Abstract
Background and objective
Previous studies indicated cancer survivors had a higher risk of developing subsequent pancreatic ductal adenocarcinoma. However, the influence of prior cancer on survival outcomes of current pancreatic cancer remains unclear.
Methods
Eligible populations were selected from the Surveillance, Epidemiology, and End Results programs from 2000 to 2012. We adopted Kaplan–Meier curves and Cox analysis to compare survival differences between patients with and without prior cancer.
Results
Overall, 67,555 pancreatic cancer patients, including 5582 (8.26%) with and 61,973 (91.74%) without prior cancer, were included. The most common types of prior cancers were prostate, breast, and colorectal cancers. The median time from diagnosis of an initial malignancy to subsequent pancreatic cancer was 59.8 months. Patients with a prior cancer had higher overall one-year and three-year survival rates compared with those without a prior cancer. Multivariable Cox analysis demonstrated that a history of prior malignancy could independently predict the better overall survival outcome of pancreatic cancer (HR = 0.92, 95% CI, 0.89–0.94, p < 0.001), especially for colorectal, breast, corpus uteri and prostate cancer survivors.
Conclusions
A history of cancer did not contribute to a poor survival outcome for patients with pancreatic cancer. More prospective trials might be warranted to validate our findings.
Keywords: Pancreatic ductal adenocarcinoma, prior cancer history, SEER, survival outcomes, population-based study
Key summary
- Summarise the established knowledge on this subject:
- Pancreatic cancer remains one of the leading causes of cancer-related deaths worldwide.
- Cancer survivors have a higher risk of developing a secondary cancer, including pancreatic cancer.
- There is a paucity of data evaluating the impact of a prior cancer on the prognosis of pancreatic cancer patients.
- What are the significant and/or new findings of this study?
- Prostate, breast, and colorectal cancer were the most common types of prior cancer in pancreatic cancer patients.
- The median time from diagnosis of an initial cancer to subsequent pancreatic ductal adenocarcinoma was 59.8 months.
- A history of cancer did not contribute to a poor prognosis for pancreatic cancer patients.
Introduction
Pancreatic cancer, a highly lethal disease, remains one of the leading causes of cancer-related deaths worldwide. In 2017 the US was estimated to see approximately 53,670 new cases and 43,090 deaths.1–3 Pancreatic ductal adenocarcinoma (PDAC), a major histological subtype, comprises 90% of pancreatic cancer cases. Due to the aggressive biology of PDAC, patients often present with local invasion and distant metastasis at the diagnosis, thus leading to its extremely poor prognosis.3 Therefore, it is important to develop screening and surveillance strategies for early detection, as well as new advanced therapies.
To date, our understanding of PDAC aetiology has not been understood completely. Several risk factors, such as obesity, tobacco, and Type II diabetes, are associated with incidence of PDAC.4–6 It is well known that cancer survivors have a higher risk of developing a secondary cancer in later life, including PDAC.7 According to a previous study, antecedent cancer history (colon cancer, biliary system and uterus malignancies) directly increases risk of subsequent PDAC.8,9 Genetic predisposition and common environmental risk factors together may contribute to this phenomenon.10–12 With increased numbers of cancer survivors, further understanding of the relative risk of developing secondary PDAC might contribute to better prognosis of this population. In addition, whether a history of prior nonpancreatic cancer could affect the survival outcome of PDAC patients is poorly understood. Therefore, we conducted a population-based study to address this gap, which might have important implications for screening and surveillance guidelines.
Method
Data source and population
Data were obtained from the Surveillance, Epidemiology and End Results (SEER) by SEER*Stat, version 8.3, which covers approximately 30% of the population in the US. Current data were based on 18 registries in the SEER released in April 2017 (San Francisco-Oakland Metropolitan Statistical Area), Connecticut, Detroit (Metropolitan), Hawaii, Iowa, New Mexico, Seattle (Puget Sound), Utah, Atlanta (Metropolitan), San Jose-Monterey, Los Angeles, Rural Georgia, California excluding San Francisco/San Jose Monterey/Los Angeles, Kentucky, Louisiana, New Jersey, Greater Georgia).13 In the present study, we identified a cohort of patients who were aged ≥ 18 with histological diagnoses of pancreatic cancer between 2000 and 2012. According to the International Classification of Diseases for Oncology, third edition (ICD-O-3),14 pancreas cancers were identified by tumour site code (C25.0, C25.1, C25.2, C25.3, C25.7, C25.8 and C25.9). Histological type of adenocarcinoma and infiltrating pancreatic adenocarcinoma (ICD-O-3 histology codes 8140 and 8500) were defined as PDAC. For patients with prior primary malignancies, we included only the common sites of prostate, breast, colon and rectum, lymphoma/leukaemia, lung and bronchus, melanoma, kidney/renal pelvis, head and neck, thyroid, stomach, liver/gallbladder/bile ducts, ovary, oesophagus, corpus uteri and bladder. To avoid the possibility of synchronous metastasis, we required a latency period of at least six months after diagnosis of initial prior cancer. Survival time was defined as the time from diagnosis to date of death, or censored at the last follow-up or alive until last follow-up. The last follow-up time for the current study was 31 December 2014. This study was exempt from institutional review board review due to the public nature and de-identification of all data.
Statistical analysis
We compared characteristics of patients with and without a history of prior cancer by Chi-square tests. The mean times between diagnosis of prior cancer and subsequent PDAC were calculated according to prior cancer site. The distributions of tumour size and stage were compared stratified by prior cancer site. Then we calculated the overall one-year and three-year survival rates of PDAC patients with and without prior cancer by Kaplan–Meier curves, and survival differences were examined by log-rank test. Furthermore, we explored the cause of death in PDAC patients with prior malignancies stratified by first cancer site. Finally, we used a multivariable Cox model to estimate hazard ratios (HRs) of prior history of cancer in survival outcomes of PDAC patients. A two-sided p < 0.05 was considered statistically significant and all analyses were performed by STATA, version 13.0 (StataCorp, College Station, TX, USA) and R, version 3.40 (http://www.r-project.org).
Results
Within the criteria set above, a total of 67,555 PDAC patients were identified from the SEER database, including 5582 (8.26%) patients with a history of prior malignancies and 61,973 (91.74%) patients without prior cancer. The detailed baseline characteristics of patients are summarised in Table 1. Compared with cases without previous cancer, individuals with previous malignancies were older and male, had smaller tumour size and were at the localised and regional stage. The percentage of surgery was similar between patients with and without a previous malignancy, and patients with prior cancer received less radiation and chemotherapy. Among 5582 PDAC patients with a history of cancer, prostate (32.9%) was the most common initial site, followed by breast (18.4%), colon and rectum (12.4%), lymphoma/leukaemia (6.9%), bladder (6.2%), lung and bronchus (4.9%), melanoma (4.4%), kidney/renal pelvis (3.5%), Corpus uteri (3.1%) head and neck (2.2%), thyroid (1.6%), liver/gallbladder/bile ducts (1.1%), stomach (1.0%), ovary (1.0%) and oesophagus (0.4%) (Table 2). The interval from the initial cancer to secondary PDAC is illustrated in Figure 1. The overall median time between the diagnosis of a prior malignancy and subsequent PDAC was 59.8 months. For cancer survivors of colon and rectum, breast, corpus uteri, prostate, and thyroid, the interval exceeded 60 months, while for survivors of ovary, lung and bronchus, oesophagus, and liver/gallbladder /bile, the interval was less than 50 months (Figure 1). Figures S1 and S2 display the distribution of stage and tumour size of PDAC stratified by initial prior cancer sites. Patients with a history of oesophagus, stomach, colon/rectum, lung/bronchus and liver/gallbladder/bile duct cancer were more likely to be diagnosed with PDAC at the localised and regional stage (Figure S1). Among survivors of oesophagus, colon/rectum, lung/bronchus, breast, corpus uteri, ovary and thyroid cancer, the proportion of small size of PDAC (≤2 cm) was higher compared with patients without a prior cancer (Figure S2).
Table 1.
Characteristics of included cohort by previous cancer status.
| Character | No previous cancer N = 61,973 | With prior cancers N = 5582 | p-value |
|---|---|---|---|
| Age (median) | 68 years | 73 years | |
| Gender | <0.001 | ||
| Male | 31,786 (51.29%) | 3334 (59.73%) | |
| Female | 30,187 (48.71%) | 2248 (40.27%) | |
| Race | <0.001 | ||
| White | 49,511 (79.89%) | 4621 (82.78%) | |
| Black | 7666 (12.37%) | 663 (11.88%) | |
| AI/AN | 362 (0.58%) | 10 (0.18%) | |
| AP | 4284 (6.91%) | 286 (5.12%) | |
| Unknown | 150 (0.24%) | 2 (0.04%) | |
| Location | <0.001 | ||
| Head | 32,379 (52.25%) | 2899 (51.93%) | |
| Body | 7003 (11.30%) | 650 (11.64%) | |
| Tail | 7342 (11.85%) | 681 (12.20%) | |
| Pancreatic duct | 415 (0.67%) | 30 (0.54%) | |
| Other specified parts of pancreas | 727 (1.16%) | 66 (1.18%) | |
| Overlapping | 4501 (7.26%) | 400 (7.17%) | |
| Pancreas, NOS | 9606 (15.50%) | 856 (15.34%) | |
| Tumour Size | <0.001 | ||
| ≤2 cm | 4636 (7.48%) | 485 (8.69%) | |
| 2 < size ≤4 cm | 22,509 (36.32%) | 2267 (40.61%) | |
| >4 cm | 17,325 (27.96%) | 1546 (27.70%) | |
| Unknown | 17,503 (28.24%) | 1284 (23.00%) | |
| SEER stage | <0.001 | ||
| Localided | 4196 (6.77%) | 558 (10.00%) | |
| Regional | 20,872 (33.68%) | 1939 (34.74%) | |
| Distant | 34,475 (55.63%) | 2828 (50.66%) | |
| Unstaged | 2430 (3.92%) | 257 (4.60%) | |
| Histologic type | 0.004 | ||
| Adenocarcinoma | 55,125 (88.95%) | 4,895 (87.69%) | |
| Infiltrating duct carcinoma | 6848 (11.05%) | 687 (12.31%) | |
| Grade | <0.001 | ||
| G1 | 2807 (4.53%) | 251 (4.50%) | |
| G2 | 11,040 (17.81%) | 930 (16.66%) | |
| G3 | 10,654 (17.19%) | 842 (15.08%) | |
| G4 | 408 (0.66%) | 29 (0.52%) | |
| Unknown | 37,064 (59.81%) | 3530 (63.24%) | |
| Surgery | 0.798 | ||
| No | 49,825 (80.40%) | 4486 (80.37%) | |
| Yes | 11,783 (19.01%) | 1067 (19.12%) | |
| Unknown | 365 (0.59%) | 29 (0.52%) | |
| Radiation | <0.001 | ||
| None | 49,209 (79.40%) | 4591 (82.25%) | |
| Radiation | 12,265 (19.79%) | 941 (16.86%) | |
| Unknown | 499 (0.81%) | 50 (0.90%) | |
| Chemotherapy | <0.001 | ||
| No | 32,283 (52.09%) | 2765 (49.53%) | |
| Chemotherapy | 29,690 (47.91%) | 2817 (50.47%) |
AI/AN: American Indian/Alaska Native; AP: Asian or Pacific Islander; G1: well differentiated; G2: moderately differentiated; G3: poorly differentiated; G4: undifferentiated; NOS: not otherwise specified; SEER: Surveillance, Epidemiology, and End Results.
Table 2.
Distribution of prior cancer sites in pancreatic cancer patients with a history of cancer.
| Prior cancer sites | Number | Percentage |
|---|---|---|
| Prostate | 1834 | 32.9% |
| Breast | 1027 | 18.4% |
| Colon and rectum | 692 | 12.4% |
| Lymphoma/Leukaemia | 385 | 6.9% |
| Bladder | 348 | 6.2% |
| Lung and bronchus | 274 | 4.9% |
| Melanoma | 247 | 4.4% |
| Kidney and renal pelvis | 195 | 3.5% |
| Corpus uteri | 173 | 3.1% |
| Head and neck | 124 | 2.2% |
| Thyroid | 87 | 1.6% |
| Liver/Gallbladder/Bile ducts | 59 | 1.1% |
| Stomach | 58 | 1.0% |
| Ovary | 55 | 1.0% |
| Oesophagus | 24 | 0.4% |
Figure 1.
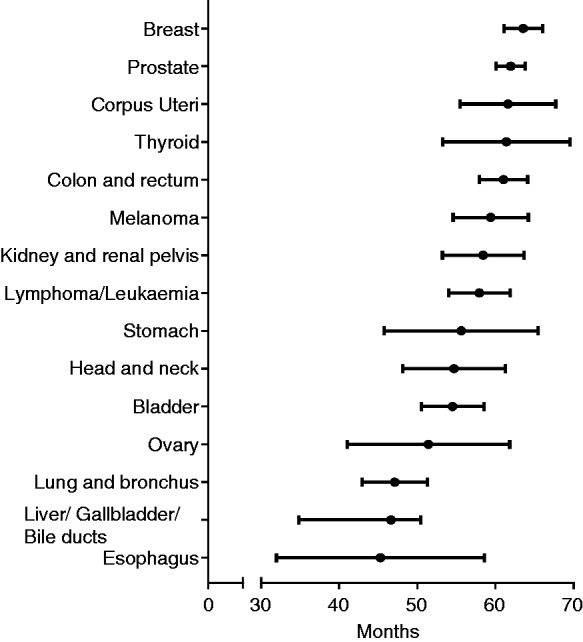
Median interval between prior cancer and subsequent pancreatic ductal adenocarcinoma stratified by initial cancer sites.
To examine whether history of cancer could influence survival, we adopted Kaplan–Meier analysis to compare survival differences. Patients with a prior cancer had significantly better overall survival than those without a prior cancer (Figure 2, p < 0.01). The overall one-year survival rate for patients with or without previous cancer were 28.66% (95% confidence interval (CI), 27.45–29.87) and 26.34% (95% CI, 25.99–26.69), respectively (Table S1). The overall three-year survival rate was 5.83% (95% CI, 5.65–6.02) for patients with no history of cancer versus 9.60% (95% CI, 8.82–10.41) for patients with a prior cancer (Table S1). When stratified by initial cancer sites, compared with PDAC patients without prior cancer, survivors of breast, ovary and corpus uteri cancers had better short-term outcomes (one-year survival rate) and survivors of colon and rectum, breast, corpus uteri, prostate, kidney and renal pelvis cancers enjoyed a better long-term prognosis (three-year survival rate). Furthermore, we also analysed the distribution of outcomes (prior cancer death, PDAC death, or alive). In patients without previous cancer, 91.37% of the population died of PDAC, while among patients with a history of cancer, 78.79% patients died of PDAC, and 8.47% died of prior cancer. Multivariable Cox regression analysis demonstrated that a history of previous malignance was independently associated with a better overall prognosis in patients with PDAC (HR, 0.92, 95% CI, 0.89–0.94) even after adjusting for other variables, such as age, gender, race, tumour size, stage, histologic type, grade, surgery, radiation and chemotherapy (Table 3). Among patients with a history of cancer of the colon and rectum (HR, 0.87, 95% CI, 0.80–0.94), breast (HR, 0.88, 95% CI, 0.82–0.94), corpus uteri (HR, 0.84, 95% CI, 0.71–0.99) and prostate (HR, 0.92, 95% CI, 0.87–0.97), the risk of all-cause mortality was significantly decreased compared with patients without a history of cancer (Table S2).
Figure 2.

Kaplan–Meier survival curves of pancreatic ductal adenocarcinoma patients with and without prior history of cancer.
Table 3.
Multivariable Cox regression analysis of overall survival in pancreatic ductal adenocarcinoma patients.
| Variables | Hazard ratio (95 CI%) | p-value |
|---|---|---|
| Age | <0.001 | |
| ≤60 years | Reference | |
| >60 years | 1.23 (1.21, 1.25) | |
| Gender | <0.001 | |
| Male | Reference | |
| Female | 0.95 (0.94, 0.97) | |
| Race | <0.001 | |
| White | Reference | |
| Black | 1.09 (1.06, 1.11) | |
| AI/AN | 0.93 (0.90, 0.95) | |
| AP | 1.08 (0.97, 1.20) | |
| Tumour size | <0.001 | |
| ≤2 cm | Reference | |
| 2 < size ≤4 cm | 1.18 (1.14, 1.21) | |
| >4 cm | 1.36 (1.32, 1.41) | |
| Prior history of cancer | <0.001 | |
| None | Reference | |
| Yes | 0.92 (0.89, 0.94) | |
| SEER stage | <0.001 | |
| Localised | Reference | |
| Regional | 1.28 (1.24,1.32) | |
| Distant | 2.07 (2.00, 2.14) | |
| Histologic type | <0.001 | |
| Adenocarcinoma | Reference | |
| Infiltrating duct carcinoma | 0.94 (0.92, 0.97) | |
| Grade | <0.001 | |
| G1 | Reference | |
| G2 | 1.30 (1.24, 1.35) | |
| G3 | 1.64 (1.57, 1.71) | |
| G4 | 1.67 (1.50, 1.85) | |
| Surgery | <0.001 | |
| No | Reference | |
| Yes | 0.44 (0.43, 0.45) | |
| Radiation | <0.001 | |
| None | Reference | |
| Radiation | 0.88 (0.86, 0.90) | |
| Chemotherapy | <0.001 | |
| No | Reference | |
| Chemotherapy | 0.47 (0.46, 0.47) |
AI/AN: American Indian/Alaska Native; AP: Asian or Pacific Islander; G1: well differentiated; G2: moderately differentiated; G3: poorly differentiated; G4: undifferentiated; SEER: Surveillance, Epidemiology, and End Results; HR: hazard ratio; CI: confidence interval.
Discussion
To our best of knowledge, the current study first addressed whether PDAC patients with a history of nonpancreatic cancer faced a worse prognosis than other patients without prior cancer. Most of our results had never been previously reported. In our study, a history of cancers occurred in 5582 (8.26%) PDAC patients, and the three most common types of prior cancer were breast, prostate and colorectal cancers. The association between nonpancreatic cancers and subsequent pancreatic cancer had also been addressed by previous studies.8,9,15 The excess risk of multiple malignancies might reflect a genetic predisposition and similar environmental factors.16,17 Up to 15% of pancreatic cancers have a genetic predisposition, such as mutations in Partner and Localiser of BRCA2 (PALB2), Ataxia telangiectasia mutated (ATM), BRCA2 and other hereditary cancer syndromes.18–24 Additionally, several environmental risk factors, such as heavy consumption of alcohol, tobacco, Western diet and insufficient physical activity, are also associated with increased incidence of malignancies.9,25 The mean interval between a prior malignancy and subsequent PDAC was 59.8 months, and the intervals were longest for breast cancer survivors and shortest for oesophagus cancer survivors. This information might provide some clue for strategies about screening intervals. Earlier stage and smaller tumour size of PDAC were detected in cancer survivors. This likely may be attributed to active surveillance and screening practices in cancer survivors. These results indicate that screening for PDAC in cancer survivors is necessary.
In addition, we also demonstrated that PDAC patients with prior history of cancer did not result in inferior survival outcomes when compared with those without previous cancer. Most patients with prior cancer also died of pancreatic cancer rather than prior cancers, which might reflect the aggressive biology of PDAC. Similar results are also supported by the results from lung cancer studies.26,27 Laccetti et al.27 reported that prior cancer did not affect the survival outcomes among patients with stage IV lung cancer. On the contrary, overall survival even significantly favoured PDAC patients with a history of colorectal, breast, corpus uteri and prostate cancer. The underlying causes were unclear but likely multifactorial. First, familial pancreatic cancer accounts for a small fraction of all pancreatic cancer cases. Most PDAC might be biologically independent of prior cancer.9 Second, reduced exposure to risk factors and intensive surveillance after the first diagnosis of a cancer might contribute to the favourable prognosis of PADC. In the current study of patients with prior cancer, PDAC stage was more likely to be localised and tumour size tended to be smaller. These findings should spur us to rethink the long-accepted and intuitive assumption that a history of cancer might negatively influence the prognosis. Thus, the practice of exclusion participants with a history of prior cancer26 from current clinical oncology trials needs to be re-evaluated. Several studies also addressed this issue recently. Gerber et al. showed no evidence that lung cancer survivors with prior cancer had a worse prognosis,26 just as our results indicated. Therefore, broader inclusion criteria of PDAC patients with prior cancer might be feasible to improve accrual and generalisability of trials.
Before considering the implications of our findings, several methodological limitations of the current study deserve mention. First, the SEER database did not provide detailed information about chemotherapy and several covariates, such as smoking status or obesity, which might influence survival outcomes of PDAC patients. Second, we were unable to totally exclude the possibility of misclassification of PDAC. Third, we included only 15 common previous cancer sites in our analysis and therefore we were unable to evaluate the prognosis of PDAC patients with less common types of malignancy. In addition, we did not analyse patients with more than two prior malignancies separately in the current study because of the limited number of participants.
In conclusion, 8.26% of PDAC patients have a history of prior cancer. More PDAC cases occurred five years after diagnosis of an initial cancer. A history of cancer did not convey an adverse effect on overall survival of PDAC patients. We hope that our results fill a critical knowledge gap concerning the prognostic impact of prior cancer and could provide some implication for clinical practice.
Acknowledgements
We thank the National Cancer Institute’s SEER Program for collection of the SEER data.
Author contributions
Xingkang He conceived and designed the study, conducted data extraction, statistical analysis and interpreted the study results, and wrote the first draft of the manuscript. Wenrui Wu extracted and analysed the data, and interpreted the study results. Yue Li interpreted the study results and wrote and revised the first draft of the manuscript. Tingting Su and Sanchuan Lai analysed and wrote and revised the first draft of the manuscript. Luyi Chen revised the secondary version of the manuscript. Jianmin Si conceived, designed, and supervised the study. Leimin Sun conceived and designed the study, interpreted the study results, wrote and revised the first draft of the manuscript, and supervised the study. All authors edited and critically revised the final version of the manuscript.
Declaration of conflicting interests
None declared.
Funding
This work was supported by the Zhejiang Natural Science Foundation of China (LY18H160019).
Ethics approval
Approval from the ethical board was not required due to the public nature of all the data.
Informed consent
This study did not involve personal identifying information or interact with human individuals. Therefore, informed consent was not required.
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9–29. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Dhakal I, Ning B, et al. Patterns and trends of pancreatic cancer mortality rates in Arkansas, 1969–2002: A comparison with the US population. Eur J Cancer Prev 2008; 17: 18–27. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD and Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed]
- 4.Inoue M, Iwasaki M, Otani T, et al. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med 2006; 166: 1871–1877. [DOI] [PubMed] [Google Scholar]
- 5.Huxley R, Ansary-Moghaddam A, Berrington de González A, et al. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005; 92: 2076–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosetti C, Lucenteforte E, Silverman DT, et al. Cigarette smoking and pancreatic cancer: An analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann Oncol 2012; 23: 1880–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donin N, Filson C, Drakaki A, et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer 2016; 122: 3075–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amin S, McBride RB, Kline JK, et al. Incidence of subsequent pancreatic adenocarcinoma in patients with a history of nonpancreatic primary cancers. Cancer 2012; 118: 1244–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen M, Boffetta P, Olsen JH, et al. A pooled analysis of second primary pancreatic cancer. Am J Epidemiol 2006; 163: 502–511. [DOI] [PubMed] [Google Scholar]
- 10.Olson SH, Kurtz RC. Epidemiology of pancreatic cancer and the role of family history. J Surg Oncol 2013; 107: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowenfels AB, Maisonneuve P, Whitcomb DC. Risk factors for cancer in hereditary pancreatitis. International Hereditary Pancreatitis Study Group. Med Clin North Am 2000; 84: 565–573. [DOI] [PubMed] [Google Scholar]
- 12.Michaud DS, Giovannucci E, Willett WC, et al. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA 2001; 286: 921–929. [DOI] [PubMed] [Google Scholar]
- 13.National Cancer Institute. Surveillance Epidemiology, and End Results (SEER) Program SEER*Stat Database: Incidence – SEER 18 Regs excluding AK Research Data, Nov 2016 Sub (2000–2014) <Katrina/Rita Population Adjustment> - Linked To County Attributes – Total U.S., 1969–2015 Counties, National Cancer Institute, Division of Cancer Control & Population Sciences, Surveillance Research Program, released April 2017, based on November 2016 submission, www.seer.cancer.gov (2017).
- 14.World Health Organization.. International Classification of Diseases for Oncology (ICD-O), 3rd ed, 1st rev Geneva: WHO Press, 2013. [Google Scholar]
- 15.Mellemkjaer L, Friis S, Olsen JH, et al. Risk of second cancer among women with breast cancer. Int J Cancer 2006; 118: 2285–2292. [DOI] [PubMed] [Google Scholar]
- 16.Hiripi E, Lorenzo Bermejo J, Li X, et al. Familial association of pancreatic cancer with other malignancies in Swedish families. Br J Cancer 2009; 101: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein AP. Genetic susceptibility to pancreatic cancer. Mol Carcinog 2012; 51: 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brentnall TA. Management strategies for patients with hereditary pancreatic cancer. Curr Treat Options Oncol 2005; 6: 437–445. [DOI] [PubMed] [Google Scholar]
- 19.Slater EP, Langer P, Fendrich V, et al. Prevalence of BRCA2 and CDKN2a mutations in German familial pancreatic cancer families. Fam Cancer 2010; 9: 335–343. [DOI] [PubMed] [Google Scholar]
- 20.Couch FJ, Johnson MR, Rabe KG, et al. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev 2007; 16: 342–346. [DOI] [PubMed] [Google Scholar]
- 21.Jones S, Hruban RH, Kamiyama M, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science 2009; 324: 217–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rulyak SJ, Brentnall TA. Inherited pancreatic cancer: Improvements in our understanding of genetics and screening. Int J Biochem Cell Biol 2004; 36: 1386–1392. [DOI] [PubMed] [Google Scholar]
- 23.Ulrich CD and Consensus Committees of the European Registry of Hereditary Pancreatic Diseases. Midwest Multi-Center Pancreatic Study Group, et al. Pancreatic cancer in hereditary pancreatitis: Consensus guidelines for prevention, screening and treatment. Pancreatology 2001; 1: 416–422. [DOI] [PubMed] [Google Scholar]
- 24.Canto MI, Harinck F, Hruban RH, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013; 62: 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barone E, Corrado A, Gemignani F, et al. Environmental risk factors for pancreatic cancer: An update. Arch Toxicol 2016; 90: 2617–2642. [DOI] [PubMed] [Google Scholar]
- 26.Gerber DE, Laccetti AL, Xuan L, et al. Impact of prior cancer on eligibility for lung cancer clinical trials. J Natl Cancer Inst 2014, pp. 106–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laccetti AL, Pruitt SL, Xuan L, et al. Effect of prior cancer on outcomes in advanced lung cancer: Implications for clinical trial eligibility and accrual. J Natl Cancer Inst 2015, pp. 107–107. [DOI] [PMC free article] [PubMed] [Google Scholar]


