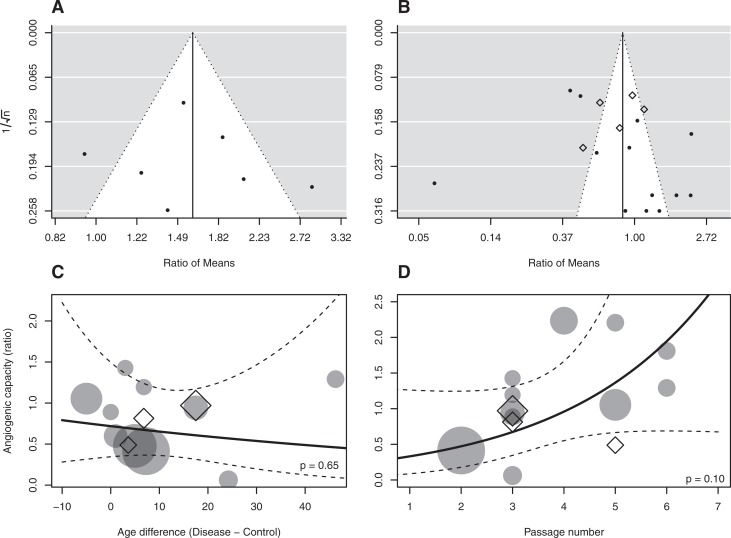
Fig. 1.
Risk of bias analyses. (A) Funnel plot depicting the distribution of senescence ratio of means. (B) Funnel plot depicting the distribution of angiogenesis ratio of means. (C) Association of delta age versus angiogenesis ratio of means (circle: in vitro; triangle: in vivo) P = 0.65. (D) Analysis of passage number versus angiogenesis ratio of means (circle: in vitro; triangle: in vivo) P = 0.10.
Risk of Bias
As no standardized criteria exist for assessing risk of bias in in vitro studies, we assessed whether the reporting or lack thereof of certain parameters is associated with positive results. These parameters encompass reporting of the study population—such as gender, age, and reporting of culture and experimental procedures such as the media used, seeding density, and the passage number used.
Various types of controls were utilized (see Table 1), from screened organ or BM transplant donors to individuals undergoing surgery not related to the CVD in question. The N of each control type was too low to allow for direct correlation with study outcomes; however, the use of screened transplant donors trended toward an association with fewer findings of dysfunction (P = 0.096).
There was no clear association between reporting of study characteristics and in vitro findings, mainly because most features were generally well reported (Table 4). Nonreporting of the seeding density was significantly associated with differentiation alterations (P = 0.018). With regard to senescence, nonreporting of the seeding density trended toward a significant association (P = 0.061), but the total N of studies that examined senescence was low.
Table 4.
Reporting of Study Characteristics.
| Parameters | # Reported | # Not Reported | Association with Any In Vitro Dysfunction (P. Fisher’s Exact Test) |
|---|---|---|---|
| N per experiment | 18 | 21 | 0.163 |
| Passage | 30 | 9 | 1 |
| Medium | 37 | 2 | 0.526 |
| Gender | 30 | 9 | 1 |
| Age | 31 | 8 | 0.682 |
| Starting seeding density | 22 | 17 | 0.494 |
| All parameters reported | 5 | 34 | 0.159 |
Analysis of a compound measure for reporting (“all parameters reported”) showed that findings of dysfunction in proliferation and angiogenesis are possibly correlated with not reporting at least one of the parameters. However, only 5 studies reported all parameters. Similarly, not all studies reported on all MSC functional characteristics. The passage number did not affect ratio of means with regard to tissue repair/angiogenesis (Fig. 1D).
The Hooijmans tool was used to assess risk of bias in animal studies. Some of the studies included blinded outcome assessment, but many of the details specified in the Hooijmans tool were lacking (data not shown). Four of the 6 studies that included healthy controls found no difference between healthy and CVD MSCs.
To assess publication bias, we created funnel plots using ratio of means data from studies assessing angiogenesis and senescence (Fig. 1A and B). No clear trend was observed that indicates publication bias with regard to either favorable or unfavorable effects of CVD on MSC function. It must be noted however that there was high heterogeneity in study results and trend toward overrepresentation of extreme findings in either direction.
Discussion
Assessment of MSC (Dys)function
We provide the first comprehensive literature review on MSC dysfunction in CVD (See figure 2 for a summary of our findings). Reliable assessment of disease-related (dys)function is essential to gain more insight into the potential clinical efficacy of MSCs in the various CVDs.
Fig. 2.
Summary of findings.
Our data show that in most studies, MSCs from CVD patients perform less well than healthy control MSCs in in vitro assays, but there were also studies in which multiple tests were used and no differences were found between healthy and CVD MSCs. In vitro assays may not fully capture the complex interactions between host and donor cells. The emphasis on the ISCT minimal criteria in many of the studies thus far is remarkable, as the ISCT minimal criteria do not bear any relationship with clinical efficacy. After all, the primary modalities of action of MSCs are through influencing the local environment by secretion of paracrine factors, and direct cell-to-cell contact, not through differentiation. No differences between diseased MSCs and control MSCs were found with regard to the ISCT extracellular marker set. In CLI, DM2 and DMG, there are indications that trilineage differentiation abnormalities are present.
Assays that do capture important aspects of MSC regenerative properties, such as immunomodulation, wound repair, and angiogenesis assays, were not often performed, and which aspect was examined varied widely. In some disease states such as DM1, MSCs were minimally affected, whereas in others profound MSC dysfunction was present in multiple aspects of regeneration, for example, in DM2 and SSc. Specific studies into disease-related dysfunction less focused on regenerative potential were also conducted, such as identification of disease-related alteration of cellular pathways or differentiation into cell types implicated in disease. For instance, in SSc and CVD, an impairment in differentiation to, respectively, endothelial and myogenic differentiation was found. As these lineages were not consistently assessed across all conditions, it is unknown whether this is unique for these disease entities. Given the profound role of immunomodulation in MSC-mediated tissue repair, it is surprising that such a small number of studies (7/41) conducted assays to assess this aspect of MSC function. Moreover, the assays used do not resemble in vivo immune function, nor do they recapitulate MSC-mediated immunomodulation, so the question remains whether the absence of dysfunction in these assays represents in vivo immunomodulatory capacity78.
Only few of the studies performed included in vivo testing of MSCs. Additionally, these studies often showed conflicting results, for instance, in DM2, where one study found DM2-associated impairment of angiogenic potential in a skin flap model and another found no impairment. Interestingly, some studies that found in vitro alterations, including reduced cytokine secretion or increased senescence in diseased MSCs, did not find a difference in in vivo efficacy44,56. However, generally, in vitro and in vivo assays were rarely combined. In vivo testing would be most useful to assess disease-related MSC (dys)function, as this allows simultaneous testing of multiple aspects of MSC function, such as tissue repair, angiogenesis, and immunomodulation. While, indeed, in vitro findings do not necessarily predict in vivo efficacy, cross-referencing may shed light on the relevance of detected in vitro abnormalities. Gremmels et al. conducted a thorough analysis of the predictive value of in vitro assays in predicting in vivo angiogenesis. The extent in which MSC CM stimulated endothelial proliferation was most predictive for in vivo results44. Such analyses are essential to gain more insight into how to gauge MSC potency. This does not only guide the decision to use autologous or allogeneic cells but can also aid in individual donor selection.
The discrepancy between in vitro and in vivo assessments of MSC function may also reflect a difference between animal models and the human body—for example, in a murine study, inappropriate differentiation of cells has been shown in cardiac tissue79, but, to date, this has not been observed in humans yet28,80. The diseased environment may also drive MSCs toward a regenerative phenotype, as has been shown previously81. On the other hand, it has also been shown that implantation in a diseased tissue can derail MSCs82. Additional indications for a negative influence of disturbed homeostasis in CVD were found in studies, in which diseased conditions such as hyperglycemia and uremia were modeled in vitro or in animal models—progenitor cell dysfunction was found83–85. Adding patient-derived serum to healthy control MSCs also induces dysfunction in several diseases86,87. In line with these findings, Cramer et al. report glucose dose-dependent dysfunction in both healthy and diseased MSCs, but interestingly Roemeling-van Rhijn et al. did not find a deleterious effect of uremia in AT-MSCs57,66.
Aging-related dysfunction in regenerative potential, such as impaired proliferation, differentiation, immunomodulation, and angiogenesis, was reported in CVD MSCs in a small number of studies, corresponding with findings in healthy, aged MSCs33,35,40,61.
Features of premature cellular aging and increased senescence were assessed in some, but not all, CVD. Cellular aging was reported in CVD MSCs in several conditions, which could reflect a true premature aging phenotype in the progenitor cell compartment. For instance, individuals with CVD have shorter telomeres than their matched controls, and telomere length has been shown to inversely correlate with cardiovascular events88. Features of increased cellular senescence, another consequence of aging, can also be detected in CVD patients89. Unfortunately, few studies assessed cellular aging parameters, and of these, even fewer correlated the results with patient age, precluding firm conclusions regarding the relationship between premature aging, patient age, and MSC dysfunction. In our analysis of ratio of means, we did not find an association between delta age and senescent phenotype, neither was delta age associated with tissue repair/angiogenesis and immunomodulation ratio of means.
We assessed correlations between CVD patient age, healthy control age, and the age difference between patient and controls and study results. None of these parameters were associated with reported MSC dysfunction. Therefore, there is no evidence that disease-related dysfunction in CVD is due to biological age rather than the disease itself.
Limitations
Our review evaluated cultured MSCs—bearing relevance for possible clinical applications—and does not allow conclusions on the native MSC population—if such a circumscript population exists at all. Since it has been established that cells can lose distinctive phenotypes while in culture90, possible differences might have been masked by the prolonged culturing required to generate a sufficient number of MSCs. However, in most studies reviewed here, cells are not cultured much beyond passages used for therapeutic applications, enabling conclusions about the final cellular therapy product.
Not all MSC harvesting locations were equally explored in each CVD; thus, it is not always clear whether reported MSC dysfunction reflects local dysfunction or a global disease phenomenon. It is not known to what extent harvesting location influences cellular characteristics, some studies found differences whereas others, mostly in oncologic populations, found no differences91–93. It has been shown that not all HSC compartments are equally perfused and that MSCs are predominantly arranged around blood vessels94,95. Differences in perfusion might thus account for differences in exposure to noxious stimuli and thereby influence cell characteristics in varying degrees. In CKD, no gene expression differences were found in BM-MSCs, but distinct alterations were present in AT-MSCs65,67.
It remains unknown why many conflicting findings have been reported. Reporting of study population characteristics is essential to identify possible confounders. For instance, healthy controls are not always completely healthy individuals but are sometimes recruited from patients who undergo orthopedic surgery. The target population for coronary bypass grafts and joint replacement overlaps, possibly creating a confounding effect. Furthermore, donors of control samples are not always assessed medically, and especially in the case of highly prevalent conditions such as the metabolic syndrome diabetes mellitus 2 or other insidious disease, this could also create confounding in otherwise matched samples. While it was not possible to analyze the relationship between each type of donor and study findings, there were no differences between studies that included screened donors versus studies that did not include screened donors.
General cell culture parameters have been shown in other studies to influence cell characteristics—for instance, culturing conditions influence paracrine secretion and gene expression in MSCs96,97. MSC passage number is inversely correlated with differentiation protocols98, and the initial seeding density influences proliferation99. Furthermore, immunomodulatory capacity has even been shown to be affected by confluence rather than initially seeded cell density100, though analysis of this variable was not possible as “confluence at the start of assays” is not a regularly reported parameter. However, reporting of specific study characteristics was not associated with MSC dysfunction. Bias may also have been introduced through mismatched control groups, as there were a few instances in which healthy gender- and age-mismatched controls were used in comparison with diseased, aged individuals; and in general, individuals in the healthy control populations were younger. However, we did not find a correlation between mismatching and findings of dysfunction, nor was there a correlation between study participant delta age and effect size or dysfunction.
Implications for Cellular Therapy
Disease-related MSC dysfunction in CVD may have important consequences for cellular therapy. One solution could be to use allogeneic donors; another solution could be to pretreat diseased cells to abrogate disease-specific dysfunction.
When opting for allogeneic donors, selection of these donors for age and comorbidity is essential. A general health screening, similar to the screening that BM transplant donors receive, should be implemented and has already been implemented in many centers. However, it should also be kept in mind that, as discussed above, diseased MSCs may function better in vivo than was expected based on in vitro findings.
The effect of preconditioning, or culturing under specific circumstances to achieve a certain effect in the cell or its efficacy, was explored in several studies, and profound effects were found. For instance, proliferation could be enhanced by using different concentrations of serum or even alternatives to “serum” (e.g., umbilical cord blood–derived serum or platelet lysate)34,45. Platelet lysate did not affect angiogenic potential of MSCs45. Culturing of CLI BM-MSCs under hypoxic circumstances stimulates cells to proliferate and secrete beneficial paracrine factors, also leading to better wound repair as assessed with a scratch wound healing assay44. Administration of insulin to DM2 AT-MSCs could rescue many disease features57. In contrast, hypoxic culturing of AT-MSCs derived from CKD patients did not result in abrogation of the disease phenotype, suggesting that depending on the specific CVD, different strategies should be applied67. Other approaches focused on repairing disease-induced dysfunction of cellular pathways. Brunt et al. were able to increase nuclear translocation of β-catenin in aged MSCs by treating them with lithium. This also enhanced myogenic differentiation, showing that influencing the WNT/β-catenin pathway can rescue disease phenotypes in CAD, such as the approach of Trinh et al. to downregulate the EGR-1 pathway in DM2 AT-MSCs and the approach of Behfar et al. to use a cocktail of small molecules that enhance “cardiopoiesis.” Both approaches improved in vivo efficacy of MSCs in an animal disease model42,55. Administration of specific growth factors may also be an option—in SSc, Cipriani et al. showed that administration of VEGF to SSc BM-MSCs enhances tubule formation (but still does not reach normal levels) and administration of PDGF-BB decreases the expression of contractile proteins70,75. Given the variable results of precondition strategies across the spectrum of CVD, any such measure taken should be carefully evaluated.
Conclusion
MSC characteristics are affected by CVD in various degrees, but studies are conflicting as to in what extent this affects regenerative potential, if at all. Linear regression analysis of pooled data did not show an association between age and in vitro dysfunction, suggesting that age is not a confounder of importance in these studies. Future studies should focus on identifying which in vitro assay best predicts in vivo or preferably clinical efficacy, and these assays should become a vital part of any study aiming to assess (dys)function of MSCs.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: Support by the ZonMw Translational Adult Stem Cell Research Programme (Grants Nos. 116003005 and 116001026) is gratefully acknowledged.
References
- 1. GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harman D. The aging process: major risk factor for disease and death. Proc Natl Acad Sci U S A. 1991;88(12):5360–5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107(1):139–146. [DOI] [PubMed] [Google Scholar]
- 4. Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012;22(17):R741–R752. [DOI] [PubMed] [Google Scholar]
- 5. Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108(4):457–463. [DOI] [PubMed] [Google Scholar]
- 6. Jie KE, Goossens MH, van Oostrom O, Lilien MR, Verhaar MC. Circulating endothelial progenitor cell levels are higher during childhood than in adult life. Atherosclerosis. 2009;202(2):345–347. [DOI] [PubMed] [Google Scholar]
- 7. Williamson KA, Hamilton A, Reynolds JA, Sipos P, Crocker I, Stringer SE, Alexander YM. Age-related impairment of endothelial progenitor cell migration correlates with structural alterations of heparan sulfate proteoglycans. Aging Cell. 2013;12(1):139–147. [DOI] [PubMed] [Google Scholar]
- 8. Hoffmann J, Haendeler J, Aicher A, Rossig L, Vasa M, Zeiher AM, Dimmeler S. Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: important role of nitric oxide. Circ Res. 2001;89(8):709–715. [DOI] [PubMed] [Google Scholar]
- 9. Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66(2):286–294. [DOI] [PubMed] [Google Scholar]
- 10. Jie KE, Zaikova MA, Bergevoet MW, Westerweel PE, Rastmanesh M, Blankestijn PJ, Boer WH, Braam B, Verhaar MC. Progenitor cells and vascular function are impaired in patients with chronic kidney disease. Nephrol Dial Transplant. 2010;25(6):1875–1882. [DOI] [PubMed] [Google Scholar]
- 11. Lau KK, Chan YH, Yiu KH, Li SW, Tam S, Lau CP, Kwong YL, Tse HF. Burden of carotid atherosclerosis in patients with stroke: relationships with circulating endothelial progenitor cells and hypertension. J Hum Hypertens. 2007;21(6):445–451. [DOI] [PubMed] [Google Scholar]
- 12. Teraa M, Sprengers RW, Westerweel PE, Gremmels H, Goumans MJ, Teerlink T, Moll FL, Verhaar MC, Group JS. Bone marrow alterations and lower endothelial progenitor cell numbers in critical limb ischemia patients. PLoS One. 2013;8(1):e55592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Teraa M, Sprengers RW, Schutgens RE, Slaper-Cortenbach IC, van der Graaf Y, Algra A, van der Tweel I, Doevendans PA, Mali WP, Moll FL, et al. Effect of repetitive intra-arterial infusion of bone marrow mononuclear cells in patients with no-option limb ischemia: the randomized, double-blind, placebo-controlled Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra-arterial Supplementation (JUVENTAS) trial. Circulation. 2015;131(10):851–860. [DOI] [PubMed] [Google Scholar]
- 14. Young PP, Schafer R. Cell-based therapies for cardiac disease: a cellular therapist’s perspective. Transfusion. 2015;55(2):441–451; quiz 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zwetsloot PP, Gremmels H, Assmus B, Koudstaal S, Sluijter JP, Zeiher AM, Chamuleau SA. Responder definition in clinical stem cell trials in cardiology: will the real responder please stand up? Circ Res. 2016;119(4):514–518. [DOI] [PubMed] [Google Scholar]
- 16. Chong MS, Ng WK, Chan JK. Concise review: endothelial progenitor cells in regenerative medicine: applications and challenges. Stem Cells Transl Med. 2016;5(4):530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bronckaers A, Hilkens P, Martens W, Gervois P, Ratajczak J, Struys T, Lambrichts I. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther. 2014;143(2):181–196. [DOI] [PubMed] [Google Scholar]
- 18. Prockop DJ, Kota DJ, Bazhanov N, Reger RL. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs). J Cell Mol Med. 2010;14(9):2190–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res. 2015;116(8):1413–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liew A, O’Brien T. Therapeutic potential for mesenchymal stem cell transplantation in critical limb ischemia. Stem Cell Res Ther. 2012;3(4):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gremmels H, Fledderus JO, Teraa M, Verhaar MC. Mesenchymal stromal cells for the treatment of critical limb ischemia: context and perspective. Stem Cell Res Ther. 2013;4(6):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luger D, Lipinski MJ, Westman PC, Glover DK, Dimastromatteo J, Frias JC, Albelda MT, Sikora S, Kharazi A, Vertelov G, et al. Intravenously delivered mesenchymal stem cells: systemic anti-inflammatory effects improve left ventricular dysfunction in acute myocardial infarction and ischemic cardiomyopathy. Circ Res. 2017;120(10):1598–1613. [DOI] [PubMed] [Google Scholar]
- 23. van Rhijn-Brouwer FC, Gremmels H, Fledderus JO, Radstake TR, Verhaar MC, van Laar JM. Cellular therapies in systemic sclerosis: recent progress. Curr Rheumatol Rep. 2016;18(2):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A, International Society for Cellular Therapy Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005;7(5):393–395. [DOI] [PubMed] [Google Scholar]
- 25. Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5(1):91–116. [DOI] [PubMed] [Google Scholar]
- 26. Choudhery MS, Badowski M, Muise A, Pierce J, Harris DT. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med. 2014;12(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, Martin H, Zeiher AM, Dimmeler S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109(13):1615–1622. [DOI] [PubMed] [Google Scholar]
- 28. Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, et al. Comparison of allogeneic vs. autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308(22):2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang XP, Sun Z, Miyagi Y, McDonald Kinkaid H, Zhang L, Weisel RD, Li RK. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation. 2010;122(23):2419–2429. [DOI] [PubMed] [Google Scholar]
- 30. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. [DOI] [PubMed] [Google Scholar]
- 31. Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cochrane Handbook for Systematic Reviews of Interventions. In: Higgins JPT, Green S, editors. The Cochrane Collaboration; 2011.
- 33. Kizilay Mancini O, Shum-Tim D, Stochaj U, Correa JA, Colmegna I. Age, atherosclerosis and type 2 diabetes reduce human mesenchymal stromal cell-mediated T-cell suppression. Stem Cell Res Ther. 2015;6(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Neef K, Choi YH, Weichel A, Rahmanian PB, Liakopoulos OJ, Stamm C, Choi CY, Jacobshagen C, Wittwer T, Wahlers T. The influence of cardiovascular risk factors on bone marrow mesenchymal stromal cell fitness. Cytotherapy. 2012;14(6):670–678. [DOI] [PubMed] [Google Scholar]
- 35. Brunt KR, Zhang Y, Mihic A, Li M, Li SH, Xue P, Zhang W, Basmaji S, Tsang K, Weisel RD. and others Role of WNT/beta-catenin signaling in rejuvenating myogenic differentiation of aged mesenchymal stem cells from cardiac patients. Am J Pathol. 2012;181(6):2067–2078. [DOI] [PubMed] [Google Scholar]
- 36. Follin B, Tratwal J, Haack-Sorensen M, Elberg JJ, Kastrup J, Ekblond A. Identical effects of VEGF and serum-deprivation on phenotype and function of adipose-derived stromal cells from healthy donors and patients with ischemic heart disease. J Transl Med. 2013;11:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dzhoyashvili NA, Efimenko AY, Kochegura TN, Kalinina NI, Koptelova NV, Sukhareva OY, Shestakova MV, Akchurin RS, Tkachuk VA, Parfyonova YV. Disturbed angiogenic activity of adipose-derived stromal cells obtained from patients with coronary artery disease and diabetes mellitus type 2. J Transl Med. 2014;12(1):337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu Y, Li Z, Liu T, Xue X, Jiang H, Huang J, Wang H. Impaired cardioprotective function of transplantation of mesenchymal stem cells from patients with diabetes mellitus to rats with experimentally induced myocardial infarction. Cardiovasc Diabetol. 2013;12:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Friis T, Haack-Sorensen M, Hansen SK, Hansen L, Bindslev L, Kastrup J. Comparison of mesenchymal stromal cells from young healthy donors and patients with severe chronic coronary artery disease. Scand J Clin Lab Invest. 2011;71(3):193–202. [DOI] [PubMed] [Google Scholar]
- 40. Efimenko A, Dzhoyashvili N, Kalinina N, Kochegura T, Akchurin R, Tkachuk V, Parfyonova Y. Adipose-derived mesenchymal stromal cells from aged patients with coronary artery disease keep mesenchymal stromal cell properties but exhibit characteristics of aging and have impaired angiogenic potential. Stem Cells Transl Med. 2014;3(1):32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grauss RW, Winter EM, van Tuyn J, Pijnappels DA, Steijn RV, Hogers B, van der Geest RJ, de Vries AA, Steendijk P, van der Laarse A, et al. Mesenchymal stem cells from ischemic heart disease patients improve left ventricular function after acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;293(4):H2438–H2447. [DOI] [PubMed] [Google Scholar]
- 42. Behfar A, Yamada S, Crespo-Diaz R, Nesbitt JJ, Rowe LA, Perez-Terzic C, Gaussin V, Homsy C, Bartunek J, Terzic A. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. J Am Coll Cardiol. 2010;56(9):721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smadja DM, d’Audigier C, Guerin CL, Mauge L, Dizier B, Silvestre JS, Dal Cortivo L, Gaussem P, Emmerich J. Angiogenic potential of BM MSCs derived from patients with critical leg ischemia. Bone Marrow Transplant. 2012;47(7):997–1000. [DOI] [PubMed] [Google Scholar]
- 44. Gremmels H, Teraa M, Quax PH, den Ouden K, Fledderus JO, Verhaar MC. Neovascularization capacity of mesenchymal stromal cells from critical limb ischemia patients is equivalent to healthy controls. Mol Ther. 2014;22(11):1960–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brewster L, Robinson S, Wang R, Griffiths S, Li H, Peister A, Copland I, McDevitt T. Expansion and angiogenic potential of mesenchymal stem cells from patients with critical limb ischemia. J Vasc Surg. 2017;65(3):826–838.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Altaner C, Altanerova V, Cihova M, Hunakova L, Kaiserova K, Klepanec A, Vulev I, Madaric J. Characterization of mesenchymal stem cells of “no-options” patients with critical limb ischemia treated by autologous bone marrow mononuclear cells. PLoS One. 2013;8(9):e73722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Minullina IR, Alexeyeva NP, Anisimov SV, Puzanov MV, Kozlova SN, Sviryaev YV, Zaritskey AY, Shlyakhto EV. Transcriptional changes in bone marrow stromal cells of patients with heart failure. Cell Cycle. 2014;13(9):1495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dmitrieva RI, Revittser AV, Klukina MA, Sviryaev YV, Korostovtseva LS, Kostareva AA, Zaritskey AY, Shlyakhto EV. Functional properties of bone marrow derived multipotent mesenchymal stromal cells are altered in heart failure patients, and could be corrected by adjustment of expansion strategies. Aging (Albany NY). 2015;7(1):14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hare JM, DiFede DL, Rieger AC, Florea V, Landin AM, El-Khorazaty J, Khan A, Mushtaq M, Lowery MH, Byrnes JJ, et al. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM trial. J Am Coll Cardiol. 2017;69(5):526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de Lima KA, de Oliveira GL, Yaochite JN, Pinheiro DG, de Azevedo JT, Silva WA, Jr, Covas DT, Couri CE, Simoes BP, Voltarelli JC, et al. Transcriptional profiling reveals intrinsic mRNA alterations in multipotent mesenchymal stromal cells isolated from bone marrow of newly-diagnosed type 1 diabetes patients. Stem Cell Res Ther. 2016;7(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yaochite JN, de Lima KW, Caliari-Oliveira C, Palma PV, Couri CE, Simoes BP, Covas DT, Voltarelli JC, Oliveira MC, Donadi EA, et al. Multipotent mesenchymal stromal cells from patients with newly diagnosed type 1 diabetes mellitus exhibit preserved in vitro and in vivo immunomodulatory properties. Stem Cell Res Ther. 2016;7(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Davies LC, Alm JJ, Heldring N, Moll G, Gavin C, Batsis I, Qian H, Sigvardsson M, Nilsson B, Kyllonen LE, et al. Type 1 diabetes mellitus donor mesenchymal stromal cells exhibit comparable potency to healthy controls in vitro. Stem Cells Transl Med. 2016;5(11):1485–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cheng NC, Hsieh TY, Lai HS, Young TH. High glucose-induced reactive oxygen species generation promotes stemness in human adipose-derived stem cells. Cytotherapy. 2016;18(3):371–83. [DOI] [PubMed] [Google Scholar]
- 55. Trinh NT, Yamashita T, Ohneda K, Kimura K, Salazar GT, Sato F, Ohneda O. Increased expression of EGR-1 in diabetic human adipose tissue-derived mesenchymal stem cells reduces their wound healing capacity. Stem Cells Dev. 2016;25(10):760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gu JH, Lee JS, Kim DW, Yoon ES, Dhong ES. Neovascular potential of adipose-derived stromal cells (ASCs) from diabetic patients. Wound Repair Regen. 2012;20(2):243–252. [DOI] [PubMed] [Google Scholar]
- 57. Cramer C, Freisinger E, Jones RK, Slakey DP, Dupin CL, Newsome ER, Alt EU, Izadpanah R. Persistent high glucose concentrations alter the regenerative potential of mesenchymal stem cells. Stem Cells Dev. 2010;19(12):1875–1884. [DOI] [PubMed] [Google Scholar]
- 58. Koci Z, Turnovcova K, Dubsky M, Baranovicova L, Holan V, Chudickova M, Sykova E, Kubinova S. Characterization of human adipose tissue-derived stromal cells isolated from diabetic patient’s distal limbs with critical ischemia. Cell Biochem Funct. 2014;32(7):597–604. [DOI] [PubMed] [Google Scholar]
- 59. Barbagallo I, Li Volti G, Galvano F, Tettamanti G, Pluchinotta FR, Bergante S, Vanella L. Diabetic human adipose tissue-derived mesenchymal stem cells fail to differentiate in functional adipocytes. Exp Biol Med (Maywood). 2017;242(10):1079–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Krawiec JT, Weinbaum JS, St Croix CM, Phillippi JA, Watkins SC, Rubin JP, Vorp DA. A cautionary tale for autologous vascular tissue engineering: impact of human demographics on the ability of adipose-derived mesenchymal stem cells to recruit and differentiate into smooth muscle cells. Tissue Eng Part A. 2015;21(3-4):426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Krawiec JT, Weinbaum JS, Liao HT, Ramaswamy AK, Pezzone DJ, Josowitz AD, D’Amore A, Rubin JP, Wagner WR, Vorp DA. In vivo functional evaluation of tissue-engineered vascular grafts fabricated using human adipose-derived stem cells from high cardiovascular risk populations. Tissue Eng Part A. 2016;22(9-10):765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Acosta L, Hmadcha A, Escacena N, Perez-Camacho I, de la Cuesta A, Ruiz-Salmeron R, Gauthier BR, Soria B. Adipose mesenchymal stromal cells isolated from type 2 diabetic patients display reduced fibrinolytic activity. Diabetes. 2013;62(12):4266–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. van de Vyver M. Intrinsic mesenchymal stem cell dysfunction in diabetes mellitus: implications for autologous cell therapy. Stem Cells Dev. 2017;26(14):1042–1053. [DOI] [PubMed] [Google Scholar]
- 64. Kim J, Piao Y, Pak YK, Chung D, Han YM, Hong JS, Jun EJ, Shim JY, Choi J, Kim CJ. Umbilical cord mesenchymal stromal cells affected by gestational diabetes mellitus display premature aging and mitochondrial dysfunction. Stem Cells Dev. 2015;24(5):575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Reinders ME, Roemeling-van Rhijn M, Khairoun M, Lievers E, de Vries DK, Schaapherder AF, Wong SW, Zwaginga JJ, Duijs JM, van Zonneveld AJ, et al. Bone marrow-derived mesenchymal stromal cells from patients with end-stage renal disease are suitable for autologous therapy. Cytotherapy. 2013;15(6):663–672. [DOI] [PubMed] [Google Scholar]
- 66. Roemeling-van Rhijn M, Reinders ME, de Klein A, Douben H, Korevaar SS, Mensah FK, Dor FJ, JN IJ, Betjes MG, Baan CC, et al. Mesenchymal stem cells derived from adipose tissue are not affected by renal disease. Kidney Int. 2012;82(7):748–758. [DOI] [PubMed] [Google Scholar]
- 67. Yamanaka S, Yokote S, Yamada A, Katsuoka Y, Izuhara L, Shimada Y, Omura N, Okano HJ, Ohki T, Yokoo T. Adipose tissue-derived mesenchymal stem cells in long-term dialysis patients display downregulation of PCAF expression and poor angiogenesis activation. PLoS One. 2014;9(7):e102311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Guiducci S, Distler O, Distler JH, Matucci-Cerinic M. Mechanisms of vascular damage in SSc—implications for vascular treatment strategies. Rheumatology (Oxford). 2008;47(Suppl 5):v18–v20. [DOI] [PubMed] [Google Scholar]
- 69. Guiducci S, Manetti M, Romano E, Mazzanti B, Ceccarelli C, Dal Pozzo S, Milia AF, Bellando-Randone S, Fiori G, Conforti ML, et al. Bone marrow-derived mesenchymal stem cells from early diffuse systemic sclerosis exhibit a paracrine machinery and stimulate angiogenesis in vitro. Ann Rheum Dis. 2011;70(11):2011–2021. [DOI] [PubMed] [Google Scholar]
- 70. Cipriani P, Di Benedetto P, Liakouli V, Del Papa B, Di Padova M, Di Ianni M, Marrelli A, Alesse E, Giacomelli R. Mesenchymal stem cells (MSCs) from scleroderma patients (SSc) preserve their immunomodulatory properties although senescent and normally induce T regulatory cells (Tregs) with a functional phenotype: implications for cellular-based therapy. Clin Exp Immunol. 2013;173(2):195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cipriani P, Marrelli A, Benedetto PD, Liakouli V, Carubbi F, Ruscitti P, Alvaro S, Pantano I, Campese AF, Grazioli P, et al. Scleroderma Mesenchymal Stem Cells display a different phenotype from healthy controls; implications for regenerative medicine. Angiogenesis. 2013;16(3):595–607. [DOI] [PubMed] [Google Scholar]
- 72. Larghero J, Farge D, Braccini A, Lecourt S, Scherberich A, Fois E, Verrecchia F, Daikeler T, Gluckman E, Tyndall A, et al. Phenotypical and functional characteristics of in vitro expanded bone marrow mesenchymal stem cells from patients with systemic sclerosis. Ann Rheum Dis. 2008;67(4):443–449. [DOI] [PubMed] [Google Scholar]
- 73. Vanneaux V, Farge-Bancel D, Lecourt S, Baraut J, Cras A, Jean-Louis F, Brun C, Verrecchia F, Larghero J, Michel L. Expression of transforming growth factor beta receptor II in mesenchymal stem cells from systemic sclerosis patients. BMJ Open. 2013;3(1): e001890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hegner B, Schaub T, Catar R, Kusch A, Wagner P, Essin K, Lange C, Riemekasten G, Dragun D. Intrinsic deregulation of vascular smooth muscle and myofibroblast differentiation in mesenchymal stromal cells from patients with systemic sclerosis. PLoS One. 2016;11(4):e0153101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cipriani P, Guiducci S, Miniati I, Cinelli M, Urbani S, Marrelli A, Dolo V, Pavan A, Saccardi R, Tyndall A, et al. Impairment of endothelial cell differentiation from bone marrow-derived mesenchymal stem cells: new insight into the pathogenesis of systemic sclerosis. Arthritis Rheum. 2007;56(6):1994–2004. [DOI] [PubMed] [Google Scholar]
- 76. Phadnis SM, Ghaskadbi SM, Hardikar AA, Bhonde RR. Mesenchymal stem cells derived from bone marrow of diabetic patients portrait unique markers influenced by the diabetic microenvironment. Rev Diabet Stud. 2009;6(4):260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Golpanian S, El-Khorazaty J, Mendizabal A, DiFede DL, Suncion VY, Karantalis V, Fishman JE, Ghersin E, Balkan W, Hare JM. Effect of aging on human mesenchymal stem cell therapy in ischemic cardiomyopathy patients. J Am Coll Cardiol. 2015;65(2):125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. de Wolf C, van de Bovenkamp M, Hoefnagel M. Regulatory perspective on in vitro potency assays for human mesenchymal stromal cells used in immunotherapy. Cytotherapy. 2017;19(7):784–797. [DOI] [PubMed] [Google Scholar]
- 79. Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, Fries JW, Tiemann K, Bohlen H, Hescheler J, et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110(4):1362–1369. [DOI] [PubMed] [Google Scholar]
- 80. Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ. ; Canadian Critical Care Trials Group. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7(10):e47559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Elman JS, Murray RC, Wang F, Shen K, Gao S, Conway KE, Yarmush ML, Tannous BA, Weissleder R, Parekkadan B. Pharmacokinetics of natural and engineered secreted factors delivered by mesenchymal stromal cells. PLoS One. 2014;9(2):e89882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Naftali-Shani N, Levin-Kotler LP, Palevski D, Amit U, Kain D, Landa N, Hochhauser E, Leor J. Left ventricular dysfunction switches mesenchymal stromal cells toward an inflammatory phenotype and impairs their reparative properties via toll-like receptor-4. Circulation. 2017;135(23):2271–2287. [DOI] [PubMed] [Google Scholar]
- 83. Noh H, Yu MR, Kim HJ, Jeon JS, Kwon SH, Jin SY, Lee J, Jang J, Park JO, Ziyadeh F, et al. Uremia induces functional incompetence of bone marrow-derived stromal cells. Nephrol Dial Transplant. 2012;27(1):218–225. [DOI] [PubMed] [Google Scholar]
- 84. de Groot K, Bahlmann FH, Sowa J, Koenig J, Menne J, Haller H, Fliser D. Uremia causes endothelial progenitor cell deficiency. Kidney Int. 2004;66(2):641–646. [DOI] [PubMed] [Google Scholar]
- 85. Krankel N, Adams V, Linke A, Gielen S, Erbs S, Lenk K, Schuler G, Hambrecht R. Hyperglycemia reduces survival and impairs function of circulating blood-derived progenitor cells. Arterioscler Thromb Vasc Biol. 2005;25(4):698–703. [DOI] [PubMed] [Google Scholar]
- 86. Rezabakhsh A, Cheraghi O, Nourazarian A, Hassanpour M, Kazemi M, Ghaderi S, Faraji E, Rahbarghazi R, Avci CB, Bagca BG, et al. Type 2 diabetes inhibited human mesenchymal stem cells angiogenic response by over-activity of the autophagic pathway. J Cell Biochem. 2017;118(6):1518–1530. [DOI] [PubMed] [Google Scholar]
- 87. Klose K, Roy R, Brodarac A, Kurtz A, Ode A, Kang KS, Bieback K, Choi YH, Stamm C. Impact of heart failure on the behavior of human neonatal stem cells in vitro. J Transl Med. 2013;11(1):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Minamino T, Komuro I. Vascular cell senescence: contribution to atherosclerosis. Circ Res. 2007;100(1):15–26. [DOI] [PubMed] [Google Scholar]
- 90. Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, et al. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci U S A. 2003;100(19):10623–10628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Schipper BM, Marra KG, Zhang W, Donnenberg AD, Rubin JP. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg. 2008;60(5):538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rubinstein MA. Aspiration of bone marrow from the iliac crest; some technical and diagnostic advantages versus sternal aspiration. Bull N Y Acad Med. 1948;24(6):400. [PMC free article] [PubMed] [Google Scholar]
- 93. Kreft A, Reimann J, Choritz H. Fibre content and cellularity of the bone marrow of the iliac crest, vertebral column and sternum in chronic myeloproliferative disorders. Leuk Lymphoma. 2000;38(1-2):165–173. [DOI] [PubMed] [Google Scholar]
- 94. Winkler IG, Barbier V, Wadley R, Zannettino AC, Williams S, Levesque JP. Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches. Blood. 2010;116(3):375–385. [DOI] [PubMed] [Google Scholar]
- 95. Lassailly F, Foster K, Lopez-Onieva L, Currie E, Bonnet D. Multimodal imaging reveals structural and functional heterogeneity in different bone marrow compartments: functional implications on hematopoietic stem cells. Blood. 2013;122(10):1730–1740. [DOI] [PubMed] [Google Scholar]
- 96. Page P, DeJong J, Bandstra A, Boomsma RA. Effect of serum and oxygen concentration on gene expression and secretion of paracrine factors by mesenchymal stem cells. Int J Cell Biol. 2014;2014:601063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wagner W, Feldmann RE, Jr, Seckinger A, Maurer MH, Wein F, Blake J, Krause U, Kalenka A, Burgers HF, Saffrich R, et al. The heterogeneity of human mesenchymal stem cell preparations—evidence from simultaneous analysis of proteomes and transcriptomes. Exp Hematol. 2006;34(4):536–548. [DOI] [PubMed] [Google Scholar]
- 98. Di Battista JA, Shebaby W, Kizilay O, Hamade E, Abou Merhi R, Mebarek S, Abdallah D, Badran B, Saad F, K Abdalla KA, et al. Proliferation and differentiation of human adipose-derived mesenchymal stem cells (ASCs) into osteoblastic lineage are passage dependent. Inflamm Res. 2014;63(11):907–917. [DOI] [PubMed] [Google Scholar]
- 99. Gregory CA, Singh H, Perry AS, Prockop DJ. The Wnt signaling inhibitor dickkopf-1 is required for reentry into the cell cycle of human adult stem cells from bone marrow. J Biol Chem. 2003;278(30):28067–28078. [DOI] [PubMed] [Google Scholar]
- 100. Lee MW, Kim DS, Ryu S, Jang IK, Kim HJ, Yang JM, Lee DH, Lee SH, Son MH, Cheuh HW, et al. Effect of ex vivo culture conditions on immunosuppression by human mesenchymal stem cells. Biomed Res Int. 2013;2013:154919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Cipriani P, Di Benedetto P, Capece D, Zazzeroni F, Liakouli V, Ruscitti P, Pantano I, Berardicurti O, Carubbi F, Alesse E, et al. Impaired Cav-1 expression in SSc mesenchymal cells upregulates VEGF signaling: a link between vascular involvement and fibrosis. Fibrogenesis Tissue Repair. 2014;7(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]