Abstract
As the most voluminous organ of the body that is exposed to the outer environment, the skin suffers from both intrinsic and extrinsic aging factors. Skin aging is characterized by features such as wrinkling, loss of elasticity, laxity, and rough-textured appearance. This aging process is accompanied with phenotypic changes in cutaneous cells as well as structural and functional changes in extracellular matrix components such as collagens and elastin. In this review, we summarize these changes in skin aging, research advances of the molecular mechanisms leading to these changes, and the treatment strategies aimed at preventing or reversing skin aging.
Keywords: skin aging, intrinsic aging, extrinsic aging, extracellular matrix, treatment strategy
Introduction
Skin is the barrier that segregates the body from the outer environment. Besides protecting the body from water loss and microorganism infection, it has an important cosmetic role. Young and beautiful appearance may have a positive influence on people’s social behavior and reproductive status1.
However, aging of organs begins from the time when one is born, and there is no exception for the skin. As the most voluminous organ of the body, the skin shows obvious and visible sign of aging when one becomes older. Therefore, for many people, especially females, a considerable amount of daily expense is occupied by cosmetics and pharmaceuticals attempting to prevent or reverse skin aging2. This vast cosmetic need continually promotes research on skin aging and its treatment.
Cutaneous aging is induced by both intrinsic and extrinsic factors. Intrinsic aging is an inevitable physiological process that results in thin, dry skin, fine wrinkles, and gradual dermal atrophy, while extrinsic aging is engendered by external environment factors such as air pollution, smoking, poor nutrition, and sun exposure, resulting in coarse wrinkles, loss of elasticity, laxity, and rough-textured appearance3,4. Notably, long-term exposure to solar ultraviolet (UV) radiation is the primary factor of extrinsic skin aging and is referred to as photoaging4. In this review, we will summarize the changes during skin aging, research advances of the molecular mechanisms leading to these changes, and treatment strategies.
Changes in Skin Aging
Exposed directly to the air, skin is not only subject to intrinsic aging but also superimposed by extrinsic aging. These aging processes are accompanied by phenotypic changes in cutaneous cells as well as structural and functional changes in extracellular matrix components such as collagens, elastin, and proteoglycans that are required to provide tensile strength, elasticity, and hydration to the skin, respectively4.
Changes in Intrinsic Aging
Intrinsic skin aging is a process of chronologically physiological change. Aging of photoprotected areas for example, the inner side of the upper arm, is mainly due to intrinsic genetic or metabolic factors, whereas exposed skin areas are additionally influenced by extrinsic factors, especially solar UV radiation5.
For the intrinsically aged skin, the most remarkable histological changes occur within the basal cell layer. Research finds that as a person ages, proliferation of cells in the basal layer reduces. The epidermis then becomes thinner, and the contact surface area between dermis and epidermis decreases, resulting in a smaller exchange surface for nutrition supply to the epidermis and further weakened ability of basal cell proliferation6,7. This process of decreased proliferative ability of cells including keratinocytes, fibroblasts, and melanocytes is called cellular senescence. In skin samples from human donors of different ages, there was an age-dependent increase in the expression of senescence marker β-galactosidase in dermal fibroblasts and epidermal keratinocytes, indicating that aged skin contains more senescent cells8.
In addition, the dermis of photoprotected aged skin shows not only fewer mast cells and fibroblasts than photoprotected young skin but also rarefied collagen fibers and elastic fibers9. It is reported that the production of type I procollagen in intrinsically aged human skin is reduced likely because of downregulation of the TGF-β/Smad signaling and its downstream connective tissue growth factor, which is regarded as a regulator of collagen expression10. Moreover, evidence supports that in intrinsically aged skin, not only fibrous extracellular matrix components including elastin, fibrillin, and collagens but also oligosaccharide are degenerated, which in turn influences the ability of skin to retain bound water11.
Changes in Extrinsic Aging
As early as 1969, it was proposed that besides intrinsic factors, sun exposure also leads to skin aging12. Exposure to UV radiation is the primary factor of extrinsic skin aging; it accounts for about 80% of facial aging13. In contrast to the thinner epidermis in intrinsically aged skin, UV-radiated epidermis thickens14. As the outermost layer of the epidermis, stratum corneum is mostly affected and thickens because of failure of degradation of corneocyte desmosomes. The expression of differentiation marker involucrin in stratum corneum is increased, which is in accord with the fact that the differentiation process of epidermal keratinocytes is impaired by UV irradiation. In basal cells, the expression of cell-surface protein β1-integrin, which interacts with extracellular matrix proteins and is regarded as one of the epidermal stem cell markers, is greatly reduced, indicating that proliferation in the aged basal keratinocytes is also impaired15,16.
The expression of type VII collagen in keratinocytes decreased in UV-radiated skin areas. Type VII collagen is the anchoring fibrils at the dermal–epidermal junction. The decrease in its production contributes to wrinkles due to the weakened connection between dermis and epidermis17. Studies have found that collagen type I diminishes in photoaged skin18,19 due to increased collagen degradation20. Various matrix metalloproteinases (MMPs), serine proteases, and other proteases participate in this degradation activity16,21,22.
For photoaged skin, a striking characteristic is the accumulation of abnormal elastic tissue deep in the dermis23, a pathologic phenotype named solar elastosis. UV-irradiation elevates the expression of elastin by 4-fold, then elastolysis occurs, characterized by elastic fiber cleavage by proteases mentioned above, resulting in severe deposition of truncated elastic fibers4,24. MMP-2, MMP-3, MMP-7, MMP-9, MMP-12, neutrophil serine proteases cathepsin G, and human leukocyte elastase are known to decompose elastin. Recent research has found that photoaging makes the N-terminal and central parts of the tropoelastin molecules more susceptible to enzymatic cleavage and, hence, accelerates the age-related degradation of elastin4.
Also, the function of the microvasculature declines with aging. This is caused by endothelial dysfunction including reduced angiogenic capacity, aberrant expression of adhesion molecules, and impaired vasodilatory function25.
Molecular Mechanisms in Skin Aging
Different models are proposed to explain the molecular basis for skin aging, including the theory of cellular senescence, decrease in cellular DNA repair capacity and loss of telomeres, point mutations of extranuclear mitochondrial DNA, oxidative stress, increased frequency of chromosomal abnormalities, single-gene mutations, reduced sugar, chronic inflammation, and so on11. Some scientists proposed that most of the effects are caused by extrinsic factors, and only 3% of aging factors have intrinsic background26. Here we highlight mainly important models and advances in molecular mechanism research on skin aging.
Oxidative Stress
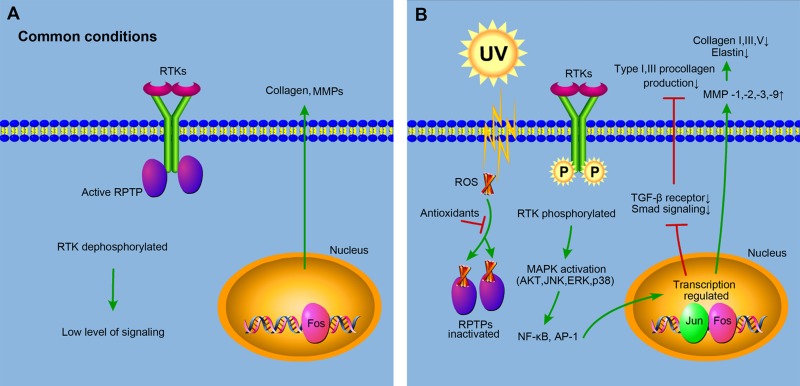
It is regarded that reactive oxygen species (ROS) play a critical role in dermal extracellular matrix alterations of both intrinsic aging and photoaging. ROS can be produced from different sources including the mitochondrial electron transport chain, peroxisomal and endoplasmic reticulum (ER) localized proteins, the Fenton reaction, and such enzymes as cyclooxygenases, lipoxygenases, xanthine oxidases, and nicotinamide-adenine dinucleotide phosphate (NADPH) oxidases27. Under common conditions without ligands, the activity of receptor tyrosine kinases (RTKs) on the cell surface is inhibited by receptor protein tyrosine phosphatases (RPTPs), which dephosphorylate RTKs (Fig. 1A). However, under UV radiation, cellular chromophores absorb the energy and get excitated, producing oxidation products and ROS. ROS inhibit the activity of RPTPs by binding to cysteine in the catalytic sites of RPTPs28, elevating the level of phosphorylated RTKs and triggering downstream signaling pathways including the activation of mitogen-activated protein kinase (MAPK) and subsequent nuclear factor-κB (NF-κB) and transcription factor activator protein-1 (AP-1). Activated NF-κB and AP-1 repress collagen production and increase MMP gene transcription, resulting in the decrease of collagen content in photoaged skin29 (Fig. 1B). It’s worth noting that NF-κB was recently found to be activated by mammalian target of rapamycin complexes 2/Akt/IκB kinase α (mTORC2/Akt/IKKα) pathway in both intrinsic aging and photoaging30.
Figure 1.
Molecular mechanisms of reactive oxygen species (ROS) in skin aging. (A) Under common conditions without ligands, the activity of receptor tyrosine kinases (RTKs) on the cell surface is inhibited by receptor protein tyrosine phosphatases (RPTPs), which dephosphorylate RTKs and keep low levels of signaling, producing a normal amount of collagen and matrix metalloproteinases (MMPs). (B) Under ultraviolet radiation, ROS are produced, which inhibit the activity of RPTPs by binding to the catalytic sites of RPTPs, elevating the level of phosphorylated RTKs and triggering downstream signaling pathways including the activation of mitogen-activated protein kinase (MAPK) and subsequent nuclear factor-κB (NF-κB) and transcription factor activator protein-1 (AP-1). NF-κB and AP-1 increase MMP gene transcription, and AP-1 downregulates the expression of transforming growth factor-β (TGF-β) type II receptor, resulting in the reduced phosphorylation of transcription factor Smads and the subsequent repression of the collagen production. Thus, the total collagen content in photoaged skin decreases. Antioxidants can neutralize ROS, prevent its binding to RPTPs, and restore the signaling back to normal levels. This diagram is revised from Rittie and Fisher28 and Kammeyer and Luiten29.
Surprisingly, other studies found that although significantly higher mitochondrial superoxide levels and oxidative damage exist in nuo-6 and isp-1 mutants of Caenorhabditis elegans31,32 Mclk1+/− mice33, or long-lived naked mole rats34,35 than in control ones, these individuals display an increased life span and reduced signs of aging that, at least in C. elegans, could be suppressed by the antioxidant treatment31. These findings are not consistent with the oxidative stress theory of aging, and researchers suggest that other mechanisms are needed to explain these phenomena34.
DNA Damage
Persistently exposing skin to UV radiation increases DNA damage and mutations and leads to premature aging or carcinogenesis36. When DNA absorbs photons from UV-B, structural rearrangement of nucleotides occurs, resulting in defects of DNA strands37. DNA damage can be repaired in lesser species through removing the lesion by photolyase enzyme, but higher mammalian and human cells don’t have this enzyme. It’s accomplished by the nucleotide excision repair pathway38. Once proteins in this pathway suffer from deficiency, DNA damage and premature skin aging will occur39. Many studies provide consistent evidence that use of sunscreen prevents DNA damage in vivo and protects the skin from squamous cell carcinoma and melanoma40, which further proves another aspect of the destructive effect of UV radiation on the DNA strand. Detailed description on the mechanism of DNA damage has already been reviewed38,41.
Telomere Shortening
Telomeres are repetitive nucleotide sequences that cap and save the ends of chromosomes from degradation and abnormal recombination. They become shorter with each cell division and ultimately result in cellular senescence and limited numbers of cell division38. Telomerase is an enzyme that adds telomere repetitions to the end to prevent telomeres from getting shorter. Telomerase-deficient mice exhibited accelerated telomere shortening, resulting in defective tissue regeneration. Moreover, proliferation capacity of epidermal stem cells with short telomeres was suppressed, whereas telomerase reintroduction in mice with critically short telomeres is sufficient to correct epidermal stem cell defects42. UV radiation leads to excessive ROS production, resulting in telomere mutations and further cell death or senescence43.
MicroRNA (miRNA) Regulation
miRNAs are a class of conserved noncoding RNAs that bind to the 3’ untranslated region of target mRNAs to promote their degradation and/or inhibit their translation. In recent years, miRNA dysregulation is found to occur in cellular senescence and organismal aging and is reviewed elsewhere5.
Besides these findings reviewed, it’s reported that miR-23a-3p causes cellular senescence by targeting hyaluronan synthase 2 (HAS2)44. Hyaluronan (HA) is a kind of polysaccharide in the extracellular matrix. Both aged and senescent fibroblasts showed increased miR-23a-3p expression and secreted significantly lower amounts of HA compared with young and nonsenescent fibroblasts. Ectopic overexpression of miR-23a-3p in nonsenescent fibroblasts led to decreased HAS2-mediated HA synthesis, upregulation of senescence-associated markers, and decreased proliferation. In vivo, miR-23a-3p was upregulated and HAS2 was downregulated in the skin of old mice compared with young ones. In addition, recent research finds that miR-126, the most abundant miRNA in endothelial cells known to protect blood vessels and induce neovascularization, is significantly downregulated in aged human skin interstitial fluid in accordance with the fact that the number and functionality of capillary structures decreases with age45. However, the detailed molecular mechanism needs further investigation.
Advanced Glycation End Product Accumulation
Advanced glycation end (AGE) products are formed by a nonenzymatic process called glycation, during which proteins, lipids, or nucleic acids are covalently bound by sugar molecules such as glucose or fructose, resulting in the inhibition of normal function of target molecules46. This is quite different from normal glycosylation, which occurs at defined sites under mediation of enzymes and is necessary for target molecules to fulfill their functions. Glycation is involved in both intrinsic and extrinsic aging. Long-lived proteins in the dermal matrix and cytoskeleton are particularly susceptible to glycation, resulting in tissue stiffening and reduced elasticity47. Among extracellular proteins, glycated elastin fibers abnormally aggregate and unusually interact with lysozyme in skin of solar elastosis but not sun-protected sites48, indicating that glycation is involved in photoaging. However, glycated collagen is highly resistant to MMP degradation49 and thus, it accumulates with age. This deteriorates the condition under the context of increased degradation of functional collagen and decreases collagen production mentioned above. For intracellular proteins, intermediate filament component vimentin was found to be the major intracellular target for Nε-(carboxymethyl)lysine glycation in fibroblasts of elder human facial skin, whose modification led to a rigorous redistribution of vimentin into a perinuclear aggregate, accompanied by loss of cellular contractile capacity50. In addition, recent research found that in sun-exposed old human skin, glyoxalase 2, which detoxifies the detrimental precursors of AGEs, showed drastic lower expression, resulting in a huge accumulation of glycated proteins51.
Notably, AGEs can also function by binding to cell surface receptors called receptors for AGEs to activate signal pathways, such as MAPKs, NF-κB, extracellular signal-regulated kinases, and phosphatidyl-inositol-3-kinase52.
Genetic Mutation
Some researchers shed light on gene mutation–caused progeroid syndromes, such as Hutchinson-Gilford progeria syndrome (HGPS), Werner syndrome, Rothmund–Thomson syndrome, Cockayne syndrome, ataxia-telangiectasia, and Down syndrome53. Gene mutation is inherited and causes progeria, a type of premature aging, often showing accelerated skin aging phenotype, including skin atrophy and sclerosis, poikiloderma, alopecia, thinning, and graying of the hair53, for example, HGPS is caused by the mutation of gene LMNA54, which produces progerin, a mutant protein that impairs many important cellular processes55. More work is being done to try to gain insight into genetic variation during the aging process. In 2014 and 2017, different groups identified single-nucleotide polymorphisms associated with skin aging in Caucasians and the Chinese Han population, respectively56,57.
Inflammaging
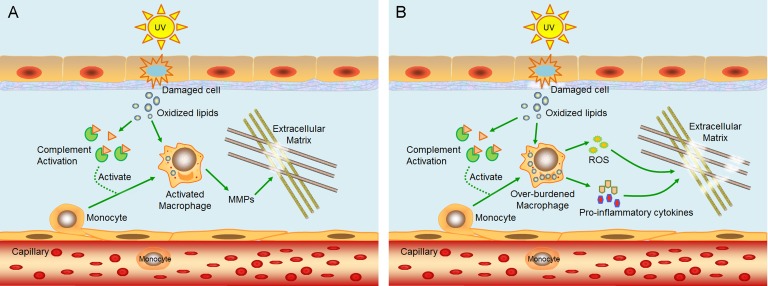
Chronic, low-grade inflammation is also recognized as a major characteristic of the aging process58. This phenomenon is called “inflammaging.” Inflammaging plays a role in the initiation and progression of age-related diseases such as type II diabetes, Alzheimer’s disease, cardiovascular disease, frailty, sarcopenia, osteoporosis, and skin aging59,60. To explain its occurrence in skin, a model is proposed in 2014 based on previous data60. Briefly, UV radiation induces oxidative stress in epidermal cells, resulting in damaged cells with oxidized lipids. Oxidation-specific epitopes on damaged cells and oxidized lipids are recognized by complement system and cause inflammation, leading to infiltration and activation of macrophages to remove the damaged cells and oxidized lipids61,62. Activated macrophages release MMPs to degrade extracellular matrix (Fig. 2A). Repeated UV radiation overactivates the complement system, causing damage to the dermis–epidermis junction, on which they deposit, and macrophages are overburdened with oxidized lipids. Overburdened macrophages release proinflammatory cytokines and ROS61,63,64, the former of which cause chronic inflammation and long-term damage to the dermis, while the latter triggers the oxidative stress-induced damage to the dermal extracellular matrix (Fig. 2B). However, many details of this model need further verification.
Figure 2.
A model proposed to explain the mechanism of inflammaging in skin. (A) Ultraviolet (UV) radiation induces oxidative stress in epidermal cells, resulting in damaged cells with oxidized lipids. Oxidation-specific epitopes on damaged cells and oxidized lipids activate complement systems and cause inflammation, leading to infiltration and activation of macrophages. Activated macrophages release matrix metalloproteinases (MMPs) to degrade extracellular matrix. (B) Repeated UV radiation overactivates the complement system, causing damage to the dermis–epidermis junction, on which they deposit, and macrophages are overburdened with oxidized lipids. Overburdened macrophages release proinflammatory cytokines and reactive oxygen species (ROS), the former of which cause chronic inflammation and long-term damage to the dermis, while the latter triggers the oxidative stress-induced damages to the dermal extracellular matrix. This schematic diagram is revised from Zhuang and Lyga60.
Treatments for Skin Aging
Antioxidants
Antioxidants as reducing agents can relieve skin aging by neutralizing ROS that have already formed. ROS activates MAPK pathway and subsequently increase MMP production that degrades collagen. This can be prevented by antioxidants (Fig. 1B), such as vitamin C and vitamin E, or antioxidative enzymes, such as superoxide dismutase, catalase, glutathione peroxidase, and coenzyme Q1065,66. Some plants can also be used as natural source of antioxidants, such as green tea and aloe vera66. A recent example is that epigallocatechin gallate (EGCG), a kind of catechin in green tea, prevents skin aging via the epidermal growth factor receptor (EGFR) pathway in an aging mouse model, resulting in better skin structure than the control67. Moreover, N-acetylcysteine, the precursor to the antioxidant glutathione, seems to be successful in the treatment of vascular and nonvascular neurological disorders as well as against age-related decline in tissue regeneration68,69, indicating its prospective antiaging application in skin.
However, it’s notable that some researchers suggest that antioxidant supplements do not possess preventive effects to chronic diseases, and excessive supplementation of β-carotene and vitamins A and E is potentially harmful with unwanted side effects70,71, especially in well-nourished populations, and the optimal source of antioxidants seems to come from our diet not from antioxidant supplements in pills or tablets72. And a previous study found that EGCG induced significant death and DNA damage in human lung and skin normal cells through a reductive mechanism73. The aim of antioxidant treatment is to restore oxygen homeostasis instead of completely eliminating all oxidants because they have their physiological functions74. Thus, for necessary clinical application of antioxidants, the doctor should evaluate the status of the patient before giving a prescription. It’s not desirable to completely inactivate all ROS, and antioxidant treatment seems to be beneficial for aging (including skin aging) only if the ROS level is reduced to those of healthy cells.
Stem Cell Therapy
Stem cell transplantation is a promising therapy for the treatment of skin aging. Adipose tissue transplantation could improve skin quality at the recipient site in addition to increasing skin volume75. Further experiments demonstrate that adipose-derived stem cells (ADSCs) contribute to the regeneration of skin during aging76,77. In recent clinical tests, autologous fat grafting rejuvenates aging skin and enhances the volume of periocular and perioral skin in recipients with an average age of 50 years78,79. Data show that ADSCs produce a series of growth factors, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), transforming growth factor (TGF)-β1, TGF-β2, hepatocyte growth factor (HGF), keratinocyte growth factor (KGF), platelet-derived growth factor AA (PDGF-AA), and placental growth factor (PGF)80, reminding us that ADSCs may influence surrounding cutaneous cells through these secretions. It seemed that ADSC may also transdifferentiate into epithelial stem cells that express epithelial stem cell marker p63 after fat grafting81. This work provides clues into the understanding of how fat grafts may rejuvenate overlying skin.
Retinoids
Retinoids are chemically similar to vitamin A, and tretinoin is the first retinoid approved for clinical use. Topical application of tretinoin inhibits AP-1, thus suppressing the expression of MMPs and preventing the degradation of collagen82. An increase in epidermal thickness and anchoring fibrils is observed, and intrinsically aged skin may also benefit from the topical application of retinoids83.
Hormone Replacement Therapy
In addition to being used in the treatment of symptoms caused by menopause, hormone replacement therapy (HRT) is used to slow the skin aging process. HRT improve skin thickness, collagen content, and elasticity and it enhances hydration. However, there are studies suggesting that HRT increases the risk of developing breast cancer84.
Telomere Modification
Although telomerase activation seems to be an ideal approach to reverse skin aging, and indeed high-level expression of telomerase reverse transcriptase (TERT) in skin fibroblasts and keratinocytes results in a significantly enhanced proliferative capacity85, but risks of epidermal carcinogenesis increase meanwhile86. Therefore, further investigation is needed to evaluate the safety of the enhanced proliferation brought by the lengthened telomere.
Diet Restriction
Because it’s still technically infeasible to reverse glycated proteins to their original state, currently the primary strategy still stays on the prevention of proteins from glycation. But the problem is that diet provides not only sugars such as glucose and fructose but also preformed AGEs, and the latter have a large amount in grilled, fried, or roasted food but very low content in foods prepared by water-based cooking such as boiling and steaming46. Therefore, low-sugar food cooked with water would decrease the intake of preformed exogenous AGEs and endogenous production of physiologically glycated proteins. In the future, finding medicines of deglycation capability would be an expected breakthrough discovery.
Some scientists believe that some culinary herbs and spices, such as cinnamon, cloves, oregano, and allspice, can inhibit fructose-induced glycation87, and some compounds, including ginger, garlic, α-lipoic acid, carnitine, taurine, carnosine, flavonoids (e.g., green tea catechins), benfotiamine, α-tocopherol, niacinamide, pyridoxal, sodium selenite, selenium yeast, riboflavin, zinc, and manganese, are also involved in the inhibition of AGE formation52,87–89. More investigation is needed to further validate these findings and reveal their inhibitory mechanisms.
Antiprogeria Strategies
Currently, therapies targeting HGPS are mostly investigated. Because the farnesyl group in the mutant protein progerin was regarded as the predominant deleterious and toxic component, the original therapies are designed to inhibit the farnesylation process90. Farnesyltransferase inhibitor lonafarnib was used in a clinical trial to treat 25 HGPS patients for 2 years, resulting in improved vascular stiffness, bone structure, and audiological status91. Another strategy using the combination of 2 compounds, statin and aminobisphosphonate, efficiently inhibited both farnesylation and geranylgeranylation of progerin and prelamin A and markedly improved the aging-like phenotypes of Zmpste24-deficient progeria mice model including growth retardation, loss of weight, lipodystrophy, hair loss, and bone defects92. A clinical trial using the same therapy was carried out, but the results are not published yet93. A recent clinical trial combining lonafarnib with prenylation inhibitor pravastatin and zoledronic acid on 37 HGPS patients reveals additional bone mineral density benefit but no added cardiovascular benefit94.
Also other alternative therapies not targeting farnesyl group have been proposed in recent years. Mammalian targets of the rapamycin (mTOR) pathway inhibitor rapamycin treatment of HGPS fibroblasts reverses premature aging and the lobulated nuclei by increasing progerin clearance through macroautophagy-related pathways95. After that, antioxidant sulforaphane is also found to enhance progerin clearance by autophagy and to reverse the cellular hallmarks of HGPS96. In addition, a small molecule called remodelin was found to improve nuclear architecture, decrease DNA damage, and reverse cell proliferation defects in HGPS cells97. More recently, retinoids were identified as a novel class of compounds that reverse aging phenotypes in HGPS patient skin fibroblasts in a high-throughput screening98.
For HGPS patients, to gain a longer life span is the biggest priority. That’s the reason why most work mentioned here wasn’t aimed at skin specifically. Nevertheless, this work has provided possible hints for future research.
Anti-inflammaging
Given that the mechanism of skin inflammaging is far from being thoroughly understood, little progress is made to develop targeted treatments. Suh et al. reported that treatment of the human fibroblast cell line using UV-absorbing compound mycosporine-like amino acids (MAAs) suppressed cyclooxygenase-2 (COX-2) gene expression, which is typically increased in response to inflammation in skin99. Moreover, the expressions of skin aging-related proteins elastin and procollagen C-proteinase enhancer, which is an important determinate of procollagen processing in the regulation of collagen deposition in the skin, are strongly suppressed after UV irradiation but restored after MAAs treatment to normal levels as in the control99. Also, there are other reported anti-inflammaging additives, such as vitamins A, C, D and E, green tea, and so forth, that are already summarized100,101.
It’s noteworthy here that although some treatments may help to relieve skin aging, prevention of extrinsic aging from occurring is still the best approach, because skin wrinkles are formed mainly by changes in the dermal part of the skin and there is still difficulty for antiaging agents in topical treatment to penetrate into deep dermis, although different delivery methods are developed.
Concluding Remarks
There is a contradiction between the irreversability of skin aging and people’s thirst for eternal young appearance. From ancient to modern times, many efforts were made trying to understand the truth of cutaneous aging and to prevent or even reverse the aging process. This review summarizes the structural changes in both intrinsically and extrinsically aged skin, main molecular mechanisms proposed to explain these phenotypes, and advances in treatment research. It seems that skin aging is brought about by the comprehensive effect of different mechanisms, and it’s difficult to develop an integrated theory to string different models together. Ambiguity in the molecular mechanism of skin aging, as well as controversy in viewpoints, retarded the progress of targeted therapy, although some therapeutic attempts have proven to be effective. As people’s cosmetic requirements increase, more research efforts should persist to fully elucidate the molecular basis of the deteriorative changes during skin aging.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Grants for Scientific Research of BSKY (No. XJ201224) from Anhui Medical University and the National Natural Science Foundation of China (31471287 and 31671568).
References
- 1. Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kazanci A, Kurus M, Atasever A. Analyses of changes on skin by aging. Skin Res Technol. 2016;23(1):48–60. [DOI] [PubMed] [Google Scholar]
- 3. Krutmann J, Bouloc A, Sore G, Bernard BA, Passeron T. The skin aging exposome. J Dermatol Sci. 2017;85(3):152–161. [DOI] [PubMed] [Google Scholar]
- 4. Mora Huertas AC, Schmelzer CE, Hoehenwarter W, Heyroth F, Heinz A. Molecular-level insights into aging processes of skin elastin. Biochimie 2016;128–129:163–173. [DOI] [PubMed] [Google Scholar]
- 5. Mancini M, Lena AM, Saintigny G, Mahe C, Di Daniele N, Melino G, Candi E. MicroRNAs in human skin ageing. Ageing Res Rev. 2014;17:9–15. [DOI] [PubMed] [Google Scholar]
- 6. Makrantonaki E, Zouboulis CC, William J. Cunliffe Scientific Awards. Characteristics and pathomechanisms of endogenously aged skin. Dermatology. 2007;214(4):352–360. [DOI] [PubMed] [Google Scholar]
- 7. Moragas A, Castells C, Sans M. Mathematical morphologic analysis of aging-related epidermal changes. Anal Quant Cytol Histol. 1993;15(2):75–82. [PubMed] [Google Scholar]
- 8. Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92(20):9363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kohl E, Steinbauer J, Landthaler M, Szeimies RM. Skin ageing. J Eur Acad Dermatol Venereol. 2011;25(8):873–884. [DOI] [PubMed] [Google Scholar]
- 10. Quan T, Shao Y, He T, Voorhees JJ, Fisher GJ. Reduced expression of connective tissue growth factor (CTGF/CCN2) mediates collagen loss in chronologically aged human skin. J Invest Dermatol. 2010;130(2):415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Naylor EC, Watson RE, Sherratt MJ. Molecular aspects of skin ageing. Maturitas. 2011;69(3):249–256. [DOI] [PubMed] [Google Scholar]
- 12. Kligman AM. Early destructive effect of sunlight on human skin. JAMA. 1969;210(13):2377–2380. [PubMed] [Google Scholar]
- 13. Friedman O. Changes associated with the aging face. Facial Plast Surg Clin North Am. 2005;13(3):371–380. [DOI] [PubMed] [Google Scholar]
- 14. Kligman LH. Photoaging. Manifestations, prevention, and treatment. Clin Geriatr Med. 1989;5(1):235–251. [PubMed] [Google Scholar]
- 15. Bosset S, Bonnet-Duquennoy M, Barre P, Chalon A, Lazou K, Kurfurst R, Bonte F, Schnebert S, Disant F, Le Varlet B, et al. Decreased expression of keratinocyte beta1 integrins in chronically sun-exposed skin in vivo. Br J Dermatol. 2003;148(4):770–778. [DOI] [PubMed] [Google Scholar]
- 16. Makrantonaki E, Zouboulis CC. Molecular mechanisms of skin aging: state of the art. Ann N Y Acad Sci. 2007;1119:40–50. [DOI] [PubMed] [Google Scholar]
- 17. Contet-Audonneau JL, Jeanmaire C, Pauly G. A histological study of human wrinkle structures: comparison between sun-exposed areas of the face, with or without wrinkles, and sun-protected areas. Br J Dermatol. 1999;140(6):1038–1047. [DOI] [PubMed] [Google Scholar]
- 18. Griffiths CE, Russman AN, Majmudar G, Singer RS, Hamilton TA, Voorhees JJ. Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid). N Engl J Med. 1993;329(8):530–535. [DOI] [PubMed] [Google Scholar]
- 19. Trautinger F, Mazzucco K, Knobler RM, Trenz A, Kokoschka EM. UVA- and UVB-induced changes in hairless mouse skin collagen. Arch Dermatol Res. 1994;286(8):490–494. [DOI] [PubMed] [Google Scholar]
- 20. Varani J, Spearman D, Perone P, Fligiel SE, Datta SC, Wang ZQ, Shao Y, Kang S, Fisher GJ, Voorhees JJ. Inhibition of type I procollagen synthesis by damaged collagen in photoaged skin and by collagenase-degraded collagen in vitro. Am J Pathol. 2001;158(3):931–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brenneisen P, Oh J, Wlaschek M, Wenk J, Briviba K, Hommel C, Herrmann G, Sies H, Scharffetter-Kochanek K. Ultraviolet B wavelength dependence for the regulation of two major matrix-metalloproteinases and their inhibitor TIMP-1 in human dermal fibroblasts. Photochem Photobiol. 1996;64(5):877–885. [DOI] [PubMed] [Google Scholar]
- 22. Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337(20):1419–28. [DOI] [PubMed] [Google Scholar]
- 23. Bonta M, Daina L, Mutiu G. The process of ageing reflected by histological changes in the skin. Rom J Morphol Embryol. 2013;54(Suppl 3):797–804. [PubMed] [Google Scholar]
- 24. Bernstein EF, Chen YQ, Kopp JB, Fisher L, Brown DB, Hahn PJ, Robey FA, Lakkakorpi J, Uitto J. Long-term sun exposure alters the collagen of the papillary dermis. Comparison of sun-protected and photoaged skin by northern analysis, immunohistochemical staining, and confocal laser scanning microscopy. J Am Acad Dermatol. 1996;34(2 Pt 1):209–218. [DOI] [PubMed] [Google Scholar]
- 25. Scioli MG, Bielli A, Arcuri G, Ferlosio A, Orlandi A. Ageing and microvasculature. Vasc Cell. 2014;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Poljsak B, Dahmane RG, Godic A. Intrinsic skin aging: the role of oxidative stress. Acta Dermatovenerol Alp Pannonica Adriat. 2012;21(2):33–36. [PubMed] [Google Scholar]
- 27. Rinnerthaler M, Bischof J, Streubel MK, Trost A, Richter K. Oxidative stress in aging human skin. Biomolecules. 2015;5(2):545–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rittie L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5(1):a015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kammeyer A, Luiten RM. Oxidation events and skin aging. Ageing Res Rev. 2015;21:16–29. [DOI] [PubMed] [Google Scholar]
- 30. Choi YJ, Moon KM, Chung KW, Jeong JW, Park D, Kim DH, Yu BP, Chung HY. The underlying mechanism of proinflammatory NF-κB activation by the mTORC2/Akt/IKKalpha pathway during skin aging. Oncotarget. 2016;7(33):52685–52694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang W, Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 2010;8(12):e1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang W, Hekimi S. Two modes of mitochondrial dysfunction lead independently to lifespan extension in Caenorhabditis elegans. Aging Cell. 2010;9(3):433–447. [DOI] [PubMed] [Google Scholar]
- 33. Lapointe J, Hekimi S. Early mitochondrial dysfunction in long-lived Mclk1+/- mice. J Biol Chem. 2008;283(38):26217–26227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Andziak B, O’Connor TP, Qi W, DeWaal EM, Pierce A, Chaudhuri AR, Van Remmen H, Buffenstein R. High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell. 2006;5(6):463–471. [DOI] [PubMed] [Google Scholar]
- 35. Labinskyy N, Csiszar A, Orosz Z, Smith K, Rivera A, Buffenstein R, Ungvari Z. Comparison of endothelial function, O2-* and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice. Am J Physiol Heart Circ Physiol. 2006;291(6): H2698–H2704. [DOI] [PubMed] [Google Scholar]
- 36. Tsatsou F, Trakatelli M, Patsatsi A, Kalokasidis K, Sotiriadis D. Extrinsic aging: UV-mediated skin carcinogenesis. Dermatoendocrinol. 2012;4(3):285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ravanat JL, Douki T, Cadet J. Direct and indirect effects of UV radiation on DNA and its components. J Photochem Photobiol B. 2001;63(1–3):88–102. [DOI] [PubMed] [Google Scholar]
- 38. Panich U, Sittithumcharee G, Rathviboon N, Jirawatnotai S. Ultraviolet radiation-induced skin aging: The role of DNA damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells Int. 2016;2016:7370642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kamenisch Y, Berneburg M. Progeroid syndromes and UV-induced oxidative DNA damage. J Investig Dermatol Symp Proc. 2009;14(1):8–14. [DOI] [PubMed] [Google Scholar]
- 40. Olsen CM, Wilson LF, Green AC, Biswas N, Loyalka J, Whiteman DC. Prevention of DNA damage in human skin by topical sunscreens. Photodermatol Photoimmunol Photomed. 2017;33(3):135–142. [DOI] [PubMed] [Google Scholar]
- 41. Nakanishi M, Niida H, Murakami H, Shimada M. DNA damage responses in skin biology—implications in tumor prevention and aging acceleration. J Dermatol Sci. 2009;56(2):76–81. [DOI] [PubMed] [Google Scholar]
- 42. Siegl-Cachedenier I, Flores I, Klatt P, Blasco MA. Telomerase reverses epidermal hair follicle stem cell defects and loss of long-term survival associated with critically short telomeres. J Cell Biol. 2007;179(2):277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Buckingham EM, Klingelhutz AJ. The role of telomeres in the ageing of human skin. Exp Dermatol. 2011;20(4):297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rock K, Tigges J, Sass S, Schutze A, Florea AM, Fender AC, Theis FJ, Krutmann J, Boege F, Fritsche E, et al. miR-23a-3p causes cellular senescence by targeting hyaluronan synthase 2: possible implication for skin aging. J Invest Dermatol. 2015;135(2):369–377. [DOI] [PubMed] [Google Scholar]
- 45. Fiedler J, Gronniger E, Pfanne A, Bronneke S, Schmidt K, Falk CS, Wenck H, Terstegen L, Thum T, Winnefeld M. Identification of miR-126 as a new regulator of skin aging. Exp Dermatol. 2017;26(3):284–286. [DOI] [PubMed] [Google Scholar]
- 46. Danby FW. Nutrition and aging skin: sugar and glycation. Clin Dermatol. 2010;28(4):409–411. [DOI] [PubMed] [Google Scholar]
- 47. Farrar MD. Advanced glycation end products in skin ageing and photoageing: what are the implications for epidermal function? Exp Dermatol. 2016;25(12):947–948. [DOI] [PubMed] [Google Scholar]
- 48. Yoshinaga E, Kawada A, Ono K, Fujimoto E, Wachi H, Harumiya S, Nagai R, Tajima S. N(varepsilon)-(carboxymethyl)lysine modification of elastin alters its biological properties: implications for the accumulation of abnormal elastic fibers in actinic elastosis. J Invest Dermatol. 2012;132(2):315–323. [DOI] [PubMed] [Google Scholar]
- 49. Dunn JA, McCance DR, Thorpe SR, Lyons TJ, Baynes JW. Age-dependent accumulation of N epsilon-(carboxymethyl)lysine and N epsilon-(carboxymethyl)hydroxylysine in human skin collagen. Biochemistry. 1991;30(5):1205–10. [DOI] [PubMed] [Google Scholar]
- 50. Kueper T, Grune T, Prahl S, Lenz H, Welge V, Biernoth T, Vogt Y, Muhr GM, Gaemlich A, Jung T, et al. Vimentin is the specific target in skin glycation. Structural prerequisites, functional consequences, and role in skin aging. J Biol Chem. 2007;282(32):23427–23436. [DOI] [PubMed] [Google Scholar]
- 51. Radjei S, Gareil M, Moreau M, Leblanc E, Schnebert S, Friguet B, Nizard C, Petropoulos I. The glyoxalase enzymes are differentially localized in epidermis and regulated during ageing and photoageing. Exp Dermatol. 2016;25(6):492–494. [DOI] [PubMed] [Google Scholar]
- 52. Nguyen HP, Katta R. Sugar sag: glycation and the role of diet in aging skin. Skin Therapy Lett. 2015;20(6):1–5. [PubMed] [Google Scholar]
- 53. Makrantonaki E, Bekou V, Zouboulis CC. Genetics and skin aging. Dermatoendocrinol. 2012;4(3):280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gonzalo S, Kreienkamp R, Askjaer P. Hutchinson-Gilford Progeria Syndrome: A premature aging disease caused by LMNA gene mutations. Ageing Res Rev. 2017;33:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vidak S, Foisner R. Molecular insights into the premature aging disease progeria. Histochem Cell Biol. 2016;145(4):401–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Naval J, Alonso V, Herranz MA. Genetic polymorphisms and skin aging: the identification of population genotypic groups holds potential for personalized treatments. Clin Cosmet Investig Dermatol. 2014;7:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gao W, Tan J, Huls A, Ding A, Liu Y, Matsui MS, Vierkotter A, Krutmann J, Schikowski T, Jin L, et al. Genetic variants associated with skin aging in the Chinese Han population. J Dermatol Sci. 2017;86(1):21–29. [DOI] [PubMed] [Google Scholar]
- 58. Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. [DOI] [PubMed] [Google Scholar]
- 59. Fougere B, Boulanger E, Nourhashemi F, Guyonnet S, Cesari M. Chronic inflammation: accelerator of biological aging. J Gerontol A Biol Sci Med Sci. 2017;72(9):1218–1225. [DOI] [PubMed] [Google Scholar]
- 60. Zhuang Y, Lyga J. Inflammaging in skin and other tissues—the roles of complement system and macrophage. Inflamm Allergy Drug Targets. 2014;13(3):153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Takahara M, Kang K, Liu L, Yoshida Y, McCormick TS, Cooper KD. iC3b arrests monocytic cell differentiation into CD1c-expressing dendritic cell precursors: a mechanism for transiently decreased dendritic cells in vivo after human skin injury by ultraviolet B. J Invest Dermatol. 2003;120(5):802–809. [DOI] [PubMed] [Google Scholar]
- 62. Yoshida Y, Kang K, Berger M, Chen G, Gilliam AC, Moser A, Wu L, Hammerberg C, Cooper KD. Monocyte induction of IL-10 and down-regulation of IL-12 by iC3b deposited in ultraviolet-exposed human skin. J Immunol. 1998;161(11):5873–5879. [PubMed] [Google Scholar]
- 63. Handoko HY, Rodero MP, Boyle GM, Ferguson B, Engwerda C, Hill G, Muller HK, Khosrotehrani K, Walker GJ. UVB-induced melanocyte proliferation in neonatal mice driven by CCR2-independent recruitment of Ly6c(low)MHCII(hi) macrophages. J Invest Dermatol. 2013;133(7):1803–1812. [DOI] [PubMed] [Google Scholar]
- 64. Hammerberg C, Duraiswamy N, Cooper KD. Active induction of unresponsiveness (tolerance) to DNFB by in vivo ultraviolet-exposed epidermal cells is dependent upon infiltrating class II MHC+ CD11bbright monocytic/macrophagic cells. J Immunol. 1994;153(11):4915–4924. [PubMed] [Google Scholar]
- 65. Masaki H. Role of antioxidants in the skin: anti-aging effects. J Dermatol Sci. 2010;58(2):85–90. [DOI] [PubMed] [Google Scholar]
- 66. Tanuja Y, Mishra S, Das S, Aggarwal S, Rani V. Anticedants and natural prevention of environmental toxicants induced accelerated aging of skin. Environ Toxicol Pharmacol. 2015;39(1):384–391. [DOI] [PubMed] [Google Scholar]
- 67. Chen J, Li Y, Zhu Q, Li T, Lu H, Wei N, Huang Y, Shi R, Ma X, Wang X, et al. Anti-skin-aging effect of epigallocatechin gallate by regulating epidermal growth factor receptor pathway on aging mouse model induced by d-Galactose. Mech Ageing Dev. 2017;164:1–7. [DOI] [PubMed] [Google Scholar]
- 68. Bavarsad Shahripour R, Harrigan MR, Alexandrov AV. N-acetylcysteine (NAC) in neurological disorders: mechanisms of action and therapeutic opportunities. Brain Behav. 2014;4(2):108–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Geissler S, Textor M, Schmidt-Bleek K, Klein O, Thiele M, Ellinghaus A, Jacobi D, Ode A, Perka C, Dienelt A, et al. In serum veritas-in serum sanitas? Cell non-autonomous aging compromises differentiation and survival of mesenchymal stromal cells via the oxidative stress pathway. Cell Death Dis. 2013;4:e970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012(3):CD007176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Marosz A, Chlubek D. The risk of abuse of vitamin supplements. Ann Acad Med Stetin. 2014;60(1):60–64. [PubMed] [Google Scholar]
- 72. Bjelakovic G, Nikolova D, Gluud C. Antioxidant supplements and mortality. Curr Opin Clin Nutr Metab Care. 2014;17(1):40–44. [DOI] [PubMed] [Google Scholar]
- 73. Lu LY, Ou N, Lu QB. Antioxidant induces DNA damage, cell death and mutagenicity in human lung and skin normal cells. Sci Rep. 2013;3:3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312(5782):1882–1883. [DOI] [PubMed] [Google Scholar]
- 75. Mojallal A, Lequeux C, Shipkov C, Breton P, Foyatier JL, Braye F, Damour O. Improvement of skin quality after fat grafting: clinical observation and an animal study. Plast Reconstr Surg. 2009;124(3):765–774. [DOI] [PubMed] [Google Scholar]
- 76. Kim WS, Park BS, Kim HK, Park JS, Kim KJ, Choi JS, Chung SJ, Kim DD, Sung JH. Evidence supporting antioxidant action of adipose-derived stem cells: protection of human dermal fibroblasts from oxidative stress. J Dermatol Sci. 2008;49(2):133–142. [DOI] [PubMed] [Google Scholar]
- 77. Zhang S, Dong Z, Peng Z, Lu F. Anti-aging effect of adipose-derived stem cells in a mouse model of skin aging induced by D-galactose. PLoS One. 2014;9(5):e97573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bernardini FP, Gennai A, Izzo L, Zambelli A, Repaci E, Baldelli I, Fraternali-Orcioni G, Hartstein ME, Santi PL, Quarto R. Superficial enhanced fluid fat injection (SEFFI) to correct volume defects and skin aging of the face and periocular region. Aesthet Surg J. 2015;35(5):504–515. [DOI] [PubMed] [Google Scholar]
- 79. Gennai A, Zambelli A, Repaci E, Quarto R, Baldelli I, Fraternali G, Bernardini FP. Skin rejuvenation and volume enhancement with the micro superficial enhanced fluid fat injection (M-SEFFI) for skin aging of the periocular and perioral regions. Aesthet Surg J. 2017;37(1):14–23. [DOI] [PubMed] [Google Scholar]
- 80. Park BS, Jang KA, Sung JH, Park JS, Kwon YH, Kim KJ, Kim WS. Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatol Surg. 2008;34(10):1323–1326. [DOI] [PubMed] [Google Scholar]
- 81. Derby BM, Dai H, Reichensperger J, Cox L, Harrison C, Cosenza N, Yang M, Bueno RA, Neumeister MW. Adipose-derived stem cell to epithelial stem cell transdifferentiation: a mechanism to potentially improve understanding of fat grafting’s impact on skin rejuvenation. Aesthet Surg J. 2014;34(1):142–153. [DOI] [PubMed] [Google Scholar]
- 82. Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379(6563):335–339. [DOI] [PubMed] [Google Scholar]
- 83. Kafi R, Kwak HS, Schumacher WE, Cho S, Hanft VN, Hamilton TA, King AL, Neal JD, Varani J, Fisher GJ, et al. Improvement of naturally aged skin with vitamin A (retinol). Arch Dermatol. 2007;143(5):606–612. [DOI] [PubMed] [Google Scholar]
- 84. Verdier-Sevrain S, Bonte F. Skin hydration: a review on its molecular mechanisms. J Cosmet Dermatol. 2007;6(2):75–82. [DOI] [PubMed] [Google Scholar]
- 85. Ramirez RD, Morales CP, Herbert BS, Rohde JM, Passons C, Shay JW, Wright WE. Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. Genes Dev. 2001;15(4):398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gonzalez-Suarez E, Geserick C, Flores JM, Blasco MA. Antagonistic effects of telomerase on cancer and aging in K5-mTert transgenic mice. Oncogene. 2005;24(13):2256–2570. [DOI] [PubMed] [Google Scholar]
- 87. Dearlove RP, Greenspan P, Hartle DK, Swanson RB, Hargrove JL. Inhibition of protein glycation by extracts of culinary herbs and spices. J Med Food. 2008;11(2):275–281. [DOI] [PubMed] [Google Scholar]
- 88. Thirunavukkarasu V, Nandhini AT, Anuradha CV. Fructose diet-induced skin collagen abnormalities are prevented by lipoic acid. Exp Diabesity Res. 2004;5(4):237–244. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89. Tarwadi KV, Agte VV. Effect of micronutrients on methylglyoxal-mediated in vitro glycation of albumin. Biol Trace Elem Res. 2011;143(2):717–725. [DOI] [PubMed] [Google Scholar]
- 90. Lo Cicero A, Nissan X. Pluripotent stem cells to model Hutchinson-Gilford progeria syndrome (HGPS): current trends and future perspectives for drug discovery. Ageing Res Rev. 2015;24(Pt B):343–348. [DOI] [PubMed] [Google Scholar]
- 91. Gordon LB, Kleinman ME, Miller DT, Neuberg DS, Giobbie-Hurder A, Gerhard-Herman M, Smoot LB, Gordon CM, Cleveland R, Snyder BD, et al. Clinical trial of a farnesyltransferase inhibitor in children with Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2012;109(41):16666–16671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Varela I, Pereira S, Ugalde AP, Navarro CL, Suarez MF, Cau P, Cadinanos J, Osorio FG, Foray N, Cobo J, et al. Combined treatment with statins and aminobisphosphonates extends longevity in a mouse model of human premature aging. Nat Med. 2008;14(7):767–772. [DOI] [PubMed] [Google Scholar]
- 93. Cau P, Navarro C, Harhouri K, Roll P, Sigaudy S, Kaspi E, Perrin S, De Sandre-Giovannoli A, Levy N. Nuclear matrix, nuclear envelope and premature aging syndromes in a translational research perspective. Semin Cell Dev Biol. 2014;29:125–147. [DOI] [PubMed] [Google Scholar]
- 94. Gordon LB, Kleinman ME, Massaro J, D’Agostino RB, Sr, Shappell H, Gerhard-Herman M, Smoot LB, Gordon CM, Cleveland RH, Nazarian A, et al. Clinical trial of the protein farnesylation inhibitors lonafarnib, pravastatin, and zoledronic acid in children with Hutchinson-Gilford progeria syndrome. Circulation. 2016;134(2):114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cao K, Graziotto JJ, Blair CD, Mazzulli JR, Erdos MR, Krainc D, Collins FS. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson-Gilford progeria syndrome cells. Sci Transl Med. 2011;3(89):89ra58. [DOI] [PubMed] [Google Scholar]
- 96. Gabriel D, Roedl D, Gordon LB, Djabali K. Sulforaphane enhances progerin clearance in Hutchinson-Gilford progeria fibroblasts. Aging Cell. 2015;14(1):78–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Larrieu D, Britton S, Demir M, Rodriguez R, Jackson SP. Chemical inhibition of NAT10 corrects defects of laminopathic cells. Science. 2014;344(6183):527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kubben N, Brimacombe KR, Donegan M, Li Z, Misteli T. A high-content imaging-based screening pipeline for the systematic identification of anti-progeroid compounds. Methods. 2016;96:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Suh SS, Hwang J, Park M, Seo HH, Kim HS, Lee JH, Moh SH, Lee TK. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar Drugs. 2014;12(10):5174–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Suggs A, Oyetakin-White P, Baron ED. Effect of botanicals on inflammation and skin aging: analyzing the evidence. Inflamm Allergy Drug Targets. 2014;13(3):168–76. [DOI] [PubMed] [Google Scholar]
- 101. Goncalves de Carvalho CM, Ribeiro SM. Aging, low-grade systemic inflammation and vitamin D: a mini-review. Eur J Clin Nutr. 2017;71(4):434–440. [DOI] [PubMed] [Google Scholar]