Abstract
Accumulating evidence has demonstrated that endothelial progenitor cells (EPCs) could facilitate the reendothelialization of injured arteries by replacing the dysfunctional endothelial cells, thereby suppressing the formation of neointima. Meanwhile, other findings suggest that EPCs may be involved in the pathogenesis of age-related vascular remodeling. This review is presented to summarize the characteristics of EPCs and age-related vascular remodeling. In addition, the role of EPCs in age-related vascular remodeling and possible solutions for improving the therapeutic effects of EPCs in the treatment of age-related diseases are discussed.
Keywords: Endothelial progenitor cell, age, vascular remodeling
Introduction
Aging is characterized by progressive degeneration of tissues and organ systems, aggravation of body functions, and decreasing ability to respond to stress, which increase the risk of age-related diseases1. Age-related diseases, such as atherosclerosis, hypertension, and type 2 diabetes mellitus, accelerate the process of aging and result in disability and premature death2,3. Among these diseases, atherosclerosis leads to the development of myocardial infarction, sudden cardiac death, ischemic heart disease, and stroke, which are the main causes of morbidity and mortality in the industrialized and some developing countries1.
Atherosclerosis is considered not only as an age-related disease but also as an age-dependent disease1. Vascular remodeling, characterized by neointimal hyperplasia, frequently accompanies atherosclerosis4. Aggravated vascular remodeling is alleviated by the process of reendothelialization, which occurs by covering the impaired neointimal surface with a functional endothelial monolayer5. The endothelial monolayer represents a dynamic structure and functional barrier between the circulating blood and surrounding tissues. It prevents platelet and leukocyte adhesion/aggregation, producing a variety of important vasoregulatory factors such as endothelins and nitric oxide6. An imbalance between endothelial cell (EC) damage and repair is the initial step in the development of age-related vascular remodeling7. Endothelial repair is accomplished by the migration and proliferation of surrounding mature ECs. However, mature ECs are terminally differentiated with a low proliferative capacity, and their ability to replace the damaged endothelium is altogether limited8. Therefore, endothelial repair may need support from other cell types8.
Endothelial progenitor cells (EPCs) are currently considered as important contributors to endogenous vascular repair by participating in endothelial regeneration9,10. Studies in animal models and humans have demonstrated that EPCs can facilitate the reendothelialization of injured arteries by replacing dysfunctional ECs, thereby suppressing the formation of neointima11,12. Meanwhile, other experimental findings have indicated that EPCs may be involved in the pathogenesis of age-related vascular remodeling12.
This review summarizes the characteristics of EPCs and age-related vascular remodeling. In addition, the role of EPCs in age-related vascular remodeling and possible solutions for improving the therapeutic effects of EPCs in the treatment of age-related diseases are discussed.
Characterization of EPCs
EPCs were initially considered as a group of cells mobilized from the bone marrow that participate in the generation and repair of the vascular endothelium13. EPCs have recently been regarded as a heterogeneous population of cells in different stages of maturation, with different origins and several residing sites, such as the spleen, vascular endothelium, and adventitia14. EPCs adhere to matrix molecules such as fibronectin, and are positive for both acetylated low-density lipoprotein (acLDL) and Ulex europaeus agglutinin I (UEA-1) lectin13,15,16. To date, there is no specific marker for identifying EPCs.
Asahara and colleagues reported that circulating CD34+ and fetal liver kinase positive (Flk-1+), also known as vascular endothelial growth factor receptor 2 (VEGFR2) or kinase insert domain receptor (KDR), mononuclear cells (MNCs) may facilitate neo-angiogenesis. These two cell surface markers were the first putative markers proposed for EPC identification13. Then, CD34, VEGFR2, and CD133 were used to characterize EPCs, and these biomarkers are the most commonly used surface markers for defining an EPC population17,18.
EPCs consist of two different subpopulations: early-outgrowth and late-outgrowth EPCs19. Early-outgrowth EPCs are also termed “circulatory angiogenic cells” (CACs) or “colony forming unit endothelial cells (CFU-EC)”, and are adherent spindle-shaped cells that develop after 4–7 days, die after 4 weeks, and have very low proliferative ability20–22. Early-outgrowth EPCs express some surface markers characteristic of progenitor cells, including CD133 and CD34, the endothelial markers CD31 and von Willebrand factor (vWF), the pan-leukocyte marker CD45, and the monocyte marker CD1423. Early-outgrowth EPCs lack impressive replicative ability but are prolific producers of several growth factors and cytokines, including VEGF, hepatocyte growth factor (HGF), granulocyte colony-stimulating factor (G-CSF), granulocyte/macrophage colony-stimulating factor (GM-CSF), and interleukin (IL)-821. Early-outgrowth EPCs cannot form a vascular network in vitro, but can adhere to mature ECs, and promote network formation and repair injured ECs through a paracrine mechanism22. Late-outgrowth EPCs, also termed “endothelial outgrowth cells” (EOCs) or “endothelial colony forming cells” (ECFCs), display a cobblestone morphology, and start to proliferate and differentiate into mature ECs after 2–3 weeks. These cells express endothelial markers such as KDR, VE-cadherin, and CD14624. Late-outgrowth EPCs can improve angiogenesis directly by incorporating into neovessels and further differentiating into mature ECs25–27. Early- and late-outgrowth EPCs may originate from different angiogenic cell types27.
Hill et al. developed a colony forming assay based on MNC culture on fibronectin-coated plates, using culture medium that was designed to promote endothelial lineage cell proliferation28. These cells (colony forming unit endothelial cells, CFU-ECs, or CFU-Hill) emerged from the cultured non-adherent human peripheral blood MNCs after 48 h of preplating on fibronectin-coated dishes28. Hill et al. identified colonies composed of multiple thin flat cells emanating from a central cluster of rounded cells28. It was apparent that CFU-ECs contain various blood cells, including hematopoietic progenitor cells, monocytes, and lymphocytes (see Richardson and Yoder29 for a comprehensive review).
Recently, Malinverno et al. identified a subpopulation of vessel-associated ECs with the characteristics of progenitor cells30. These PW1-positive cells are highly proliferative and form colonies when cultured at clonal dilution. PW1-positive cells can proliferate to efficiently form new vessels in vivo30.
Age-related vascular remodeling
Aging refers to the biological and physiological processes that involve organs, tissues, and cells throughout life, gradually causing a decline of normal functions31. Aging is one of the main risk factors for the development of cardiovascular diseases (CVD), which might be due to the structural changes that emerge in the systems and organs, such as complicated alterations in the vasculature, with age32,33. ECs, located at the interface between blood vessels and tissues, stand poised to respond to the environment and modulate the vascular function to maintain homeostasis and host defenses against microbial invaders and injury34. Inappropriate signaling from vascular ECs that leads to endothelial dysfunction induces common diseases characterized by arterial remodeling, notably atherosclerosis35.
Recently, the concept of endothelial dysfunction has changed from a pure “damage model” to a more dynamic process, where the effects of endothelial repair are outpaced by local injury36. Alteration in the damage/repair balance causes endothelial dysfunction, which is considered the main cause of initiation and development of atherosclerosis23. In healthy subjects, a low basal level of endothelial turnover has been unveiled6,37. However, acute injury or chronic immuno-inflammatory endothelial dysfunction contributes to the loss of anti-thrombotic function as well as enhanced arrest and transmigration of circulating leukocytes8. This pathological vascular remodeling gradually leads to redundant sub-endothelial accumulation of lipids and immune cells, neointimal hyperplasia, excessive proliferation of smooth muscle cells (SMCs), matrix deposition, and foam cell formation38. Recently, a growing body of studies have highlighted the involvement of myofibroblasts (MFs) in the neointima induced by vascular injury39. MFs are derived from adventitial fibroblasts, the transdifferentiation of SMCs residing in the tunica media, and ECs through an endothelial–mesenchymal transition40. The major functions of MFs are production and modification of extracellular matrix (ECM), secretion of pro-inflammatory and angiogenic factors, and generation of tensile force39. MFs contribute not only to the formation of neointima but also to the thickening of tunica media, adventitial fibrosis, and deposition of the ECM, a process that can lead to late lumen stenosis after vascular injury39.
Consequently, occlusive atherosclerotic plaques with lumen stenosis of the arterial wall aggravate and clinically lead to chronic distal tissue ischemia, often complicated by acute myocardial infarction38,41. Beyond acute complications, sufficient endothelial regeneration appears to be crucial for attenuating arterial stenosis secondary to injury (e.g. balloon angioplasty or stent placement). It is also thought to prevent endothelial dysfunction and initiation of corresponding atheromatous plaque growth by replacing the injured ECs12.
EPCs and age-related vascular remodeling
EPC status in age-related vascular remodeling
Previous study has shown that the number of circulating EPCs decreases reversibly with aging, especially in patients with coronary artery disease42. For example, Scheubel et al. reported an age-related reduction of circulating EPCs in aged patients undergoing coronary artery bypass grafting43. The number of EPC-CFUs in culture was found to be inversely correlated with cardiovascular risks in adults28. Among patients with low, intermediate, and high numbers of CFU-ECs, those with the highest levels were considered to be the healthiest28. Indeed, decreased ability of EPCs to proliferate in vitro and to express the endothelial phenotype was associated with the risk factors for coronary artery disease and endothelial dysfunction28,44.
The circulating number of EPCs can serve as a predicting factor for the patient’s outcome45. Dysfunctional EPCs may lead to impaired ability to restore endothelial damage46. EPC number was found to be a strong and independent negative predictor of atherosclerotic plaque occurrence in the common carotid artery47,48. Meanwhile, it has also been demonstrated that EPC number is reduced with the presence and progression of preclinical atherosclerosis, and the risk factors constitute a decrease in aortic and femoral sites, but not in carotid circulation49. Peripheral arterial disease was associated with lower cell counts of CD34+ and CD34+/VEGFR2+ 50. A decrease of EPCs to below 0.0038% of total circulating peripheral blood MNCs represents a six-fold higher risk for the development of CVDs46. Coronary artery disease patients with the highest EPC number have the highest likelihood of remaining event-free44.
Therefore, EPCs serve as promising biomarkers of cardiovascular health48. However, some investigators reported no correlation between EPC subsets and vascular remodeling, with no direct association of EPC number change with CVD progression51,52. Differences in methods used for identifying EPCs may lead to such disparity.
Involvement of EPCs in age-related vascular remodeling
Endothelial damage is an important early step in the initiation and development of atherosclerosis, a hallmark of aging1. Structural and functional endothelial damage contributing to atherosclerosis is a common event, and endothelial regeneration is critical for maintaining endothelial homeostasis53,54. In the context of regeneration, animal studies have shown that EPCs efficiently contribute to restoring endothelial function and decrease neointimal formation after arterial injury55–60. An adequate homing of EPCs plays a central role in this regenerative arterial remodeling57. The process of EPC homing, including mobilization, recruitment, and adhesion, is regulated by key angiogenic chemokines (CXCL1, CXCL7, CXCL12, CCL2) and their respective receptors (CXCR2, CXCR4, CCR2)57. Hristov et al. showed that CXCR2 is crucial for the homing of circulating EPCs to sites of arterial injury and for endothelial repair55. It was also found that rat bone marrow-derived EPC functional activity could be ameliorated by decreasing cellular senescence via AKT/endothelial nitric oxide synthase (eNOS) pathways and improving homing capacity via increasing CXCR4 expression levels61. Walter et al. reported that the use of statins increased circulating rat EPCs and promoted adhesion of cultured human EPCs by augmentation of integrin subunits α5, αv, β1, and β5 of human EPCs58. Augmentation of integrin receptor expression may thus promote adhesion and enhance homing of EPCs to foci of ischemia or vascular injury58. Meanwhile, EPCs play an important role in the neovascularization of ischemic tissue by promoting the formation of new vessels and releasing angiogenic growth factors11,12. Currently, the common clinical concept claims a protective role for EPCs even during the initiation and development of atherosclerosis, further suggesting that EPCs may reflect the endogenous vascular repair ability46,62. Intramyocardial injection by synergistic local co-administration of angiogenic compounds may help to further promote the homing of EPCs and neovascularization after myocardial infarction63. The therapeutic effects are exerted even in the chronic stage, when acute inflammation and oxidative stress are attenuated63,64. Kaushal et al. coated vascular grafts with endogenous EPCs and found that EPCs can exert functions similar to arterial ECs, thereby conferring longer vascular-graft survival65. Thus, the coating of stents with EPC-attracting peptides or antibodies to capture EPCs in terms of promoting endothelialization and diminishing in-stent stenosis remains an exciting alternative for clinical application66,67.
Although therapeutic effects of EPCs in the treatment of atherosclerotic diseases have been observed in animal models and humans, the crucial involvement of EPCs in the pathogenesis of atherosclerosis has newly emerged. Interestingly, transplanted EPCs increase the lipid content and decrease collagen amounts in atherosclerotic plaques of Apoe−/− mice68. Furthermore, higher serum concentrations of IL-6 and monocyte chemoattractant protein-1, and lower serum concentration of IL-10, were found in mice transfused with EPCs68. Increased plasma CXCR2 receptor ligands such as CXCL1 and CXCL7 were clinically related to plaque destabilization, while blocking of CXCR2 was associated with a more stable plaque phenotype in experimental models69–71. The influx of CXCR2+ monocyte subsets containing putative endothelial precursors with inflammatory, proteolytic, and angiogenic properties may partly contribute to these findings55,71. Vega et al. found that the atherosclerotic plaque secretome promotes EPC proliferation, mobilization, permeability, contraction, and adhesion72. Furthermore, the up-regulated expression of proteins that are mostly involved in cell proliferation, migration, and vascular remodeling was observed in the atherosclerotic plaque secretome treated cells72. It was also found that increased circulating CD34+ cells after coronary stenting may serve as an independent risk factor for predicting in-stent restenosis and indicate the involvement of CD34+ subpopulations in neointimal hyperplasia73. Thus, the dual contribution of EPC subpopulations to vascular remodeling in atherosclerosis needs a critical reevaluation8.
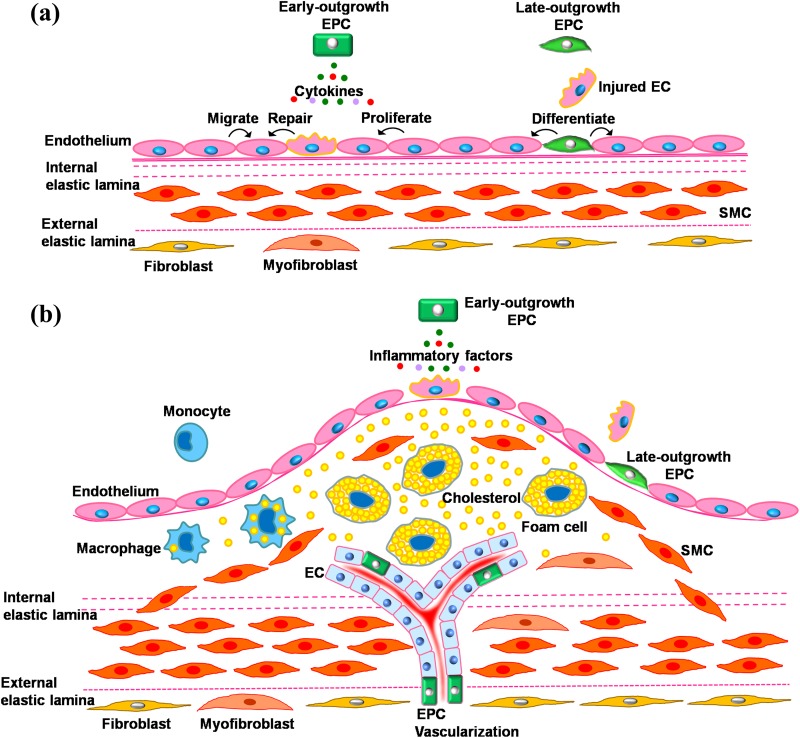
In the early stage of primary and secondary atherosclerosis after injury, which is characterized by endothelial dysfunction, EPCs (mainly as late-outgrowth EPCs) mobilize to the injured area, penetrate the site of vessel injury, and differentiate into mature ECs. This in turn replaces the dysfunctional endothelium, further avoiding the development of atherosclerosis (Figure 1(a)). Hence, EPCs may provide a circulating pool of cells that could generate a cellular patch at the site of denuding injury or serve as a cellular reservoir to substitute the injured endothelium28. In recent years, accumulating evidence has indicated that activation of tissue-resident ECs through paracrine mechanisms may become more crucial for EPC-based neovascularization than direct differentiation and incorporation into the vasculature74,75. Early-outgrowth EPCs secrete several cytokines, such as VEGF, HGF, G-CSF, and GM-CSF20,21. Therefore, EPCs (mainly as early-outgrowth EPCs) could also repair the injured ECs by secreting growth factors (Figure 1(a)). Advanced atherosclerosis is characterized by redundant sub-endothelial accumulation of lipids and immune cells, neointimal hyperplasia, excessive proliferation of SMCs, matrix deposition, and foam cell formation38. It involves widespread mobilization of EPCs associated with that of monocytes in response to inflammatory factors, such as monocyte chemoattractant protein 1, and may promote plaque instability/vascularization12 (Figure 1(b)). Again, the contribution of EPCs during vascular remodeling in the early and advanced disease stages, as well as primary and secondary atherosclerosis, requires a more careful and critical reevaluation76.
Figure 1.
Involvement of EPCs in age-related vascular remodeling. (a) In the early stage of primary and secondary atherosclerosis after injury, characterized by endothelial dysfunction, endothelial progenitor cells (EPCs, mainly as late-outgrowth EPCs) can be mobilized to the injured area, penetrate the site of vessel injury, and differentiate into mature endothelial cells (ECs), replacing the dysfunctional endothelium and avoiding further atherosclerosis development. EPCs (mainly as early-outgrowth EPCs) could also repair injured ECs by secreting growth factors. (b) In advanced atherosclerosis, characterized by redundant sub-endothelial accumulation of lipids and immune cells, neointimal hyperplasia, excessive proliferation of smooth muscle cells (SMCs), matrix deposition, and foam cell formation, a widespread EPC mobilization concomitant with that of monocytes in response to inflammatory factors may, rather, promote plaque instability/vascularization.
In addition, it should be mentioned that aging may also affect the pathways involved in the contribution of EPCs to vascular remodeling. A significant reduction in the expression of CXCR4 was found in the CD34+ cell population with aging77. It was also found that the surface CXCR4 expression on bone marrow-derived cells was significantly reduced in aged mice compared with young mice78,79. Xia et al. showed no difference in the surface expression of CXCR4 receptor in EPCs between older and younger men80. However, phosphorylation of JAK-2, a downstream signaling of CXCR4, is markedly decreased in EPCs derived from elderly men80. It was suggested that bone marrow-derived EPC functional activity could be ameliorated by decreasing cellular senescence and improving homing capacity through increasing CXCR4 expression levels61. So, aging may impair the protective effects of EPCs in vascular remodeling via affecting CXCR4-JAK-2 pathways.
Obstacles and possible solutions
There is no specific marker for EPCs, and many studies assessing EPCs have included limited analyses regarding the cell phenotypes. The use of an often poorly defined label “progenitor cells” for heterogeneous therapeutic cell subtypes complicates study comparisons, making it quite challenging to reach definitive conclusions concerning their efficacy81. Defining and standardizing EPC surface markers are extremely important for comparing more EPC studies82. In addition, the methods for culturing EPCs should be more standardized and, if possible, this should be done in a uniform manner.
Convincing evidence has emerged that EPCs from the elderly are impaired in terms of number, function, and survival83–87. Clinical application of cardiovascular cell repair therapy showed some limitations in older patients88,89. The major obstacles include degradation of functionality of autologous stem or progenitor cells in older individuals, and difficulties in engraftment and survival of transplanted cells in the hostile host microenvironment7. EPC-based therapy showed that an increase in the number or function of circulating EPCs may be effective in the treatment of atherosclerotic diseases90. However, large-scale use of cell-based therapy was limited due to the poor viability of EPCs after transplantation90. Therefore, enhancement strategies to reactivate the proliferation and function of EPCs in aged patients need to be explored urgently91.
EPC function enhancement was observed after administration of growth factors such as HGF and insulin-like growth factor (IGF)-192,93. Recombinant bone morphogenetic protein 4 also markedly improved the migration and adhesion capacity of human EPCs94. In clinical application, the most feasible method to improve EPC number and function is drug treatment. To date, the most practicable strategies applied in the clinic are pharmacological treatments with anti-hypertensive and anti-hyperglycemic effects91. Previous studies reported that beta blockers, calcium channel blockers, and angiotensin II receptor antagonists significantly increased EPC counts95–99. In diabetic and non-diabetic patients, pharmacological products, such as anti-diabetic peroxisome proliferator-activated receptor gamma (PPARG) agonists, have been shown to enhance EPC number and function100,101. Other drugs, for example rosuvastatin and cilostazol, also exerted positive effects on circulating EPC levels102. As well as Western medicine, traditional Chinese drugs have recently shown beneficial effects on EPC function. Tanshinone IIA may have the potential to protect EPCs against damage induced by tumor necrosis factor-α103. Danhong injection, extracted from Radix Salvia miltiorrhiza and Flos Carthamus tinctorius L, is effective in repairing endothelial lesions by mobilizing EPCs104.
Although the drug treatment mentioned earlier could increase the number and function of EPCs, adverse effects of the drugs, such as headache, nausea, and asthenia, may also occur, especially in the elderly population. Non-drug therapies may be good choices to enhance EPC function and avoid adverse effects. Interestingly, exercise has direct beneficial effects on EPC number and function in the aged population80,105–108. Meanwhile, other interesting studies have shown that Mediterranean diets and black tea exert protective effects on EPC level and function109–112. As concomitant strategies to enhance EPC number and function, lifestyle and diet modifications should be strongly encouraged in aged patients91.
Recent attempts to improve the number and function of transplanted EPCs with gene modification may facilitate repair of the injured endothelium and accelerate reendothelialization113. Transplantation of genetically modified EPCs that overexpress PDGFR-β, β2AR, and CXCR7, or with reduced Lnk levels, significantly enhanced the vascular repair ability of EPCs, improving the inhibition of adverse remodeling after vascular injury113–117. EPC transplantation combined with gene transfer may be a promising EPC therapeutic strategy in the future for age-related vascular remodeling.
Conclusions
Endothelial damage is a critical early step in the initiation and development of atherosclerosis. EPCs may repair and replace the injured ECs, and avoid initiation and development of atherosclerosis, through differentiating into mature ECs and the release of protective paracrine factors. However, widespread EPC mobilization may, rather, cause plaque instability in advanced atherosclerosis. Selectively controlling the mobilization and homing of EPCs helps to increase their therapeutic potential and avoid promoting the development of atherosclerotic diseases. EPCs from the elderly are impaired in terms of number, function, and survival. Thus, improving the effectiveness of EPC treatments to delay the progression of age-related vascular remodeling and diseases remains an urgent necessity. Drug regimens, gene transfer, lifestyle, and diet modifications are effective approaches, and may constitute promising therapeutic strategies for the treatment of age-related vascular remodeling.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by research grants from the National Natural Science Foundation of China [NSFC 81400192, 81170270], Natural Science Foundation of Zhejiang Province [LQ14H020006] and Science and Technology Department of Zhejiang Province [grant number 2015C33212].
References
- 1. Altabas V, Altabas K, Kirigin L. Endothelial progenitor cells (EPCs) in ageing and age-related diseases: how currently available treatment modalities affect EPC biology, atherosclerosis, and cardiovascular outcomes. Mech Ageing Dev. 2016;159:49–62. [DOI] [PubMed] [Google Scholar]
- 2. Tian XL, Li Y. Endothelial cell senescence and age-related vascular diseases. J Genet Genomics. 2014;41(9):485–95. [DOI] [PubMed] [Google Scholar]
- 3. Bao Q, Pan J, Qi H, Wang L, Qian H, Jiang F, Shao Z, Xu F, Tao Z, Ma Q, Nelson P, Hu X. Aging and age-related diseases—from endocrine therapy to target therapy. Mol Cell Endocrinol. 2014;394(1–2):115–18. [DOI] [PubMed] [Google Scholar]
- 4. Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–74. [DOI] [PubMed] [Google Scholar]
- 5. Carmeliet P, Moons L, Stassen JM, De Mol M, Bouche A, van den Oord JJ, Kockx M, Collen D. Vascular wound healing and neointima formation induced by perivascular electric injury in mice. Am J Pathol. 1997;150(2):761–76. [PMC free article] [PubMed] [Google Scholar]
- 6. Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91(10):3527–61. [PubMed] [Google Scholar]
- 7. Madonna R, Novo G, Balistreri CR. Cellular and molecular basis of the imbalance between vascular damage and repair in ageing and age-related diseases: as biomarkers and targets for new treatments. Mech Ageing Dev. 2016;159:22–30. [DOI] [PubMed] [Google Scholar]
- 8. Hristov M, Weber C. Endothelial progenitor cells in vascular repair and remodeling. Pharmacol Res. 2008;58(2):148–51. [DOI] [PubMed] [Google Scholar]
- 9. Balistreri CR, Buffa S, Pisano C, Lio D, Ruvolo G, Mazzesi G. Are endothelial progenitor cells the real solution for cardiovascular diseases? Focus on controversies and perspectives. Biomed Res Int. 2015;2015:835934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim SW, Kim H, Cho HJ, Lee JU, Levit R, Yoon YS. Human peripheral blood-derived CD31+ cells have robust angiogenic and vasculogenic properties and are effective for treating ischemic vascular disease. J Am Coll Cardiol. 2010;56(7):593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rabelink TJ, de Boer HC, de Koning EJ, van Zonneveld AJ. Endothelial progenitor cells: more than an inflammatory response? Arterioscler Thromb Vasc Biol. 2004;24(5):834–8. [DOI] [PubMed] [Google Scholar]
- 12. Hristov M, Weber C. Ambivalence of progenitor cells in vascular repair and plaque stability. Curr Opin Lipidol. 2008;19(5):491–7. [DOI] [PubMed] [Google Scholar]
- 13. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–7. [DOI] [PubMed] [Google Scholar]
- 14. Yoder MC. Is endothelium the origin of endothelial progenitor cells? Arterioscler Thromb Vasc Biol. 2010;30(6):1094–103. [DOI] [PubMed] [Google Scholar]
- 15. Yang JX, Pan YY, Ge JH, Chen B, Mao W, Qiu YG, Wang XX. Tanshinone II A attenuates TNF-alpha-induced expression of VCAM-1 and ICAM-1 in endothelial progenitor cells by blocking activation of NF-kappaB. Cell Physiol Biochem. 2016;40(1–2):195–206. [DOI] [PubMed] [Google Scholar]
- 16. Yang JX, Chen B, Pan YY, Han J, Chen F, Hu SJ. Zoledronate attenuates angiogenic effects of angiotensin II-stimulated endothelial progenitor cells via RhoA and MAPK signaling. PLoS One. 2012;7(10):e46511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95(3):952–8. [PubMed] [Google Scholar]
- 18. Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95(4):343–53. [DOI] [PubMed] [Google Scholar]
- 19. Prater DN, Case J, Ingram DA, Yoder MC. Working hypothesis to redefine endothelial progenitor cells. Leukemia. 2007;21(6):1141–9. [DOI] [PubMed] [Google Scholar]
- 20. Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24(2):288–93. [DOI] [PubMed] [Google Scholar]
- 21. Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107(8):1164–9. [DOI] [PubMed] [Google Scholar]
- 22. Sieveking DP, Buckle A, Celermajer DS, Ng MK. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: insights from a novel human angiogenesis assay. J Am Coll Cardiol. 2008;51(6):660–8. [DOI] [PubMed] [Google Scholar]
- 23. Recchioni R, Marcheselli F, Antonicelli R, Lazzarini R, Mensa E, Testa R, Procopio AD, Olivieri F. Physical activity and progenitor cell-mediated endothelial repair in chronic heart failure: is there a role for epigenetics? Mech Ageing Dev. 2016;159:71–80. [DOI] [PubMed] [Google Scholar]
- 24. Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28(9):1584–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Avogaro A, Albiero M, Menegazzo L, de Kreutzenberg S, Fadini GP. Endothelial dysfunction in diabetes: the role of reparatory mechanisms. Diabetes Care. 2011;34(Suppl 2):S285–S290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee PS, Poh KK. Endothelial progenitor cells in cardiovascular diseases. World J Stem Cells. 2014;6(3):355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Madonna R, De Caterina R. Circulating endothelial progenitor cells: do they live up to their name? Vascul Pharmacol. 2015;67–69:2–5. [DOI] [PubMed] [Google Scholar]
- 28. Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593–600. [DOI] [PubMed] [Google Scholar]
- 29. Richardson MR, Yoder MC. Endothelial progenitor cells: quo vadis? J Mol Cell Cardiol. 2011;50(2):266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Malinverno M, Corada M, Ferrarini L, Formicola L, Marazzi G, Sassoon D, Dejana E. Peg3/PW1 is a marker of a subset of vessel associated endothelial progenitors. Stem Cells. 2017;35(5):1328–40. [DOI] [PubMed] [Google Scholar]
- 31. Abdelmagid SM, Barbe MF, Safadi FF. Role of inflammation in the aging bones. Life Sci. 2015;123:25–34. [DOI] [PubMed] [Google Scholar]
- 32. Yildiz O. Vascular smooth muscle and endothelial functions in aging. Ann N Y Acad Sci. 2007;1100:353–60. [DOI] [PubMed] [Google Scholar]
- 33. Ferrari AU, Radaelli A, Centola M. Invited review: Aging and the cardiovascular system. J Appl Physiol (1985). 2003;95(6):2591–7. [DOI] [PubMed] [Google Scholar]
- 34. Gimbrone MA, Jr, Garcia-Cardena G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc Pathol. 2013;22(1):9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heusch G, Libby P, Gersh B, Yellon D, Bohm M, Lopaschuk G, Opie L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet. 2014;383(9932):1933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van Craenenbroeck EM, Conraads VM. Mending injured endothelium in chronic heart failure: a new target for exercise training. Int J Cardiol. 2013;166(2):310–4. [DOI] [PubMed] [Google Scholar]
- 37. Dignat-George F, Sampol J. Circulating endothelial cells in vascular disorders: new insights into an old concept. Eur J Haematol. 2000;65(4):215–20. [DOI] [PubMed] [Google Scholar]
- 38. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–95. [DOI] [PubMed] [Google Scholar]
- 39. Forte A, Della Corte A, De Feo M, Cerasuolo F, Cipollaro M. Role of myofibroblasts in vascular remodelling: focus on restenosis and aneurysm. Cardiovasc Res. 2010;88(3):395–405. [DOI] [PubMed] [Google Scholar]
- 40. Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112(12):1776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Libby P, Aikawa M. Stabilization of atherosclerotic plaques: new mechanisms and clinical targets. Nat Med. 2002;8(11):1257–62. [DOI] [PubMed] [Google Scholar]
- 42. Werner N, Wassmann S, Ahlers P, Schiegl T, Kosiol S, Link A, Walenta K, Nickenig G. Endothelial progenitor cells correlate with endothelial function in patients with coronary artery disease. Basic Res Cardiol. 2007;102(6):565–71. [DOI] [PubMed] [Google Scholar]
- 43. Scheubel RJ, Zorn H, Silber RE, Kuss O, Morawietz H, Holtz J, Simm A. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2003;42(12):2073–80. [DOI] [PubMed] [Google Scholar]
- 44. Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353(10):999–1007. [DOI] [PubMed] [Google Scholar]
- 45. Kiewisz J, Kaczmarek MM, Pawlowska A, Kmiec Z, Stompor T. Endothelial progenitor cells participation in cardiovascular and kidney diseases: a systematic review. Acta Biochim Pol. 2016;63(3):475–82. [DOI] [PubMed] [Google Scholar]
- 46. Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111(22):2981–7. [DOI] [PubMed] [Google Scholar]
- 47. Lau KK, Chan YH, Yiu KH, Li SW, Tam S, Lau CP, Kwong YL, Tse HF. Burden of carotid atherosclerosis in patients with stroke: relationships with circulating endothelial progenitor cells and hypertension. J Hum Hypertens. 2007;21(6):445–51. [DOI] [PubMed] [Google Scholar]
- 48. Fadini GP, de Kreutzenberg S, Albiero M, Coracina A, Pagnin E, Baesso I, Cignarella A, Bolego C, Plebani M, Nardelli GB, Sartore S, Agostini C, Avogaro A. Gender differences in endothelial progenitor cells and cardiovascular risk profile: the role of female estrogens. Arterioscler Thromb Vasc Biol. 2008;28(5):997–1004. [DOI] [PubMed] [Google Scholar]
- 49. Friedrich EB, Walenta K, Scharlau J, Nickenig G, Werner N. CD34-/CD133+/VEGFR-2+ endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circ Res. 2006;98(3):e20–e25. [DOI] [PubMed] [Google Scholar]
- 50. Hayek SS, MacNamara J, Tahhan AS, Awad M, Yadalam A, Ko YA, Healy S, Hesaroieh I, Ahmed H, Gray B, Sher SS, Ghasemzadeh N, Patel R, Kim J, Waller EK, Quyyumi AA. Circulating progenitor cells identify peripheral arterial disease in patients with coronary artery disease. Circ Res. 2016;119(4):564–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sibal L, Aldibbiat A, Agarwal SC, Mitchell G, Oates C, Razvi S, Weaver JU, Shaw JA, Home PD. Circulating endothelial progenitor cells, endothelial function, carotid intima-media thickness and circulating markers of endothelial dysfunction in people with type 1 diabetes without macrovascular disease or microalbuminuria. Diabetologia. 2009;52(8):1464–73. [DOI] [PubMed] [Google Scholar]
- 52. Xiao Q, Kiechl S, Patel S, Oberhollenzer F, Weger S, Mayr A, Metzler B, Reindl M, Hu Y, Willeit J, Xu Q. Endothelial progenitor cells, cardiovascular risk factors, cytokine levels and atherosclerosis—results from a large population-based study. PLoS One. 2007;2(10):e975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mannarino E, Pirro M. Endothelial injury and repair: a novel theory for atherosclerosis. Angiology 2008;59(Suppl 2):69S–72S. [DOI] [PubMed] [Google Scholar]
- 54. Zhang L, Xu Q. Stem/Progenitor cells in vascular regeneration. Arterioscler Thromb Vasc Biol. 2014;34(6):1114–9. [DOI] [PubMed] [Google Scholar]
- 55. Hristov M, Zernecke A, Bidzhekov K, Liehn EA, Shagdarsuren E, Ludwig A, Weber C. Importance of CXC chemokine receptor 2 in the homing of human peripheral blood endothelial progenitor cells to sites of arterial injury. Circ Res. 2007;100(4):590–7. [DOI] [PubMed] [Google Scholar]
- 56. Wang CH, Cherng WJ, Yang NI, Kuo LT, Hsu CM, Yeh HI, Lan YJ, Yeh CH, Stanford WL. Late-outgrowth endothelial cells attenuate intimal hyperplasia contributed by mesenchymal stem cells after vascular injury. Arterioscler Thromb Vasc Biol. 2008;28(1):54–60. [DOI] [PubMed] [Google Scholar]
- 57. Hristov M, Zernecke A, Liehn EA, Weber C. Regulation of endothelial progenitor cell homing after arterial injury. Thromb Haemost. 2007;98(2):274–7. [PubMed] [Google Scholar]
- 58. Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation 2002;105(25):3017–24. [DOI] [PubMed] [Google Scholar]
- 59. Gulati R, Jevremovic D, Peterson TE, Witt TA, Kleppe LS, Mueske CS, Lerman A, Vile RG, Simari RD. Autologous culture-modified mononuclear cells confer vascular protection after arterial injury. Circulation. 2003;108(12):1520–6. [DOI] [PubMed] [Google Scholar]
- 60. He T, Smith LA, Harrington S, Nath KA, Caplice NM, Katusic ZS. Transplantation of circulating endothelial progenitor cells restores endothelial function of denuded rabbit carotid arteries. Stroke. 2004;35(10):2378–84. [DOI] [PubMed] [Google Scholar]
- 61. de Nigris F, Balestrieri ML, Williams-Ignarro S, D’Armiento FP, Lerman LO, Byrns R, Crimi E, Palagiano A, Fatigati G, Ignarro LJ, Napoli C. Therapeutic effects of autologous bone marrow cells and metabolic intervention in the ischemic hindlimb of spontaneously hypertensive rats involve reduced cell senescence and CXCR4/Akt/eNOS pathways. J Cardiovasc Pharmacol. 2007;50(4):424–33. [DOI] [PubMed] [Google Scholar]
- 62. Leor J, Marber M. Endothelial progenitors: a new Tower of Babel? J Am Coll Cardiol. 2006;48(8):1588–90. [DOI] [PubMed] [Google Scholar]
- 63. Schuh A, Liehn EA, Sasse A, Hristov M, Sobota R, Kelm M, Merx MW, Weber C. Transplantation of endothelial progenitor cells improves neovascularization and left ventricular function after myocardial infarction in a rat model. Basic Res Cardiol. 2008;103(1):69–77. [DOI] [PubMed] [Google Scholar]
- 64. Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1210–21. [DOI] [PubMed] [Google Scholar]
- 65. Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, Rabkin E, Moran AM, Schoen FJ, Atala A, Soker S, Bischoff J, Mayer JE., Jr Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7(9):1035–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Aoki J, Serruys PW, van Beusekom H, Ong AT, McFadden EP, Sianos G, van der Giessen WJ, Regar E, de Feyter PJ, Davis HR, Rowland S, Kutryk MJ. Endothelial progenitor cell capture by stents coated with antibody against CD34: the HEALING-FIM (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth-First In Man) Registry. J Am Coll Cardiol. 2005;45(10):1574–9. [DOI] [PubMed] [Google Scholar]
- 67. Blindt R, Vogt F, Astafieva I, Fach C, Hristov M, Krott N, Seitz B, Kapurniotu A, Kwok C, Dewor M, Bosserhoff AK, Bernhagen J, Hanrath P, Hoffmann R, Weber C. A novel drug-eluting stent coated with an integrin-binding cyclic Arg-Gly-Asp peptide inhibits neointimal hyperplasia by recruiting endothelial progenitor cells. J Am Coll Cardiol. 2006;47(9):1786–95. [DOI] [PubMed] [Google Scholar]
- 68. George J, Afek A, Abashidze A, Shmilovich H, Deutsch V, Kopolovich J, Miller H, Keren G. Transfer of endothelial progenitor and bone marrow cells influences atherosclerotic plaque size and composition in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2005;25(12):2636–41. [DOI] [PubMed] [Google Scholar]
- 69. Smith C, Damas JK, Otterdal K, Oie E, Sandberg WJ, Yndestad A, Waehre T, Scholz H, Endresen K, Olofsson PS, Halvorsen B, Gullestad L, Froland SS, Hansson GK, Aukrust P. Increased levels of neutrophil-activating peptide-2 in acute coronary syndromes: possible role of platelet-mediated vascular inflammation. J Am Coll Cardiol. 2006;48(8):1591–9. [DOI] [PubMed] [Google Scholar]
- 70. Breland UM, Halvorsen B, Hol J, Oie E, Paulsson-Berne G, Yndestad A, Smith C, Otterdal K, Hedin U, Waehre T, Sandberg WJ, Froland SS, Haraldsen G, Gullestad L, Damas JK, Hansson GK, Aukrust P. A potential role of the CXC chemokine GROalpha in atherosclerosis and plaque destabilization: downregulatory effects of statins. Arterioscler Thromb Vasc Biol. 2008;28(5):1005–11. [DOI] [PubMed] [Google Scholar]
- 71. Boisvert WA, Santiago R, Curtiss LK, Terkeltaub RA. A leukocyte homologue of the IL-8 receptor CXCR-2 mediates the accumulation of macrophages in atherosclerotic lesions of LDL receptor-deficient mice. J Clin Invest. 1998;101(2):353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vega FM, Gautier V, Fernandez-Ponce CM, Extremera MJ, Altelaar AFM, Millan J, Tellez JC, Hernandez-Campos JA, Conejero R, Bolivar J, Pardal R, Garcia-Cozar FJ, Aguado E, Heck AJR, Duran-Ruiz MC. The atheroma plaque secretome stimulates the mobilization of endothelial progenitor cells ex vivo. J Mol Cell Cardiol. 2017;105:12–23. [DOI] [PubMed] [Google Scholar]
- 73. Schober A, Hoffmann R, Opree N, Knarren S, Iofina E, Hutschenreuter G, Hanrath P, Weber C. Peripheral CD34+ cells and the risk of in-stent restenosis in patients with coronary heart disease. Am J Cardiol. 2005;96(8):1116–22. [DOI] [PubMed] [Google Scholar]
- 74. Kim JY, Song SH, Kim KL, Ko JJ, Im JE, Yie SW, Ahn YK, Kim DK, Suh W. Human cord blood-derived endothelial progenitor cells and their conditioned media exhibit therapeutic equivalence for diabetic wound healing. Cell Transplant. 2010;19(12):1635–44. [DOI] [PubMed] [Google Scholar]
- 75. Zhang M, Malik AB, Rehman J. Endothelial progenitor cells and vascular repair. Curr Opin Hematol. 2014;21(3):224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hristov M, Zernecke A, Schober A, Weber C. Adult progenitor cells in vascular remodeling during atherosclerosis. Biol Chem. 2008;389(7):837–44. [DOI] [PubMed] [Google Scholar]
- 77. Hernandez-Lopez C, Varas A, Sacedon R, Martinez VG, Hidalgo L, Valencia J, Zapata AG, Vicente A. The CXCL12/CXCR4 pair in aged human thymus. Neuroimmunomodulation. 2010;17(3):217–20. [DOI] [PubMed] [Google Scholar]
- 78. Shao H, Xu Q, Wu Q, Ma Q, Salgueiro L, Wang J, Eton D, Webster KA, Yu H. Defective CXCR4 expression in aged bone marrow cells impairs vascular regeneration. J Cell Mol Med. 2011;15(10):2046–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Xu Q, Wang J, He J, Zhou M, Adi J, Webster KA, Yu H. Impaired CXCR4 expression and cell engraftment of bone marrow-derived cells from aged atherogenic mice. Atherosclerosis 2011;219(1):92–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Xia WH, Li J, Su C, Yang Z, Chen L, Wu F, Zhang YY, Yu BB, Qiu YX, Wang SM, Tao J. Physical exercise attenuates age-associated reduction in endothelium-reparative capacity of endothelial progenitor cells by increasing CXCR4/JAK-2 signaling in healthy men. Aging Cell. 2012;11(1):111–9. [DOI] [PubMed] [Google Scholar]
- 81. Rehman J. Feeling the elephant of cardiovascular cell therapy. Circulation. 2010;121(2):197–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yang JX, Pan YY, Zhao YY, Wang XX. Endothelial progenitor cell-based therapy for pulmonary arterial hypertension. Cell Transplant. 2013;22(8):1325–36. [DOI] [PubMed] [Google Scholar]
- 83. Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45(9):1441–8. [DOI] [PubMed] [Google Scholar]
- 84. Jie KE, Goossens MH, van Oostrom O, Lilien MR, Verhaar MC. Circulating endothelial progenitor cell levels are higher during childhood than in adult life. Atherosclerosis 2009;202(2):345–7. [DOI] [PubMed] [Google Scholar]
- 85. Xia WH, Yang Z, Xu SY, Chen L, Zhang XY, Li J, Liu X, Qiu YX, Shuai XT, Tao J. Age-related decline in reendothelialization capacity of human endothelial progenitor cells is restored by shear stress. Hypertension 2012;59(6):1225–31. [DOI] [PubMed] [Google Scholar]
- 86. Wang CH, Lee MF, Yang NI, Mei HF, Lin SY, Cherng WC. Bone marrow rejuvenation accelerates re-endothelialization and attenuates intimal hyperplasia after vascular injury in aging mice. Circ J. 2013;77(12):3045–53. [DOI] [PubMed] [Google Scholar]
- 87. Felice F, Barsotti MC, Poredos P, Balbarini A, Di Stefano R. Effect of aging on metabolic pathways in endothelial progenitor cells. Curr Pharm Des. 2013;19(13):2351–65. [DOI] [PubMed] [Google Scholar]
- 88. Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, Kim YJ, Soo Lee D, Sohn DW, Han KS, Oh BH, Lee MM, Park YB. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet. 2004;363(9411):751–6. [DOI] [PubMed] [Google Scholar]
- 89. Nasseri BA, Ebell W, Dandel M, Kukucka M, Gebker R, Doltra A, Knosalla C, Choi YH, Hetzer R, Stamm C. Autologous CD133+ bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: the Cardio133 trial. Eur Heart J. 2014;35(19):1263–74. [DOI] [PubMed] [Google Scholar]
- 90. Yang J, Yu J, Li D, Yu S, Ke J, Wang L, Wang Y, Qiu Y, Gao X, Zhang J, Huang L. Store-operated calcium entry-activated autophagy protects EPC proliferation via the CAMKK2-MTOR pathway in ox-LDL exposure. Autophagy. 2017;13(1):82–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rurali E, Bassetti B, Perrucci GL, Zanobini M, Malafronte C, Achilli F, Gambini E. BM ageing: implication for cell therapy with EPCs. Mech Ageing Dev. 2016;159:4–13. [DOI] [PubMed] [Google Scholar]
- 92. Sanada F, Taniyama Y, Azuma J, Iekushi K, Dosaka N, Yokoi T, Koibuchi N, Kusunoki H, Aizawa Y, Morishita R. Hepatocyte growth factor, but not vascular endothelial growth factor, attenuates angiotensin II-induced endothelial progenitor cell senescence. Hypertension. 2009;53(1):77–82. [DOI] [PubMed] [Google Scholar]
- 93. Thum T, Hoeber S, Froese S, Klink I, Stichtenoth DO, Galuppo P, Jakob M, Tsikas D, Anker SD, Poole-Wilson PA, Borlak J, Ertl G, Bauersachs J. Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Circ Res. 2007;100(3):434–43. [DOI] [PubMed] [Google Scholar]
- 94. Xia WH, Chen L, Liang JW, Zhang XY, Su C, Tong X, He J, Li Y, Cao Z, Lin XF, Tao J. BMP4/Id2 signaling pathway is a novel therapeutic target for late outgrowth endothelial progenitor cell-mediated endothelial injury repair. Int J Cardiol. 2017;228:796–804. [DOI] [PubMed] [Google Scholar]
- 95. Sorrentino SA, Doerries C, Manes C, Speer T, Dessy C, Lobysheva I, Mohmand W, Akbar R, Bahlmann F, Besler C, Schaefer A, Hilfiker-Kleiner D, Luscher TF, Balligand JL, Drexler H, Landmesser U. Nebivolol exerts beneficial effects on endothelial function, early endothelial progenitor cells, myocardial neovascularization, and left ventricular dysfunction early after myocardial infarction beyond conventional beta1-blockade. J Am Coll Cardiol. 2011;57(5):601–11. [DOI] [PubMed] [Google Scholar]
- 96. Pelliccia F, Pasceri V, Cianfrocca C, Vitale C, Speciale G, Gaudio C, Rosano GM, Mercuro G. Angiotensin II receptor antagonism with telmisartan increases number of endothelial progenitor cells in normotensive patients with coronary artery disease: a randomized, double-blind, placebo-controlled study. Atherosclerosis. 2010;210(2):510–5. [DOI] [PubMed] [Google Scholar]
- 97. De Ciuceis C, Rossini C, Tincani A, Airo P, Scarsi M, Agabiti-Rosei C, Ruggeri G, Caimi L, Ricotta D, Agabiti-Rosei E, Rizzoni D. Effect of antihypertensive treatment with lercanidipine on endothelial progenitor cells and inflammation in patients with mild to moderate essential hypertension. Blood Press. 2016;25(6):337–43. [DOI] [PubMed] [Google Scholar]
- 98. Peixiao S, Ningyuan F, Haiya W. Lercanidipine effect on circulating CD34+ progenitor cells in elderly patients: a randomized study. Curr Med Res Opin. 2016;32(suppl 2):9–12. [DOI] [PubMed] [Google Scholar]
- 99. Sun J, Xie J, Kang L, Ferro A, Dong L, Xu B. Amlodipine ameliorates ischemia-induced neovascularization in diabetic rats through endothelial progenitor cell mobilization. Biomed Res Int. 2016;2016:3182764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Redondo S, Hristov M, Gumbel D, Tejerina T, Weber C. Biphasic effect of pioglitazone on isolated human endothelial progenitor cells: involvement of peroxisome proliferator-activated receptor-gamma and transforming growth factor-beta1. Thromb Haemost. 2007;97(6):979–87. [PubMed] [Google Scholar]
- 101. Werner C, Kamani CH, Gensch C, Bohm M, Laufs U. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone increases number and function of endothelial progenitor cells in patients with coronary artery disease and normal glucose tolerance. Diabetes. 2007;56(10):2609–15. [DOI] [PubMed] [Google Scholar]
- 102. Chantzichristos VG, Agouridis AP, Moutzouri E, Stellos K, Elisaf MS, Tselepis AD. Effect of rosuvastatin or its combination with omega-3 fatty acids on circulating CD34(+) progenitor cells and on endothelial colony formation in patients with mixed dyslipidaemia. Atherosclerosis. 2016;251:240–7. [DOI] [PubMed] [Google Scholar]
- 103. Wang XX, Yang JX, Pan YY, Zhang YF. Protective effects of tanshinone A on endothelial progenitor cells injured by tumor necrosis factor-alpha. Mol Med Rep. 2015;12(3):4055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hu Z, Wang H, Fan G, Zhang H, Wang X, Mao J, Zhao Y, An Y, Huang Y, Li C, Chang L, Chu X, Li L, Li Y, Zhang Y, Qin G, Gao X, Zhang B. Effect of danhong injection on the mobilisation of endothelial progenitor cells to vascular repair after percutaneous coronary intervention: a randomised controlled trial. Lancet. 2016;388(Suppl 1):S34. [Google Scholar]
- 105. Paul JD, Powell TM, Thompson M, Benjamin M, Rodrigo M, Carlow A, Annavajjhala V, Shiva S, Dejam A, Gladwin MT, McCoy JP, Zalos G, Press B, Murphy M, Hill JM, Csako G, Waclawiw MA, Cannon RO., 3rd Endothelial progenitor cell mobilization and increased intravascular nitric oxide in patients undergoing cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2007;27(2):65–73. [DOI] [PubMed] [Google Scholar]
- 106. Hoetzer GL, Van Guilder GP, Irmiger HM, Keith RS, Stauffer BL, DeSouza CA. Aging, exercise, and endothelial progenitor cell clonogenic and migratory capacity in men. J Appl Physiol (1985). 2007;102(3):847–52. [DOI] [PubMed] [Google Scholar]
- 107. Yang Z, Xia WH, Su C, Wu F, Zhang YY, Xu SY, Liu X, Zhang XY, Ou ZJ, Lai GH, Liao XX, Jin YF, Tao J. Regular exercise-induced increased number and activity of circulating endothelial progenitor cells attenuates age-related decline in arterial elasticity in healthy men. Int J Cardiol. 2013;165(2):247–54. [DOI] [PubMed] [Google Scholar]
- 108. Guo Y, Peng R, Liu Q, Xu D. Exercise training-induced different improvement profile of endothelial progenitor cells function in mice with or without myocardial infarction. Int J Cardiol. 2016;221:335–41. [DOI] [PubMed] [Google Scholar]
- 109. Marin C, Yubero-Serrano EM, Lopez-Miranda J, Perez-Jimenez F. Endothelial aging associated with oxidative stress can be modulated by a healthy Mediterranean diet. Int J Mol Sci. 2013;14(5):8869–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Fernandez JM, Rosado-Alvarez D, Da Silva Grigoletto ME, Rangel-Zuniga OA, Landaeta-Diaz LL, Caballero-Villarraso J, Lopez-Miranda J, Perez-Jimenez F, Fuentes-Jimenez F. Moderate-to-high-intensity training and a hypocaloric Mediterranean diet enhance endothelial progenitor cells and fitness in subjects with the metabolic syndrome. Clin Sci (Lond). 2012;123(6):361–73. [DOI] [PubMed] [Google Scholar]
- 111. Maiorino MI, Bellastella G, Petrizzo M, Gicchino M, Caputo M, Giugliano D, Esposito K. Effect of a Mediterranean diet on endothelial progenitor cells and carotid intima-media thickness in type 2 diabetes: follow-up of a randomized trial. Eur J Prev Cardiol. 2017;24(4):399–408. [DOI] [PubMed] [Google Scholar]
- 112. Grassi D, Draijer R, Schalkwijk C, Desideri G, D’Angeli A, Francavilla S, Mulder T, Ferri C. Black tea increases circulating endothelial progenitor cells and improves flow mediated dilatation counteracting deleterious effects from a fat load in hypertensive patients: a randomized controlled study. Nutrients. 2016;8(11):727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ke X, Shu XR, Wu F, Hu QS, Deng BQ, Wang JF, Nie RQ. Overexpression of the beta2AR gene improves function and re-endothelialization capacity of EPCs after arterial injury in nude mice. Stem Cell Res Ther. 2016;7(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wang H, Yin Y, Li W, Zhao X, Yu Y, Zhu J, Qin Z, Wang Q, Wang K, Lu W, Liu J, Huang L. Over-expression of PDGFR-beta promotes PDGF-induced proliferation, migration, and angiogenesis of EPCs through PI3K/Akt signaling pathway. PLoS One 2012;7(2):e30503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wang H, Yin YG, Huang H, Zhao XH, Yu J, Wang Q, Li W, Cai KY, Ding SF. Transplantation of EPCs overexpressing PDGFR-beta promotes vascular repair in the early phase after vascular injury. BMC Cardiovasc Disord. 2016;16(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lee JH, Ji ST, Kim J, Takaki S, Asahara T, Hong YJ, Kwon SM. Specific disruption of Lnk in murine endothelial progenitor cells promotes dermal wound healing via enhanced vasculogenesis, activation of myofibroblasts, and suppression of inflammatory cell recruitment. Stem Cell Res Ther. 2016;7(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Dai X, Yan X, Zeng J, Chen J, Wang Y, Li Y, Barati MT, Wintergerst KA, Pan K, Nystoriak MA, Conklin DJ, Rokosh G, Epstein PN, Li X, Tan Y. Elevating CXCR7 improves angiogenic function of EPCs via Akt/GSK-3beta/Fyn-mediated Nrf2 activation in diabetic limb ischemia. Circ Res. 2017;120(5):e7–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]